Abstract
Impulsivity is a defining characteristic of adolescence. Compared to adults, for example, adolescents engage in higher rates of drug and alcohol experimentation, risky sexual practices, and criminal activity. Such behavior may reflect reduced sensitivity to long-term consequences for behavior during adolescence. Recently, our lab has attempted to refine mouse procedures to study developmental trends in decision making in the laboratory. In the present experiment, we examined sensitivity to delayed rewards in C57BL/6J (B6) and DBA/2J (D2) mice during adolescence and adulthood using an adaptation of a two-week delay discounting procedure developed by Adriani & Laviola (2003) [Behavioral Neuroscience, 117, 695-703]. During training, mice could choose between a 20- or 100-ul drop of milk delivered after a 1-s delay. During testing, the delay to the large drop of milk was increased from 1 to 100 seconds. As the delay to the larger volume increased, preference shifted to the smaller, more immediate option. In adolescence, both strains showed similar shifts in preference. In contrast, adult B6 mice were less sensitive to increasing delays than were adult D2 mice, who continued to perform much as their adolescent counterparts. A subsequent resistance-to-extinction test ruled out the possibility that the slower change in the adult B6 mice was due to perseverative responding. The present findings suggest that B6 and D2 strains may be differentially suited to uncovering the biological mechanism of short-term and long-term patterns of impulsive behavior.
Keywords: Impulsivity, Delay Discounting, Development, Resistance to Extinction, C57BL6/6J, DBA/2J
The adolescent undergoes profound neural, physiological, behavioral, and social changes during in the transition from childhood to adulthood. Although the adolescent period is important for establishing the individual as an independent adult, it is also a period of developmental vulnerability (Casey, Jones, & Hare, 2008; Steinberg, 2005). Adolescent behavior is often described as impulsive, risky, and sensation-driven, reflecting increased tendencies for adolescents to engage in unsafe sexual practices, dangerous driving, criminal behavior, and experimentation with controlled substances (Arnett, 1992; Chambers, Taylor, & Potenza, 2003; Crews, He, & Hodge, 2007; Spear, 2000). In fact, potentially maladaptive behavior is so pervasive that Moffitt (1993) has suggested social deviancy often observed during adolescence is the norm, not the exception.
Laboratory preparations provide important opportunities to study adolescent behavior in transition. In particular, such preparations have revealed that “risky” and “impulsive” behavior are not unitary phenomena, but are comprised of multiple, distinct processes (Evenden, 1999). Recently, our lab has focused on sensitivity to delayed reward as one dimension of impulsive behavior. Sensitivity to delay has been measured in the laboratory most often with the use of delay discounting tasks. In delay discounting tasks, an individual is given the opportunity to choose between smaller rewards delivered sooner or larger rewards delivered later (de Wit, 2009; Madden & Johnson, 2010). As the delay to the large reward increases, preference often shifts to the smaller, sooner reward. This shift in preference occurs at shorter delays in more impulsive individuals, who are more sensitive to reward delay.
Increased sensitivity to reward delay has been linked empirically to engagement in impulsive and maladaptive behavior in adult humans, such as drug and alcohol abuse (Bickel et al., 2007; Coffey et al., 2003; Madden et al., 1997; Petry, 2001; Mitchell, 1999) and pathological gambling (Petry & Madden, 2010). With regard to developmental trends in decision making, moreover, adolescents tend to shift preference to the smaller, sooner reward more quickly than adults (Green, Myerson, & Ostaszewski, 1999; Olson et al., 2007; Steinberg et al., 2009), that is, adolescents tend to be more sensitive to reward delay than are adults. Moffitt (1993) has suggested that maladaptive behavior patterns characteristic of adolescence often subside upon entering adulthood; for a few others, however, the tendency for such behavior persists. Given the correlation between maladaptive behavior and sensitivity to reward delay, it is interesting to speculate that developmental trends in sensitivity to delay may be responsible for the developmental trends reported by Moffitt (1993). That is, some individuals may become less sensitive to delay as they mature, resembling normal adults, while others may continue to make choices based upon immediate gratification, which continues to produce the rash, impulsive, and potentially maladaptive behavior more characteristic of adolescence.
