SUMMARY
From the realization that cell number homeostasis is fundamental to the biology of all metazoans, and that deregulation of this process leads to human diseases, enormous interest has been devoted over the past two decades to map the requirements of cell death and cell survival. This effort has led to tangible progress, and we can now chart with reasonable accuracy complex signaling circuitries controlling cell fate decisions. Some of this knowledge has translated into novel therapeutics, and the outcome of these strategies, especially in cancer, is eagerly awaited. However, the function of cell death modifiers have considerably broadened over the past few years, and these molecules are increasingly recognized as arbiters of cellular homeostasis, from cell division, to intracellular signaling, to cellular adaptation. This panoply of functions is best exemplified by members of the Inhibitor of Apoptosis (IAP) gene family, molecules originally narrowly defined as endogenous caspase inhibitors, but now firmly positioned at the crossroads of multiple normal and transformed cellular responses.
Keywords: IAP, survivin, mitochondria, NFκB, cancer therapy
INTRODUCTION
Although there are various morphologically distinct, and biochemically separate forms of cell death, only apoptosis embodies an orderly genetic program of cellular suicide [1]. This process is designed to sculpt the developing organism [2], and maintain the cell number homeostasis of tissues and organs throughout adult life [3]. Deregulation of apoptosis is a pathogenic factor of many human diseases, and aberrantly increased cell survival is a hallmark of virtually every human tumor [4].
Extensive studies over the past two decades have identified two main pathways by which mammalian cells commit suicide [5]. An extrinsic apoptotic pathway is activated by ligand binding to so-called death receptors at the cell surface, molecules structurally reminiscent of the tumor necrosis factor (TNF) receptor. This results in the assembly of a multiprotein complex associated with the cytosolic tail of the receptor, and culminates with the activation of upstream caspase-8 [6]. An intrinsic pathway of apoptosis is activated by genotoxic, metabolic and other stimuli, and is centered on a sudden loss of mitochondrial integrity [7]. Dubbed “mitochondrial permeability transition” [8], this process ultimately leads to the rupture of the organelle outer membrane with discharge of apoptogenic proteins normally stored in the mitochondrial intermembrane space, in particular cytochrome c [7]. Once released in the cytosol, cytochrome c assembles in a large supramolecular complex called apoptosome that promotes the activation of initiator caspase 9 via induced proximity [9]. Regardless of the triggering stimulus, active initiator caspases promote the downstream processing of executioner caspases, which dismantle a cell's architecture imparting the classical morphological features of apoptosis [9]. There is extensive crosstalk between the two apoptotic pathways of apoptosis, and mechanisms for signal amplification in selected cell types have been described [9].
Among the regulators of cell death, the Bcl-2 gene family comprises both apoptosis inducers and apoptosis inhibitors [10]. These molecules are structurally diverse, and form heteromeric complexes to control mitochondrial integrity, especially at the level of outer membrane permeability [10]. In contrast, the Inhibitor of Apoptosis (IAP) family of proteins was originally characterized as physical inhibitors of caspases [11], providing a cytoprotective step downstream of death receptor or mitochondrial apoptosis. However, studies over the past few years have uncovered a far more complex biology of IAPs with broadened roles in various facets of cellular homeostasis [12, 13]. The review of these multiple IAP functions is the main theme of the present article. Excellent contributions covering virtually every aspect of cell death regulation, including mechanisms of death receptor activation [6], mitochondrial permeability transition [7, 8], apoptosis modifiers [10, 13], or caspases [14] have been published in the premiere literature, and the reader is directed to those articles for a more in depth perspective.
The biochemistry of IAPs: the “old” caspase inhibitors
IAPs are recognized by the presence of a ~70 amino acid Baculovirus IAP Repeat (BIR), a zinc finger fold present at least once in each family member [13] (Fig. 1). The eight IAPs in humans contain one to three BIRs, typically arranged in the protein's amino-terminus. Several mammalian IAPs, for instance c-IAP1, c-IAP2 and XIAP contain additional structural domains, including a carboxyl-terminus RING, which functions as an E3 ubiquitin ligase, a ubiquitin-associated domain implicated in binding to ubiquitinated proteins, and a caspase-recruitment domain (CARD, in c-IAP1 and c-IAP2), of less clear function (Fig. 1). There is extensive modularity in the assembly of these domains, and different IAPs can variously display BIRs as well as other protein domains (Fig. 1).
Figure 1. Schematic diagram of domain structure in representative IAP proteins.
The individual domains found in IAPs and how they are variously assembled in representative members of the IAP gene family are shown. BIR, Baculovirus IAP repeat; CARD, caspaserecruitment domain; DIAP1, Drosophila IAP1, UBA, binding site for poly-ubiquitinated proteins.
Compared to other IAPs, survivin is structurally unique. At 142 amino acids, survivin is the smallest mammalian IAP, containing a single BIR and a long carboxyl-terminus α-helix, but no other identifiable protein domain. Structural data suggest that survivin forms a stable homodimer in solution [15], but definitive evidence that this organization is required for function(s) is still lacking. Conversely, certain aspects of survivin nucleo-cytoplasmic trafficking [16], and key protein recognition, for instance binding to the chromosomal passenger protein, Borealin [17, 18], appear to require the monomeric protein.
