Abstract
Yeast cells undergo diploid-specific developments such as spore formation via meiosis and pseudohyphal development under certain nutrient-limited conditions. Studies on these aspects require homozygous diploid mutants, which are generally constructed by crossing strains of opposite mating-type with the same genetic mutation. So far, there has been no direct way to generate and select diploids from haploid cells. Here, we developed a method for efficient construction of homozygous diploids using a PGAL1-HO gene (galactose-inducible mating-type switch) and a PSTE18-URA3 gene (counter selection marker for diploids). Diploids are generated by transient induction of the HO endonuclease, which is followed by mating of part of the haploid population. Since the STE18 promoter is repressed in diploids, diploids carrying PSTE18-URA3 can be selected on 5-fluoroorotic acid (5-FOA) plates where the uracil prototrophic haploids cannot grow. To demonstrate that this method is useful for genetic studies, we screened suppressor mutations of the complex colony morphology, strong agar invasion and/or hyper-filamentous growth caused by lack of the Hog1 MAPK in the diploid Σ1278b strain background. Following this approach, we identified 49 suppressor mutations. Those include well-known positive regulator genes for filamentous growth signaling pathways, genes involved in mitochondrial function, DNA damage checkpoint, chromatin remodeling, and cell cycle, and also previously uncharacterized genes. Our results indicate that combinatorial use of the PGAL1-HO and PSTE18-URA3 genes is suitable to efficiently construct and select diploids and that this approach is useful for genetic studies especially when combined with large-scale screening.
Introduction
Over the last decades genetic studies using the budding yeast Saccharomyces cerevisiae have led to discovery of a variety of cellular signaling components as well as many other fundamental cellular processes. One of the advantages of yeast genetics is that it is straightforward to isolate desired mutant strains and identify the underlying mutations. In principle, such genetic approaches can be applied only in the haploid backgrounds because it is difficult to isolate recessive mutations in diploids due to complementation of the phenotype by the second copy of the gene. This becomes an issue when mutant strains defective in diploid-specific developments such as meiosis, sporulation, spore germination, bipolar budding pattern, and pseudohyphal development need to be isolated. Although a yeast homozygous knockout library of the S288C background is available [1], this genetic background has lost some of these specific phenotypes and hence those are commonly studied in other strain backgrounds. Therefore, a method for efficient construction of homozygous double mutants is required.
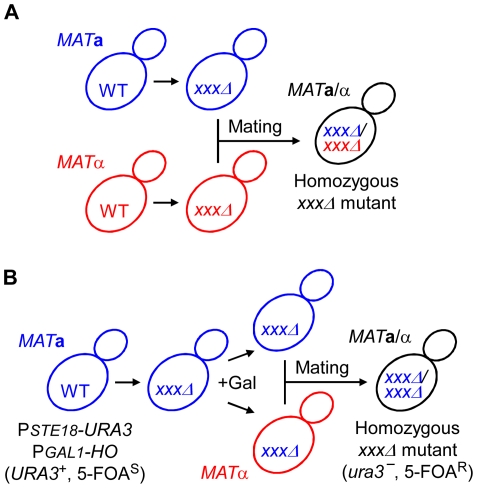
The yeast sexual cell types are designated a and α, which are conferred by the MAT a and MAT α alleles of the Mating-Type Locus (MAT), respectively [2]. In general, homozygous diploid mutant strains (i.e. MAT a / α xxxΔ/xxxΔ) are constructed by crossing strains of the opposite mating-type, which need to be constructed individually. When the two haploids have different prototrophic or antibiotic resistance markers, the diploids can be easily selected on plates lacking both nutrients or containing both antibiotics because auxotrophy or antibiotic sensitivity are complemented by each genotype (Figure 1A). The HO endonuclease, which mediates mating-type switch, can be used to obtain diploids via mating of MAT a and MAT α cells within colonies [3]. Alternatively, zygotes (dumbbell-shaped cells) can be isolated by micromanipulation during conjugation of two cells. However, these methods are unsuitable for large-scale analysis. Thus, there has been no easy way to construct and select diploid strains from single haploids at high throughput so far.
Figure 1. Strategy for construction of homozygous diploid strains.
(A) The traditional method requires individual construction of MAT a and MAT α haploid strains carrying different selection markers (linked to mutations or each strain). The diploid strains can be selected on plates lacking nutrients or containing antibiotics. (B) The new method proposed in this study generates homozygous diploids from a single haploid strain by subsequent use of the PGAL1-HO (galactose-inducible mating-type switch) and PSTE18-URA3 (counter selection marker for diploids) genes. The diploid strains are selected on plates containing 5-FOA, where non-mated haploid strains cannot grow.