The present research was designed to bring developmental transitions in decision making under study with a rodent laboratory preparation. Laboratory procedures with rodents have been important tools for exploration into factors determining complex behavior (Koot et al., 2009; McKinney, 2001). The refinement of laboratory techniques to the study of lifespan trends in decision making stand to advance our ability to ask experimental questions of the relationship between biology and behavior during critical periods of development. As a first examination, DBA/2J (D2) mice were compared to C57BL/6J (B6) mice. The two strains were selected because they have been compared across a variety of drug self-administration and reinforcement paradigms (e.g., Crabbe et al., 1982; Cunningham et al., 1992; Fish et al., 2010; Helms et al., 2006; Orsini et al., 2005; Risinger et al., 1998). As the adolescent period is relatively brief for rodents, compared to humans, most mainstream procedures for assessing delay discounting are not suitable for developmental studies because it may take several weeks or months to establish baseline performance (see Madden & Johnson, 2010 for a review of common procedures). For the present experiment, we adapted our procedure from an across-session delay discounting procedure reported by Adriani & Laviola (2003). The procedure was selected because it has potential as a high-throughput assessment of decision making and it has been shown previously to distinguish patterns of decision making between adolescent and adult outbred CD-1 mice (Adriani & Laviola, 2003).
Method
Subjects
Subjects were male mice drawn from C57BL/6J and DBA/2J strains (Jackson Laboratories, Bar Harbor, ME). Different groups of mice were studied during adolescence (n=14 per strain) and adulthood (n=12 per strain). Adolescent mice arrived at 4 weeks of age; adult mice arrived at 10 weeks of age. Mice were allowed to acclimate to the colony room for the first week. Training commenced in the second week, and testing commenced in the third week. So, discounting was assessed during the 6th week of age for the adolescents and 12th week for the adults; the specific time points were chosen because they correspond to late adolescence and adulthood, respectively. Upon arrival, mice were housed individually in a humidity- and temperature-controlled colony room. Mice were handled and weighed daily. Lights in the room were on a 12 h : 12 h light:dark cycle. Mice were tested during the light portion of the cycle.
During the first five days in the colony room, mice had ad libitum access to standard lab chow. Two days prior to the start of the experiment, each mouse was placed on a restricted diet of 2.5 g of chow per day. The amount of food was chosen to ensure that mice maintained a relatively constant weight across the experiment. Weights at the beginning of the experiment are given (mean ±SEM g): Adolescent B6, 18.3 ± 0.3g; Adolescent D2, 19.0 ± 0.2g; Adult B6 22.1 ± 0.1g; Adult D2 21.5 ± 0.1g. The consistency of deprivation levels across the experiment can be confirmed by comparing the beginning weights to those obtained at the end of the 14-day experiment (mean ±SEM g): Adolescent B6, 17.9 ± 0.2g; Adolescent D2, 19.4 ± 0.1g; Adult B6 22.3 ± 0.1g; Adult D2 21.3 ± 0.1g. Finally, a mixed model repeated-measures ANOVA found no significant effect of Age or Strain (between factors) or Day (repeated factor) on body weight, though the effect of Age approached significance (p = 0.088). All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Apparatus
Experiments were conducted in eight, commercially available operant chambers for mice (Model #ENV-307W, Med Associates, St. Albans, VT) housed in a ventilated enclosure (Model #ENV-022MD, Med Associates, St. Albans, VT). Each chamber was equipped with two nose poke apertures (Model #ENV-313W, Med Associates, St. Albans, VT), located on opposite sides of one wall of the chamber. A single 24-V bulb was mounted near the ceiling on the wall opposite the two apertures and served as a house light. A speaker mounted in the enclosure behind the apertures provided auditory stimulation via a tone generator (Model #ANL-926, Med Associates, St. Albans, VT). Nose pokes into the aperture that interrupted an infrared beam passing through the opening were counted as responses. Yellow LEDs located inside the aperture served as discriminative stimuli. Each aperture allowed access to a dipper providing condensed milk mixed with water in a 1:1 ratio. One dipper delivered milk solution at a volume of 20 ul (small volume); the other dipper delivered 100 ul of solution (large volume). Experimental events and data recording was accomplished by a PC running Med State™ IV software.
Assessment of Choice Under Increasing Delays to Milk Delivery
The procedure consisted of a week of training on a two-alternative free choice task followed by a week of testing. During the 7-day training program, mice could choose freely between the two volumes of milk. At the start of the session, LED stimuli were lit in each nose poke hole. Nose pokes into either aperture extinguished both LED's and started a 1-s delay. The delay was signaled by the illumination of the house light and an 800 Hz tone. At the end of the delay, the tone and house light were turned off and the respective dipper (either 20 or 100 ul) was presented for 10 s. Following every choice, a 15-s blackout was in effect where all lights were extinguished and nose pokes had no programmed consequences. At the end of the blackout, the choice options were reinstated. Sessions lasted 30 min.