BIRs mediate protein recognition and protein-protein interactions [13]. Accordingly, a deep peptide-binding groove in the BIRs of XIAP, c-IAP1 and c-IAP2 serves as a hydrophobic recognition site for proteins containing a IAP-binding motif (IBM). The IBM is a tetrapeptide region with an invariant amino-terminal Ala and other conserved residues found in initiator (caspase 9), and effector (caspase 7) caspases [19], as well as in certain apoptosis inducers, for instance Smac/DIABLO [20]. Not all BIRs contain a “canonical” IBM recognition motif [21]. For instance, BIR1 in XIAP does not bind IBM-containing proteins, but recognizes molecules implicated in Nuclear Factor-κB (NF-κB) activation (see below) [22, 23]. Similarly, the BIRs in survivin and some of its likely orthologs in yeast, C.elegans or Drosophila do not appear to contain an IBM-binding motif. However, this is clearly not a rigid rule, as survivin binds IBM-containing Smac/DIABLO [24], in a complex that resembles Smac interaction with XIAP BIR3 [25].
One of the most studied IBM-dependent complexes is the interaction between IAPs and caspases [19], which obliterates their enzymatic activity. Historically, this has been the role proposed for all IAPs [26], expanding a cytoprotective function first observed with the viral orthologs of these proteins [27]. However, we now know that only one mammalian IAP, XIAP is truly a physiologic inhibitor of caspases, in vivo [12]. Other IAPs, for instance c-IAP-1 and c-IAP-2 bind caspases in vitro, but these interactions are unlikely to be physiologically meaningful, in vivo. Conversely, XIAP associates with executioner caspase-3 and -7, as well as initiator caspase-9 with high affinity, shutting off their cell killing ability. The structural requirements of these interactions have been worked out in detail [28]. With respect to executioner caspases, it is the XIAP linker region upstream of BIR2 that inserts into the catalytic cleft of the enzymes, preventing substrate accessibility, and thus blocking activity [29-31]. Instead, XIAP binds caspase-9 through its BIR3, associating with the homo-dimerization domain of the enzyme, and preventing the conformational change that is necessary for activity [32].
In addition, XIAP contains a RING domain (Fig. 1) involved in cell death regulation [13]. How this happens, however, has not been conclusively elucidated. Earlier work with IAPs orthologs in Drosophila suggested that the E3 ligase activity of the RING catalyzed a nondegradative ubiquitination step of bound caspase [33], blocking substrate access to their catalytic sites [34]. A similar paradigm has been proposed for mammalian IAPs, but in this case RING-mediated poly-ubiquitination of caspase-3 and -7 was degradative, and resulted in proteasomal destruction of the modified caspase [35]. Recent evidence reinforced the role of the RING in cytoprotection, as mice expressing a BIR-only form of XIAP, thus deleted in the RING, exhibited higher caspase activity, and increased cell death, in vivo [36].
Similar to all other IAPs except XIAP, survivin does not directly bind caspases [13]. Instead, a prevailing model is that survivin inhibits apoptosis via cooperative interactions with other partners, in vivo. An example of these interactions is an IAP-IAP complex between survivin and XIAP [37]. The structure of this recognition is not yet available, but biochemical data suggest that survivin BIR residues 15-38 [38] associate with discontinuous sites in XIAP BIR1 and BIR3 [37]. IAP-IAP complexes may provide a general mechanism to expand the functional repertoire of these molecules, as survivin also interacts with the large IAP, BRUCE [39], as well as c-IAP1 [37], in the control of cytokinesis and the mitotic spindle checkpoint [40].
The biological implications of a survivin-XIAP interaction are complex (Fig. 2). Current evidence suggests that only a pool of survivin compartmentalized in mitochondria, and released in the cytosol in response to cell death stimuli [41], has the ability to associate with XIAP, and this recognition is inhibited by survivin phosphorylation on Ser20 by protein kinase A (Fig. 2) [38]. Functionally, a survivin-XIAP complex enhances XIAP stability against ubiquitin-dependent degradation, synergistically increases the activity of XIAP for caspase inhibition [37, 38], promotes tumor growth, in vivo [38], and directly participates in XIAP-mediated intracellular signaling, in particular NF-κB activation (see below) [42] (Fig. 2). This IAP-IAP complex may also reciprocally control survivin stability, as a XIAP-associated molecule, XAF-1, promotes RING-mediated poyubiquitination and proteasomal destruction of survivin [43]. Other mechanisms of survivin cytoprotection have been proposed, including the ability of mitochondria-localized survivin to sequester pro-apoptotic Smac/DIABLO away from XIAP [24], or altogether prevent its release from mitochondria [44], although the functional implications of this pathway have not been clearly defined [45].
Figure 2. Survivin cytoprotection involves a pathway of cytoplasmic-mitochondrial shuttling and intermolecular cooperation with XIAP.