Decreasing gene dosage by RNAi (restored by introducing Dicer and Argonaute from S. castellii) [4] or by haplo-insufficiency (heterozygous mutant) [5] may be useful for studying diploid-specific developments. Since these methods do not completely abolish gene function and consequently might give false negative or positive results, an efficient method to create homozygous deletion mutants is desired. In this study, we present an efficient method for construction of diploid strains using a galactose-inducible mating-type switch gene (PGAL1-HO) and a counter selection marker gene (PSTE18-URA3).
We applied our method to the study of yeast morphological developments. In the S. cerevisiae Σ1278b background, diploid cells develop pseudohyphae (filamentous growth) under nitrogen starvation. Since filamentous growth is essential for virulence of yeast pathogens such as Candida albicans [6], discovery of positive regulators for filamentous growth using S. cerevisiae as a model organism can contribute to understanding common conserved mechanisms. The high-osmolarity glycerol (HOG) response MAPK pathway, which plays a central role in osmoadaptation [7], [8], negatively regulates filamentous growth and deletion of the HOG1 MAPK gene leads to hyper-filamentous phenotype even under nutrient-rich conditions [9], [10]. In order to identify positive regulators essential for filamentous growth, we performed large-scale construction of homozygous double mutants in theΣ1278b hog1Δ/hog1Δ background. The screen identified 49 suppressor mutations, showing that our method is useful for genetic study.
Results and Discussion
Efficient Construction of Yeast Homozygous Diploid Strains
Our strategy for construction of homozygous diploid cells is shown in Figure 1B. As a host strain, we used either a MAT a or MAT α haploid strain carrying; i) PSTE18-URA3, which expresses the URA3 gene under the control of haploid specific STE18 (G-protein γ subunit) promoter [11] and ii) PGAL1-HO, which expresses the HO endonuclease under the control of GAL1 promoter (repressed by glucose and induced by galactose [12]). Once the host strain is transiently incubated on galactose plates (HO induction), the mating-type switch (MAT a→MAT α or MAT α→MAT a) occurs and consequently diploid cells are formed by mating of MAT a and MAT α cells within colonies. Following short induction times of HO (<12 hours), the colonies contain three cell types, MAT a, MAT α, and MAT a / α. Our strategy can select diploids by counter selection using 5-FOA, where haploids cannot grow because Ura3 (orotidine-5′-phosphate decarboxylase) converts 5-FOA into a toxic compound [13], while diploids are resistant to 5-FOA because URA3 is not expressed. Leaky expression of HO does not matter as long as the host strain is maintained on plates lacking uracil.
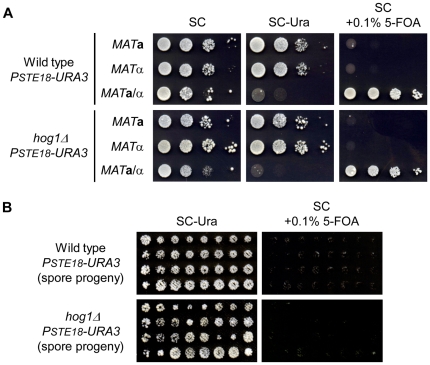
First, we investigated whether the PSTE18-URA3 gene allows selecting for diploid cells. We constructed wild-type PSTE18-URA3 and hog1Δ PSTE18-URA3 strains in the three cell types (MAT a, MAT α, and MAT a / α) and grew them on SC plates lacking uracil or containing 0.1% 5-FOA. As shown in Figure 2A, the haploid and diploid PSTE18-URA3 strains showed the expected phenotypes, i.e. the haploid strains were uracil prototrophic and 5-FOA sensitive, and the diploid strains were uracil auxotrophic and 5-FOA resistant. Moreover, the haploid cells that germinated from spore progeny of the diploid PSTE18-URA3 strains displayed uracil prototrophy and 5-FOA sensitivity (Figure 2B). Taken together, these results demonstrate that the PSTE18-URA3 gene can be used as a diploid selection marker.
Figure 2. Effect of the PSTE18-URA3 gene on growth of haploids and diploids.
(A) The haploid and diploid PSTE18-URA3 strains display opposite growth phenotypes on plates lacking uracil or containing 5-FOA. The strains were grown for 2–3 days at 30°C. (B) The growth phenotype of the diploid PSTE18-URA3 strain can revert to that of haploid after sporulation. The indicated diploid strains were sporulated, tetrads were dissected and spore progeny was grown on YPD plate for 3 days at 30°C. Then, the cells were replicated on the indicated plates and grown for 2–3 days at 30°C.
Next, we determined the efficiency of construction of diploids by our strategy. The wild-type PSTE18-URA3 and hog1Δ PSTE18-URA3 strains (MAT a and MAT α) carrying pJH283 (PGAL1-HO) were grown overnight on galactose plate, and then cells were restreaked on SC plate containing 0.1% 5-FOA. The single colonies obtained on the 5-FOA plate were analyzed by two methods: i) observation of pseudohyphal growth on SLAD plate, and ii) determination of mating type by PCR. All of the single colonies analyzed (10 colonies for each strain) showed diploid specific patterns: i) strongly enhanced pseudohyphal growth (data not shown), and ii) two PCR bands corresponding to diploids (Figure S1). These results indicate that our method is highly efficient for construction of diploid strains.
