Abstract
Background
Effective wound healing has been a lasting and challenging topic in health care. Various strategies have been used to accelerate and perfect the healing process. One such strategy has involved the application of an exogenous electrical stimulus to chronic wounds with the aim of stimulating healing responses.
The Problem
The biology of electric stimulation to instigate healing, however, is very poorly understood. How does electric stimulation induce healing responses?
Basic/Clinical Science Advances
Recent research shows that the electric fields (EFs) activate multiple signaling pathways that are critical for wound healing. Importantly, the EFs provide a powerful, sometimes an overriding, directional signal for cell migration in wound healing. Unlike other stimuli, EFs have the intrinsic property of being directional. The EF-directed cell migration (electrotaxis/galvanotaxis) appears to be a consequence of EF-induced polarized signaling of epidermal growth factor receptors, integrins, and phosphoinositide 3 kinase/Pten, and may be mediated by protein kinase C, intracellular Ca2+, and cyclic adenosine monophosphate (cAMP). Because directional cell migration is a key component in wound healing, galvanotaxis may represent an important mechanism of wound healing.
Clinical Care Relevance
With the constantly enlarging diabetic and aging population, chronic or nonhealing wounds pose increasing health and economic problems, and currently there is no effective therapy available. Electric stimulation activates important intracellular signaling pathways that are polarized in the EF direction, resulting in enhanced and stimulated directional cell migration. Electric stimulation offers a novel approach to achieve better and accelerated wound healing.
Conclusion
Experimental evidence suggests a significant role of endogenous EFs in cell migration in wound healing. Most importantly, EFs are a very powerful signal to direct cell migration. Electric stimulation therefore may represent a promising and unique strategy to induce cell and tissue growth in a directional manner, to enhance wound healing, and to achieve better wound healing.
BACKGROUND
Injury elicits multiple signaling pathways.1–3 To restore tissue integrity and homeostasis effectively, those signaling pathways have to be well coordinated. Impairment of signaling orchestration may lead to defective healing, and chronic and nonhealing wounds.4 Many strategies have been used in an attempt to accelerate the healing response. One strategy is application of an exogenous electrical stimulus to activate healing.5–8 Despite encouraging results being reported and approval of Medicare and other insurance policies for use of electric stimulation in some types of chronic and nonhealing wounds, the advancement in using electric stimulation to achieve effective wound healing is limited.9 Yet, recent research has provided significant insights into electrical stimulation of wound healing.
Endogenous wound electric fields (EFs) are present at human skin wounds, generated immediately upon wounding. Application of EFs, in dose ranges of those generated naturally, activates signaling molecules critical for wound healing in many types of exposed cells, including epidermal growth factor receptors (EGFRs), integrins, and phosphoinositide 3 (PI3) kinases and Pten (phosphatase and tensin homolog).8,10–13 Significantly, these signaling molecules are activated in a directional manner, often on the side of the cell facing the cathodal pole of the EF. This provides a unique prohealing advantage to application of EF as a therapeutic modality, since the directional cueing of the repair response is maintained as long as the EF is present—unlike the topical application of growth factors or other stimulatory compounds, which normally are difficult to maintain as a stable gradient to sustain directional migratory or proliferative responses. Importantly, the electric signaling appears to be a predominant signaling mechanism in guiding cell migration in epithelial wound healing.11
Clinically, both direct current (DC) stimulation and alternating current (AC) stimulation have been tested for wound healing. The stimulation schemes include constant DCs, DC pulses, and ACs. The electric stimulation comes with a huge number of choices of different amplitudes, frequencies (AC and pulsed DC), duty cycles, durations, current strengths, and so on. Current is usually very small for DC stimulation (hundreds of μA–μA). Low-voltage pulsed currents are pulses with durations up to 1 s and voltages up to 150 V. Monophasic and biphasic pulsed currents can be low voltage and high voltage up to several hundred volts with short duration (μs).7,14–17 For example, a recent clinical study in Germany used Dermapulse® (Gerromed, Hamburg, Germany) with varied polarity, a pulse frequency of 128 Hz, and an average current strength of 300 μA. This double-blinded, placebo-controlled clinical study demonstrated pain relief, improved perfusion, and a tendency of improved healing in chronic venous leg ulcers.18 Because there are many modalities of using pulsed DC and AC, understanding the effects of those modalities on wound-healing signaling pathways will offer better choice of those many parameters.