A pool of survivin is recruited to mitochondria, mostly of tumor cells and released in the cytosol in response to cell death stimuli. Mitochondrially released survivin forms a complex with XIAP that is negatively regulated by protein kinase A (PKA) phosphorylation of survivin on Ser20, and results in increased XIAP stability against proteasomal degradation, enhanced gene expression, i.e. NF-κB, and synergistic inhibition of effector and initiator caspases (a schematic diagram of caspase 9 is shown).
More than caspase inhibition: other IAP functions
The idea that IAPs could have functions beyond the control of cell death was first inferred from work with survivin [46], as it became clear that, in addition to cytoprotection, the molecule had additional roles in cellular homeostasis. Characterized by a sharp cell cycle-regulated expression that peaked at mitosis, and subcellular localization to various compartments of the mitotic apparatus [47], survivin is now unanimously recognized as an indispensable regulator of cell division [48, 49]. Differently from all other IAPs, except BRUCE [39], homozygous deletion of the survivin gene caused early embryonic lethality [50], and, similarly, conditional deletion of survivin in adult tissues triggered mitotic defects, cell death and tissue involution [51, 52]. Evidence collected in other model systems supports this scenario, as putative survivin orthologs in C.elegans [53, 54], and yeast [55] have key roles in mitosis, especially with respect to chromosomal segregation and cytokinesis.
However, teasing out how survivin controls mitosis proved challenging [48, 49]. A unifying, albeit not completely satisfying model for this pathway is that independent pools of survivin localized to various aspects of the mitotic apparatus orchestrate different phases of cell division. As an essential member of the chromosomal passenger complex [56], survivin physically interacts with Aurora B, Borealin and INCENP [18] to regulate chromosomal alignment, chromatin-associated spindle assembly, and cytokinesis [49]. A second pool of survivin has been implicated in stabilization of the mitotic spindle [57], by binding to polymerized microtubules via its –COOH terminus α-helices (Fig. 1), and actively repressing microtubule dynamics [58]. Independent evidence suggests that this pool of survivin may also participate in the spindle assembly checkpoint and kinetochore-microtubule attachment [48]. How the multiple pools of survivin work together in a seamless continuum at mitosis is not entirely clear, but post-translational modifications play an important role in this pathway. Accordingly, monoubiquitination of survivin by both Lys48 and Lys63 regulates its mitotic trafficking in the context of the chromosomal passenger complex [59], whereas phosphorylation of survivin by mitotic kinases, including p34cdc2/Cdk1 [60, 61], Aurora B [62, 63], and Polo-like kinase-1 [64], controls protein stability, subcellular localization, association with protein partners and cytoprotection during the cell cycle.
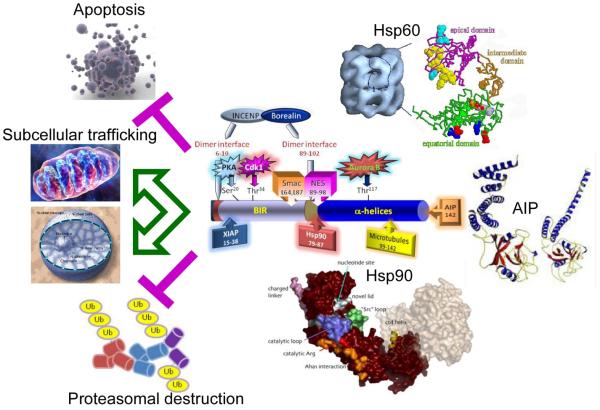
Another emerging function of IAPs is in the cellular stress response (Fig. 3). So far, this has been studied in some detail only for survivin, and whether a similar function applies to other IAPs remains to be explored. With respect to survivin, biochemical studies combined with proteomics screenings identified at least three molecular chaperones, Heat Shock Protein-90 (Hsp90) [65], Hsp60 [66], and the aryl hydrocarbon receptor-interacting protein (AIP) [67], that physically interact with survivin, in vivo (Fig. 3). Based on initial mapping studies, survivin may simultaneously accommodate the binding of at least two of these chaperones, Hsp90 [65] and AIP [67], as they engage spatially distinct sites, but the cellular implications of a potential survivin-multi-chaperone complex have not yet been established. Functionally, these interactions preserve survivin stability against proteasomal degradation, and inhibit mitochondrial apoptosis [65-67]. However, it is also possible that chaperones help localize survivin to specific subcellular compartments (Fig. 3), including mitochondria, as both AIP [68], and Hsp90 [69], have been implicated in organelle preprotein import.
Figure 3. Role of survivin in the cellular stress response.
The various functional motifs in survivin are indicated, including the binding sites for protein partners, XIAP (residues 15-38), Hsp90 (residues 79-87), polymerized microtubules (residues 99-142), and AIP (residue 142), and the position of experimentally validated phosphorylation sites for PKA (Ser20) p34cdc2/Cdk1 (Thr34) and Aurora B (Thr117). The survivin binding site for Hsp60 has not yet been identified. Formation of complexes between survivin and molecular chaperones Hsp60, Hsp90 and AIP has been associated with increased survivin stability against proteasomal degradation, nuclear and mitochondrial subcellular trafficking, and inhibition of apoptosis.