Screening Suppressor Mutations of Enhanced Morphological Developments of hog1Δ/hog1Δ
To demonstrate that our strategy for construction of diploid strains is useful for genetic studies of diploid-specific developments, we applied it to yeast filamentous growth in the Σ1278b background. Transposon insertion mediated-random mutagenesis and -systematic gene disruption have previously been employed to dissect the genetic bases of filamentous growth [14], [15]. However, these studies used haploid strain backgrounds in which filamentous growth was ectopically induced by an extra copy of the opposite mating-type locus and the PHD1 gene (transcriptional activator for filamentation) or by adding 1% butanol to the growth medium. In addition, however, homozygous diploid strains must be used to analyze the genetics of filamentous growth. We have recently reported that hyperosmotic stress inhibits all of the yeast morphological developments and that the Hog1 MAPK is a central negative regulator of these developments [10]. Moreover, the effect of HOG1 deletion is reflected in diploids more clearly than in haploids [16]. Therefore, suppressor mutations of the enhanced morphological developments of hog1Δ/hog1Δ are expected to lead to the identification of genes involved in controlling those phenotypes. In the present study, we screened suppressor mutations of complex colony morphology, strong invasive growth, and hyper-filamentous growth in the Σ1278b hog1Δ/hog1Δ background by constructing homozygous double mutants (i.e. xxx::mTn/xxx::mTn hog1Δ/hog1Δ).
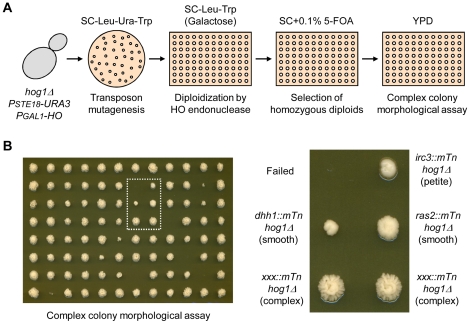
Following the strategy shown in Figure 3A, a transposon insertion mutagenesis was performed using the haploid hog1Δ PSTE18-URA3 strain carrying pJH283 (PGAL1-HO) as a host strain. Since the diploid hog1Δ/hog1Δ strain displays complex colony morphology even on YPD plates while the diploid wild-type strain does not [10], [16], mutant strains defective in formation of complex colony morphology were first screened by visual inspection (Figure 3B). From more than six thousand 5-FOA resistant strains (candidates for homozygous diploids) which were obtained at 93% success rate of randomly picked transformants, we isolated more than 100 mutant strains that showed smooth- or less complex-colony morphology. The diploid state in those was confirmed by PCR analysis of the mating-type locus. We amplified the transposon insertion regions of these strains by vectorette PCR and sequencing of the PCR products identified 49 unique genes (Table 1 and Table S1). Morphological developments (complex colony morphology, invasive growth, and filamentous growth) of all 49 homozygous double mutant strains on YPD plates were characterized, and the results are discussed below.
Figure 3. Screening suppressor mutations of the complex colony morphology or hyper-filamentous growth phenotype in the hog1Δ/hog1Δ backgrounds.
(A) Strategy for screening the suppressor mutations. Using the haploid hog1Δ PSTE18-URA3 strain carrying pJH283 (PGAL1-HO::TRP1) as a host strain, transposon insertion mutagenesis was performed and mutant strains defective in complex colony morphology were screened by visual inspection. The details are described in Materials and Methods. (B) One example of the screening results is shown. The candidates, smooth colony or less complex colony, were further analyzed: identification of the transposon insertion position, mating-type PCR, and morphological assay for invasive growth and filamentous growth.
Table 1. Identified mutations that suppress enhanced morphological developments of the homozygous hog1Δ/hog1Δγstrain.
