Abstract
Physalis peruviana, commonly known as Cape gooseberry, is an Andean Solanaceae fruit with high nutritional value and interesting medicinal properties. In the present study we report the development and characterization of microsatellite loci from a P. peruviana commercial Colombian genotype. We identified 932 imperfect and 201 perfect Simple Sequence Repeats (SSR) loci in untranslated regions (UTRs) and 304 imperfect and 83 perfect SSR loci in coding regions from the assembled Physalis peruviana leaf transcriptome. The UTR SSR loci were used for the development of 162 primers for amplification. The efficiency of these primers was tested via PCR in a panel of seven P. peruviana accessions including Colombia, Kenya and Ecuador ecotypes and one closely related species Physalis floridana. We obtained an amplification rate of 83% and a polymorphic rate of 22%. Here we report the first P. peruviana specific microsatellite set, a valuable tool for a wide variety of applications, including functional diversity, conservation and improvement of the species.
Introduction
Physalis peruviana commonly known as Cape gooseberry or golden berry is an Andean tropical fruit from the Solanaceae family native to South American countries including Colombia, Ecuador and Peru. Physalis peruviana grows wild in various parts of the Andes, typically 2,200 meters above sea level. The Cape gooseberry was known to the Incas but their origins are not clear, after Christopher Columbus the Cape gooseberry was introduced into Africa and India [1]. In Colombia, over the last three decades, P. peruviana went from being a neglected species to be the most promissory and successful exotic fruit for national and international markets; thus, since 1991, the Cape gooseberry market has been growing annually and in 2007 exports brought USD 34 million into the country. The main consumers of the Colombian Cape gooseberry are Europe with 97%, along with Asia and the United States with the remaining 3% [2]. The commercial interest in this fruit has grown due to its nutritional properties related to high vitamins content, minerals and antioxidants as well as its anti-inflammatory, anti-cancer and other medicinal properties [3], [4], [5], [6], [7], [8].
Despite growing interest in the Cape gooseberry, little is known about its genetic diversity and population structure. The collections kept in germplasm banks have been partially evaluated for morphologic and agronomic traits [9], [10], [11]. Although it has been reported that Cape gooseberry is a diploid species with 2n = 48 [12]; different chromosome numbers might exist among genotypes since 2n = 24 has been reported for wild ecotypes, 2n = 32 for the cultivated Colombia ecotype and 2n = 48 for the cultivated Kenya ecotype [13]. The genetic diversity of the Cape gooseberry at the molecular level has been poorly studied, to our knowledge there is only one report applying dominant markers RAMs (Random Amplified Microsatellites) in 43 individuals from five geographical regions in Colombia suggesting high heterozigocity and genetic diversity [14]. Additionally, in our experience, the use of heterologous microsatellite markers previously developed for several other Solanaceae species have not been successful in identifying polymorphic markers in Cape gooseberry.
Microsatellites or SSRs are defined as highly variable DNA sequences composed of tandem repeats of 1–6 nucleotides with co-dominant inheritance which have become the markers of choice for a variety of applications including characterization and certification of plant materials, identification of varieties with agronomic potential, genetic mapping, assistance in plant-breeding programs, among others [15], [16], [17], [18], [19]. However, no SSR markers specific for P. peruviana have been developed. The genetic analysis with microsatellites is simple and robust, although their identification and development present significant challenges in emerging species [16], [20]. According to the origin of the sequences used for the initial identification of simple repeats, SSRs are divided in two categories: Genomic SSRs which are derived from random genomic sequences and EST-SSRs derived from expressed sequence tags or from coding sequences. Genomic SSRs are not expected to have neither genic function nor close linkage to transcriptional regions, while EST-SSRs and coding-SSRs are tightly linked with functional genes that may influence certain important agronomic characters. The de novo identification of simple sequence repeats has usually involved large-scale sequencing of genomic, SSR-enriched genomic or EST libraries, which are expensive, laborious and time-consuming. Next generation sequencing technologies have enabled rapid identification of SSR loci derived from ESTs which can be identified in any emergent species [17], [19], [21].
