Abstract
SWAN is a NIH-funded, multi-disciplinary, longitudinal observational study of middle-aged US women of multiple ethnicities in or near Boston, Newark, Pittsburgh, Detroit, Chicago, Oakland, and Los Angeles. SWAN measures multiple variables annually, including spine and hip areal bone mineral density (aBMD), serum and urine indices of bone remodeling, and pituitary, ovarian, and adrenal hormones. Published SWAN data show:
ethnic differences in pre-menopausal aBMD and peri-menopausal bone loss are greatly affected by body weight;
aBMD of pre-menopausal and early peri-menopausal women is inversely correlated with serum FSH, not estradiol, when these are measured once-yearly on menstrual cycle day 2-5;
rates of bone loss in middle-aged women correlate with once-yearly measurements of serum FSH, not serum estradiol;
middle-aged women do not lose significant bone until their menses become less frequent;
at that time, bone loss is as rapid as during the years immediately after the final menses.
Keywords: menopause, bone, osteoporosis, weight, bone turnover
The Study of Women's Health Across the Nation (SWAN) is a cross-sectional and longitudinal observational study of middle-aged women of multiple ethnicities, at seven sites across the United States of America. SWAN's investigators are multi-disciplinary and are evaluating many physiological, psychological, and social aspects of middle-aged women.
The SWAN Bone Study is being carried out in 2,176 women sampled from Boston, Pittsburgh, Detroit, Oakland and Los Angeles areas, who were self-defined as Caucasian, African-American, Chinese, or Japanese (approximately half the women in each locale were self-defined Caucasians). At entry all SWAN participants were age 42-52 and either pre-menopausal or early peri-menopausal. SWAN classifies women pre-menopausal if they have had monthly bleeding during each of the last three months and have noted no change in their prior individual menstrual pattern, but classifies them early peri-menopausal if they have had menstrual bleeding in one, two, or three of the last three months and have also noted a change in bleeding pattern from their prior menstrual pattern. Women in the SWAN Bone Study have annual measurements of lumbar spine and hip aBMD by Hologic dual energy x-ray absorptiometry (DXA). A local Hologic anthropomorphic spine phantom is measured daily on each densitometer and a circulating Hologic anthropomorphic spine phantom is measured periodically on all densitometers. If retroactive analyses of these phantom measurements reveal significant longitudinal and/or cross-sectional deviation in the calibration of any densitometer, participant measurements from that densitometer during that interval are retroactively adjusted by the SWAN quality control center to eliminate this effect. Each SWAN Bone Study participant also has annual measurements of fasting morning urine type I collagen N-telopeptide (NTx), and fasting morning serum FSH, estradiol, testosterone, sex hormone binding globulin (SHBG), and dehydroepiandrosterone-sulfate (DHEA-S), usually on day 2-5 of the woman's menstrual cycle (at least during the time when her menses are still regular). Serum osteocalcin was measured on samples from the first 2 to 3 visits. These urine and serum measurements are made in a central laboratory with extensive quality control procedures to prevent longitudinal assay drift and bias.
SWAN Bone Study Baseline Analyses
SWAN baseline DXA measurements in 2,176 women show a striking effect of ethnicity on spine aBMD, which is highest in the African-Americans and lowest in the Japanese. The other ethnic groups have intermediate values and all groups are significantly (p < 0.05) different from each other.
To interpret these ethnic differences in aBMD in a study with such diverse participants, one must take into account various other ethnic-specific differences. For example, smoking incidence differs significantly by ethnicity (lower in SWAN Asian-Americans) and the percentage of women menstruating regularly (premenopausal) at baseline is significantly higher in SWAN Japanese than in the other three ethnic groups. Body weight is substantially higher in SWAN's African-American participants than in the other ethnic groups, and significantly lower in SWAN's Asian-American participants: the average African-American participant weighs approximately 27 kilograms more than the average Asian-American participant, and there is almost no overlap in body weight between these two ethnic groups.