IAPs as intracellular signal transducers and signal integrators
Building on pioneering work that linked XIAP to various intracellular signaling pathways [70, 71], it is now clear that IAPs have diverse functions in signal transduction, independently of caspase inhibition [72]. Much emphasis has focused on the role of IAPs as modulators of NF-κB, a pleiotropic gene expression program [73], which is pivotal for inflammation, immunity, and cell survival [74, 75].
Similar to model organisms, for instance, Drosophila, where IAP orthologs activate NF-κB [76], mammalian XIAP is also now recognized as a physiologic activator of NF-κB. This pathway is centered on a non-IBM, BIR1-dependent recruitment of an activator complex comprising the TGFβ-activating kinase (TAK1) and its adapter protein, TAB1 [22]. In turn, this complex facilitates dimerization and activation of TAK1 with subsequent phosphorylation-dependent ubiquitination and proteasomal degradation of the NF-κB inhibitor, IκBα [22]. There is also a postulated role of the XIAP RING in NF-κB activation, potentially via a nondegradative ubiquitination step [70], but this activity has not been characterized in detail. Because NF-κB triggers the transcriptional upregulation of the same IAPs [77], as well as survivin [78], this pathway functions as an amplification loop ideally suited to enhance cell survival [79], especially in cancer, where high NF-κB activity correlates with aggressive disease [80]. In addition, recent evidence has suggested that IAP-mediated NF-κB activation may directly contribute to tumor progression, in particular metastasis [42]. Accordingly, assembly of a survivin-XIAP complex in tumor cells functions as a better activator of NFκB than XIAP alone, resulting in NF-κB-dependent transcription of the extracellular matrix protein, fibronectin [42]. In turn, the newly produced fibronectin engages β1 integrins at the cell surface, with activation of cell motility kinases, Src and FAK, and dramatically increased tumor cell migration, invasion, and metastatic dissemination, in vivo, independently of cytoprotection [42].
Further studies on the role of cIAPs in NF-κB regulation have uncovered an even greater degree of complexity, with implications for tumor cell survival and novel cancer therapeutics. It had been known that c-IAP1 and c-IAP2 form a complex with the TNF receptor 1 (TNFR1), and promote TNFα-induced NF-κB activation [81, 82], via ubiquitin-dependent stabilization of Receptor-Interacting Protein-1 (RIP-1) kinase [83]. Functionally, this pathway protects cells from the noxious effects of TNFα, as loss of both c-IAPs attenuated TNFα-mediated NF-κB activation [81, 82], but also unhindered the assembly of a pro-apoptotic, caspase 8-activating complex in the cytosol [84].
However, it was the more recent characterization of so-called “Smac mimetics” that unraveled a second function of c-IAPs in NF-κB signaling. Smac mimetics are a class of small molecules that reproduce the physical competition of Smac/DIABLO for the caspase-binding site(s) of XIAP, thus eliminating its anti-apoptotic function [85]. Unexpectedly, a brief exposure of tumor cells to these compounds caused sudden degradation of c-IAP1 and c-IAP2 [86, 87], with concomitant loss of RIP-1 ubiquitination [81, 83]. In turn, this activated NF-κB via the non-canonical pathway [86, 87], a mechanism used by certain TNF receptor family members that involves stabilization of NFκB-inducing kinase (NIK) [88]. When induced by Smac mimetics in certain tumor cells, non-canonical NF-κB activity enhances the production of TNFα [89], causing TNFR1- and caspase-8-dependent apoptosis [86, 87]. Such response is attractive for cancer therapy, as production of TNFα confined to the tumor cells may avoid systemic toxicity, in vivo. Unfortunately, at least in vitro, only a minority of tumor cells produce TNFα in response to Smac mimetics, and the so-called “resistant” cells do not die unless challenged with exogenous TNFα [89]. Therefore, unexpectedly, c-IAP1 and c-IAP2 act as both activators and repressors of canonical and non-canonical NF-κB signaling, respectively, and the balance between these two activities likely controls a broad survival threshold in tumor cells.
IAPs in cancer
Given their role in cellular homeostasis, it is not surprising that deregulated IAP expression or function is frequently associated with human diseases, most notably cancer. In this context, the survivin locus on 17q25 is often amplified in neuroblastoma [90], whereas the c-IAP1 and c-IAP2 locus on 11q22 is amplified in several epithelial malignancies [91]. Aside from copy number increase, the expression of IAPs is deregulated in many types of cancer, with aberrantly increased protein levels in transformed cells. In this context, survivin is a striking cancer gene, over-expressed in virtually every human tumor examined, whereas largely undetectable or expressed at very low levels in normal tissues [46]. The sharp differential distribution of survivin is unique among IAPs, which are typically found in normal tissues as well, and occasionally further upregulated in cancer [46].