In addition to wound healing, electric stimulation has been used in orthopedics, sports medicine, and neurology.19–22 For bone and cartilage cells, a specific, defined capacitively coupled electrical signal can result in significant upregulation of cartilage matrix protein production, and nitric oxide–dependent chondrocyte proliferation.14,19
This short perspective focuses on activation of signaling pathways by DC EFs. Because cells are responsive to DC EFs, exogenously applied electric stimulation is likely to affect cells through same or similar mechanisms. Those responses are likely to be cell-type specific, and stimulation parameter dependent.
CLINICAL PROBLEM ADDRESSED
The clinical problems presented by chronic, nonhealing wounds are familiar to the readers of this review. While acute wounds normally heal without many complications, the comorbidities of diabetes, malnutrition, pressure, infection, chronic inflammation, and aging can create a scenario that leads to nonhealing wounds. The biologic impediments that prevent healing are poorly understood.23 Chronic wounds generate significant economic burden to our health care system.4,24 Even with the best standard of care, up to 30% of venous leg ulcers and 65% of diabetic foot ulcers fail to heal25 and few therapeutic advances have significantly altered these figures. Thus, new treatment paradigms are needed to address this burgeoning problem.
RELEVANT BASIC SCIENCE CONTEXT
Du-Bois Reymond, a founder of electrophysiology, detected wound EFs on a cut in his fingertip 160 years ago.26 The existence of endogenous wound EFs has been confirmed in all wounds that have been measured using various modern techniques. When guinea pig skin is wounded, a large and steady EF arises, measuring ~ 140 mV/mm.12,27,28 The vibrating probe technique has allowed nonin-vasive measurements with great temporal and spatial resolution of endogenous electric currents.11,29,30 The electric currents (flow of positive charge) are oriented toward the wound and, therefore, are well positioned to guide and stimulate the migration and other healing responses of cells at wounds (Fig. 1A).
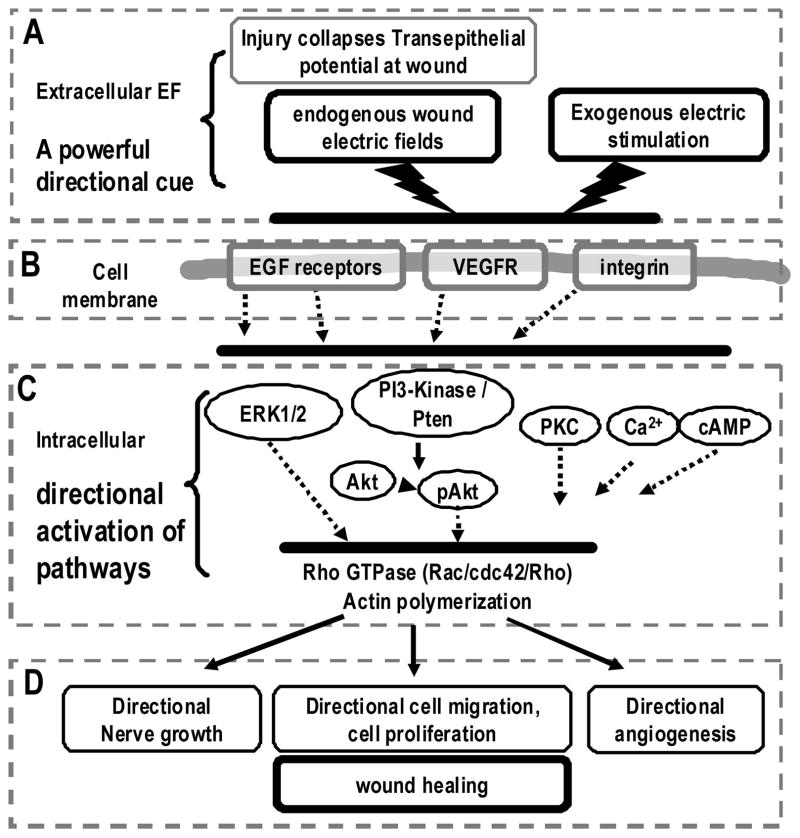
Figure 1.