| Gene | CCM | IG | FG | Description of gene product | Reference |
| ADE6 | – – | – – | – | Formylglycinamidine-ribonucleotide (FGAM)-synthetase | This study |
| AMN1 | – – | – | – | Protein required for daughter cell separation, multiple mitotic checkpoints, and chromosome stability | [15] |
| ATO2 | – – | – – | + | Putative transmembrane protein involved in export of ammonia | This study |
| CLN1 | – – | – | – – | G1 cyclin involved in regulation of the cell cycle | [16], [23] |
| DHH1 | – – | – – | – | Cytoplasmic DExD/H-box helicase | [24] |
| FLO8 | – – | – – | – – | Transcription factor required for flocculation, diploid filamentous growth, and haploid invasive growth | [15], [20] |
| GCN2 | – – | – | + | Protein kinase that phosphorylates the alpha-subunit of translation initiation factor eIF2 | [19] |
| HIR2 | – – | – | – | Subunit of the HIR complex | [15] |
| HIR3 | – – | – | – | Subunit of the HIR complex | This study |
| IES1 | – | + | – | Subunit of the INO80 chromatin remodeling complex | This study |
| IMP2′ | – | – | – | Transcriptional activator involved in maintenance of ion homeostasis and protection against DNA damage | This study |
| KRE11 | – | – | + | Subunit of the TRAPP II (transport protein particle) complex | This study |
| KSS1 | – – | – – | – – | Mitogen-activated protein kinase involved in filamentous growth and pheromone response | [21] |
| MSN1 | – | – – | – | Transcriptional activator involved in invertase expression and invasive growth/pseudohyphal differentiation | [15], [26] |
| MTC5 | – – | – – | – – | Subunit of the SEA (Seh1-associated) complex | This study |
| RAD1 | – – | – – | – | Single-stranded DNA endonuclease | This study |
| RAD24 | – | – | + | Checkpoint protein involved in the activation of the DNA damage and meiotic pachytene checkpoints | This study |
| RAS2 | – – | – – | – – | GTP-binding protein that regulates the nitrogen starvation response, sporulation, and filamentous growth | [15], [16] |
| RDI1 | – | + | – | Rho GDP dissociation inhibitor involved in the localization and regulation of Cdc42 | [22] |
| RIM9 | – – | – – | – – | Protein of unknown function involved in the proteolytic activation of Rim101p in response to alkaline pH | [15], [27] |
| SHE10 | – – | – | + | Putative glycosylphosphatidylinositol (GPI)-anchored protein of unknown function | This study |
| SIR3 | – – | – – | – | Silencing protein that interacts with Sir2p and Sir4p, and histone H3 and H4 tails | This study |
| SLP1 | – | – | – | Member of the SUN-like family of proteins | This study |
| SPT2 | – | – – | – | Protein involved in negative regulation of transcription | This study |
| STE7 | – – | – – | – – | MAPK kinase involved in pheromone response and pseudohyphal/invasive growth | [16], [21] |
| TCO89 | – | – | – | Subunit of TORC1, a complex that regulates growth in response to nutrient availability | This study |
| TEC1 | – – | – – | – – | Transcription factor required for haploid invasive and diploid pseudohyphal growth (TEA/ATTS family) | [14], [15], [16] |
| UBP6 | – | – | – | Ubiquitin-specific protease | This study |
| YDR306C | – – | – – | + | F-box protein of unknown function | This study |
| YHR177W | – – | – – | – – | Putative protein of unknown function | This study |
CCM: complex colony morphology, IG: invasive growth, FG: filamentous growth.
– –: severe defect, –: intermediate defect, +: similar to control (hog1Δ/hog1Δ).
Mitochondrial Function Is Essential for Complex Colony Morphology
Sixteen of the 49 strains displayed petite and smooth colony morphology (Figure S2) and were unable to grow on plates containing glycerol as a sole carbon source (data not shown). These phenotypes suggest impaired respiratory growth, and all of these mutations are indeed linked to mitochondrial functions (Table S1). We confirmed that a rho 0 mutant (lacking mitochondria DNA) in the hog1Δ/hog1Δ background also displays petite and smooth colony morphology (Figure S2). Moreover, we identified three additional genes encoding proteins related to mitochondrial functions, ILM1, SCO1, and SDH1 (Table S1). These results indicate that mitochondrial function is essential for complex colony morphology. Jin et al. have previously shown that mitochondrial dysfunction inhibits filamentous growth through the retrograde signaling pathway, which is a mitochondria-to-nucleus pathway transducing changes in mitochondrial function to specific adaptive changes in nuclear gene expression [15]. ILM1 is known to be required for both mitochondrial function and slowed DNA synthesis-induced filamentous growth [17]. However, all of the mitochondria-related mutants identified by our screen poorly suppressed hyper-filamentous growth and strong invasive growth of the hog1Δ/hog1Δ strain (data not shown), suggesting that the hyper-filamentous phenotype of HOG1 deletion involves multiple mechanisms that are not simply suppressed by mitochondrial dysfunction. Alonso-Monge et al. have recently reported that the Candida albicans hog1 mutant shows an enhanced basal respiratory rate compared to the wild-type strain and suggested a link between Hog1 and mitochondrial function [18]. Therefore, it would be interesting to investigate whether and how Hog1 activated by hyperosmotic stress inhibits mitochondrial function during filamentous growth.