The basis for such “cancer-specific” expression of survivin is not completely understood. There is compelling evidence that this reflects transcriptional changes, and several oncogenic pathways have been identified that independently turn on survivin gene expression [92]. Conversely, many tumor suppressor networks have also been shown to exert the opposite effect, and actively silence transcription of the survivin gene, by various mechanisms [92]. It is possible that this finely-tuned balance maintains survivin levels low in normal tissues, where tumor suppression mechanisms dominate [4], whereas transformed cells characterized by oncogene activation and/or loss of tumor suppression may exhibit early deregulation, i.e. induction of survivin gene expression, in vivo [92]. Non-transcriptional mechanisms that deregulate survivin expression in cancer have also been described, for instance stabilization of survivin mRNA in a mammalian Target of Rapamycin (mTOR)-mediated pathway in prostate cancer [93]. Once over-expressed in tumors, retrospective analysis of patient series and genome-wide microarray studies have consistently identified survivin as a risk-associated gene for resistance to therapy, disseminated disease and overall unfavorable disease outcome [46].
Although there may be one function of survivin pivotally important for disease progression, a more likely scenario is that tumors globally exploit the multifaceted biology of the protein for the broadest advantage in cell proliferation, survival, and adaptation. Consistent with this model, deregulation of survivin profoundly affects mitotic transitions in tumor cells, maintaining viability of aneuploid cells [94], bypassing cell cycle checkpoints [95], promoting resistance to microtubule-targeting agents [96], and cooperating with oncogenes, i.e. myc, for disease progression [97]. The link between survivin and molecular chaperones (Fig. 3) may similarly be important to preserve cell proliferation and cell survival in face of the highly unfavorable environments characteristic of tumor growth, in vivo [98], a concept further reinforced by the over-expression of Hsp60 in tumors versus normal tissues [66], and the differential subcellular recruitment of Hsp90 to mitochondria of transformed cells [99]. And, finally, there is evidence from transgenic animals that survivin upregulation during tumor progression, in vivo may also occur independently of the cell cycle [100, 101], suggesting that the non-mitotic functions of survivin in blocking apoptosis in interphase cells may be also prominently exploited, in vivo.
Although these findings reinforce the model that survivin and XIAP confer a broad advantage for tumor growth, the situation for other IAPs, in particular c-IAP1 and c-IAP2 is seemingly more complex. In particular, genomic deletions of the c-IAP1 and c-IAP2 locus have been observed in some types of cancer, for instance multiple myeloma, a condition that would be expected to produce unbridled non-canonical NF-κB activation [102]. While it is too soon to conclude that c-IAPs contribute to a yet-to-be-elucidated tumor suppression pathway, it is intriguing that unrestrained non-canonical NF-κB activation is observed in other tumors, in vivo [103], suggesting a role of this response in disease progression.
Concluding remarks
Over the past decade and half, unraveling the biology of IAPs has produced important insights into disparate cellular circuitries of cell survival, adaptation, mitosis and intracellular signaling. Although considered at first somewhat redundant endogenous caspase inhibitors, it is now clear that IAPs serve unique and cornerstone functions in cellular homeostasis. In a little over ten years, significant progress has also been made in exploiting IAP biology for novel cancer therapeutics [104, 105]: no small feat when one considers the excruciatingly long timeline for bringing new agents to the clinic. However it is also clear that important questions about IAP function remains, for instance how these molecules intersect other signaling pathways, participate in adaptation or regulate the cell cycle, just to name a few. Given the fast pace of IAP research, the answer to some of these questions is undoubtedly forthcoming, helping frame new, more rationally-grounded strategies for targeting IAPs in human diseases, especially cancer.
ACKNOWLEDGMENTS
I apologize to all the colleagues whose work could not be cited for reasons of space constraints. This work was supported by National Institutes of Health grants CA118005, CA90917, CA78810 and HL54131.
REFERENCES
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meier P, Finch A, Evan GI. Apoptosis in development. Nature. 2000;407:796–801. doi: 10.1038/35037734. [DOI] [PubMed] [Google Scholar]
- 3.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 6.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 7.Bouchier-Hayes L, Lartigue L, Newmeyer DD. Mitochondria: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2640–2647. doi: 10.1172/JCI26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y. A structural view of mitochondria-mediated apoptosis. Nat Struct Biol. 2001;8:394–401. doi: 10.1038/87548. [DOI] [PubMed] [Google Scholar]