Electrical activation of wound-healing pathways. Schematic diagram shows the generation of the endogenous wound electric signals and the electrical activation of some important signaling pathways for cell migration and wound healing. (A) Injury breaks the epithelial barrier and induces endogenous wound electric fields (EFs). Electric stimulation can also be exogenously applied; (B) extracellular EFs may activate epidermal growth factor receptors (EGFRs), vascular endothelial growth factor receptors (VEGFRs), and integrins; (C) polarized activation of membrane receptors induces asymmetric intracellular signaling, notably extracellular-signaling regulated kinase 1/2 (ERK1/2), phosphoinositide 3 (PI3) kinase/Akt, Rac, and protein kinase C (PKC) to induce downstream cascades; (D) the orchestration of the polarized intracellular signaling results in directional cell migration and proliferation, and perhaps directional nerve growth and new blood vessel formation. These effects may lead to improved wound-healing response.
Impaired cell migration into wounds is characteristic of chronic wounds in the elderly, pressure ulcers, and venous stasis ulcers of the skin.2,24,31 To heal a wound effectively, cells must not only become motile, but also migrate in the correct direction. Many types of cells migrate directionally in an EF, a phenomenon called electrotaxis or galvano-taxis.32 Because the cells have to migrate directionally to heal a wound, and because endogenous EFs naturally occur at wounds and cells migrate directionally in EFs, it has long been postulated that the wound EFs might have a role in wound healing in directing cell and tissue growth.33–35
EXPERIMENTAL MODEL OR MATERIAL—ADVANTAGES AND LIMITATIONS
We have used a vibrating probe system to measure the endogenous wound electric currents at epithelial wounds of skin and cornea. While not a chronic wound, the acute wound offers the advantage of being easier to manipulate. Using the vibrating probe system requires specialized instrument and special expertise, which limits the use of this technique in the clinical situation. A noninvasive device currently being developed may offer opportunities to measure EFs in patients and determine to what extent the changes in wound EFs correlate to impaired wound healing.28 This may lead to diagnostic and prognostic application of measuring EFs and indicate manipulation of wound EFs to achieve better wound healing.
We use digital video microscopy to observe EF-induced cellular responses, for example, cell migration, division, and signaling mechanisms. This allows us to mimic the effects of endogenous EFs on the cells. Our results show that keratinocytes, corneal epithelial cells, fibroblasts, endothelial cells, and neutrophils—when cultured in an EF—all migrate directionally. The advantages of this approach include precise control of the experimental conditions, the stimulating parameters, and direct evaluation of the cellular response and signaling mechanisms. We have identified some important signaling mechanisms that are activated by EFs. Most importantly, the electric signaling gives cells a sense of direction by activating EGFR, extracellular-signaling regulated kinase 1/2 (ERK1/2), and PI3 kinase in a polarized manner. The directionality of EFs when combined with promising therapies of using biochemical stimulators such as growth factors may achieve better wound healing, especially in chronic, nonhealing wounds. However, the extrapolation and translation of the results to clinical application are still a major challenge.
With further understanding of the molecular and cellular mechanisms, and development in techniques of application of EFs, electric stimulation may lead to effective therapies.
DISCUSSION OF FINDINGS AND RELEVANT LITERATURE
Newly developed tools such as micro-needle arrays and a bioelectric imager have confirmed and greatly extended our understanding of the existence of wound EFs. A shallow slit wound (~1 mm in depth and ~1–2 mm in length) on human finger tip produces an electric current of 8–10 μA/cm2.29 The microneedle arrays and bioelectric imager have measured very similar wound EFs (100–177 mV/mm) on human skin immediately upon wounding.27,28 EFs of similar strength activate multiple signaling pathways important to wound healing (Fig. 1).
EFs relocalize and activate EGFRs and downstream signaling pathways
EFs induce polarization and activation of EGFRs in keratinocytes and corneal epithelial cells. We have observed cathodal redistribution of EGFRs (Fig. 1B) on keratinocytes and corneal epithelial cells as early as 10 min in an EF. The downstream signaling–dua-phosphorylated ERK1/2 and actin filament are also polarized to the cathodal side.8,12,41 Long-time exposure in combination with extracellular matrix (ECM) upregulates expression of EGFRs in corneal epithelial cells in culture.36 EGFR signaling is a ubiquitous signaling mechanism following wounding, and its activation results in many important prohealing responses, such as enhanced directional cell motility and proliferation. Activation of EGFRs also has antiapoptotic effects.