Multiple Mechanisms Are Necessary for the Enhanced Morphological Developments of hog1Δ/hog1Δ
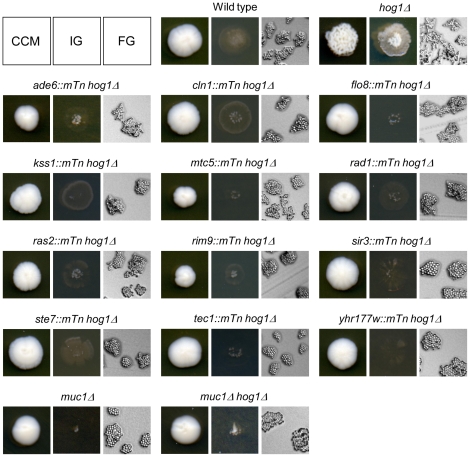
In addition to the mitochondria-related mutations, we identified 30 mutations that suppress at least two of the enhanced morphological developments of hog1Δ/hog1Δ (Table S1) and representative mutants are shown in Figure 4. Thirteen of the 30 genes have previously been reported to be involved in at least one of the three morphological developments in the S. cerevisiae Σ1278b background [14], [15], [16], [19], [20], [21], [22], [23], [24]. Those genes include the well-known STE7, KSS1, TEC1, RAS2, and FLO8 that regulate filamentous growth via the MAPK or cAMP-PKA signaling pathway. These two signaling pathways converge on the regulation of the MUC1 (also known as FLO11) gene [25], which encodes a GPI-anchored cell surface mucin required for morphological developments. DHH1 is involved in translational regulation of the Ste12 transcription factor which is regulated under the Kss1 MAPK pathway and essential for MUC1 expression [24]. GCN2 (general amino acid control system) and MSN1 (transcriptional activator) are involved in the regulation of MUC1 under certain nutrient conditions [19], [26]. Presumably, RIM9 is also important for the regulation of MUC1 through the pH-responsive Dfg16-Rim101 pathway [27]. Thus, our screen implies that impaired MUC1 expression is sufficient to suppress enhanced morphological developments of hog1Δ/hog1Δ. Indeed, deletion of the MUC1 gene in the hog1Δ/hog1Δ background lost the enhanced morphological developments and resulted in morphology similar to a muc1Δ/muc1Δ strain (Figure 4).
Figure 4. Morphological assay of homozygous double mutant strains which suppress enhanced morphological developments of hog1Δ/hog1Δ.
CCM: complex colony morphology, IG: invasive growth, FG: filamentous growth. All other suppressor mutants identified are shown in Table 1.
Seventeen of the 30 mutations occurred in genes not previously implicated in filamentous growth in S. cerevisiae. These gene products are involved in various cellular mechanisms, especially DNA damage checkpoint control (IMP2′, RAD1, and RAD24), gene expression via chromatin remodeling or histone-nucleosome assembly (HIR2, HIR3, IES1, SIR3, and SPT2), control of cell cycle or cell division (AMN1 and YHR177W), and other functions. Although the present study did not reveal the morphological suppressing mechanism by deletion of these genes or physical interactions between Hog1 and the identified targets, our screen could highlight the genetic networks that are required for the enhanced morphological developments independent of the filamentous growth signaling pathways. Since the active Hog1 (Hog1-D170A/F318S) strain inhibits all of the morphological developments in a hyperosmotic stress-independent manner [10], the mechanisms identified in our screen might be negatively regulated by Hog1. While crosstalk between the HOG and filamentous growth MAPK pathways is well established [28], the present mutant collection will enable further analyses of connections between the HOG pathway and other mechanisms. Such efforts can contribute to understanding mechanisms of filamentous growth common between model yeasts and pathogenic yeasts as well as devising novel antifungal targets.
In conclusion, we demonstrate that combinatorial use of the PGAL1-HO and PSTE18-URA3 genes is effective for construction of homozygous diploid strains and a useful tool when combined with large-scale screening. Genetic information of S. cerevisiae homozygous diploids obtained by the method proposed in this study may be useful for the studies of other organisms such as diploid Candida albicans in which construction of homozygous mutant strains is a difficult task.
Materials and Methods
Yeast Strains and Plasmids
The yeast strains used in this study are listed in Table 2. Standard yeast manipulations were performed as described previously [29]. To generate the PSTE18-URA3 strains, a PCR product of PSTE18-URA3 amplified from pPSTE18-URA3 (see below) using primers (AATGTGGCTGTGGTTTCAGGGTCCATAAAGCTTTTCAATTCATCTTTTTTCGTTGGCCGATTCATTCCCGAATTGGG and CATGCATTTAGAGCTCATACAGTTTTTTAGTTTTGC) was integrated into the ura3-52 locus. Correct integration was verified by PCR using the URA3 flanking primers (GGTGAAGGATAAGTTTTGACCATCAAAGAAGG and CGACCGAGATTCCCGGGTAATAACTG). To construct pPSTE18-URA3, a STE18 promoter region (from –630 to +6) was amplified by PCR using primers (ATGCTGGATGAAGCTTAGTGTGATCTGATGTTCC and GAGAGTTTTGGGATCCTGTCATTCTTAGAATTATTG), and inserted into the HindIII-BamHI sites of pPAQY2-URA3 [10], resulting in replacement of the AQY2 promoter by the STE18 promoter. To generate the homozygous PSTE18-URA3/PSTE18-URA3 strains, the haploid PSTE18-URA3 strains (MAT a and MAT α) were crossed, and then (hyper-)filamentous strains were isolated on synthetic low ammonia dextrose (SLAD; 2% glucose, 50 µM ammonium sulfate, 0.17% yeast nitrogen base without amino acids/ammonium sulfate, supplemented with amino acids to satisfy nutritional requirements) plate. To generate rho 0 mutant strains, the YSH2381 and YSH2386 strains were grown on YPD plate containing 10 µg/ml ethidium bromide for 3 days. The resulting respiratory deficiency was confirmed by complete lack of growth on YPGlycerol (1% yeast extract, 2% peptone and 2% glycerol) plate. pJH283 (original name pFH800 [30]; CEN, TRP1, PGAL1-HO) was used to induce mating type switch of haploids.