The goal of the present study was to identify polymorphic SSR loci using the assembled leaf transcriptome sequences from a commercial Colombian ecotype of P. peruviana developed in our laboratory (http://www.ncbi.nlm.nih.gov/bioproject/67621). Imperfect as well as perfect repeat searches in non-coding or untranslated regions (UTRs) were performed. From these loci, primers were designed for amplification of UTR SSR loci. The effectiveness of these primers was tested via PCR in seven P. peruviana accessions, among them, the ecotypes Colombia, Kenya and Ecuador, as well as one closely related species Physalis floridana. The molecular markers developed here are valuable tools for assessing functional diversity, aid in species conservation and plant breeding programs.
Materials and Methods
SSR loci identification and marker development
A collection of Physalis peruviana leaf transcript sequences was used as the source for SSR development (Transcriptome Shotgun Assembly (TSA) Database, GenBank Accession numbers JO124085-JO157957). The transcripts were compared for sequence similarity with the non-redundant protein sequences database from NCBI using BLASTX. SSR loci were searched in both coding and non-coding sequences. Candidate SSR loci were identified using Phobos [22] in both coding and non-coding sequences using perfect and imperfect repeat searches with a minimum length of 18 bp for dinucleotides, 24 bp for tri and tetranucleotides, 30 bp for pentanucleotides and 36 bp for hexanucleotide repeats.
Primer design and amplification of SSR loci by PCR
Primer3 version 0.4.0 [23] was used to design primers for microsatellite amplification in P. peruviana. In addition, the oligocalculator - SIGMA Aldrich (http://www.sigma-genosys.com/calc/DNACalc.asp) was used to predict secondary structures (i.e. hairpins, primer dimers) for each primer pair designed. To determine the success of the microsatellite primer design, we carried out PCR tests to amplify the SSR loci in seven P. peruviana accessions (including Kenya, Ecuador and Colombia ecotypes) and one Physalis floridana accession, a closely related species (Table 1). The following PCR conditions were used: 1X PCR buffer: 1.5 to 3 mM MgCl2 depending on the primer pair, 0.2 µM dNTPs, 0.2 to 0.3 µM of each primer (depending on the primer pair), 0.05 U/µl Taq polymerase and 25 ng of genomic DNA, in a 15 µl reaction volume. The temperature conditions were 95°C for 3 minutes followed by 35 cycles of 95°C for 30 seconds, 50 to 52°C (depending on the primer pair) for 30 seconds and 72°C for 90 seconds, and a final extension of 72°C for 8 minutes. The PCR amplification products were analyzed by polyacrylamide gel electrophoresis (PAGE).
Table 1. Plant material used for SSR development and characterization.
| Species | Work Code | Accession/Common Name | Accession Code | Origin | |
| Source/region | Country | ||||
| P. peruviana | 1 | ILS 3804* | 09U086-1 | CORPOICA/Ambato | Ecuador |
| P. peruviana | 2 | Ecotype Kenia | 09U215-1 | Universidad de Nariño/+NA | Colombia |
| P. peruviana | 3 | Ecotype Colombia | 09U216-1 | Universidad de Nariño/NA | Colombia |
| P. floridana | 4 | ILS 1437* | 09U139-1 | Botanical Garden of Birmingham/NA | U.K. |
| P. peruviana | 5 | Novacampo (commercial) | 09U 274-1 | CORPOICA/Cundinamarca | Colombia |
| P. peruviana | 6 | ILS 3807* | 09U089-1 | CORPOICA/Antioquia | Colombia |
| P. peruviana | 7 | ILS 3826* | 09U108-1 | CORPOICA/Antioquia | Colombia |
| P. peruviana | 8 | ILS 3817* | 09U099-1 | CORPOICA/Caldas | Colombia |
ILS* = Introduction maintained at La Selva Research Center, CORPOICA; NA = Not available; +NA = Not available (in vitro propagated material).
Gene Ontology analysis of SSR loci
A gene ontology (GO) analysis was performed using blast2go [24] with the assembled transcript sequences containing the 30 polymorphic SSRs described here. These sequences were compared with the UniProtKB/Swiss-Prot database with a cutoff e-value of 1×10−5.