Following training, the 7-day testing component was conducted wherein the delay to the large volume of milk was systematically increased across days. Parameters remained constant for the small-volume option. Across testing sessions, the delay following selection of the 100 ul volume was changed systematically each day. The series of delays studied was 1, 10, 20, 40, 60, 80, and 100 s. The replication of the 1 s delay during testing served as a control observation. Note, given the parameters of the procedure, mice could always earn the most milk by choosing the large-volume option, even when the delay was 100 s. Taking the longest delay as an example, mice could earn 100 ul of milk every 125 s by choosing the larger delayed option (100 s delay + 10 s dipper delivery + 15 s blackout). In that same amount of time, only 4 choices of the small volume could be completed, yielding 80 ul of milk. So, even at the longest delay, amount of milk per unit time would be maximized by choosing the larger volume.
Assessment of Response Perseveration
The results of the testing phase showed that adult B6 mice were least sensitive to increasing delays to the large volume of milk (see Results). As it has been reported that B6 mice may show perseverative response patterns, which may reflect control by stimulus features of the environment rather than control by food consequences (e.g., Olsen & Winder, 2009), we assessed the role of the contingency in the maintenance of nose poking. Adult B6 and D2 mice were studied an additional week following the delay discounting procedure. Each mouse was placed on an FR 1 schedule of milk delivery for 4 sessions. The only active nose poke hole was the one that produced the large volume of milk during the delay discounting procedure, though milk was present in both dipper wells to keep olfactory cues consistent with the previous phase. Responses were recorded in the inactive hole (i.e., the hole that previously provided access to the 20-ul volume of milk) but had no programmed consequences. Each nose poke in the active hole presented a 100-ul drop of milk for 10 seconds. Sessions ended after 30 milk presentations; in general, sessions were completed in approximately 6-8 min, including the time the dipper was presented. Mice always earned the total number of presentations possible during this time. Time to complete the session did not differ by strain on any day, as confirmed by t-tests (p > .05 for all tests). Nose poking was placed on extinction during the final session. During extinction, milk still was present in the dipper wells, but nose poking did not raise the dipper, again to control for olfactory cues. The extinction session lasted 30 min.
Results
During training, some mice failed to sample both choice alternatives. Specifically, failure to sample both alternatives was observed in adolescent D2 (n=3), adult D2 (n=1), and adult B6 (n=1) mice. Mice that did not sample both alternatives during training did not participate in the testing phase and their data were excluded from the analysis of the training segment, yielding 11-14 mice per group. Mice that sampled both options during training sessions continued to do so during each testing session, as has been commonly reported for the across-session discounting task (Koot et al., 2009; Adriani & Laviola, 2006; Adriani et al., 2004).
Over the course of training, a preference for the large volume of milk emerged in both strains Table 1 shows the mean percentage of large-volume choices for each session of training. A mixed-model ANOVA was used to analyze the data. The model included two between-subject factors (Age and Strain) and one within-subject factor (Session). The data were log-transformed prior to the analysis of delay discounting measures. The ANOVA revealed a significant main effects of Age (F(1,43) = 9.2, p < .004) and Session (F(6,258) = 12.8, p < .0001). There was no main effect of Strain, nor of any interaction term. The effect of age appeared to be due to greater preference for the larger volume in the choices of adult mice compared to those of adolescent mice. Age-related differences likely reflect different rates of acquiring preference, rather than any absolute difference in preference, as the data in Table 1 show that values for all strains converged by the end of training. As we were most interested in the data immediately prior to testing, we examined choice proportions from session 7 of training in greater detail. First, choice of the larger volume of milk exceeded 50% for all groups by session 7 (Adolescent D2, t = 3.6, df = 10, p < .002; Adolescent B6, t = 5.4, df = 13, p < .0003, Adult D2, t = 3.3, df = 10, p < .002; Adult B6, t = 6.3, df = 10, p < .003, Bonferroni corrected t-tests). Second, post-hoc analysis of the data from session 7 failed to identify differences among any of the groups (p > 0.05 for all comparisons).
Table 1.
Mean (SEM) percentage of choice of large milk volume across training sessions.