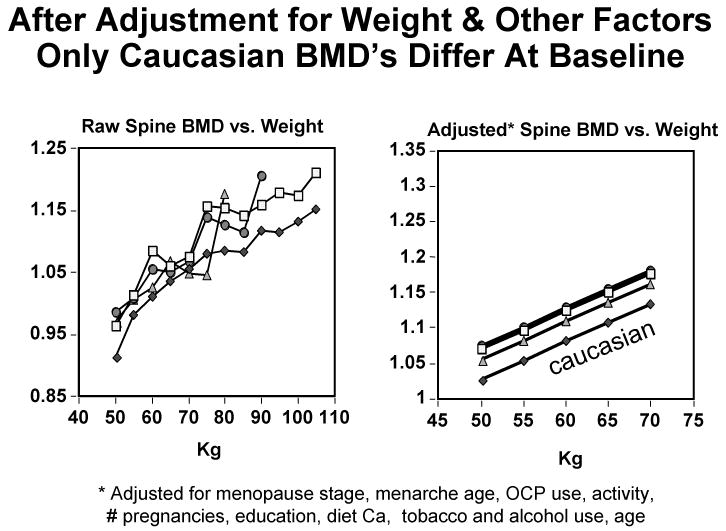
After adjustment for body weight, baseline spine aBMD is similar in African-American, Chinese, and Japanese SWAN participants, and only Caucasians differ significantly (having lower aBMD than the other three groups: Figure 1, left panel). This remains true if the comparison is restricted to weight-matched participants, even after additional adjustments for menopausal stage, menarche age, oral contraceptive use, physical activity, number of pregnancies, education, diet calcium, tobacco and alcohol use, and age (Figure 1, right panel). Body weight thus has a dramatic effect on the aBMD of pre-menopausal and early peri-menopausal women in the spine. The same is true of hip aBMD.1
Figure 1.
Left panel: Mean unadjusted baseline PA spine aBMD vs. body weight in African-American (□), Japanese (△), Chinese (○), and Caucasian (◆) SWAN participants. Right panel: Mean baseline PA spine aBMD vs. body weight in a sub-set of weight-matched participants, following adjustment for their other significant characteristics.1
Baseline spine aBMD is also significantly (p < 0.05) related to baseline serum FSH: the higher the serum FSH, the lower the spine aBMD. In contrast, serum estradiol is not significantly correlated with spine or hip bone mineral density at baseline.2 This calls into question the assumption that high serum FSH levels are related to low bone mineral density because FSH increases in response to estrogen lack (see Discussion below). To explore further the relationship between FSH, estrogen lack, and bone, baseline urine NTx and serum osteocalcin were compared with baseline serum FSH and estradiol. Both urine NTx and serum osteocalcin show a threshold relationship with serum FSH, with uniform mean values across the first, second, and third quartiles of baseline serum FSH, but 10% higher mean values in women whose baseline serum FSH is in the highest (fourth) quartile. Urine NTx and serum osteocalcin show a weaker correlation with serum estradiol at the inception of SWAN, and no threshold relationship.3
To evaluate the effect of ethnicity on serum osteocalcin and urine NTx in pre-menopausal and early peri-menopausal middle-aged women, we made comparisons across ethnic groups after adjusting for body weight or body mass index, diabetes, TSH, menopause age, FSH, alcohol intake, medications, smoking, age, season of the year, and geographic location (we did not correct for serum estradiol because it was not significantly related to bone turnover).4 After these adjustments, serum osteocalcin and urine NTx are highest in Caucasians and lowest in Chinese, with intermediate or similar values in African-American or Japanese, but all these inter-ethnic differences are small.
In summary, SWAN baseline analyses show that pre-menopausal and peri-menopausal DXA spine and hip bone mineral density are strongly influenced by weight, which accounts for 21 percent and 7 percent of the variation respectively; are similar in African-American, Chinese, and Japanese, but lower in Caucasians, after adjustment for weight and other factors; and are inversely correlated with serum FSH, but not with serum estradiol. The baseline SWAN analyses also show that pre-menopausal and peri-menopausal indices of bone turnover (serum osteocalcin and urine NTx) vary for unclear reasons (only 9-16 percent of their total variation can be attributed to the numerous variables we tested). In addition, serum osteocalcin and urine NTx show a threshold relationship with serum FSH, and not with serum estradiol, but this is not very meaningful because serum FSH accounts for only 1-3 percent of the inter-individual variation in serum osteocalcin and urine NTx.