Results
Identification of SSR loci in P. peruviana
A total of 1,520 SSR loci were identified and a large fraction were located in UTRs (74%) as compared to coding sequences (CDS) with 26%. The highest number of SSR loci found contained trinucleotide and hexanucleotide repeats with 544 (36%) and 530 (35%) respectively (Table 2).
Table 2. SSR loci identified in Physalis peruviana leaf Expressed Sequence Tags (ESTs).
| Repeat Type | Perfect | Imperfect | Frequency | |||||
| CDS | UTRs | Total | CDS | UTRs | Total | |||
| Dinucleotide | - | 34 | 34 | 2 | 98 | 100 | 134 | 8% |
| Trinucleotide | 36 | 81 | 117 | 178 | 249 | 427 | 544 | 36% |
| Tetranucleotide | 1 | 16 | 17 | 13 | 69 | 82 | 99 | 7% |
| Pentanucleotide | - | 6 | 6 | 47 | 160 | 207 | 213 | 14% |
| Hexanucleotide | 46 | 64 | 110 | 64 | 356 | 420 | 530 | 35% |
| Total | 83 | 201 | 284 | 304 | 932 | 1236 | 1520 | - |
| Frequency | 6% | 13% | 19% | 20% | 61% | 81% | ||
The number of SSR loci identified at coding sequences (CDS) and Untranslated Regions (UTRs) by using perfect and imperfect repeat search criteria.
Microsatellite primer design and PCR analysis
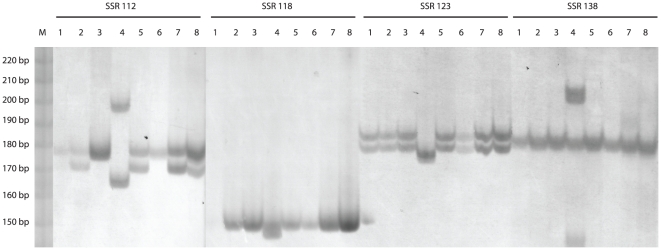
The SSR loci selected for primer design were located at UTRs and identified with an imperfect repeat search to increase the probabilities for finding polymorphisms within the individuals analyzed. Using this strategy a total of 162 primers pairs were designed. A successful PCR amplification was obtained for 138 (83%) of the 162 primers designed from microsatellite loci using seven P. peruviana and one P. floridana genotype (Table 1). Polymorphisms among the eight genotypes were observed for 30 (22%) loci whereas the remaining 108 loci were monomorphic (Figure 1, Tables 3 and 4).
Figure 1. SSR alleles in eight Physalis genotypes and four polymorphic loci.
The polymorphic SSR loci were visualized in 6% polyacrylamide gels, samples 1–8 correspond to the work code shown in Table 1. M = Molecular size marker, 10 bp DNA Ladder (Invitrogen, Carlsbad, CA).
Table 3. Polymorphisms in Physalis peruviana SSR loci.
| SSR Type | Polymorphic | Monomorphic | Total |
| Dinucleotide | 19 | 53 | 72 |
| Trinucleotide | 10 | 39 | 49 |
| Tetranucleotide | - | 5 | 5 |
| Pentanucleotide | 1 | 1 | 2 |
| Hexanucleotide | - | 10 | 10 |
| Total | 30 | 108 | 138 |
Table 4. Allelic variation in 30 Physalis peruviana SSR loci.