- 10.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvesen GS, Duckett CS. Apoptosis: IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 12.Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasula SM, Ashwell JD. IAPs: what's in a name? Mol Cell. 2008;30:123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 15.Verdecia MA, Huang H, Dutil E, Kaiser DA, Hunter T, Noel JP. Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat. Struct. Biol. 2000;7:602–608. doi: 10.1038/76838. [DOI] [PubMed] [Google Scholar]
- 16.Engelsma D, Rodriguez JA, Fish A, Giaccone G, Fornerod M. Homodimerization antagonizes nuclear export of survivin. Traffic. 2007;8:1495–1502. doi: 10.1111/j.1600-0854.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- 17.Bourhis E, Hymowitz SG, Cochran AG. The mitotic regulator Survivin binds as a monomer to its functional interactor Borealin. J Biol Chem. 2007;282:35018–35023. doi: 10.1074/jbc.M706233200. [DOI] [PubMed] [Google Scholar]
- 18.Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP Core Complex Reveals How Chromosomal Passengers Travel Together. Cell. 2007;131:271–285. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 19.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Sun C, Olejniczak ET, Meadows RP, Betz SF, Oost T, Herrmann J, Wu JC, Fesik SW. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature. 2000;408:1004–1008. doi: 10.1038/35050006. [DOI] [PubMed] [Google Scholar]
- 21.Lin SC, Huang Y, Lo YC, Lu M, Wu H. Crystal structure of the BIR1 domain of XIAP in two crystal forms. J Mol Biol. 2007;372:847–854. doi: 10.1016/j.jmb.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu M, Lin SC, Huang Y, Kang YJ, Rich R, Lo YC, Myszka D, Han J, Wu H. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 24.Song Z, Yao X, Wu M. Direct interaction between survivin and Smac/DIABLO is essential for the anti-apoptotic activity of survivin during taxol-induced apoptosis. J Biol Chem. 2003;278:23130–23140. doi: 10.1074/jbc.M300957200. [DOI] [PubMed] [Google Scholar]
- 25.Sun C, Nettesheim D, Liu Z, Olejniczak ET. Solution structure of human survivin and its binding interface with Smac/Diablo. Biochemistry. 2005;44:11–17. doi: 10.1021/bi0485171. [DOI] [PubMed] [Google Scholar]
- 26.Deveraux QL, Reed JC. IAP family proteins-suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 27.Clem RJ, Miller LK. Control of programmed cell death by the baculovirus genes p35 and iap. Mol Cell Biol. 1994;14:5212–5222. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Park YC, Rich RL, Segal D, Myszka DG, Wu H. Structural basis of caspase inhibition by XIAP: differential roles of the linker versus the BIR domain. Cell. 2001;104:781–790. [PubMed] [Google Scholar]
- 29.Chai J, Shiozaki E, Srinivasula SM, Wu Q, Datta P, Alnemri ES, Shi Y. Structural basis of caspase-7 inhibition by XIAP. Cell. 2001;104:769–780. doi: 10.1016/s0092-8674(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 30.Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 31.Scott FL, Denault JB, Riedl SJ, Shin H, Renatus M, Salvesen GS. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 2005;24:645–655. doi: 10.1038/sj.emboj.7600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519–527. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 33.Wilson R, Goyal L, Ditzel M, Zachariou A, Baker DA, Agapite J, Steller H, Meier P. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat Cell Biol. 2002;4:445–450. doi: 10.1038/ncb799. [DOI] [PubMed] [Google Scholar]
- 34.Ditzel M, Broemer M, Tenev T, Bolduc C, Lee TV, Rigbolt KT, Elliott R, Zvelebil M, Blagoev B, Bergmann A, Meier P. Inactivation of effector caspases through nondegradative polyubiquitylation. Mol Cell. 2008;32:540–553. doi: 10.1016/j.molcel.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci U S A. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schile AJ, Garcia-Fernandez M, Steller H. Regulation of apoptosis by XIAP ubiquitin-ligase activity. Genes Dev. 2008;22:2256–2266. doi: 10.1101/gad.1663108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dohi T, Okada K, Xia F, Wilford CE, Samuel T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, Salvesen GS, Reed JC, Altieri DC. An IAP-IAP complex inhibits apoptosis. J Biol Chem. 2004;279:34087–34090. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]
- 38.Dohi T, Xia F, Altieri DC. Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection. Mol Cell. 2007;27:17–28. doi: 10.1016/j.molcel.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohl C, Jentsch S. Final stages of cytokinesis and midbody ring formation are controlled by BRUCE. Cell. 2008;132:832–845. doi: 10.1016/j.cell.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Samuel T, Okada K, Hyer M, Welsh K, Zapata JM, Reed JC. cIAP1 Localizes to the nuclear compartment and modulates the cell cycle. Cancer Res. 2005;65:210–218. [PubMed] [Google Scholar]
- 41.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC. IAP regulation of metastasis. Cancer Cell. 2010;17:53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arora V, Cheung HH, Plenchette S, Micali OC, Liston P, Korneluk RG. Degradation of survivin by the XIAP-XAF1 complex. J Biol Chem. 2007 doi: 10.1074/jbc.M700776200. [DOI] [PubMed] [Google Scholar]
- 44.Ceballos-Cancino G, Espinosa M, Maldonado V, Melendez-Zajgla J. Regulation of mitochondrial Smac/DIABLO-selective release by survivin. Oncogene. 2007;26:7569–7575. doi: 10.1038/sj.onc.1210560. [DOI] [PubMed] [Google Scholar]