SWAN Bone Study Follow-Up Analyses
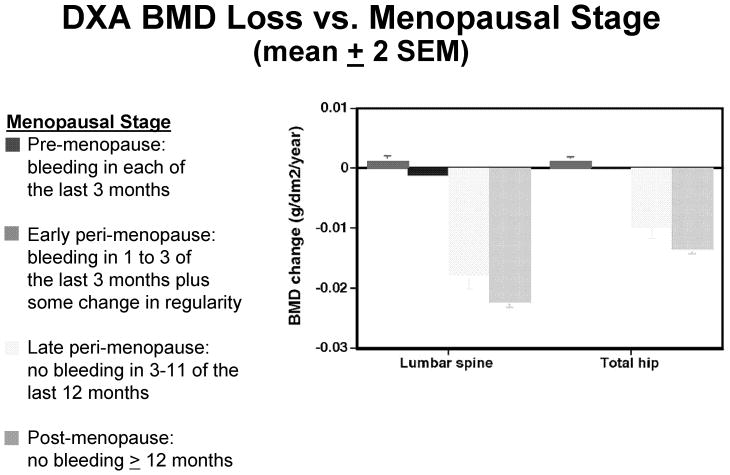
SWAN Bone Study investigators have to date reported in the medical literature up to five annual follow-up measurements. A SWAN participant can contribute data to one or more menopausal categories, and the reported data include 2,451 observations in women when they were pre-menopausal, 5,080 observations in women when they were early peri-menopausal, 548 observations in women when they were late peri-menopausal, and 486 observations in women when they were post-menopausal at the time of their evaluation. SWAN defines pre-menopausal and early peri-menopausal as above; late peri-menopausal as bleeding in at least 1 of the last 11 months but not in the last 3 months, and post-menopausal as no bleeding in the past twelve or more months. In the SWAN analyses reviewed here, all data are censored after hysterectomy, use of estrogen or drugs that alter circulating estrogen, use of progestin, or prescription drug treatment for osteoporosis. Mean annual changes in aBMD are estimated using repeated measures linear mixed models to account for the correlation among each individual woman's serial aBMD measurements. These estimates of mean annual bone loss are then adjusted for baseline weight, weight change, age, ethnicity, intake of calcium and vitamin D and alcohol, smoking, physical activity, and geographic location. Figure 2 shows that spine and hip bone loss are insignificant in pre-menopausal and early peri-menopausal women, but very rapid in late peri-menopausal and post-menopausal women.5 In fact, the rate of bone loss in late peri-menopause is as rapid as in newly post-menopausal women. These differences persist when the spine aBMD loss is examined as a function of menopausal stage within each ethnicity. In women of every ethnicity measured, SWAN data reported to date show late peri-menopausal bone loss is rapid and essentially equivalent to post-menopausal bone loss, whereas pre-menopausal and early peri-menopausal bone loss are either not occurring, or occurring at rates too low to be medically significant.5
Figure 2.