| Session | |||||||
|---|---|---|---|---|---|---|---|
| Age/Strain | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Adolescent | |||||||
| D2 | 49.4 (3.8) | 51.0 (4.3) | 54.6 (3.0) | 63.5 (2.8) | 61.6 (4.3) | 59.4 (5.5) | 64.8 (4.1) |
| B6 | 53.2 (2.5) | 55.0 (3.6) | 59.6 (2.4) | 63.4 (2.8) | 70.6 (2.6) | 63.4 (3.0) | 65.0 (2.8) |
| Adult | |||||||
| D2 | 56.3 (5.0) | 58.0 (8.2) | 67.3 (6.5) | 70.4 (6.7) | 65.7 (7.3) | 71.5 (4.8) | 69.8 (6.0) |
| B6 | 63.7 (3.4) | 69.1 (2.7) | 78.0 (1.7) | 76.1 (2.3) | 76.5 (3.3) | 74.6 (3.3) | 72.6 (3.6) |
In addition to changes in preference, the absolute number of choices made changed with training; in general, mice made more choices in later training sessions than in earlier sessions. Table 2 shows mean number of choices made during each session for each group. A mixed-model ANOVA revealed a significant main effect of Session (F(6,258) = 21.1 p < .0001) and main effect of Strain (F(1,43) = 20.3, p < .0001). Additionally, there were significant Strain × Session (F(6,258) = 5.7, p < .0001) and Session × Age (F(6,258) = 2.6, p < .016) interactions. Primarily, our concern was that groups might be different at the end of training, and so we were interested in comparisons among groups on the 7th training session. Post-hoc t-tests showed that adults of both strains and adolescent B6 mice made more choices than adolescent D2 mice (p's ranged from p < .047 − p < .005, uncorrected t-tests), but the number of nose pokes did not differ significantly among the remaining groups. Collectively, the data show that during training preference came under control of the large milk volume to a similar extent in all strains, though adolescent D2 mice made fewer absolute choices.
Table 2.
Mean (SEM) number of choices made during each session across training and testing phases. The number in parenthesis by each day indicates the delay to the large volume of milk; the delay to the small volume was always 1 s.
| Training Day | |||||||
|---|---|---|---|---|---|---|---|
| Age/Strain | 1 (1 s) | 2 (1 s) | 3 (1 s) | 4 (1 s) | 5 (1 s) | 6 (1 s) | 7 (1 s) |
| Adolescent | |||||||
| D2 | 18.9 (2.1) | 21.9 (3.6) | 27.6 (2.7) | 37.6 (3.3) | 35.4 (2.1) | 38.8 (3.5) | 38.2 (3.2) |
| B6 | 35.7 (2.4) | 45.1 (2.4) | 40.2 (2.7) | 47.1 (3.0) | 45.5 (3.6) | 49.8 (2.4) | 49.2 (2.6) |
| Adult | |||||||
| D2 | 31.1 (2.7) | 36.1 (4.9) | 38.7 (3.4) | 42.9 (3.8) | 46.8 (4.0) | 46.6 (3.5) | 47.3 (2.9) |
| B6 | 43.9 (1.5) | 51.6 (1.6) | 49.0 (2.0) | 48.8 (2.2) | 48.9 (2.7) | 50.0 (2.6) | 48.3 (2.9) |
| Testing Day | |||||||
| Strain-Age | 1 (1 s) | 2 (10 s) | 3 (20 s) | 4 (40 s) | 5 (60 s) | 6 (80 s) | 7 (100 s) |
| Adolescent | |||||||
| D2 | 38.1 (3.2) | 43.8 (1.5) | 40.8 (1.5) | 38.0 (2.9) | 38.7 (2.2) | 39.2. (2.8) | 40.1 (3.4) |
| B6 | 53.4 (3.2) | 47.7 (1.7) | 43.0 (1.6) | 40.7 (2.6) | 41.1 (3.1) | 41.6 (3.8) | 46.4 (5.3) |
| Adult | |||||||
| D2 | 47.7 (2.8) | 47.0 (1.5) | 43.3 (1.9) | 38.7 (2.4) | 40.4 (2.9) | 37.3 (3.3) | 41.5 (4.1) |
| B6 | 48.1 (2.5) | 43.2 (1.5) | 38.1 (0.8) | 29.5 (1.5) | 25.6 (1.3) | 26.8 (2.2) | 24.5 (2.7) |
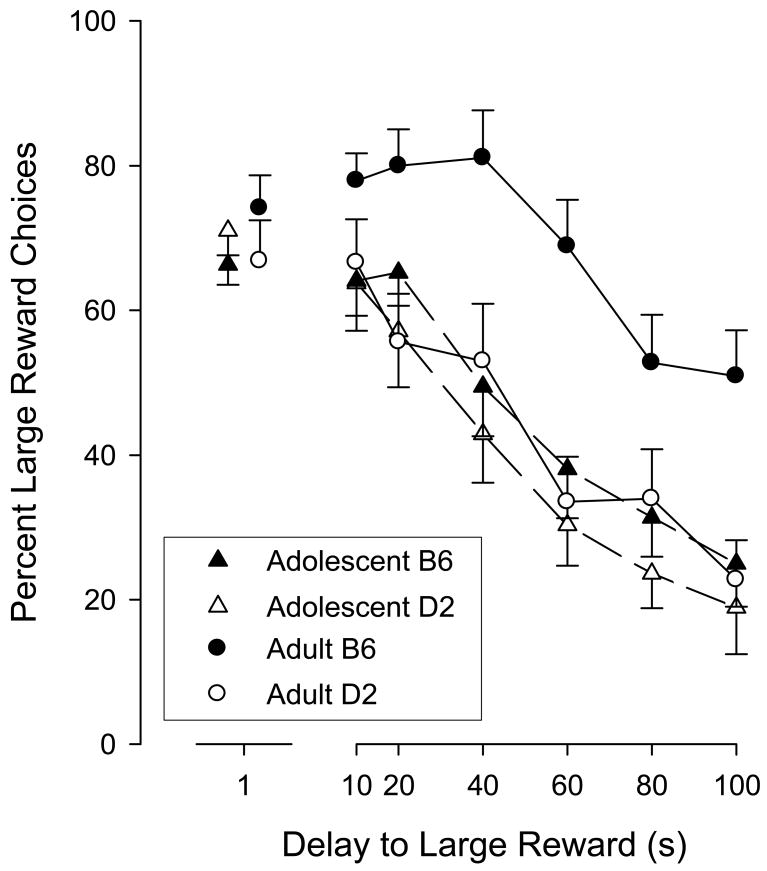
