Abstract
Testosterone has been previously shown to enhance adult neurogenesis within the dentate gyrus of adult male rats, whereas social isolation has been shown to cause a decrease in adult neurogenesis under some conditions. The current study tested the combined effects of testosterone and social isolation upon adult neurogenesis using two experiments involving adult male rats. For both experiments, half of the subjects were pair-housed and half were housed individually for the duration of the experiments (34 days). For experiment 1, the subjects were divided into four groups (n=8/group): 1) sham/pair-housed, 2) sham/isolated, 3) castrate/pair-housed, and 4) castrate/isolated. Rats in the castrate groups were bilaterally castrated, and rats in the sham groups were sham castrated. For experiment 2, all rats were castrated and the effects of testosterone were tested using daily injections of testosterone propionate (0.500 mg/rat for 15 days) or the oil vehicle. Subjects were divided into four groups (n =8/group): 1) oil/pair-housed, 2) oil/isolated, 3) testosterone/pair-housed, and 4) testosterone/isolated. All rats were injected with 5-Bromo-2’-deoxyuridine (BrdU, 200 mg/kg body mass) and immunohistochemistry was used to determine levels of neurogenesis following a 16-day cell survival period. For experiment 1, castrated subjects had significantly fewer BrdU-labeled cells along the granule cell layer and sub-granular zone (GCL+SGZ) of the dentate gyrus than did intact subjects, and this effect was mainly due to low levels of neurogenesis in the castrate/isolated group. For experiment 2, social isolation caused a significant decrease in neurogenesis within the GCL+SGZ relative to the pair-housed groups. Testosterone injections did not buffer against this effect but instead tended to cause a decrease in neurogenesis. Thus, social isolation reduced hippocampal neurogenesis, but the effects of testosterone were inconsistent. This suggests that normal circulating levels of testosterone may buffer against the neurogenesis-impairing effects of isolation, whereas high doses of testosterone do not.
Keywords: adult neurogenesis, androgen, bromodeoxyuridine, hippocampus, social isolation, testosterone
INTRODUCTION
Neurogenesis occurs continuously in the mammalian forebrain throughout an adult’s life (Abrous et al., 2005; Kempermann, 2006). Adult neurogenesis involves proliferation, migration, and differentiation of new neurons within the adult brain. Among mammals, the subgranular zone of the dentate gyrus sub-region of the hippocampal formation is one of the primary sites of adult neurogenesis. Newly proliferated neurons from the subgranular zone migrate a short distance into the granule cell layer (GCL) of the dentate gyrus, where they extend functional axons into the CA3 region of the hippocampus (van Praag et al., 2002; Jessberger and Kempermann, 2003; Zhao et al., 2006). Young hippocampal neurons exhibit enhanced excitability, increased Ca2+ conductance, and a lower threshold for induction of long-term potentiation (LTP) than do mature granule cells (Schmidt-Hieber et al., 2004; Ambrogini et al., 2010). These attributes may make young neurons a particularly good substrate for memory formation. Therefore, adult neurogenesis may be a mechanistic link that allows a variety of endogenous and environmental factors to influence cognitive ability. Research on adult neurogenesis has provided insights regarding how memories are formed (Aimone et al., 2006; Kempermann, 2008; Deng et al., 2010) and has offered great promise for treatment of neurodegenerative diseases, including age-related dementia (Duman and Monteggia, 2006; Steiner et al., 2006) and chronic depression (Eisch et al., 2008; Perera et al., 2008).
Two components of adult neurogenesis are routinely measured: the number of newly proliferated cells produced, and the number of cells that survive to specific time points. Hippocampal cell proliferation levels are higher in female rodents than in males (Galea and McEwen, 1999), whereas survival of newly proliferated cells is higher among male rats than among females (Westenbroek et al., 2004; Dalla et al., 2009). Given these sex differences, it is not surprising that that steroidal sex hormones influence adult neurogenesis in both males and females (Galea et al., 2006). Estradiol, the primary female sex steroid, has been shown to increase cell proliferation and decrease cell survival among female rats, and these effects are dependent on dose and timing of exposure (Ormerod et al., 2003; Barker and Galea, 2008; Barha et al., 2009). A growing number of studies indicate that testosterone, the primary male sex steroid, influences adult neurogenesis among male rodents. Castrated male rats show reduced hippocampal neurogenesis compared to intact males due to decreased survival of new neurons (Spritzer and Galea, 2007; Wainwright et al., 2011). Injections of relatively high doses of testosterone (0.5 or 1.0 mg/rat) given to castrated male rats for 30 days caused an increase in neurogenesis compared to castrated males that were not given hormone replacement (Spritzer and Galea, 2007). In contrast, another study found no effect of testosterone implants upon neurogenesis (Buwalda et al., 2010), but the hormone replacement was given prior to BrdU injection making it impossible to determine whether testosterone might be influencing cell survival independently of any effects on cell proliferation. The effects of castration upon cell proliferation within the dentate gyrus of male rats have been inconsistent, with two studies indicating no effect of castration (Spritzer and Galea, 2007; Buwalda et al., 2010) and another study indicating that castration decreases cell proliferation (Wainwright et al., 2011). Among male mice, castration had no effect on cell proliferation but it did cause a decrease in the number of immature neurons in the dentate gyrus as indexed by the endogenous marker doublecortin (Benice and Raber, 2010). Another study with mice found that injections of testosterone during the cell proliferation stage of development had no effect on subsequent neurogenesis levels (Zhang et al., 2010). Among meadow voles (Microtus pennysylvanicus), reproductively (active males had higher levels of hippocampal neuron survival than did reproductively inactive individuals (Ormerod and Galea, 2003). This difference was likely due to differences in testosterone because androgen levels increase dramatically during the breeding season among voles. Contrasting the effects of testosterone on cell survival, multiple studies indicate that testosterone has no effect on cell proliferation among voles (Ormerod and Galea, 2003; Fowler et al., 2003). The primary metabolites of testosterone are estradiol, which binds estrogen receptors, and dihydrotestosterone (DHT), which binds androgen receptors. A few studies have tested how these hormones influence neurogenesis among male rodents. Rats injected with DHT showed an increase in neurogenesis comparable to that observed with testosterone, whereas estradiol injections had no effect on neurogenesis (Spritzer and Galea, 2007; Barker and Galea, 2008). Thus, the effects of testosterone on neurogenesis have been somewhat inconsistent across rodent species and studies, but testosterone generally seems to enhance neurogenesis through an androgen-dependent enhancement of cell survival. Additionally, most studies indicate that testosterone has no effect on cell proliferation in the dentate gyrus.
In contrast to the apparent neurogenesis-enhancing effects of testosterone, acute and chronic stress generally cause a decrease in adult neurogenesis (Mirescu and Gould, 2006), and evidence indicates that this effect is caused by elevated corticosterone levels associated with stress (Gould et al., 1992; Cameron and Gould, 1994; Brummelte and Galea, 2010). Social isolation is stressful for rats, inducing an increase in the stress hormone corticosterone (Ruis et al., 1999; Weiss et al., 2004). Among both male mice and male rats, social isolation for four weeks immediately after weaning caused reduced neurogenesis and impaired spatial memory relative to group-housed individuals (Lu et al., 2003; Ibi et al., 2008). There is currently not evidence for comparable effects of social isolation among adult rodents, but interactions between social isolation and other variables have been shown to influence adult neurogenesis. For example, Stranahan et al. (2006) found that exercise reduced neurogenesis among male rats that were socially isolated, whereas exercise enhanced neurogenesis among rats that were group housed. Similar effects were obtained with adult female rats (Leasure and Decker, 2009), but contradictory findings were obtained with mice (Kannangara et al., 2009). There is some evidence for sex differences in the effects of social isolation on adult neurogenesis. Socially isolated female Flinder’s Sensitive Line rats (a model of depression) showed an increase in neurogenesis relative to group-housed females (Bjornebekk et al., 2007). Chronic footshock stress decreased neurogenesis among socially isolated male rats, and group housing prevented this decrease (Westenbroek et al., 2004). In contrast, chronic stress increased neurogenesis among isolated female rats, and group housing prevented this increase (Westenbroek et al., 2004). This sex difference in response to social isolation suggests that sex steroids, such as testosterone, may influence the way in which social isolation affects neurogenesis. Thus, social isolation and testosterone may interact to influence hippocampal neurogenesis.