| Polymorphic loci | Forward primer (5′-3′) | Reverse primer (5′-3′) | PCR conditions | Alleles (pb) | Repeat type | Location | ||||
| Primer [µM] | MgCl2 [mM] | °Tm | Expected size | Range size observed | ||||||
| SSR1 | AGAGGACTCCATTTGTTTGCT | TGAGGGTGTTGGATGTTTTCT | 0,2 | 2 | 50 | 206 | 170 | 210 | AT | 3′ UTR |
| SSR2 | CATTGGGTTTCGCATCCAT | AGACAAGCCTAGGGGAAAGG | 0,2 | 2 | 50 | 237 | 230 | 250 | AG | 3′ UTR |
| SSR9 | TGCTCCGAGTTTTAGGGTTC | GCAGTTGGTAAAGTTGAGAGACG | 0,2 | 2 | 50 | 193 | 220 | 240 | AG | 5′ UTR |
| SSR10 | GCTTCCTATTGTGTTGCCTGA | ACTTTGGGTTTCGGGAATTG | 0,2 | 2 | 50 | 185 | 170 | 190 | AT | 3′ UTR |
| SSR11 | CAGCTGAAATAAGAGAGTGATTGG | CCCTCTTTTTCTCCTCCGAGT | 0,2 | 2 | 50 | 180 | 180 | 210 | AG | 3′ UTR |
| SSR13 | GCGGAATCCATTGTTTTTCA | CCGATGAGATATAGTCACGCAAA | 0,2 | 2 | 50 | 190 | 160 | 210 | AC | 5′ UTR |
| SSR14 | TGAAACCCATCTAGCTGAACG | TGGGTTGTTCCTTACAATCCAT | 0,2 | 1,5 | 50 | 204 | 200 | 220 | AT | 3′ UTR |
| SSR15 | GCTTGTTGATCAGCTTTCTTTG | TGGATCATAACCTTGCTAATGC | 0,2 | 1,5 | 50 | 172 | 160 | 180 | AT | 3′ UTR |
| SSR18 | CAGAGTGATTACCTTGGACGAA | TGTCCATTTTAGTCGCCAAT | 0,2 | 1,5 | 50 | 179 | 180 | 230 | AC | 3′ UTR |
| SSR20 | GCACATCACATAAAGTATCTTTCTCA | TTGCCTGGTGTCTTGCTATG | 0,2 | 1,5 | 50 | 270 | 170 | 220 | AT | 3′ UTR |
| SSR36 | ATGAACCACATGTCGGAGGA | GGGGATCCAAACGAAGTGTA | 0,2 | 1,5 | 52 | 211 | 170 | 240 | AG | 3′ UTR |
| SSR37 | CCAACTGAATCAACACACAGC | CCACACTGAAAAAGGGATCTG | 0,3 | 2 | 50 | 212 | 260 | 330 | AG | 3′ UTR |
| SSR54 | CGGCTGGTATGCTTACAAAGAT | GCACTTCCACTGTTTTTAACTTCC | 0,2 | 1,5 | 50 | 197 | 190 | 210 | AC | 3′ UTR |
| SSR55 | CACCTACATAGGCAGCCAAAA | ATTTGTGGGCGGAGGAAG | 0,2 | 1,5 | 50 | 183 | 200 | 210 | AG | 5′ UTR |
| SSR57 | AGTGAAAAGCAGCCCATTCT | GGCGAAGCTGAATTGAAAAA | 0,2 | 1,5 | 50 | 183 | 200 | 210 | AT | 3′ UTR |
| SSR67 | GCTTCTGTTCCATTATTCACCA | GCAGTGTGGGATCAATCAAT | 0,2 | 1,5 | 50 | 207 | 180 | 240 | AG | 3′ UTR |
| SSR68 | GAAGCAAACAACTACACCCAAA | AAGCCTCGGATTTCATAGCA | 0,2 | 1,5 | 50 | 187 | 160 | 220 | AG | 3′ UTR |
| SSR72 | GTGCTCGCAGTTTCTTCAAA | CCGCCGTTACTTCCTAATCA | 0,2 | 1,5 | 50 | 158 | 130 | 170 | AG | 3′ UTR |
| SSR77 | CATACCATAACTCCCCATCTCTC | TGCCGATTCTGATTTCTTCC | 0,2 | 1,5 | 50 | 216 | 170 | 200 | AT | 5′ UTR |
| SSR92 | TGGTTTGAGGATCAAGAAAGAA | GTGGTATCAACGCAGAGTGG | 0,25 | 2,5 | 50 | 205 | 180 | 210 | AAG | 3′ UTR |
| SSR107 | CATCCAACACCAGAAATACGC | TCCAACTTTATCATTTCTTCCAC | 0,2 | 1,5 | 50 | 206 | 220 | 250 | AAG | 5′ UTR |
| SSR110 | CACCCATATCCCAATCTTCTTC | GGGTAATTTTCACGGGGAAT | 0,2 | 1,5 | 50 | 198 | 170 | 200 | CTT | 3′ UTR |
| SSR112 | CTACGCCTACCACTTGCACA | CAGTGGAAGCCTCAAGATCC | 0,2 | 1,5 | 50 | 203 | 200 | 220 | TCT | 3′ UTR |
| SSR118 | AATCAAGGGTCAGAAGAAATGG | GCAAGAATGGATGTGGGTGT | 0,2 | 1,5 | 50 | 180 | 130 | 180 | AAG | 5′ UTR |
| SSR121 | AGCAACCTCCCAATCAGCTA | TGGTGAGTAAATGGGGGAAA | 0,2 | 1,5 | 50 | 189 | 170 | 190 | ATC | 3′ UTR |
| SSR123 | TCAGTGGAGCGCGTATATCT | GCGATCTCACCAAACCTCTC | 0,2 | 1,5 | 50 | 216 | 190 | 210 | ATC | 5′ UTR |