- 45.McNeish IA, Lopes R, Bell SJ, McKay TR, Fernandez M, Lockley M, Wheatley SP, Lemoine NR. Survivin interacts with Smac/DIABLO in ovarian carcinoma cells but is redundant in Smac-mediated apoptosis. Exp Cell Res. 2005;302:69–82. doi: 10.1016/j.yexcr.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 46.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 47.Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 48.Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Lens SM, Vader G, Medema RH. The case for Survivin as mitotic regulator. Curr Opin Cell Biol. 2006;18:616–622. doi: 10.1016/j.ceb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 50.Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ, Vaux DL, Choo KH. Survivin and the inner centromere protein INCENP show similar cell- cycle localization and gene knockout phenotype. Curr. Biol. 2000;10:1319–1328. doi: 10.1016/s0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- 51.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 52.Gurbuxani S, Xu Y, Keerthivasan G, Wickrema A, Crispino JD. Differential requirements for survivin in hematopoietic cell development. Proc Natl Acad Sci U S A. 2005;102:11480–11485. doi: 10.1073/pnas.0500303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraser AG, James C, Evan GI, Hengartner MO. Caenorhabditis elegans inhibitor of apoptosis protein (IAP) homologue BIR-1 plays a conserved role in cytokinesis. Curr. Biol. 1999;9:292–301. doi: 10.1016/s0960-9822(99)80137-7. [DOI] [PubMed] [Google Scholar]
- 54.Speliotes EK, Uren A, Vaux D, Horvitz HR. The survivin-like C. elegans BIR-1 protein acts with the Aurora-like kinase AIR-2 to affect chromosomes and the spindle midzone. Mol. Cell. 2000;6:211–223. doi: 10.1016/s1097-2765(00)00023-x. [DOI] [PubMed] [Google Scholar]
- 55.Huang HK, Bailis JM, Leverson JD, Gomez EB, Forsburg SL, Hunter T. Suppressors of Bir1p (Survivin) identify roles for the chromosomal passenger protein Pic1p (INCENP) and the replication initiation factor Psf2p in chromosome segregation. Mol Cell Biol. 2005;25:9000–9015. doi: 10.1128/MCB.25.20.9000-9015.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruchaud S, Carmena M, Earnshaw WC. The chromosomal passenger complex: one for all and all for one. Cell. 2007;131:230–231. doi: 10.1016/j.cell.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Giodini A, Kallio MJ, Wall NR, Gorbsky GJ, Tognin S, Marchisio PC, Symons M, Altieri DC. Regulation of microtubule stability and mitotic progression by survivin. Cancer Res. 2002;62:2462–2467. [PubMed] [Google Scholar]
- 58.Rosa J, Canovas P, Islam A, Altieri DC, Doxsey SJ. Survivin modulates microtubule dynamics and nucleation throughout the cell cycle. Mol Biol Cell. 2006;17:1483–1493. doi: 10.1091/mbc.E05-08-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vong QP, Cao K, Li HY, Iglesias PA, Zheng Y. Chromosome alignment and segregation regulated by ubiquitination of survivin. Science. 2005;310:1499–1504. doi: 10.1126/science.1120160. [DOI] [PubMed] [Google Scholar]
- 60.O'Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, Altieri DC. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13103–13107. doi: 10.1073/pnas.240390697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barrett RM, Osborne TP, Wheatley SP. Phosphorylation of survivin at threonine 34 inhibits its mitotic function and enhances its cytoprotective activity. Cell Cycle. 2009;8:278–283. doi: 10.4161/cc.8.2.7587. [DOI] [PubMed] [Google Scholar]
- 62.Wheatley SP, Barrett RM, Andrews PD, Medema RH, Morley SJ, Swedlow JR, Lens SM. Phosphorylation by Aurora-B Negatively Regulates Survivin Function During Mitosis. Cell Cycle. 2007;6 doi: 10.4161/cc.6.10.4179. [DOI] [PubMed] [Google Scholar]
- 63.Wheatley SP, Henzing AJ, Dodson H, Khaled W, Earnshaw WC. Aurora-B Phosphorylation in Vitro Identifies a Residue of Survivin That Is Essential for Its Localization and Binding to Inner Centromere Protein (INCENP) in Vivo. J Biol Chem. 2004;279:5655–5660. doi: 10.1074/jbc.M311299200. [DOI] [PubMed] [Google Scholar]
- 64.Colnaghi R, Wheatley SP. Liaisons between survivin and PLK1 during cell division and cell death. J Biol Chem. doi: 10.1074/jbc.M109.065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fortugno P, Beltrami E, Plescia J, Fontana J, Pradhan D, Marchisio PC, Sessa WC, Altieri DC. Regulation of survivin function by Hsp90. Proc Natl Acad Sci U S A. 2003;100:13791–13796. doi: 10.1073/pnas.2434345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. J Biol Chem. 2008;283:5188–5194. doi: 10.1074/jbc.M705904200. [DOI] [PubMed] [Google Scholar]
- 67.Kang BH, Altieri DC. Regulation of survivin stability by the aryl hydrocarbon receptor-interacting protein. J Biol Chem. 2006;281:24721–24727. doi: 10.1074/jbc.M603175200. [DOI] [PubMed] [Google Scholar]
- 68.Yano M, Terada K, Mori M. AIP is a mitochondrial import mediator that binds to both import receptor Tom20 and preproteins. J Cell Biol. 2003;163:45–56. doi: 10.1083/jcb.200305051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. doi: 10.1016/s0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- 70.Lewis J, Burstein E, Reffey SB, Bratton SB, Roberts AB, Duckett CS. Uncoupling of the signaling and caspase-inhibitory properties of X-linked inhibitor of apoptosis. J Biol Chem. 2004;279:9023–9029. doi: 10.1074/jbc.M312891200. [DOI] [PubMed] [Google Scholar]