Some complex interactions occur between the hypothalamic-pituitary-adrenal (HPA) axis and the hypothalamic-pituitary-gonadal (HPG) axis. Specifically, testosterone inhibits arginine vasopressin synthesis in the hypothalamus, which reduces basal adrenocorticotropic hormone (ACTH) levels, which in turn causes reduced corticosterone (Viau, 2002). Increased corticosterone levels following a stressor suppress testosterone production by Leydig cells within the testes (Sapolsky et al., 2000; Hardy et al., 2002). Therefore, the neurogenesis-enhancing effects of testosterone and the neurogenesis-impairing effects of corticosterone may be partially explained by the effects these hormones have on one another.
The current study tested the hypothesis that elevated testosterone may prevent the neurogenesis-suppressing effects of stress. A previous study tested this hypothesis using repeated social defeat as the stressor, and testosterone implants given to intact male rats were shown to prevent the effects of stress on initial cell proliferation but testosterone had no effect on subsequent neurogenesis (Buwalda et al., 2010). Another recent study showed that castration and chronic mild stress had a synergistic effect that suppressed both cell proliferation and neurogenesis (Wainwright et al., 2011). Our experiments were distinct from these past studies in that we tested the effects of both castration and androgen replacement in order to produce clear differences in testosterone levels among groups and we used social isolation as the stressor. Specifically, experiment 1 compared castrated to intact males, and experiment 2 compared castrated males to castrated males that received testosterone replacement. We used social isolation as our stressor for the reasons described above: 1) social isolation has been previously shown to interact with other variables (i.e., exercise) to influence neurogenesis, and 2) sex differences in the effects of social isolation suggest possible interactions with testosterone.
EXPERIMENTAL PROCEDURES
Subjects
Adult male Sprague Dawley rats (approximately 55 days old) were obtained from Charles River Laboratory (St. Corustant, Quebec, Canada). All subjects were housed in standard polypropylene cages (21 × 42 × 21 cm) with Tek-Fresh Bedding (Harlan Laboratories, Indianapolis, IN) and free access to water and rodent chow (Harlan Teklad Diet #7012). The housing room was temperature controlled (21 ± 1 °C) with a 12:12 h light/dark cycle (lights on at 0800 h). Immediately upon arrival, half of the animals were pair-housed and half housed individually. All animal procedures were approved by the Middlebury College Institutional Animal Care and Use Committee and were carried out in accordance with ethical guidelines set by the National Institutes of Health.
Surgery
For each experiment, surgeries were conducted 7–8 days after the animals arrived in the facility, with half the surgeries conducted each day (Fig. 1). Aseptic technique was used throughout and isoflurane was used as the anesthesia (3.5–4.0% in oxygen during induction, 2.0–2.5% in oxygen during maintenance). The analgesic Ketofen was administered just prior to starting surgery (5 mg/kg body mass, s.c.), and the topical analgesic Fougera (2.5% lidocaine, 2.5% prilocaine) was applied to the incision site immediately after surgery. For castrations, each testis was excised through a small incision at the posterior end of the scrotum and ligated with chromic gut suture material (Ethicon, Somerville, NJ, USA). The muscular sheath was closed with chromic gut sutures, and the skin layer was closed with ethilon sutures (Ethicon). Sham castrations involved incisions into the skin and muscle layers, which were then sutured without removing the testes. Pair-housed rats were separated for 24 h after surgery. Animals were checked daily for one week following surgery to ensure proper recovery. Seven days after surgery, the average body mass of the subjects was 335.2 ± 2.6 g, and there were no significant differences in the mass of the rats used for the two experiments (P=0.56).
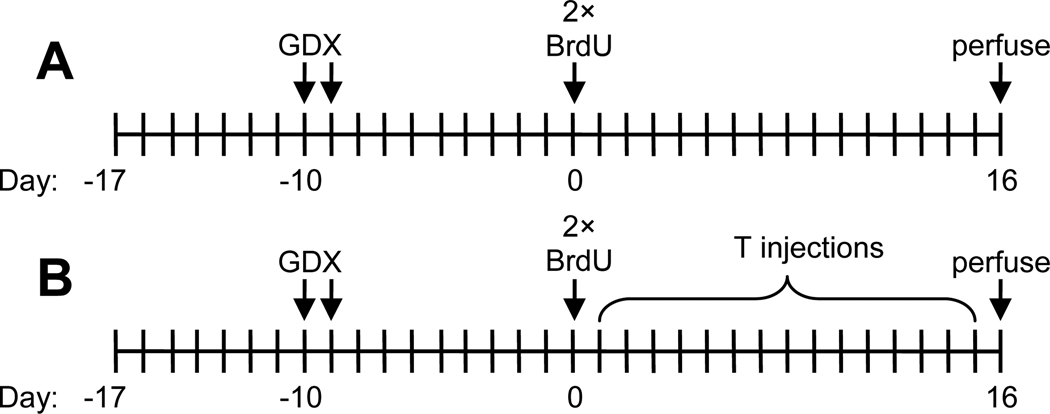
Fig. 1.
Timeline for experiment 1 (A) and experiment 2 (B). Pair housing or social isolation began when animals arrived in the facility 7 days prior to castration (GDX) surgeries. Two injections of BrdU occurred 12 h apart on the same day, which was 9–10 days after castration surgeries. For both experiments, all rats were perfused and brains extracted 16 days after BrdU injections. For experiment 2, some rats received 15 days of testosterone (T) injections.
Procedure
To habituate animals to the researchers, all animals were handled for 5 min/day for four days prior to any further experimental manipulation. Nine or ten days after surgery, all rats received two i.p. injections of 5-Bromo-2’-deoxyuridine (BrdU, 200 mg/kg body mass) spaced 12 h apart. BrdU (Sigma-Aldrich, St. Louis, MO, USA) was dissolved to 20 mg/ml in warm, sterile 0.9% saline containing 0.7% NaOH and filtered using a 0.22 µm syringe filter. BrdU is a thymidine analog that is incorporated into dividing cells during the S-phase, thereby acting as a marker of cells that were actively proliferating at the time of BrdU injection. The dose and frequency of BrdU injections vary widely among neurogenesis studies, and we chose to use a double injection of a relatively high dose of BrdU in order to label a large population of cells that were all dividing on the same day. We wanted to label enough cells to detect the potentially subtle effects of pair-housing upon neurogenesis. BrdU is taken up by proliferating cells in the dentate gyrus of rats during the 8–9 h of S-phase, and the entire cell cycle is approximately 25 h (Cameron and McKay, 2001). Injections were spaced 12 h apart to avoid re-labeling the same cells while at the same time labeling the majority of cells that were actively dividing during a 24 h period. An identical injection protocol has been used in past studies of neurogenesis in rats (McDonald and Wojtowicz, 2005; Snyder et al., 2005), and doses as high as 300 mg/kg are non-toxic (Cameron and McKay, 2001).