| SSR126 | TCCAAAAAGAAAACAAAAACACT | TTGAATGCATGTTTGATGGA | 0,2 | 1,5 | 50 | 202 | 190 | 200 | AGC | 5′ UTR |
| SSR127 | TTGGTTTGGCATAACTGCAA | GGTTTGCAACTCTCATGCTG | 0,2 | 1,5 | 50 | 180 | 140 | 160 | AAT | 5′ UTR |
| SSR138 | TCCGATCACTACTTCAGCACG | CAATTCGGGTTGTGAATCGGGT | 0,2 | 1,5 | 50 | 138 | 130 | 160 | AAT | 3′ UTR |
| SSR146 | AGGCTAATGAGGACGAAGCA | GGTTGCATTACAAAGCACTGA | 0,2 | 1,5 | 50 | 187 | 160 | 210 | AAAAG | 3′ UTR |
Functional relationships of polymorphic SSR markers
A significant GO annotation was found for 10 of the 30 markers, which are related to 43 different ontology terms, of these 27 (67%) were related to biological process, 11 (25%) to molecular function and 5 (8%) to cellular component (Table 5).
Table 5. Functional annotation of 10 P. peruviana contigs containing polymorphic SSR markers.
| SSR Marker | GO Category: ID | Functional Annotation |
| SSR2 | P:0006350 | Transcription |
| SSR37 | F:0016301 | Kinase activity |
| C:0005886 | Plasma membrane | |
| SSR54 | P:0006952 | Defense response |
| P:0012501 | Programmed cell death | |
| C:0044464 | Cell part | |
| F:0000166 | Nucleotide binding | |
| SSR55 | P:0051865 | Protein autoubiquitination |
| F:0004842 | Ubiquitin-protein ligase activity | |
| P:0048437 | Floral organ development | |
| P:0046621 | Negative regulation of organ growth | |
| SSR77 | P:0009789 | Positive regulation of abscisic acid mediated signaling pathway |
| P:0006979 | Response to oxidative stress | |
| P:0052544 | Callose deposition in cell wall during defense response | |
| P:0009753 | Response to jasmonic acid stimulus | |
| P:0031348 | Negative regulation of defense response | |
| P:0008219 | Cell death | |
| P:0009651 | Response to salt stress | |
| P:0042742 | Defense response to bacterium | |
| P:0009926 | Auxin polar transport | |
| P:0010119 | Regulation of stomatal movement | |
| P:0009408 | Response to heat | |
| F:0005515 | Protein binding | |
| P:0010150 | Leaf senescence | |
| P:0048765 | Root hair cell differentiation | |
| P:0009871 | Jasmonic acid and ethylene-dependent systemic resistance, ethylene mediated signaling pathway | |
| P:0001736 | Establishment of planar polarity | |
| P:0050832 | Defense response to fungus | |
| P:0010182 | Sugar mediated signaling pathway | |
| SSR92 | F:0004674 | Protein serine/threonine kinase activity |
| P:0045449 | Regulation of transcription | |
| P:0007169 | Transmembrane receptor protein tyrosine kinase signaling pathway | |
| F:0005524 | ATP binding | |
| F:0003700 | Transcription factor activity | |
| P:0010030 | Positive regulation of seed germination | |
| P:0006468 | Protein amino acid phosphorylation | |
| SSR110 | C:0044444 | Cytoplasmic part |
| SSR126 | F:0005488 | Binding |
| F:0003824 | Catalytic activity | |
| SSR138 | F:0016740 | Transferase activity |
| SSR146 | C:0005730 | Nucleolus |
| C:0016020 | Membrane | |
| F:0003677 | DNA binding |
Gene ontology (GO) functional Categories: C = Cellular component, F = Molecular function, P = Biological process.