- 71.Sanna MG, Duckett CS, Richter BW, Thompson CB, Ulevitch RJ. Selective activation of JNK1 is necessary for the anti-apoptotic activity of hILP. Proc Natl Acad Sci U S A. 1998;95:6015–6020. doi: 10.1073/pnas.95.11.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Riordan MX, Bauler LD, Scott FL, Duckett CS. Inhibitor of apoptosis proteins in eukaryotic evolution and development: a model of thematic conservation. Dev Cell. 2008;15:497–508. doi: 10.1016/j.devcel.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bianchi K, Meier P. A tangled web of ubiquitin chains: breaking news in TNF-R1 signaling. Mol Cell. 2009;36:736–742. doi: 10.1016/j.molcel.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 74.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 75.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 76.Leulier F, Lhocine N, Lemaitre B, Meier P. The Drosophila inhibitor of apoptosis protein DIAP2 functions in innate immunity and is essential to resist gram-negative bacterial infection. Mol Cell Biol. 2006;26:7821–7831. doi: 10.1128/MCB.00548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin HS, Lee DH, Kim DH, Chung JH, Lee SJ, Lee TH. cIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-kappaB activation. Cancer Res. 2009;69:1782–1791. doi: 10.1158/0008-5472.CAN-08-2256. [DOI] [PubMed] [Google Scholar]
- 78.Guha M, Xia F, Raskett CM, Altieri DC. Caspase 2-mediated tumor suppression involves survivin gene silencing. Oncogene. 2010;29:1280–1292. doi: 10.1038/onc.2009.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baud V, Karin M. Is NF-[kappa]B a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 82.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J, Korneluk RG. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 85.Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, Shi Y. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 86.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 87.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 88.Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, Korneluk RG, Cheng G. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smacmimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bown N. Neuroblastoma tumour genetics: clinical and biological aspects. J Clin Pathol. 2001;54:897–910. doi: 10.1136/jcp.54.12.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma O, Cai WW, Zender L, Dayaram T, Shen J, Herron AJ, Lowe SW, Man TK, Lau CC, Donehower LA. MMP13, Birc2 (cIAP1), and Birc3 (cIAP2), amplified on chromosome 9, collaborate with p53 deficiency in mouse osteosarcoma progression. Cancer Res. 2009;69:2559–2567. doi: 10.1158/0008-5472.CAN-08-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guha M, Altieri DC. Survivin as a global target of intrinsic tumor suppression networks. Cell Cycle. 2009;8:2708–2710. doi: 10.4161/cc.8.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vaira V, Lee CW, Goel HL, Bosari S, Languino LR, Altieri DC. Regulation of survivin expression by IGF-1/mTOR signaling. Oncogene. 2007;26:2678–2684. doi: 10.1038/sj.onc.1210094. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen HG, Ravid K. Tetraploidy/aneuploidy and stem cells in cancer promotion: The role of chromosome passenger proteins. J Cell Physiol. 2005 doi: 10.1002/jcp.20565. [DOI] [PubMed] [Google Scholar]
- 95.Vogel C, Hager C, Bastians H. Mechanisms of mitotic cell death induced by chemotherapy-mediated G2 checkpoint abrogation. Cancer Res. 2007;67:339–345. doi: 10.1158/0008-5472.CAN-06-2548. [DOI] [PubMed] [Google Scholar]
- 96.Lu J, Tan M, Huang WC, Li P, Guo H, Tseng LM, Su XH, Yang WT, Treekitkarnmongkol W, Andreeff M, Symmans F, Yu D. Mitotic deregulation by survivin in ErbB2-overexpressing breast cancer cells contributes to Taxol resistance. Clin Cancer Res. 2009;15:1326–1334. doi: 10.1158/1078-0432.CCR-08-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goga A, Yang D, Tward AD, Morgan DO, Bishop JM. Inhibition of CDK1 as a potential therapy for tumors over-expressing MYC. Nat Med. 2007;13:820–827. doi: 10.1038/nm1606. [DOI] [PubMed] [Google Scholar]
- 98.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 99.Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 100.Xia F, Altieri DC. Mitosis-independent survivin gene expression in vivo and regulation by p53. Cancer Res. 2006;66:3392–3395. doi: 10.1158/0008-5472.CAN-05-4537. [DOI] [PubMed] [Google Scholar]
- 101.Li F, Cheng Q, Ling X, Stablewski A, Tang L, Foster BA, Johnson CS, Rustum YM, Porter CW. Generation of a novel transgenic mouse model for bioluminescent monitoring of survivin gene activity in vivo at various pathophysiological processes: survivin expression overlaps with stem cell markers. Am J Pathol. 2010;176:1629–1638. doi: 10.2353/ajpath.2010.090414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, Braggio E, Henry T, Zhu YX, Fogle H, Price-Troska T, Ahmann G, Mancini C, Brents LA, Kumar S, Greipp P, Dispenzieri A, Bryant B, Mulligan G, Bruhn L, Barrett M, Valdez R, Trent J, Stewart AK, Carpten J, Bergsagel PL. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wharry CE, Haines KM, Carroll RG, May MJ. Constitutive noncanonical NFkappaB signaling in pancreatic cancer cells. Cancer Biol Ther. 2009;8:1567–1576. doi: 10.4161/cbt.8.16.8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 105.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]