Two experiments were conducted to test the effects of testosterone and social isolation on adult neurogenesis. For both of the experiments, half of the subjects were pair-housed and half were housed individually (i.e., isolated) throughout the experiments. Beginning the day that the animals arrived in our facility, the subjects remained in their respective housing condition for a total of 34 days (Fig. 1). For experiment 1, the subjects were divided into four groups (n=8/group): 1) sham/pair-housed, 2) sham/isolated, 3) castrate/pair-housed, and 4) castrate/isolated. Rats in the castrate groups were bilaterally castrated, and rats in the sham groups were sham castrated as described above. Experiment 2 was similar to experiment 1 except that all rats were castrated and the effects of testosterone were tested using injections. The subjects used for experiment 2 were divided into four groups (n =8/group): 1) oil/pair-housed, 2) oil/isolated, 3) testosterone/pair-housed, and 4) testosterone/isolated. The testosterone groups received 15 daily s.c. injections of 0.500 mg of testosterone propionate dissolved in 0.1 ml sesame oil beginning the day after BrdU injections. This timeline allowed us to test the effects of testosterone upon primarily the survival of newly proliferated cells rather than on the process of cell proliferation itself. As a control for the effects of injection, the oil groups received daily s.c. injections of 0.1 ml sesame oil during the same time period. The testosterone dose used was previously shown to enhance adult neurogenesis when rats were given 30 days of injections (Spritzer and Galea, 2007). We chose to use the 15-day time period because this is a duration that had been previously used to test the effects of estradiol on neurogenesis in male rodents (Barker and Galea, 2008; Ormerod et al., 2004), and by this point approximately 75% of BrdU-labeled cells in the rat hippocampus express mature neuronal markers allowing quantification of neurogenesis (Snyder et al., 2009).
Sixteen days after the BrdU injections, rats were euthanized using a lethal dose of Nembutal (sodium pentabartibol, 150 mg/kg) and perfused transcardially with 60 ml of 0.9% saline followed by 120 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were extracted and post-fixed with 4% paraformaldehyde at 4°C overnight, then transferred to 30% sucrose in 0.1 M TBS (0.08 M Tris-HCL, 0.02 M Tris-base, 0.9% saline, pH 7.4) for cryoprotection and kept at 4°C until sectioning. Using a vibrating blade microtome (Vibratome 3000, St. Louis, MO), each brain was sliced in a bath of 0.1 M TBS into 40-µm-thick coronal sections through the entire rostro-caudal extent of the hippocampus. Tissue was collected and stored in an antifreeze solution (0.05 M TBS, 30% ethylene glycol and 20% glycerol) at −20°C until immunohistochemical processing.
Immunohistochemistry
Peroxidase immunohistochemistry was performed on free-floating tissue in a series of every 10th section (i.e., 400 µm intervals) through the rostro-caudal extent of the hippocampus to visualize BrdU-labeled cells. Sections were rinsed in 0.1 M TBS (pH 7.4) three times for 10 min between steps unless otherwise noted. Tissue was initially incubated for 30 min in 0.6% H2O2 to eliminate endogenous peroxidase activity. DNA was denatured by applying 2 N HCl for 30 min at 37°C. This step was immediately followed by a 10 min incubation in 0.1 M borate buffer (pH 8.5) to neutralize the acid. Tissue was next blocked for 30 min in a solution of 0.1 M TBS, 0.1% Triton-X 100, and 3.0% normal horse serum (Vector Laboratories, Burlingame, CA, USA), followed by a 16 h incubation at 4°C on a platform shaker in mouse monoclonal antibody against BrdU (Roche Diagnostics, Indianapolis, IN, USA) at a concentration of 1:400 in blocking solution. Sections were then incubated in horse-anti-mouse secondary antibodies (1:100 in 0.1 M TBS; Vector Laboratories) for 4 h, followed by a 1.5 h incubation in avidin-biotin horseradish peroxidase solution (ABC Elite Kit; 1:50; Vector Laboratories). Sections were reacted for 3–5 min in a solution of 3,3’diaminobenzidine (0.5 mg/ml; Sigma-Aldrich, Atlanta, GA) and 0.003% H2O2 in 0.1 M TBS. The sections were mounted onto Superfrost/Plus microscope slides (Fisher Scientific, Suwanee, GA, USA) and dried overnight. Finally, slides were counterstained with cresyl violet acetate, dehydrated with ethanol, cleared with xylene, and coverslipped using Permount (Sigma-Aldrich).
Immunofluorescent double-labeling was performed on free-floating tissue (n=3 brains/group) to determine the percentage of BrdU-labeled cells that were mature neurons. For each brain, one series of every 10th section was labeled for expression of BrdU and neuronal nuclei (NeuN), which is a maker of mature neurons (Mullen et al., 1992). Sections were rinsed in 0.1 M TBS (pH 7.4) three times for 10 min between steps unless otherwise noted. As for peroxidase labeling, DNA was denatured by incubating sections in 2 N HCl for 30 min at 37°C, followed immediately by 10 min in 0.1 M borate buffer. Tissue was next blocked for 30 min in a solution of 0.1M TBS, 0.1% Triton-X 100, and 3.0% normal goat serum (Sigma-Aldrich), followed by a 16 h incubation at 4°C on a platform shaker in primary antibodies: rat anti-BrdU monoclonal antibody (1:200, AbD Serotec, Raleigh, NC, USA) and mouse anti-NeuN monoclonal antibody (1:200, Millipore, Temecula, CA, USA) in blocking solution. Sections were blocked again for 30 min, followed by 16 h incubation at 4°C on a platform shaker in secondary antibodies: Alexa Fluor488 goat anti-mouse IgG (1:200, Invitrogen, Eugene, OR) and Alexa Fluor568 goat anti-rat IgG (1:200, Invitrogen) in blocking solution. Sections were mounted on Superfrost/Plus slides, coverslipped with the anti-fading agent diazobicyclooctane (0.1 M TBS, 2.5% DABCO, 10% polyvinyl alcohol and 20% glycerol) and stored at −20°C.
Microscopy
All BrdU-labeled cells in the dentate gyrus were counted by an experimenter blind to the subjects’ group assignments. For experiment 1, one subject in the castrate/pair-housed group had no visible BrdU-labeled cells and was dropped from further analysis. Every 10th section was counted through the entire rostro-caudal extent of the dentate gyrus (10–12 sections per brain) at 1000× magnification using a light microscope (Zeiss Axio Imager D1). Labeled cells in the uppermost focal plane were excluded to avoid oversampling (i.e., optical dissector method). Cells observed within 20 µm of the inner edge of the GCL were considered the subgranular zone (SGZ), and these cell counts were combined with the GCL counts. Cells were considered labeled if they exhibited a dark brown punctate stain. Labeled cells were counted in the hilus and compared to counts in the GCL+SGZ to determine whether any experimentally induced effects influenced cell division in the brain more broadly rather than being specific to the neurogenic niche along the SGZ. Progeny from progenitor cells in the hilus give rise to a population of ectopic cells that are morphologically and physiologically distinct from the granule cells produced along the SGZ (McCloskey et al., 2006; Scharfman et al., 2007).
Total numbers of labeled cells per brain was estimated by multiplying the number of cells counted by ten (i.e., inverse of the sampling ratio). Digital images were made of all sections at 25× magnification using a light microscope (Axio Imager D1, Zeiss, Germany) and imaging software (Axiovision, ver. 4.6; Zeiss). Areas of the GCL+SGZ and hilus were measured using the program ImageJ (ver. 1.42; National Institutes of Health, Bethesda, MD, USA). Volumes (mm3) of the GCL+SGZ and hilus were estimated using Cavalieri’s principle (Gundersen et al., 1988): the sum of the areas (mm2) was multiplied by the distance between sections (0.4 mm).