Figure 1 shows the effects of increasing the delay to the large milk volume on preference. As shown in the figure, increasing the delay to the large volume resulted in a shift in preference for the smaller, more immediate volume in all groups. There were, however, differences in the rate of preference change. Most noticeably, adult B6 mice were more tolerant of the increasing delay than the remaining groups, which were more similar to each other. A mixed-model ANOVA was used to analyze the data. The model included two between-subject factors (Age and Strain) and one within-subject factor (Delay). The ANOVA revealed a significant main effect of Strain (F(1,43) = 8.1, p < .01) and Delay (F(6,258) = 26.3, p < .0001). Importantly, the analysis also identified a significant Age × Strain interaction (F(1,43) = 11.9, p < .003) and a Age × Strain × Delay interaction (6, 258) = 3.8, p < .001). The significant three-way interaction reflects the increased tolerance to delay observed in B6 adult mice when compared to the other three strains.
Figure 1.
Age and strain differences in tolerance to delayed to reward. The y-axis shows the percentage of large-reward choices as a function of delay. Data from one-second delay test has been separated because it also constitutes a replication of the training segment. Error bars denote ± 1 SEM.
One consequence of the fixed session length is that the number of choice opportunities necessarily decreases with increasing delays to the large volume, and the number of choices could have fallen below a value that permits meaningful comparisons. Table 2 shows the average number of choices made for each session during the testing phase. Although the number of choices declined for the adult B6 mice, the data show that on average approximately 25 choices were still made, which approximates or exceeds the number of free choices commonly measured in other tests of delay discounting (e.g., Green, Myerson, & Calvert, 2010; Madden et al. 2010). So, the number of choices remained adequate enough for meaningful analysis of decision making, even under the longest delay.
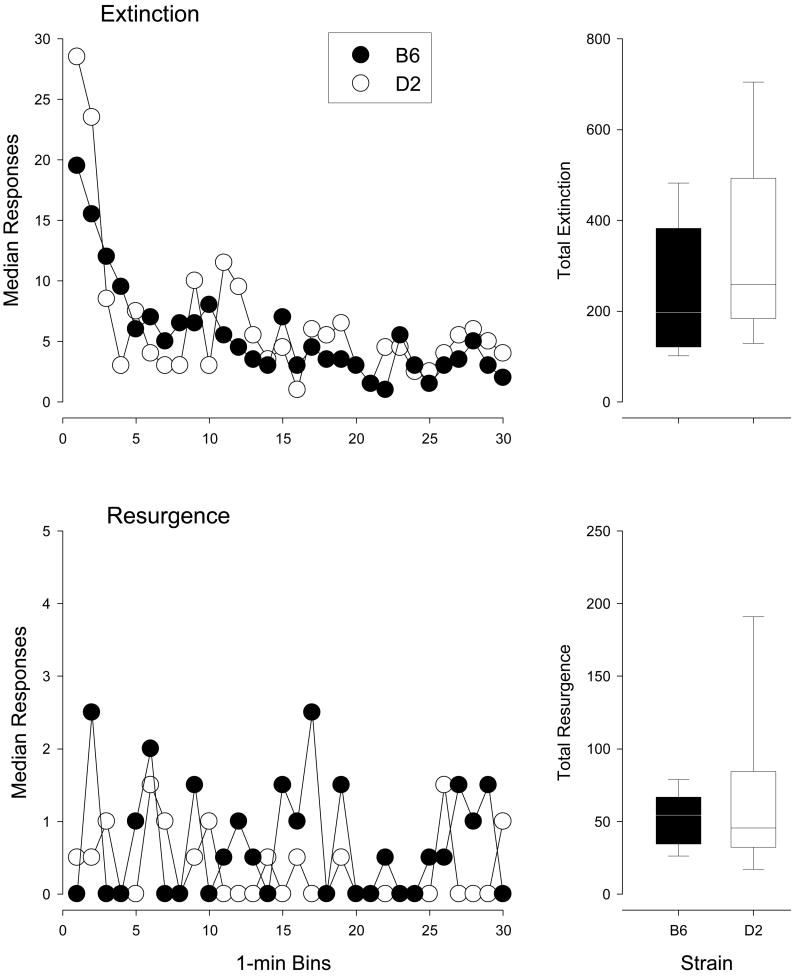
The relative insensitivity of adult B6 mice to increasing reward delay could have been due to perseverative response patterns. That is, choice may have been insensitive to the reinforcement contingency, and instead was under the control of some other stimulus feature of the environment. To address such possibilities, adult mice were examined for perseverative behavior in a resistance to extinction task (see Methods). As the data from the extinction sessions were not normally distributed, we analyzed the results in terms of group medians, rather than means. Prior to extinction, median responses per minute were 15.3 (adult D2) and 14.6 (adult B6) responses per minute; rates of responding were not different between strains (Wilcoxon test, p > .05). The results of the extinction test are shown in Figure 2. The upper left graph shows median responses per minute for each minute of extinction. Response rate was increased early in the extinction component, consistent with extinction “bursting” commonly observed upon reinforcement withdrawal (Minor, 1987; Weissman, 1959). Rate of responding declined across the remainder of the extinction test. Over the last 5 minutes of extinction, median rates were 4.2 (adult D2) and 3.4 (adult B6) responses per minute; these values were significantly less than values observed prior to extinction for both the D2 (Wilcoxon test, Z = -2.134, p < .033) and B6 (Wilcoxon test, Z = -2.432, p < .015) mice. The upper right graph shows box plots summarizing total response output across the 30-min extinction session. Total responses emitted during extinction did not differ between adult D2 and B6 mice (Mann-Whitney U, p > .05).