Annual decrease in PA spine (left) and total hip (right) aBMD (mean ± 2 SEM) as a function of menopausal stage.5
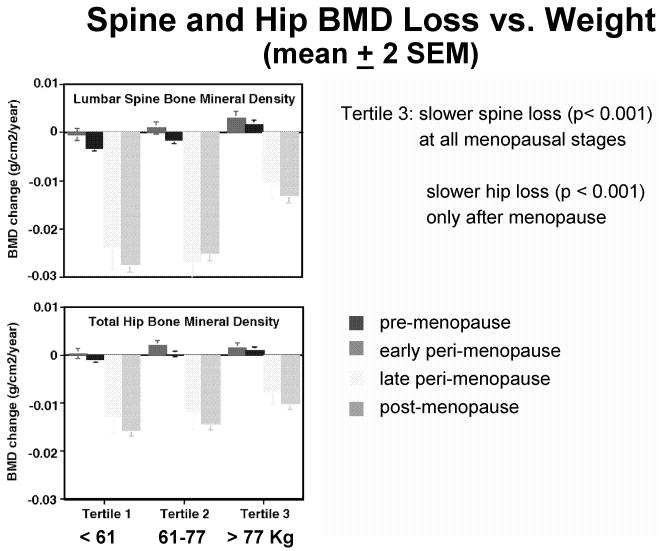
To assess the effects of body weight, ethnicity, or other variables on mid-life bone loss, SWAN investigators therefore adjust participants' yearly bone loss for the simultaneous menopausal status. Figure 3 shows the mean annual loss of bone at spine and hip (± SEM) in women of the lowest, highest, and middle weight tertiles, broken down by menopausal stage.5 In the heaviest women (tertile 3, weighing ≥ 77 kg), spine aBMD declines slower (p < 0.001) at all menopausal stages, and this difference may become medically-significant during late-peri-menopause and post-menopause. In these heaviest women (tertile 3), hip aBMD also declines slower (p < 0.001) post-menopause. After body weight is taken into account, ethnicity no longer has any significant impact on the rate of bone loss at the spine or at the hip, as demonstrated by multi-variate statistical analysis, and by comparisons of serial bone loss in women of various ethnicities matched for weight.5
Figure 3.
Mean annual decrease (± 2 SEM) in PA spine (top) and total hip (bottom) aBMD as a function of body weight and menopausal stage.5
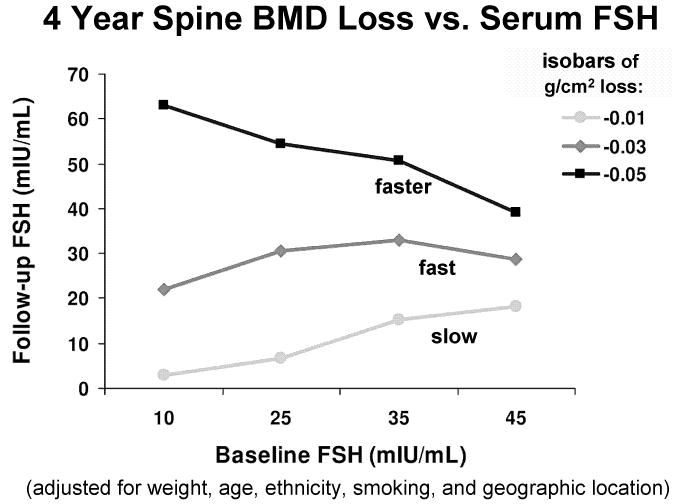
The rate of decline in aBMD is not related to the serum estradiol (measured as above) but is related to serum FSH.6 Bone loss is fastest in SWAN participants with a low baseline serum FSH and a high final serum FSH, or a high serum FSH throughout the study (Figure 4). Bone loss is slowest in SWAN participants with a low serum FSH throughout the study, or a high baseline FSH that subsequently decreases substantially (Figure 4).
Figure 4.
Isobars of 4-year decline in PA spine aBMD, as a function of initial serum FSH (horizontal axis) and final serum FSH (vertical axis).6 Note different scales of the two axes.
In summary, SWAN follow-up analyses to date show that spine and hip aBMD decrease rapidly during late-perimenopause, at annual rates as fast as post-menopause, and these rates of bone loss are slower in the women of highest weight. Although the rates of bone loss vary across ethnic groups, these differences are mostly attributable to ethnic differences in weight. Rates of bone loss correlate with serum FSH and changes in serum FSH, but not with serum estradiol, testosterone, SHBG, DHEA-sulfate, or changes therein. Inter-individual variations in serum osteocalcin and urine NTx largely reflect unknown variables.