Discussion
Here we present the first collection of EST-derived microsatellite markers in Physalis peruviana. The highest number of SSR loci found contained trinucleotide and hexanucleotide repeats (Table 2), which is consistent with results reported in Solanaceae and other plant species [19], [20], [25], [26], [27], [28], [29], [30], [31]. 1,236 out of 1,520 SSR loci are composed of imperfect repeats increasing the probability of polymorphism among Physalis species. This inference is bolstered by the fact that 30 of the 162 imperfect SSRs (22%) were polymorphic in the panel of 8 accessions from P. peruviana and the related species P. floridana (Table 1), suggesting the potential utility of these genetic based SSR markers for future studies. i.e. germplasm diversity and breeding applications [17], [19], [32].
Our results show that most of the SSR loci were located at UTRs (Table 2) in agreement with the results reported by Morgante and others [27] who hypothesize that in plants most of the SSR loci from transcribed regions are distributed along the UTRs. Increased numbers of SSR loci at UTRs could be related to changes in transcription (5′UTRs) or RNA silencing (3′UTRs), which are sources of variation among species [18], [19], [20], [29], [30]. Cereal species appear to have a different SSR distribution; Yu and others [33] found that most of the 444 EST derived SSR markers (62%) were located at coding regions, while 38% were located at UTRs.
Since the SSR loci found in this study were derived from genes, they may be related to some traits of interest [18], [20], [27] such as resistance to Fusaruim oxysporum, which is one of the main constraints for Cape gooseberry production at the commercial level. According to the functional annotation obtained by the GO analysis, two polymorphic SSR markers (SSR54 and SSR77 respectively) were related with proteins involved in defense responses to pathogens such as programed cell death and ethylene as well as jasmonic acid pathways. These two polymorphic SSR makers would be useful in P. peruviana breeding programs focused on F. oxysporum resistance.
The high rate of successful PCR amplification for the primer pairs designed (84%, Table 4) is related to the fact that these loci are specific to P. peruviana and they were also developed from genes, increasing the transferability within species of the same genus i.e. P. floridana. These results are in agreement with Zeng et al. and Csencsics et al. [19], [21], who used full-length cDNA and ESTs and found rates of successful PCR amplification larger than 80%.
This study reports the first set of microsatellite markers developed for P. peruviana and related species. A total of 1,520 SSR loci were identified, including 932 imperfect SSRs located at UTRs. From these loci a total of 162 SSR primers were developed to assay their utility as microsatellite markers in a panel of seven accessions of P. peruviana and one accession of P. floridana by PCR amplification. A total of 138 (83%) primer markers amplified, with a polymorphism rate of 22%. The markers developed here can be used in plant breeding programs that may ultimately lead to superior phenotypic characteristics such as increase in fruit size, reduction in the tendency to split during transport, reduction in the plant susceptibility to pests and diseases, and improvement of fruit quality.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Support for this research was provided by a grant from the Colombian Ministry of Agriculture Contract Nos. 054/08072-2008L4787-3281 to LSB and 054/08190-2008L7922-3322 to VMNZ. This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Library of Medicine, and National Center for Biotechnology Information. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Popenoe H, King S, Leon J, Kalinowski L. Goldenberry (cape gooseberry). Lost Crops of The Incas: Little-known Plants of the Andes with Promise for Worldwide Cultivation, ed by National Research Council National Academy Press, Washington DC. 1990. pp. 241–252.