Figure 2.
Effects of extinction on fixed-ratio maintained behavior of adult mice. The top set of graphs show the effects of the removal of the reinforcement contingency on nose poking. The top left figure shows the median number of responses per minute of a 30-min extinction period for D2 (filled circles) and B6 (open circles) mice. The top right graph shows boxplots of the total number of responses emitted under extinction for each strain. The line is the median, the box indicates upper and lower quartiles, the error bars denote the 5th and 95th percentiles. The bottom set of graphs show data for nose poking on the inactive hole. Details are the same as in the top set of graphs.
Extinction of one operant response can result in recovery or “resurgence” of previously reinforced responses (e.g., Lieving & Lattal, 2003). So, it was of interest to examine responding on the inactive hole during extinction. On the session prior to extinction, median rates were 0.8 (D2) and 0.4 (B6) responses per minute. During extinction, responding on the active hole increased somewhat, reaching 1.6 (D2) and 1.5 (B6) responses per minute over the last 5 minutes of extinction, but these increases were not significantly different from the prior session when response rates were calculated over the last 5 minutes or over the entire session (Wilcoxon test, p > .05 in all cases). Total number of inactive hole responses are shown in box plots in the bottom right of Figure 2. Total number of responses did not differ under the extinction test (Mann-Whitney U, p > .05).
Collectively, the data show that removal of reinforcement resulted in increases in response rate early in the extinction test followed by decreasing rates over the remainder of the session. By the end of the extinction test, rates of responding were reduced compared to level under the FR 1 contingency. There was some increase in responding on the inactive hole, but those effects were not significant. Most importantly, B6 evinced no greater propensity than D2 mice to persist responding during the extinction test. Responding by both strains fell below levels maintained by the contingency once it was removed. Thus, it seems unlikely that the apparent insensitivity to delayed reinforcement observed in B6 mice in the prior condition was due to perseverative responding.
Discussion
The present experiment examined developmental changes in decision making in two inbred strains commonly used in substance abuse research. The data showed that during increasing delays to the large volume of milk, preference shifted to the smaller, sooner option, even though this reduced the overall amount of milk earned. Adolescent mice of both strains showed similar patterns of decision making and were similarly intolerant to delayed reinforcement. In contrast, there was a marked separation between strains tested during adulthood. Adult B6 mice were more tolerant to increasing delays and were slower to shift preference to the smaller immediate option when compared to adult D2 mice.