Discussion
It is important to consider why aBMD, and serial decreases in aBMD, are related to serum FSH but not serum estradiol. Serum estradiol varies dramatically across the menstrual cycle, and is at its nadir on days 2-5 of the cycle, when SWAN measurements are made. The skeleton presumably responds to the average serum estradiol (which is much higher than the serum estradiol on days 2-5). If this average correlates poorly with the serum estradiol on cycle days 2-5, then SWAN's serum estradiol measurements will not adequately reflect the average serum estradiol to which the bones are exposed. A second possibility is that elevated serum FSH may accelerate bone loss directly, as recently suggested by others.7,8 If so, then bone loss should be faster in animals and humans after ovariectomy (which lowers serum estradiol and raises serum FSH) than after long-acting GnRH agonist or antagonist treatment (which lowers both). Because GnRH agonists and antagonists initially stimulate gonadal function, and inhibit it only later, it is necessary to make such comparisons long after the induction of estrogen deficiency in animals and/or humans. In humans it is also necessary to make this comparison after adjusting for residual serum estradiol, weight, smoking, and other variables.
It is also important to understand why body weight is correlated with basal aBMD and with serial declines in aBMD in middle-aged women (SWAN's data exclude secretion of estradiol by adipose tissue as a cause). Because DXA corrects for the cross-sectional area but not the depth of a bone, aBMD measurements conflate true bone mineral density and bone size. To the extent that heavy women have isometrically-larger bones, this could account for some or all of their higher aBMD at baseline. But this artifact cannot account for the fact that women in the highest tertile lose bone more slowly, even after correcting for other variables. DXA assumes that skeletal pixels have the same fat:cell ratio as neighboring para-skeletal pixels, and subtracts this as a background. If neighboring para-skeletal pixels in fact have a higher fat:cell ratio than the bone pixels, too little background will be subtracted and aBMD will be erroneously inflated.9,10 Similarly, if progressive obesity increases peri-renal fat selectively, and thereby increases the fat:cell ratio of para-spinal tissues more than it increases the fat:cell ratio in the neighboring lumbar spine pixels, this artifact will increase calculated lumbar spine aBMD even if the true lumbar spine BMD remains constant.9,10 But this artifact cannot explain slower decreases in aBMD among heavy women whose tissues have a stable fat:cell ratio. The impact of such technical artifacts on baseline aBMD and serial changes in aBMD can be examined by using CT, MRI, or other techniques that correct for bone depth and are insensitive to the fat:cell ratio of extra-skeletal tissues.
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495).
The content of this review is solely the responsibility of the author and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
References
- 1.Finkelstein JS, Lee ML, Sowers M, Ettinger B, Neer RM, Kelsey JL, Cauley JA, Huang MH, Greendale GA. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002 Jul;87(7):3057–67. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 2.Sowers MR, Finkelstein JS, Ettinger B, Bondarenko I, Neer RM, Cauley JA, Sherman S, Greendale GA, Study of Women's Health Across the Nation The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int. 2003 Jan;14(1):44–52. doi: 10.1007/s00198-002-1307-x. [DOI] [PubMed] [Google Scholar]
- 3.Sowers MR, Greendale GA, Bondarenko I, Finkelstein JS, Cauley JA, Neer RM, Ettinger B. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003 May;14(3):191–7. doi: 10.1007/s00198-002-1329-4. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein JS, Sowers M, Greendale GA, Lee ML, Neer RM, Cauley JA, Ettinger B. Ethnic variation in bone turnover in pre- and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002 Jul;87(7):3051–6. doi: 10.1210/jcem.87.7.8480. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, Neer RM. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008 Mar;93(3):861–8. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, Neer RM, Johnston J, Ettinger B. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006 Apr;91(4):1261–7. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006 Apr 21;125(2):247–60. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci U S A. 2006 Oct 3;103(40):14925–30. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolotin HH, Sievänen H, Grashuis JL, Kuiper JW, Järvinen TL. Inaccuracies inherent in patient-specific dual-energy X-ray absorptiometry bone mineral density measurements: comprehensive phantom-based evaluation. J Bone Miner Res. 2001 Feb;16(2):417–26. doi: 10.1359/jbmr.2001.16.2.417. [DOI] [PubMed] [Google Scholar]
- 10.Bolotin HH, Sievänen H, Grashuis JL. Patient-specific DXA bone mineral density inaccuracies: quantitative effects of non-uniform extra-osseous fat distributions. J Bone Miner Res. 2003 Jun;18(6):1020–7. doi: 10.1359/jbmr.2003.18.6.1020. [DOI] [PubMed] [Google Scholar]