The percentage of BrdU-labeled cells that co-expressed NeuN was assessed for 50 randomly selected BrdU-labeled cells within the GCL+SGZ of each brain (n=3/group). Eight to ten sections were sampled for each brain, such that cells were sampled evenly across dorsal and ventral sections. BrdU-labeled cells were visualized and photographed at 630× magnification using a confocal microscope (Zeiss, LSM 510 META) and associated software (Zeiss LSM Image Browser, ver. 4.2.0.121). Images were collected in sequential scanning mode to prevent cross-bleeding between detection channels. Confocal z-stacks were collected at 1 µm intervals, and cells were considered double-labeled if: 1) co-expression was observed throughout the z-stack, and 2) the NeuN label was intense relative to background staining intensity. Staining intensity was determined using the program ImageJ (ver. 1.42).
Testosterone radioimmunoassay
Total serum testosterone was measured for each subject to confirm the elimination of testosterone in castrated and oil-injected animals and to determine the effects of testosterone injections on circulating testosterone levels. Approximately 3 ml of blood were collected on ice from the chest cavity of each subject at the time of perfusion. Samples were stored at 4°C overnight to coagulate, and centrifuged at 9.3 g for 15 min. Serum was extracted and stored at −20°C until running assays. All samples were assayed in duplicate using coated-tube radioimmunoassay (RIA) kits (Diagnostic Systems Laboratories, Webster, TX, USA). The testosterone antibody had some cross-reactivity with dihydrotestosterone (5.8%), 11-oxotestosterone (4.2%), and androstenedione (2.3%), and other androgens (< 1.0%), but had no detectable cross-reactivity with progesterone, estrogens, or glucocorticoids. The lower limit of detection for the kits was 0.08 ng/ml, and any samples with values below this limit were given a value of 0 ng/ml for data analyses. Based on our data, the intra-assay coefficient of variation was 9.6% and the inter-assay coefficient of variation was 6.5%.
Statistical analysis
The estimated total number of BrdU-labeled cells and volumes of brain regions were analyzed using univariate ANOVA with housing (i.e., pair-housed or isolated) and condition as fixed factors. Condition varied between experiments: sham vs. castrate for experiment 1 and oil vs. testosterone injections for experiment 2. Cell counts and volumes were analyzed separately for the GCL+SGZ and the hilus. We hypothesized that the combined effect of castration and social isolation would reduce neurogenesis within the GCL+SGZ below that observed in any of the other groups. Therefore, planned contrasts were used to compare the number of BrdU-labeled cells in the castrate/isolated group (experiment 1) or oil/isolated group (experiment 2) to that observed in each of the other groups. Serum testosterone levels of isolated and pair-housed rats were compared for each experiment using independent-samples t-tests. Linear regression was used to analyze relationships between serum testosterone levels and the total number of BrdU-labeled cells in the GCL+SGZ. SPSS (ver. 16.0; Chicago, IL, USA) was used for all analyses, and the significance level was set at α = 0.05 for all tests.
RESULTS
Adult neurogenesis
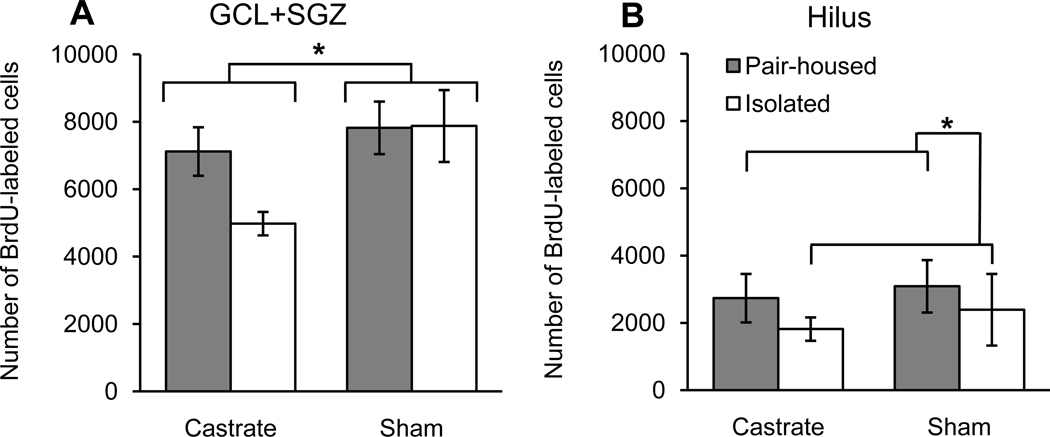
For experiment 1, castrated rats had significantly fewer BrdU-labeled cells in the GCL+SGZ than did the sham-castrated rats (Fig. 2A; F1,27=5.35, P=0.029). There was no significant main effect of housing or a housing × condition interaction for number of BrdU-labeled cells in the GCL+SGZ (both P>0.16). Planned contrasts revealed that the castrate/isolated group had significantly fewer BrdU-labeled cells in the GCL+SGZ than did either the sham/pair-housed group (P=0.014) or the sham/isolated group (P=0.012), but the difference between the castrate/isolated group and the castrate/pair-housed group was not quite significant (P=0.065). Isolated rats had significantly fewer BrdU-labeled cells in the hilus than did pair-housed rats (Fig. 2B; F1,27=4.33, P=0.047). There was no significant main effect of condition or a housing × condition interaction for number of BrdU-labeled cells in the hilus (both P>0.24).
Fig. 2.
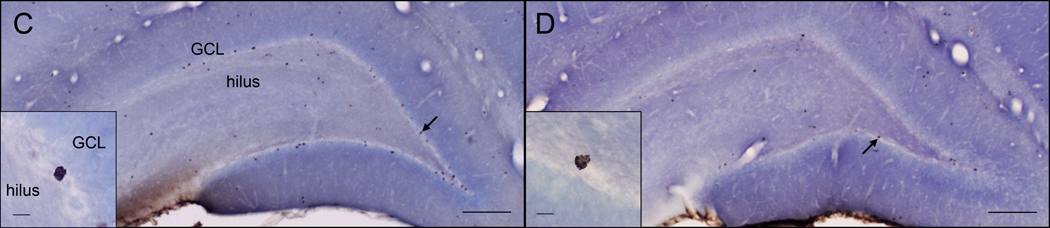
Total number of BrdU-labeled cells (mean ± SEM) in the dentate gyrus among the four groups of rats (n=7–8/group). Within the GCL+SGZ (A), rats in the sham group had more BrdU-labeled cells than did castrated rats (*P<0.05), while there was no significant effect of housing. Within the hilus (B), pair-housed males had significantly more BrdU-labeled cells than did isolated males (*P<0.05), while there was no significant effect of condition. Photomicrographs of representative sections show dark brown BrdU-labeled cells mainly along the subgranular zone (SGZ) between the granule cell layer (GCL) and the hilus. Sections are shown for a rat from the sham/pair-housed group (C) and for a rat from the castrate/isolated group (D). The main images were taken at 40× magnification (scale bars = 200 µm). The inserts show BrdU-labeled cells at 1000× magnification (scale bars = 10 µm) with their positions indicated by arrows on the main images.