Though our findings were not explicitly anticipated, certain features of the data mirror prior results. First, the procedure, adopted from Adriani & Laviola (2003), has been shown to be sensitive to age-related differences in delay discounting. Similarly, the present findings revealed that adolescents B6 are more impulsive than their adult counterparts. Second, the strain differences obtained between adult mice has been reported previously using a different procedure. Helms et al. (2006) compared delay discounting performance between B6 and D2 mice using an adjusting amount procedure and found that adult D2 mice were more impulsive than adult B6 mice. As we have been able to demonstrate both developmental differences in impulsivity (adolescent B6 more impulsive than adult B6) and replicate between strain differences observed in adult mice (D2 mice more impulsive than B6 mice), it seems unlikely that the present findings are due to some idiosyncratic aspect of our design.
We also examined resistance to extinction in the adult mice to examine the possibility that the apparent tolerance to delay seen in the adult B6 mice was due perseverative responding. When adult mice could earn the large volume of milk from the same hole as it was earned the delay discounting task, rates of responding were similar between the two strains. When milk delivery was terminated, responding of both strains underwent extinction. For both strains, suspension of the reinforcement contingency resulted in increased levels of responding or extinction bursts (Minor, 1987; Weissman, 1959) early during the session, followed by a clear decline in responding across the remainder of the session. By the end of the session, response rates were reduced significantly compared to those maintained by the operant contingency. The two features of behavior under extinction, the extinction bursts and the decline in operant rate, are typical findings following the withdrawal of food reward and show that behavior established by the large volume of milk was sensitive to the contingency and was not preseverative in nature. Importantly, there were no discernable differences between D2 and B6 mice in terms of resistance to extinction in our preparation, suggesting that such processes played no role in the delayed shift in preference for the immediate reward seen in adult B6 mice. Thus, the resistance-to-extinction test indicates that responding by the adult B6 mice was sensitive to the consequences of nose poking.
On the other hand, our general findings may appear at odds with some previous reports. If greater sensitivity to immediate reward is associated with greater likelihood to use and abuse of substances, B6 mice may be expected to be more impulsive than D2 mice as they have been shown to display more propensity to consume and self-administer drugs of abuse such as ethanol (Belknap et al., 1993) and cocaine (Grahame & Cunningham, 1995). Other research, however, suggests previously reported strain differences may be due to specific motor differences and taste sensitivities. In conditioned place preference paradigms, for example, D2 mice are more sensitive to ethanol (Cunningham et al., 1992) and cocaine (Orsini et al., 2005) reward, and under some circumstances may self-administer more cocaine than B6 mice (van der Veen et al., 2007). Additionally, although D2 mice drink less alcohol via the oral route than B6 mice, when allowed to self-administer ethanol intravenously, D2 mice self-administer as much ethanol as B6 mice (Grahame & Cunningham, 1997), suggesting findings obtained with the oral route reflect a particular sensitivity of D2 mice to the orosensory properties of ethanol. More recently, Fish et al., (2010) showed that D2 mice are more sensitive than B6 mice to the rewarding effects of both ethanol and cocaine as measured by changes in brain-reward stimulation thresholds. Thus, a consideration of other factors suggests that D2 mice may be more sensitive to the rewarding effects of drugs of abuse than B6 mice, and in that regard, our data are in line with the general tendency for D2 mice to exhibit greater sensitivity to reward delay across the lifespan.
Finally, the differences in developmental trends observed between strains may parallel some features of human development. Moffitt (1993) identified two distinct populations of adolescent delinquency. Adolescent-limited deviancy reflects normative trends in impulsivity and decision making associated with adolescence. In the adolescent-limited type, adult roles are adopted as the individual matures and the rash, impulsive behavior of adolescence subsides. Life-course persistent delinquency, on the other hand, continues into adulthood; those individuals continue displaying maladaptive behavior patterns, such as drug use and criminal activity. Given the link between sensitivity to delayed reward and maladaptive behavior, it may be that the persistent behavior patterns shown by the D2 strain may provide a foundation for exploration into the biological mechanisms of more persistent forms of impulsive decision making, while the B6 strain may be better suited to exploration of more normative developmental patterns where impulsivity is elevated during adolescence and attenuates during adulthood (Green et al., 1999; Olson et al., 2007; Steinberg et al., 2009).