Table 2. Yeast strains used in this study.
| Strain | Genotype | Source |
| 10560-6B | MATα leu2::hisG trp1::hisG his3::hisG ura3-52 | Lab collection |
| 10560-4A | MATa leu2::hisG trp1::hisG his3::hisG ura3-52 | Lab collection |
| YSH1772 | MATα leu2::hisG trp1::hisG his3::hisG ura3-52 hog1::kanMX4 | [10] |
| YSH2049 | MATa leu2::hisG trp1::hisG his3::hisG ura3-52 hog1::kanMX4 | [10] |
| YSH2377 | MATα leu2::hisG trp1::hisG his3::hisG ura3-52::PSTE18-URA3 | This study |
| YSH2379 | MATa leu2::hisG trp1::hisG his3::hisG ura3-52::PSTE18-URA3 | This study |
| YSH2381 | MATa/α leu2::hisG/leu2::hisG trp1::hisG/trp1::hisG his3::hisG/his3::hisG ura3-52::PSTE18-URA3/ura3-52::PSTE18-URA3 | This study |
| YSH2382 | MATα leu2::hisG trp1::hisG his3::hisG ura3-52::PSTE18-URA3 hog1::kanMX | This study |
| YSH2384 | MATa leu2::hisG trp1::hisG his3::hisG ura3-52::PSTE18-URA3 hog1::kanMX | This study |
| YSH2386 | MATa/α leu2::hisG/leu2::hisG trp1::hisG/trp1::hisG his3::hisG/his3::hisG ura3-52::PSTE18-URA3/ura3-52::PSTE18-URA3 hog1::kanMX4/hog1::kanMX4 | This study |
| YSH2443 | MATa/α leu2::hisG/leu2::hisG trp1::hisG/trp1::hisG his3::hisG/his3::hisG ura3-52::PSTE18-URA3/ura3-52::PSTE18-URA3 rho 0 | This study |
| YSH2445 | MATa/α leu2::hisG/leu2::hisG trp1::hisG/trp1::hisG his3::hisG/his3::hisG ura3-52::PSTE18-URA3/ura3-52::PSTE18-URA3 hog1::kanMX4/hog1::kanMX4 rho 0 | This study |
| YSH2447 | MATa leu2::hisG trp1::hisG his3::hisG ura3-52::PSTE18-URA3 hog1::LEU2 | This study |
| YSH2449 | MATa/α leu2::hisG/leu2::hisG trp1::hisG/trp1::hisG his3::hisG/his3::hisG ura3-52::PSTE18-URA3/ura3-52::PSTE18-URA3 muc1::kanMX/muc1::kanMX | This study |
| YSH2450 | MATa/α leu2::hisG/leu2::hisG trp1::hisG/trp1::hisG his3::hisG/his3::hisG ura3-52::PSTE18-URA3/ura3-52::PSTE18-URA3 muc1::kanMX/muc1::kanMX hog1::LEU2/hog1::LEU2 | This study |
Transposon Insertion Mutagenesis
Mutant screening was performed using a genomic library mutagenized by random insertion of the transposon mTn-lacZ/LEU2 [31]. The genomic library was digested by NotI, and the resulting DNA fragments were transformed into the haploid hog1Δ PSTE18-URA3 strain carrying pJH283, and transformants were selected on synthetic complete (SC) plate lacking Leu, Ura, and Trp. The transformants were transferred on 1% galactose plates using a toothpick, and incubated overnight to induce diploidization. The cells were replicated on plates containing 0.1% 5-FOA using a 96-pin replicator (Singer RoToR) to select only diploids. Next, the 5-FOA resistant cells were replicated on YPD (1% yeast extract, 2% peptone and 2% glucose) plates and grown for 2 days at 30°C and for additional 5 days at room temperature. Suppressor mutant strains that showed no (smooth) or less complex colony morphology were screened by visual inspection.