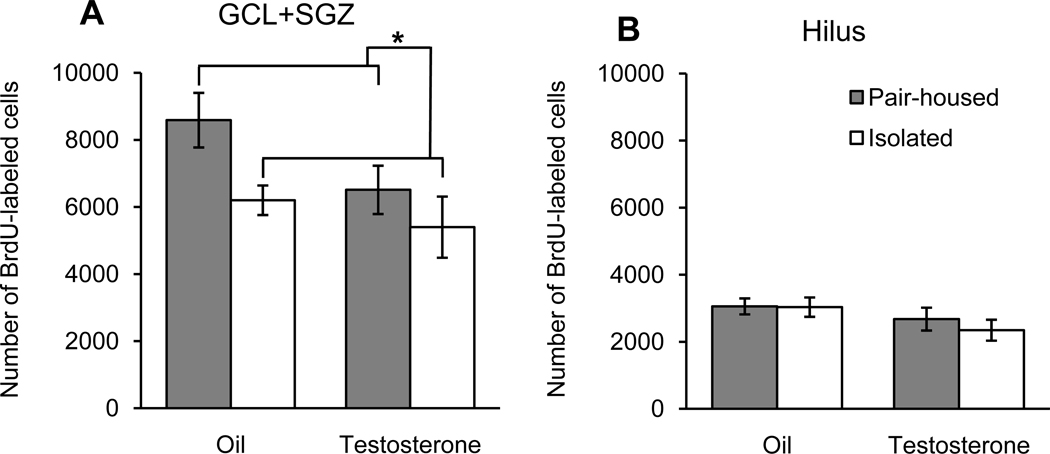
For experiment 2, isolated rats had significantly fewer BrdU-labeled cells in the GCL+SGZ than did pair-housed rats (Fig 3; F1,28=5.56, P=0.026). Testosterone-injected rats had fewer BrdU-labeled cells in the dentate gyrus than did oil-injected controls, but the main effect of condition did not quite reach statistical significance (F1,28=3.76, P=0.063). The housing × condition interaction was not significant (P=0.40). Planned contrasts revealed that the oil/isolated group had significantly fewer BrdU-labeled cells in the GCL+SGZ than did the oil/pair-housed group (P=0.031), but the oil/isolated group was not significantly different from the two groups that received testosterone injections (both P>0.45). Housing and condition had no significant effects on the number for BrdU-labeled cells in the hilus (both P>0.08), and the housing × condition interaction was not significant (P=0.61).
Fig. 3.
Total number of BrdU-labeled cells (mean ± SEM) in the dentate gyrus among the four groups of rats (n=8/group). Pair-housed rats had more BrdU-labeled cells than did isolated rats in the GCL+SGZ (A; *P<0.05) but not in the hilus (B). Daily injections with testosterone (0.5 mg/rat) had no statistically significant effects on the number of BrdU-labeled cells in the GCL+SGZ or the hilus.
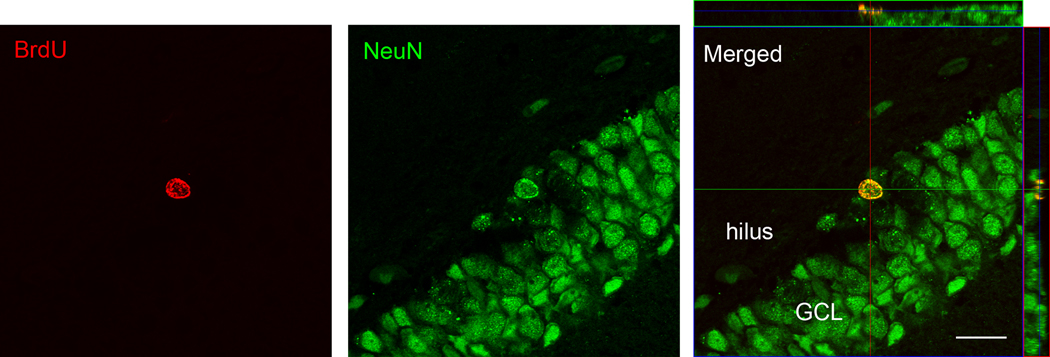
For both experiments, housing and condition had no significant main effects or interaction effect on the volume of the GCL+SGZ or the hilus (Table 1; all P≥0.11). For experiment 1, 70–88% of BrdU-labeled cells within the GCL+SGZ were co-labeled with NeuN (Table 2; Fig. 4), and housing and condition had no significant main effects or interaction effect on the percentage of double-labeled cells (all P≥0.19). For experiment 2, 66–86% of BrdU-labeled cells within the GCL+SGZ were co-labeled with NeuN (Table 2), and isolated rats had a significantly higher percentage of cells developing into neurons than did pair-housed rats (F1,8=18.67, P=0.003). There was no significant housing × condition interaction (P=0.77) or main effect of condition (P=0.10).
Table 1.
Volumes (mean ± SEM) of the combined granule cell layer and subgranular zone (GCL+SGZ) and hilus for each group of rats from both experiments.
| Experiment | Group | n | GCL+SGZ (mm3) | hilus (mm3) |
|---|---|---|---|---|
| 1 | Castrate/pair-housed | 7 | 2.52 ± 0.14 | 6.00 ± 0.43 |
| Castrate/isolated | 8 | 2.45 ± 0.13 | 5.42 ± 0.29 | |
| Sham/pair-housed | 8 | 2.57 ± 0.15 | 6.14 ± 0.28 | |
| Sham/isolated | 8 | 2.32 ± 0.18 | 5.52 ± 0.49 | |
| 2 | Oil/pair-housed | 8 | 2.46 ± 0.18 | 4.48 ± 0.36 |
| Oil/isolated | 8 | 2.37 ± 0.13 | 4.32 ± 0.17 | |
| Testosterone/pair-housed | 8 | 2.23 ± 0.17 | 3.97 ± 0.42 | |
| Testosterone/isolated | 8 | 2.11 ± 0.17 | 3.79 ± 0.24 |
Table 2.
Percentage (mean ± SEM) of BrdU-labeled cells in the GCL+SGZ that were co-labeled with NeuN for both experiments (n=3/group).
| Experiment | Group | Percent cells co-labeled |
|---|---|---|
| 1 | Castrate/pair-housed | 83.3 ± 1.8 |
| Castrate/isolated | 75.0 ± 3.0 | |
| Sham/pair-housed | 79.3 ± 5.2 | |
| Sham/isolated | 78.0 ± 1.2 | |
| 2 | Oil/pair-housed | 69.3 ± 1.3 |
| Oil/isolated | 78.5 ± 1.7* | |
| Testosterone/pair-housed | 72.7 ± 3.5 | |
| Testosterone/isolated | 82.7 ± 1.8* |
For Experiment 2, socially isolated rats had a significantly higher percentage of co-labeled cells than did pair-housed rats (P=0.003).
Fig. 4.
Confocal images (630× magnification) of a representative cell within the granule cell layer (GCL) labeled with BrdU and NeuN. The image at the right includes orthogonal planes to show that the cell was double labeled throughout (scale bar = 20 µm).
Testosterone levels
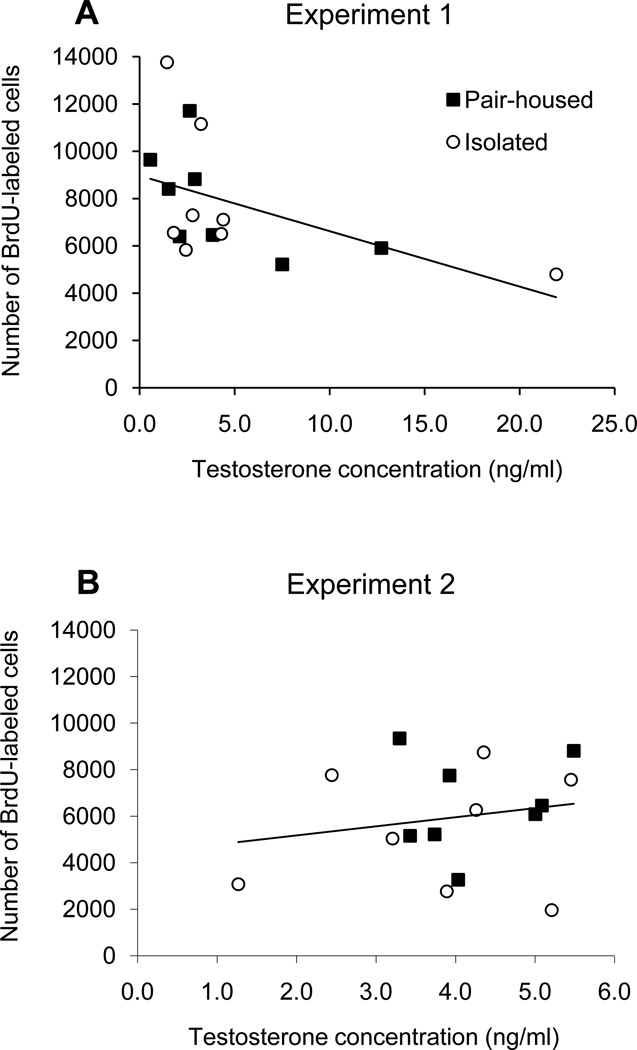
All blood samples from castrated rats (experiment 1) and castrated rats injected with oil (experiment 2) had serum testosterone levels below the limit of detection (< 0.08 ng/ml). For both experiments, there was no significant difference in serum testosterone levels between pair-housed and socially isolated groups (Table 3; both P>0.40). Considering only the sham-castrated males used in experiment 1, there was a significant negative relationship between serum testosterone concentration and the total number of BrdU-labeled cells in the GCL+SGZ (Fig. 5A; F1,14=4.61, r2=0.25, P=0.050). However, this relationship was non-linear (Fig. 5A), and a log transformation of testosterone concentrations was used to establish linearity. This transformation resulted in a stronger negative relationship (F1,14=6.41, r2=0.31, P=0.024). Considering only the males that were injected with testosterone in experiment 2, there was no significant relationship between serum testosterone levels and total number of BrdU-labeled cells in the GCL+SGZ (Fig. 5B; F1,15=0.54, r2=0.037, P=0.48). Note that experiment 2 involved a much narrower range of serum testosterone concentrations than did experiment 1 (Fig. 5).