- 2.Bonilla MH, Arias PA, Landínez LM, Moreno JM, Cardozo F, et al. 2009. Agenda prospectiva de investigación y desarrollo tecnológico para la cadena productiva de la uchuva en fresco para exportación en Colombia- Ministerio De Agricultura y Desarrollo Rural; Proyecto Transición De La Agricultura; Universidad Nacional De Colombia CCDIAC, editor. BOGOTÁ D.C., Colombia: Ministerio de Agricultura y Desarrollo Rural.
- 3.Yen CY, Chiu CC, Chang FR, Chen JY, Hwang CC, et al. 4beta-Hydroxywithanolide E from Physalis peruviana (golden berry) inhibits growth of human lung cancer cells through DNA damage, apoptosis and G2/M arrest. BMC Cancer. 2010;10:46. doi: 10.1186/1471-2407-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu SJ, Chang SP, Lin DL, Wang SS, Hou FF, et al. Supercritical carbon dioxide extract of Physalis peruviana induced cell cycle arrest and apoptosis in human lung cancer H661 cells. Food Chem Toxicol. 2009. [DOI] [PubMed]
- 5.Pinto Mda S, Ranilla LG, Apostolidis E, Lajolo FM, Genovese MI, et al. Evaluation of antihyperglycemia and antihypertension potential of native Peruvian fruits using in vitro models. J Med Food. 2009;12:278–291. doi: 10.1089/jmf.2008.0113. [DOI] [PubMed] [Google Scholar]
- 6.Ramadan MF, Morsel JT. Oil goldenberry (Physalis peruviana L.). J Agric Food Chem. 2003;51:969–974. doi: 10.1021/jf020778z. [DOI] [PubMed] [Google Scholar]
- 7.Franco LA, Matiz GE, Calle J, Pinzon R, Ospina LF. [Antiinflammatory activity of extracts and fractions obtained from Physalis peruviana L. calyces]. Biomedica: revista del Instituto Nacional de Salud. 2007;27:110–115. [PubMed] [Google Scholar]
- 8.Martinez W, Ospina LF, Granados D, Delgado G. In vitro studies on the relationship between the anti-inflammatory activity of Physalis peruviana extracts and the phagocytic process. Immunopharmacol Immunotoxicol. 2010;32:63–73. doi: 10.1080/08923970903143957. [DOI] [PubMed] [Google Scholar]
- 9.Lagos Burbano TC, Criollo Escobar H, Ibarra A, Hejeile H. Caracterización morfológica de la colección Nariño de uvilla o uchuva Physalis peruviana L. Fitotecnia Colombiana. 2003;3:1–9. [Google Scholar]
- 10.Ligarreto GA, Lobo M, Correa A. Recursos genéticos del género Physalis en Colombia. In: Fischer G, Miranda D, Piedrahita W, Romero J, editors. Avances en cultivo, poscosecha y exportación de la uchuva (Physalis peruviana L) en Colombia. Universidad Nacional de Colombia (Sede Bogotá) Facultad de Agronomía ed. Bogotá: Unibiblos, Universidad Nacional de Colombia; 2005. pp. 9–27. [Google Scholar]
- 11.Trillos González O, Cotes Torres JM, Medina Cano CI, Lobo Arias M, Navas Arboleda AA. Caracterización morfológica de cuarenta y seis accesiones de uchuva (Physalis peruviana L.), en Antioquia (Colombia). Revista Brasileira de Fruticultura. 2008;30:708–715. [Google Scholar]
- 12.Menzel MY. The cytotaxonomy and genetics of Physalis. Proceedings of the American Philosophical Society. 1951;95:132–183. [Google Scholar]
- 13.Nohra C, Rodriguez C, Bueno A. Study of the cytogenetic diversity of Physalis peruviana L.(Solanaceae). Acta biol Colomb. 2006;11:75–85. [Google Scholar]
- 14.Muñoz Flórez JE, Morillo Coronado AC, Morillo Coronado Y. Random amplified microsatellites (RAMs) in plant genetic diversity studies. Acta Agron. 2008;57:219–226. [Google Scholar]
- 15.Goldstein DB, Schlötterer C. Microsatellites: evolution and applications (POD) 1999.