Acknowledgments
This work was supported by Grant AA012337. The authors thank Gerardo Martinez for his assistance.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behavioral Neuroscience. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Delay aversion but preference for large and rare rewards in two choice tasks: implications for the measurement of self-control parameters. BMC Neuroscience. 2006;23:52. doi: 10.1186/1471-2202-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriani W, Rea M, Baviera M, Invernizzi W, Carli M, Ghirardi O, Caprioli A, Laviola G. Acetyl-L-carnitine reduces impulsive behavior in adolescent rats. Psychopharmacology. 2004;176:296–304. doi: 10.1007/s00213-004-1892-9. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Developmental Review. 1992;12:339–373. [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug & Alcohol Dependence. 2007;90:S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Science. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental & Clinical Psychopharmacology. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Jr, Johnson NA, Gray DK, Kosobud A, Young ER. Biphasic effects of ethanol on open-field activity: sensitivity and tolerance in C57BL/6N and DBA/2JN mice. Journal of Comparative & Physiological Psychology. 1982;96:440–451. doi: 10.1037/h0077898. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology, Biochemistry, & Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology. 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fish EW, Riday TT, McGuigan MM, Faccidomo S, Hodge CW, Malanga CJ. Alcohol, cocaine, and brain stimulation-reward in C57Bl6/J and DBA2/J mice. Alcoholism: Clinical & Experimental Research. 2010;34:81–89. doi: 10.1111/j.1530-0277.2009.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. Intravenous ethanol self-administration in C57BL/6J and DBA/2J mice. Alcoholism: Clinical & Experimental Research. 1997;21:56–62. [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. Genetic differences in intravenous cocaine self-administration between C57BL/6J and DBA/2J mice. Psychopharmacology. 1995;122:281–291. doi: 10.1007/BF02246549. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, Calvert AL. Pigeons' discounting of probabilistic and delayed reinforcers. Journal of the Experimental Analysis of Behavior. 2010;94:113–123. doi: 10.1901/jeab.2010.94-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Ostaszewski P. Discounting of delayed rewards across the life span: age differences in individual discounting functions. Behavioural Processes. 1999;46:89–96. doi: 10.1016/S0376-6357(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and d-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology. 2006;188:144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Koot S, Adriani W, Saso L, van den Bos R, Laviola G. Home cage testing of delay discounting in rats. Behavior Research Methods. 2009;41:1169–1176. doi: 10.3758/BRM.41.4.1169. [DOI] [PubMed] [Google Scholar]
- Lieving GA, Lattal KA. Recency, repeatability, and reinforcer retrenchment: an experimental analysis of resurgence. Journal of the Experimental Analysis of Behavior. 2003;80:217–233. doi: 10.1901/jeab.2003.80-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Johnson PS. A delay-discounting primer. In: Madden GJ, Bickel's WK, editors. Impulsivity: the behavioral and neurological science of discounting. Washington, DC: APA Books; 2010. pp. 11–37. [Google Scholar]
- Madden GJ, Johnson PS, Brewer AT, Pinkston JW, Fowler SC. Effects of pramipexole on impulsive choice in male wistar rats. Experimental & Clinical Psychopharmacology. 2010;18:267–276. doi: 10.1037/a0019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opiod-dependent and non-drug-using control patients: drug and monetary rewards. Experimental & Clinical Psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- McKinney WT. Overview of the past contributions of animal models and their changing place in psychiatry. Seminars in Clinical Neuropsychiatry. 2001;6:68–78. doi: 10.1053/scnp.2001.20292. [DOI] [PubMed] [Google Scholar]
- Minor TR. Stimulus- and pellet-induced drinking during a successive discrimination. Journal of the Experimental Analysis of Behavior. 1987;48:61–80. doi: 10.1901/jeab.1987.48-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course persistent antisocial behavior: a developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34:1685–1694. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EA, Hooper CJ, Collins P, Luciana M. Adolescents' performance on delay and probability discounting tasks: contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Personality and Individual Differences. 2007;43:1886–1897. doi: 10.1016/j.paid.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2J inbred strains. Psychopharmacology. 2005;181:327–336. doi: 10.1007/s00213-005-2259-6. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. Journal of Abnormal Psychology. 2001;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Petry NM, Madden GJ. Discounting and pathological gambling. In: Madden GJ, Bickel's WK, editors. Impulsivity: the behavioral and neurological science of discounting. Washington, DC: APA Books; 2010. pp. 273–294. [Google Scholar]
- Risinger FO, Brown MM, Doan AM, Oakes RA. Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol, Clinical & Experimental Research. 1998;22:677–684. doi: 10.1111/j.1530-0277.1998.tb04311.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O'Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- van der Veen R, Piazza PV, Deroche-Gamonet V. Gene-environment interactions in vulnerability to cocaine intravenous self-administration: a brief social experience affects intake in DBA/2J but not in C57BL/6J. Psychopharmacology. 2007;193:179–186. doi: 10.1007/s00213-007-0777-0. [DOI] [PubMed] [Google Scholar]
- Weissman A. The behavioral effects of repeated exposure to three mixed extinction schedules. Journal of the Experimental Analysis of Behavior. 1959;2:255. doi: 10.1901/jeab.1960.3-115. [DOI] [PMC free article] [PubMed] [Google Scholar]