Table 3.
Testosterone concentrations (mean ± SEM) in serum collected at the time of perfusion (n=8/group).
| Experiment | Group | Serum testosterone (ng/ml) |
|---|---|---|
| 1 | Castrate/pair-housed | 0.00 ± 0.00a |
| Castrate/isolated | 0.00 ± 0.00a | |
| Sham/pair-housed | 4.22 ± 1.42 | |
| Sham/isolated | 5.28 ± 2.41 | |
| 2 | Oil/pair-housed | 0.00 ± 0.00a |
| Oil/isolated | 0.00 ± 0.00a | |
| Testosterone/pair-housed | 4.25 ± 0.30 | |
| Testosterone/isolated | 3.76 ± 0.50 |
All samples below the detection limit of the assay (0.08 ng/ml) were assigned a value of 0 ng/ml.
Fig. 5.
Linear regressions of the total number of BrdU-labeled cells in the GCL+SGZ against serum testosterone levels from blood collected at the time of perfusion from rats that were either socially isolated or pair-housed throughout the experiments. For experiment 1 (A), there was a significant negative relationship (r2=0.25, P=0.050), whereas for experiment 2 (B) there was no significant relationship (r2=0.037, P=0.48). Note that there was a much wider range of testosterone levels observed in experiment 1 than in experiment 2, likely because experiment 1 involved intact males while experiment 2 involved testosterone injections given to castrated males.
DISCUSSION
Social isolation is a mild stressor that has been shown to cause a decrease in neurogenesis within the dentate gyrus (Ibi et al., 2008; Stranahan et al., 2006), whereas testosterone has been shown to enhance adult neurogenesis (Spritzer and Galea, 2007; Wainwright et al., 2011; Benice and Raber, 2010). We therefore predicted that testosterone would prevent the neurogenesis-suppressing effects of social isolation. The results of our two experiments only partially support this hypothesis, and indicate mainly independent effects of testosterone and social isolation on neurogenesis. Experiment 1 showed that castration caused a significant reduction in neurogenesis, with the lowest level of neurogenesis occurring in the castrate/isolated group. Experiment 2 produced somewhat contradictory results: isolated rats had significantly less neurogenesis than did pair-housed individuals, and there was a trend for testosterone-injected males to have less neurogenesis than oil-injected controls. In combination, the results suggest that social isolation reduces neurogenesis and testosterone can either enhance or possibly reduce neurogenesis depending on the dose.
Effects of testosterone on adult neurogenesis
The difference in the effects of testosterone on neurogenesis observed in our two experiments may be explained by the different ways in which testosterone was manipulated: for experiment 1 castrated rats were compared to intact rats, whereas for experiment 2 all rats were castrated and some were given daily injections of testosterone (0.5 mg/rat). Unlike the sham-castrated males used in experiment 1, the testosterone-injected males used in experiment 2 probably experienced supraphysiological surges in testosterone each day. Among the testosterone-injected subjects, our testosterone assay indicated serum testosterone levels that were relatively high but within the normal physiological range. However, blood samples were collected approximately 24 h after the last testosterone injections. In another study using the same injection dose (0.5 mg/rat) and a similar duration (14 days of injections), we observed much higher testosterone levels (about 12 ng/ml) in serum samples that were collected 6 h after injection (Spritzer et al., 2011). Therefore, while normal circulating levels seem to sustain or enhance adult neurogenesis, supraphysiological testosterone may have no effect or possibly a negative effect on neurogenesis. The hypothesis that there may be an optimal dose of testosterone for maximizing hippocampal neurogenesis is supported by our finding that there was a negative relationship between circulating testosterone and number of BrdU-labeled cells among the sham-castrated rats in experiment 1. Further support for the optimal-dose hypothesis comes from some in vitro studies that have shown that lower concentrations of testosterone enhance neurite outgrowth, while relatively high concentrations induce apoptosis (Estrada et al., 2006a; Estrada et al., 2006b). Similarly, relatively low doses of testosterone reduced the infarct volume among male mice with focal cerebral ischemia, while high doses of testosterone increased infarct volume (Uchida et al., 2009).
Some previous studies support the conclusion that testosterone does not necessarily enhance adult neurogenesis. Buwalda et al. (2010) demonstrated that testosterone implants given to intact male rats had no effect on hippocampal neurogenesis. Similarly, a study involving male mice found that three days of supraphysiological injections of testosterone (4 mg/kg) had no effect on hippocampal neurogenesis (Zhang et al., 2010). The results of experiment 2 directly contradict one previous study in which testosterone injections of 0.5 and 1.0 mg/rat were shown to increase neurogenesis among castrated male rats (Spritzer and Galea, 2007). The main difference between the two experiments is that Spritzer et al. (2007) used 30 days of testosterone injections, while the current study involved only 15 days of injections. Therefore, prolonged periods of elevated testosterone may be needed to enhance neurogenesis. Among rats, most newly proliferated cells in the dentate gyrus express mature neuronal markers within 14 days of birth (Snyder et al., 2009), and that is one reason why we chose a relatively short 16-day maturation period for our study. However, expression of immediate early genes, indicative of neural activity, does not become common in new cells until approximately 4 weeks after birth (Snyder et al., 2009), and studies with mice indicate that maturation of dendritic spines does not occur until 28–56 days after birth (Zhao et al., 2006). Thus, high doses of testosterone may have neuroprotective effects during the later stages of cell development while possibly having a negative effect on neurogenesis during earlier stages of cell development.
In spite of the unexpected findings of experiment 2, the results of experiment 1 support two previous studies in which castration reduced neurogenesis among adult male rats (Spritzer and Galea, 2007; Wainwright et al., 2011). The physiological mechanisms underlying this effect remain unknown, but may involve the direct effects of testosterone or its metabolites on the hippocampus. Testosterone can be aromatized to estradiol in the brain (Stoffel-Wagner, 2003), and estradiol has been shown to enhance cell proliferation and neurogenesis in other rodent species (Ormerod et al., 2004; Saravia et al., 2007). In contrast, neither 15 days nor 30 days of estradiol injections influenced neurogenesis among male rats (Barker and Galea, 2008; Spritzer and Galea, 2007). However, 30 days of injections of the non-aromatizable androgen DHT enhanced neurogenesis in castrated rats (Spritzer and Galea, 2007). Thus, testosterone may have its neurogenesis-enhancing effects by binding to androgen receptors, which have been isolated from neural stem cells and have been detected in the dentate gyrus of male rats (Brännvall et al., 2005; Tabori et al., 2005). In vitro studies have shown that androgens can activate both the Mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase/Akt (PI-3k/Akt) pathways, both of which regulate cell survival (Nguyen et al., 2005; Gatson et al., 2006). Downstream of these pathways, DHT has also been shown to activate cyclic AMP response element binding protein (CREB), which is known to regulate a wide range of neurotrophic effects (Nguyen et al., 2009).