- 16.Scott KD, Eggler P, Seaton G, Rossetto M, Ablett EM, et al. Analysis of SSRs derived from grape ESTs. TAG Theoretical and Applied Genetics. 2000;100:723–726. [Google Scholar]
- 17.Bozhko M, Riegel R, Schubert R, Muller-Starck G. A cyclophilin gene marker confirming geographical differentiation of Norway spruce populations and indicating viability response on excess soil-born salinity. Mol Ecol. 2003;12:3147–3155. doi: 10.1046/j.1365-294x.2003.01983.x. [DOI] [PubMed] [Google Scholar]
- 18.Varshney RK, Graner A, Sorrells ME. Genic microsatellite markers in plants: features and applications. Trends Biotechnol. 2005;23:48–55. doi: 10.1016/j.tibtech.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Zeng S, Xiao G, Guo J, Fei Z, Xu Y, et al. Development of a EST dataset and characterization of EST-SSRs in a traditional Chinese medicinal plant, Epimedium sagittatum (Sieb. Et Zucc.) Maxim. BMC Genomics. 2010;11:94. doi: 10.1186/1471-2164-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujimori S, Washio T, Higo K, Ohtomo Y, Murakami K, et al. A novel feature of microsatellites in plants: a distribution gradient along the direction of transcription. FEBS Lett. 2003;554:17–22. doi: 10.1016/s0014-5793(03)01041-x. [DOI] [PubMed] [Google Scholar]
- 21.Csencsics D, Brodbeck S, Holderegger R. Cost-effective, species-specific microsatellite development for the endangered Dwarf Bulrush (Typha minima) using next-generation sequencing technology. J Hered. 2010;101:789–793. doi: 10.1093/jhered/esq069. [DOI] [PubMed] [Google Scholar]
- 22.Mayer C. Phobos, a Tandem Repeat Search Tool for Complete Genomes. Version. 2008.
- 23.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 24.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 25.Lu FH, Cho MC, Park YJ. Transcriptome profiling and molecular marker discovery in red pepper, Capsicum annuum L. TF68. Mol Biol Rep. 2011. [DOI] [PubMed]
- 26.Barchi L, Lanteri S, Portis E, Acquadro A, Vale G, et al. Identification of SNP and SSR markers in eggplant using RAD tag sequencing. BMC Genomics. 2011;12:304. doi: 10.1186/1471-2164-12-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgante M, Hanafey M, Powell W. Microsatellites are preferentially associated with nonrepetitive DNA in plant genomes. Nat Genet. 2002;30:194–200. doi: 10.1038/ng822. [DOI] [PubMed] [Google Scholar]
- 28.Varshney RK, Thiel T, Stein N, Langridge P, Graner A. In silico analysis on frequency and distribution of microsatellites in ESTs of some cereal species. Cell Mol Biol Lett. 2002;7:537–546. [PubMed] [Google Scholar]
- 29.Eujayl I, Sledge MK, Wang L, May GD, Chekhovskiy K, et al. Medicago truncatula EST-SSRs reveal cross-species genetic markers for Medicago spp. Theor Appl Genet. 2004;108:414–422. doi: 10.1007/s00122-003-1450-6. [DOI] [PubMed] [Google Scholar]
- 30.La Rota M, Kantety RV, Yu JK, Sorrells ME. Nonrandom distribution and frequencies of genomic and EST-derived microsatellite markers in rice, wheat, and barley. BMC Genomics. 2005;6:23. doi: 10.1186/1471-2164-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luro FL, Costantino G, Terol J, Argout X, Allario T, et al. Transferability of the EST-SSRs developed on Nules clementine (Citrus clementina Hort ex Tan) to other Citrus species and their effectiveness for genetic mapping. BMC Genomics. 2008;9:287. doi: 10.1186/1471-2164-9-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toth G, Gaspari Z, Jurka J. Microsatellites in different eukaryotic genomes: survey and analysis. Genome Res. 2000;10:967–981. doi: 10.1101/gr.10.7.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu JK, La Rota M, Kantety RV, Sorrells ME. EST derived SSR markers for comparative mapping in wheat and rice. Mol Genet Genomics. 2004;271:742–751. doi: 10.1007/s00438-004-1027-3. [DOI] [PubMed] [Google Scholar]