Characterization of Suppressor Mutant Strains
To determine the sites of transposon insertion in the isolated mutants, vectorette PCR was performed following the manual of the Yale Genome Analysis Center (http://ygac.med.yale.edu/). The purified PCR products were sequenced by Eurofins MWG Operon (Germany). To confirm diploidization of the mutant strains, mating-type was determined by PCR using three primers (AGTCACATCAAGATCGTTTATGG, GCACGGAATATGGGACTACTTCG, and ACTCCACTTCAAGTAAGAGTTTG) as described previously [32]. For colony morphological assay, yeast cells were grown on YPD plates for 2 days at 30°C and for additional 5 days at room temperature. For invasive growth assay, yeast cells (obtained for colony morphological assay) were washed off under flowing water and rubbed with a wet finger to remove cells that did not invade the agar. For filamentous growth assay, yeast cells were streaked on YPD plates and grown for 16 hours at 30°C, and the cells were visualized by light microscopy. To verify that the observed phenotype is due to the transposon insertion, homozygous double mutant strains were created again by deleting genes with a kanMX marker in the YSH2447 background carrying pJH283.
Supporting Information
Confirmation of diploidized yeast strains by mating-type PCR. The mating-type PCR of MAT a, MAT α, and MAT a/α (diploid) cells provides 544-bp, 404-bp, and both PCR products, respectively. All of the 5-FOA resistant single colonies which were generated from the indicated haploid PSTE18-URA3 strains carrying pJH283 provided the diploid specific PCR pattern. M: 100-bp DNA ladder.
(TIF)
Identified mitochondria-related mutations that suppress complex colony morphology of the homozygous hog1Δ/hog1Δ strain. Cells were grown on YPD plates for 2 days at 30°C and for additional 5 days at room temperature. A rho 0 mutation in the hog1Δ/hog1Δ background resulted in the same phenotype as the identified mitochondria-related mutations.
(TIF)
Identified mitochondria-related mutations that suppress complex colony morphology of the homozygous hog1Δ/hog1Δ strain.
(DOC)
Acknowledgments
We are grateful to Michael Snyder (Yale University) for the mTn-lacZ/LEU2 transposon library and James E. Haber (Brandeis University) for pJH283. We also thank all members of the Hohmann laboratory, especially Peter Dahl for valuable discussions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Swedish Research Council, the Science Faculty at the University of Gothenburg Platform program, the European Commission funded projects FP6 CELLCOMPUT (Contract No. 043310) and FP7 UNICELLSYS (Contract No. 201142). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Giaever G, Chu AM, Ni L, Connelly C, Riles L, et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 2.Klar AJ. The yeast mating-type switching mechanism: a memoir. Genetics. 2010;186:443–449. doi: 10.1534/genetics.110.122531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herskowitz I, Jensen RE. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 1991;194:132–146. doi: 10.1016/0076-6879(91)94011-z. [DOI] [PubMed] [Google Scholar]
- 4.Drinnenberg IA, Weinberg DE, Xie KT, Mower JP, Wolfe KH, et al. RNAi in budding yeast. Science. 2009;326:544–550. doi: 10.1126/science.1176945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deutschbauer AM, Jaramillo DF, Proctor M, Kumm J, Hillenmeyer ME, et al. Mechanisms of haploinsufficiency revealed by genome-wide profiling in yeast. Genetics. 2005;169:1915–1925. doi: 10.1534/genetics.104.036871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, et al. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 7.Hohmann S, Krantz M, Nordlander B. Yeast osmoregulation. Methods Enzymol. 2007;428:29–45. doi: 10.1016/S0076-6879(07)28002-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen RE, Thorner J. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1773:1311–1340. doi: 10.1016/j.bbamcr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Rourke SM, Herskowitz I. The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev. 1998;12:2874–2886. doi: 10.1101/gad.12.18.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa K, Sidoux-Walter F, Hohmann S. Expression of the yeast aquaporin Aqy2 affects cell surface properties under the control of osmoregulatory and morphogenic signalling pathways. Mol Microbiol. 2009;74:1272–1286. doi: 10.1111/j.1365-2958.2009.06933.x. [DOI] [PubMed] [Google Scholar]
- 11.de Godoy LM, Olsen JV, Cox J, Nielsen ML, Hubner NC, et al. Comprehensive mass-spectrometry-based proteome quantification of haploid versus diploid yeast. Nature. 2008;455:1251–1254. doi: 10.1038/nature07341. [DOI] [PubMed] [Google Scholar]