Effects of social isolation on adult neurogenesis
The results of experiment 2 indicated that 15 days of social isolation caused a decrease in adult neurogenesis. This adds to growing evidence indicating that social interactions can maintain or increase levels of adult neurogenesis in the dentate gyrus (Gheusi et al., 2009). Sexual interactions have been shown to increase hippocampal neurogenesis among male rats (Spritzer et al., 2009; Leuner et al., 2010). Among female rats, a combination of group housing and environmental enrichment enhanced neurogenesis compared to that observed in socially isolated rats living without enrichment (Nilsson et al., 1999). Group housing alone increased neurogenesis among juvenile rats relative to socially isolated individuals (Lu et al., 2003), but similar differences due to housing have not been previously observed among adult rats (Westenbroek et al., 2004; Leasure and Decker, 2009; Stranahan et al., 2006). Our results indicate that social isolation may be sufficient to reduce neurogenesis among adult rats, but the effects were strongest among castrated males.
Prolonged social isolation causes an increase in basal corticosterone levels (Núñez et al., 2002; Dronjak et al., 2004; Gavrilovic and Dronjak, 2005) and hyperactive HPA functioning (Ruis et al., 1999). Testosterone implants have been shown to reduce corticosterone release in response to an acute stressor among castrated male rats (Viau and Meaney, 1996). Social isolation also causes a significant reduction in hippocampal brain-derived neurotrophic factor (BDNF) compared that observed in to pair-housed rats (Scaccianoce et al., 2006), but male rats have higher basal BDNF levels in the dentate gyrus than do females (Franklin and Perrot-Sinal, 2006). These past results led us to hypothesize that elevated testosterone levels might reduce HPA axis activity and in turn prevent the neurogenesis-reducing effects of social isolation. In both of our experiments, we observed that testosterone seemed to attenuate the effects of social isolation. In experiment 1 the castrate/isolated group had less neurogenesis than any of the other groups, but the difference between the castrate/isolated and castrate/pair-housed group was only marginally significant. In experiment 2, the difference between the pair-housed and isolated groups was strongest among the oil-injected rats. Similarly, Wainwright et al. (2011) found that intact male rats were less impacted by the neurogenesis impairing effects of chronic mild stress than were castrated males. In contrast, Buwalda et al. (2010) found that testosterone implants did not prevent a decrease in hippocampal cell proliferation caused by social-defeat stress. Discrepancies between studies may be due to differences in the nature of the stressors. Buwalda et al. (2010) exposed rats to 5 days of 1-hour social defeat sessions, whereas Wainwright et al. (2011) exposed rats to 21 days of a variety of mild stressors. In our experiments, rats were exposed to 34 days of social isolation, which may have produced a milder stress response than that induced by the chronic mild stress paradigm employed by Wainwright et al. (2011). This could explain why we observed a more subtle effect of stress upon neurogenesis than did Wainwright et al. (2011).
Relative to pair-housed rats, social isolation caused an approximately 10% increase in the percentage of cells that were double labeled with BrdU and NeuN in experiment 2. It is unclear whether this fairly small percent difference in cellular differentiation has functional significance, and there are multiple possible explanations for this result. Isolation may bias cells to differentiate into neurons rather than glial cells, or isolation may speed the rate at which newly proliferated cells differentiate into neurons relative to pair housing. Alternatively, cells that remain undifferentiated among isolated animals may be more likely to undergo cell death. No previous studies of the effects of social interactions on neurogenesis have reported differences in cellular differentiation, but the percent of double-labeled cells that we observed was similar to that reported in past studies for cells of similar age (Snyder et al., 2009; Leuner et al., 2010).
Unexpectedly, social isolation resulted in a significant decrease in the number of BrdU-labeled cells within the hilus for experiment 1. Although the rate of cell division is much lower in the hilus than in the neurogenic niche along the GCL+SGZ, BrdU-labeled cells are commonly observed in the hilus. Increased cell division among ectopic granule cells within the hilus is robustly induced by epileptic seizures (McCloskey et al., 2006), and neurotrophic factors also increase the expression of BrdU-labeled cells in the hilus (Jin et al., 2002; Scharfman et al., 2005). Given that social isolation reduces hippocampal BDNF (Scaccianoce et al., 2006), reduced BDNF may be the mechanism by which social isolation reduced the number of BrdU-labeled cells in the hilus. The function of ectopic granule cells in the hilus remains unclear, but they produce synchronous burst discharges that distinguish them from the granule cells in the dentate gyrus (Scharfman et al., 2007).
Conclusion
We have demonstrated that testosterone and social isolation both influence adult neurogenesis within the dentate gyrus of male rats. Socially isolated rats had decreased neurogenesis relative to pair-housed individuals, which highlights the need to carefully consider housing conditions in future studies of adult neurogenesis. Social isolation provides a useful model for testing the effects of mild chronic stress on neural plasticity in the adult brain. The effects of testosterone seem to be dose dependent: physiological levels of testosterone consistently increase neurogenesis above that observed in castrated males, whereas the effects of supraphysiological doses of testosterone have been inconsistent. Endogenous testosterone may buffer against the neurogenesis-impairing effects of social isolation, whereas supraphysiological testosterone may reduce neurogenesis irrespective of housing conditions.
Research Highlights.
Castration significantly reduced neurogenesis in the dentate gyrus of male rats.
Isolation significantly reduced neurogenesis in the dentate gyrus of male rats.
Serum testosterone was negatively correlated with neurogenesis among intact males.
Testosterone may dose-dependently buffer against the effects of social isolation.
Acknowledgments
We thank Josh Chan, Robert Hawkins, Alyssa Panning, Graeme Rosenberg, Leanne Shulman, Priscilla Sinclair, Julia Stern, QiaQia Wu, and Jae-hee Jane Yoon for their assistance during various stages of this study. We thank Vicki Major, Sarah Froebel, and the rest of the Middlebury animal facility staff for providing excellent care for our animals. We also thank Marilyn Wadsworth and the rest of the staff at the University of Vermont Microscopy Imaging Center for assistance with confocal microscopy. This project was funded by Middlebury College and the Vermont Genetics Network (P20 RR16462) from the INBRE Program of the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The contents of this project are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Abbreviations
- ANOVA
analysis of variance
- BrdU
5-Bromo-2’deoxyuridine
- GCL
granule cell layer
- HPA
hypothalamic-pituitary-adrenal
- HPG
hypothalamic-pituitary-gonadal
- PVA DABCO
Polyvinyl alcohol 1,4-diazabicyclo-[2.2.2]octane
- SEM
standard error of the mean
- SGZ
sub-granular zone
- SVZ
subventricular zone
- TBS
Tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mark D. Spritzer, Email: mspritze@middlebury.edu.
Erin Ibler, Email: erin.ibler@gmail.com.
William Inglis, Email: winglis09@gmail.com.
Molly G. Curtis, Email: molly.gail.curtis@gmail.com.
REFERENCES
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Cuppini R, Ciuffoli S, Frontini A, Fanelli M. Synaptogenesis in adult-generated hippocampal granule cells is affected by behavioral experiences. Hippocampus. 2010;20:799–810. doi: 10.1002/hipo.20679. [DOI] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Galea LAM. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J Neuroendocrinol. 2009;21:155–166. doi: 10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- Barker JM, Galea LAM. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- Benice TS, Raber J. Castration and training in a spatial task alter the number of immature neurons in the hippocampus of male mice. Brain Res. 2010;1329:21–29. doi: 10.1016/j.brainres.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Gruber SH, Brene S. Social isolation increases number of newly proliferated cells in hippocampus in female flinders sensitive rats. Hippocampus. 2007;17:1193–1200. doi: 10.1002/hipo.20352. [DOI] [PubMed] [Google Scholar]
- Brännvall K, Bogdanovic N, Korhonen L, Lindholm D. 19-nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur J Neurosci. 2005;21:871–878. doi: 10.1111/j.1460-9568.2005.03942.x. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LAM. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience. 2010;168:680–690. doi: 10.1016/j.neuroscience.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Buwalda B, van der Borght K, Koolhass JM, McEwen BS. Testosterone decrease does not play a major role in the suppression of hippocampal cell proliferation following social defeat stress in rats. Physiol Behav. 2010;101:719–725. doi: 10.1016/j.physbeh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Dalla C, Papachristos EB, Whetstone AS, Shors TJ. Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neuron in their hippocampi. Proc Natl Acad Sci U S A. 2009;106:2927–2932. doi: 10.1073/pnas.0809650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neruosci. 2010;11:339–349. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronjak S, Gavrilovic L, Filopovic D, Radojcic MB. Immobilization and cold stress effect sympatho-adrenomedullary system and pituitary-adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol Behav. 2004;81:409–415. doi: 10.1016/j.physbeh.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming G, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada M, Uhlen P, Ehrlich BE. Ca2+ oscillations induced by testosterone enhance neurite outgrowth. J Cell Sci. 2006a;119:733–743. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- Estrada M, Varshney A, Ehrlich BE. Elevated testosterone induces apoptosis in neuronal cells. J Biol Chem. 2006b;281:25492–25501. doi: 10.1074/jbc.M603193200. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Freeman ME, Wang ZX. Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J Neurobiol. 2003;57:257–269. doi: 10.1002/neu.10273. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neruotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology. 2006;31:38–48. doi: 10.1016/j.psyneuen.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience. 1999;89:955–964. doi: 10.1016/s0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Kaur P, Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phophoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology. 2006;147:2028–2034. doi: 10.1210/en.2005-1395. [DOI] [PubMed] [Google Scholar]
- Gavrilovic L, Dronjak S. Activation of rat pituitary-andrenocortical and symaptho-adrenmedullary system in response to different stressors. Neuro Endocrinol Lett. 2005;26:515–520. [PubMed] [Google Scholar]
- Gheusi G, Ortega-Perez I, Murray K, Lledo P- A niche for adult neurogenesis in social behavior. Behav Brain Res. 2009;200:315–322. doi: 10.1016/j.bbr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Besterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwen BS, Haider SG, Markham CM, Blanchard RJ, Blanchard DC, Saki RR. Trends of reproductive hormone of male rats during psychosocial stress: role of glucocorticoid metabolism on behavioral dominance. Biol Reprod. 2002;67:1750–1755. doi: 10.1095/biolreprod.102.006312. [DOI] [PubMed] [Google Scholar]
- Ibi D, Kazuhiro T, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, Mamei H, Nagai T, Yoneda Y, Nabeshima T, Yamada K. Social isolation rearing-induced impairment of hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurosci. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara TS, Webber A, Gil-Mohapel J, Christie BR. Stress differentially regulates the effects of voluntary exercise on cell proliferation in the dentate gyrus of mice. Hippocampus. 2009;19:889–897. doi: 10.1002/hipo.20514. [DOI] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neruosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Adult Neurogenesis: Stem Cells and Neuronal Development in the Adult Brain. USA: Oxford University Press; 2006. p. 448. [Google Scholar]
- Leasure JL, Decker L. Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus. 2009;19:907–912. doi: 10.1002/hipo.20563. [DOI] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PloS One. 2010;5:e11597. doi: 10.1371/journal.pone.0011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, Pei G, Ma L. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183:600–609. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- McCloskey DP, Hintz TM, Pierce JP, Scharfman HE. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilecticus in the adult rat. Eur J Neurosci. 2006;24:2203–2210. doi: 10.1111/j.1460-9568.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald HY, Wojtowicz JM. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci Lett. 2005;385:70–75. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ. Dihydrotestosterone activates CREB signaling in cultured hippocampal neurons. Brain Res. 2009;1298:1–12. doi: 10.1016/j.brainres.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Núñez MJ, Rivas M, Riverio P, Suárez J, Balboa J, Núñez LA, Rey-Méndez M, Freire-Garabal M. Effects of nefazodone on voluntary ethanol consumption induced by isolation stress in young and aged rats. Pharmacol Biochem Behav. 2002;73:689–696. doi: 10.1016/s0091-3057(02)00875-4. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT-, Galea LAM. Estradiol enhances neurogenesis in the dentate gyri of adult male meadow voles by increasing the survival of young granule neurons. Neuroscience. 2004;128:645–654. doi: 10.1016/j.neuroscience.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LAM. Reproductive status influences the survival of new cells in the dentate gyrus of adult male meadow voles. Neurosci Lett. 2003;346:25–28. doi: 10.1016/s0304-3940(03)00546-9. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LAM. Estradiol initially enhances but subsequently suppressed (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- Perera TD, Park S, Nemirovaskaya Y. Cognitive role of neurogenesis in depression and antidepressant treatment. Neuroscientist. 2008;14:326–338. doi: 10.1177/1073858408317242. [DOI] [PubMed] [Google Scholar]
- Ruis MAW, te Brake JHA, Buwalda B, D SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuoendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? integrating permissive suppressive, stimulatory, and preparative actions. Endo Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Saravia F, Beauquis J, Pietranera L, De Nicola AF. Neuroprotective effects of estradiol in hippocampal neurons and glia of middle age mice. Psychoneuroendocrinology. 2007;32:480–492. doi: 10.1016/j.psyneuen.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Scaccianoce S, Del Bianco P, Paolone G, Caprioli D, Modafferi AME, Nencini P, Badiani A. Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behav Brain Res. 2006;168:323–325. doi: 10.1016/j.bbr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, McCloskey D. Ectopic granule cells of the rat dentate gyrus. Dev Neurosci. 2007;29:14–27. doi: 10.1159/000096208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jones P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurline SI, Hurley PB, A, Kamhi F, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Galea LAM. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Weinberg A, Viau V, Galea LAM. Prior sexual experience increases hippocampal cell proliferation and decreases risk assessment behavior in response to acute predator odor stress in male rats. Behav Brain Res. 2009;200:106–112. doi: 10.1016/j.bbr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodriguez-Wisdom KN. Effects of testosterone on spatial learning and memory in adult male rats. Horm Behav. 2011;59:484–496. doi: 10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B, Wolf SA, Kempermann G. Adult neurogenesis and neurodegenerative disease. Regenerative Medicine. 2006;1:15–28. doi: 10.2217/17460751.1.1.15. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteriod biosynthesis in the human brain and its clinical implications. Ann N Y Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabori NE, stewart LS, Znamensky V, Rmeo RD, Alves SE, McEwen BS, Milner TA. Ultrastrutural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29:1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress in mediated by the medial preoptic area. J Neurobiol. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright SR, Lieblich SE, Galea LAM. Hypogonadism predisposes males to the development of behavioural and neuroplastic depressive phenotypes. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.03.004. In Press. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303–308. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yang R, Zhou R, Li L, Sakobe M, Chen L. Progesterone promotes survival of newborn neurons in the dentate gyrus of adult male mice. Hippocampus. 2010;20:402–412. doi: 10.1002/hipo.20642. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, summers RG, Ming G, Gage FH. Distinct morphological stages of dentate granule neuron maturation in adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]