- 12.Johnston M, Davis RW. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boeke JD, LaCroute F, Fink GR. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 14.Mösch HU, Fink GR. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics. 1997;145:671–684. doi: 10.1093/genetics/145.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin R, Dobry CJ, McCown PJ, Kumar A. Large-scale analysis of yeast filamentous growth by systematic gene disruption and overexpression. Mol Biol Cell. 2008;19:284–296. doi: 10.1091/mbc.E07-05-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granek JA, Magwene PM. Environmental and genetic determinants of colony morphology in yeast. PLoS Genet. 2010;6:e1000823. doi: 10.1371/journal.pgen.1000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang CM, Jiang YW. Genome-wide survey of non-essential genes required for slowed DNA synthesis-induced filamentous growth in yeast. Yeast. 2005;22:79–90. doi: 10.1002/yea.1195. [DOI] [PubMed] [Google Scholar]
- 18.Alonso-Monge R, Carvaihlo S, Nombela C, Rial E, Pla J. The Hog1 MAP kinase controls respiratory metabolism in the fungal pathogen Candida albicans. Microbiology. 2009;155:413–423. doi: 10.1099/mic.0.023309-0. [DOI] [PubMed] [Google Scholar]
- 19.Braus GH, Grundmann O, Brückner S, Mösch HU. Amino acid starvation and Gcn4p regulate adhesive growth and FLO11 gene expression in Saccharomyces cerevisiae. Mol Biol Cell. 2003;14:4272–4284. doi: 10.1091/mbc.E03-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Styles CA, Fink GR. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics. 1996;144:967–978. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts RL, Fink GR. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- 22.Tiedje C, Sakwa I, Just U, Höfken T. The Rho GDI Rdi1 regulates Rho GTPases by distinct mechanisms. Mol Biol Cell. 2008;19:2885–2896. doi: 10.1091/mbc.E07-11-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loeb JD, Kerentseva TA, Pan T, Sepulveda-Becerra M, Liu H. Saccharomyces cerevisiae G1 cyclins are differentially involved in invasive and pseudohyphal growth independent of the filamentation mitogen-activated protein kinase pathway. Genetics. 1999;153:1535–1546. doi: 10.1093/genetics/153.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park YU, Hur H, Ka M, Kim J. Identification of translational regulation target genes during filamentous growth in Saccharomyces cerevisiae: regulatory role of Caf20 and Dhh1. Eukaryot Cell. 2006;5:2120–2127. doi: 10.1128/EC.00121-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rupp S, Summers E, Lo HJ, Madhani H, Fink G. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 1999;18:1257–1269. doi: 10.1093/emboj/18.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagiano M, van Dyk D, Bauer FF, Lambrechts MG, Pretorius IS. Msn1p/Mss10p, Mss11p and Muc1p/Flo11p are part of a signal transduction pathway downstream of Mep2p regulating invasive growth and pseudohyphal differentiation in Saccharomyces cerevisiae. Mol Microbiol. 1999;31:103–116. doi: 10.1046/j.1365-2958.1999.01151.x. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Mitchell AP. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics. 1997;145:63–73. doi: 10.1093/genetics/145.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito H. Regulation of cross-talk in yeast MAPK signaling pathways. Curr Opin Microbiol. 2010;13:677–683. doi: 10.1016/j.mib.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Amberg DC, Burke DJ, Strathern JN. Cold Spring Harbor Laboratory Press; 2005. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. [Google Scholar]
- 30.Nickoloff JA, Singer JD, Hoekstra MF, Heffron F. Double-strand breaks stimulate alternative mechanisms of recombination repair. J Mol Biol. 1989;207:527–541. doi: 10.1016/0022-2836(89)90462-2. [DOI] [PubMed] [Google Scholar]
- 31.Burns N, Grimwade B, Ross-Macdonald PB, Choi EY, Finberg K, et al. Large-scale analysis of gene expression, protein localization, and gene disruption in Saccharomyces cerevisiae. Genes Dev. 1994;8:1087–1105. doi: 10.1101/gad.8.9.1087. [DOI] [PubMed] [Google Scholar]
- 32.Huxley C, Green ED, Dunham I. Rapid assessment of S. cerevisiae mating type by PCR. Trends Genet. 1990;6:236. doi: 10.1016/0168-9525(90)90190-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Confirmation of diploidized yeast strains by mating-type PCR. The mating-type PCR of MAT a, MAT α, and MAT a/α (diploid) cells provides 544-bp, 404-bp, and both PCR products, respectively. All of the 5-FOA resistant single colonies which were generated from the indicated haploid PSTE18-URA3 strains carrying pJH283 provided the diploid specific PCR pattern. M: 100-bp DNA ladder.
(TIF)
Identified mitochondria-related mutations that suppress complex colony morphology of the homozygous hog1Δ/hog1Δ strain. Cells were grown on YPD plates for 2 days at 30°C and for additional 5 days at room temperature. A rho 0 mutation in the hog1Δ/hog1Δ background resulted in the same phenotype as the identified mitochondria-related mutations.
(TIF)
Identified mitochondria-related mutations that suppress complex colony morphology of the homozygous hog1Δ/hog1Δ strain.
(DOC)