EFs have the intrinsic property of being directional, and do not easily dissipate like soluble chemicals. EFs thus provide a persistent directional signal through activation of intracellular pathways in a polarized manner, different from activation of intracellular signaling by diffusible molecules. This gives the therapeutic approach of electrical stimulation an advantage over conventional approaches with diffusing agents.
Signaling through integrins—Rac
EFs redistribute integrins in fibroblasts.37 Integrins are cell surface receptors that cells use to bind and respond to the ECM. Integrins have diverse functions. They regulate cell adhesion to ECM and to neighbor cells. They also contribute to the control of cell proliferation, shape, and motility. Integrins also mediate many important signal pathways. Knockout of β4 integrin abrogated the electrotaxis of keratinocytes in the absence of EGF, which can be recovered by transfection and expression of β4 integrin.13 Two distinct but synergistic signaling pathways—EGFR and integrin β4—coordinate galvanotaxis and are required for the activation of Rac that was essential for directional migration of keratinocytes in response to an EF (Fig. 1B,C).
PI3 kinase and Pten are key molecules that mediate electrotaxis
PI3 kinase/Akt and Pten are compass molecules in directional sensing and migration of cells in response to chemical gradients. EFs activate PI3 kinase/Akt signaling pathway in neutrophils and keratinocytes that are cultured in serum-free medium. The activation of PI3 kinase/Akt and several other kinases in keratinocytes is rapid and specific.11 Most strikingly, the activation of those signaling pathways is polarized in the direction of cell migration. When the polarity of EF is reversed, PI3 kinase signaling is activated at the new cathode-facing side, membrane protrusion, and directional migration in the new direction ensue.10,11 Genetic disruption or pharmacological inhibition of PI3 kinase abrogated electrotaxis of corneal epithelial cells and keratinocytes in wound healing in monolayer and organ cultures. Those results suggest that the EF-induced directional activation of PI3 kinase underlies the directional cell migration (Fig. 1C).
EFs are an overriding guidance cue directing cell migration in wound healing
It is generally accepted that the following cues give cells a sense of direction and the cells migrate and grow directionally: (i) injury stimulation per se; (ii) gradients of chemoattractants; (iii) contact inhibition release; (iv) wound void; (v) population pressure—growth of adjacent cells; and (vi) changes in mechanical force after injury.1–3,38 However, when an EF of physiological strength is applied in the opposite direction of those cues, cell migration simply follows the direction of EF,11 suggesting that EFs are able to override other cues in directing cell migration.
EFs induce and direct proangiogenic responses and nerve growth
In addition to guiding epithelial cells, EFs may direct angiogenesis and nerve growth.39 An EF upregulates the expression of vascular endothelial growth factor (VEGF). EFs as low as 75–100 mV/mm direct the reorientation, elongation, and migration of endothelial cells in culture. These proangiogenic responses require VEGF receptor (VEGFR) activation and are mediated through PI3 kinase-Akt and the small GTPase Rho kinase (ROCK) signaling pathways, resulting in reorganization of the actin cytoskeleton. Thus, the endogenous EFs might play a role in angiogenesis in vivo by stimulating the VEGFR signaling pathway, to induce angiogenic responses. Nerve sprouting at corneal wounds increases in number and grows more directionally when the endogenous would EFs are increased.12
Manipulation of endogenous EFs
As the endogenous wound EFs are generated because of transport of ions by ion channels and pumps, we have used pharmacological agents to increase wound EFs, which indeed significantly enhanced wound healing and nerve growth.11,30
CAUTION, CRITICAL REMARKS, AND RECOMMENDATIONS
Currently, electric stimulation is being used in certain refractory and nonhealing wounds. While there are reports with encouraging outcomes, there is no FDA-approved device specifically for the indication of skin wound healing. Great discrepancy exists in the reported clinical protocols that claim to have achieved improved wound healing using electric stimulation. Well-controlled, randomized clinical trials and standardization of the device and protocols for electric stimulation are needed.
Practical application of electrical stimulation itself may need to be carefully considered and designed. The endogenous EFs are generated at the leading edge, very focally because of tightly regulated ion transport at the breach to skin electrical resistant barrier. Experimentally applied EFs in culture dish can be precisely controlled. Exogenously applied electric stimulation at wounds in patients, however, can generate highly uneven current density, path, and voltage distribution. Those are significantly confounding factors in electric stimulation for wound healing in patients. These confounding factors may explain the discrepant results reported in some of the clinical trials.
At the same time, electric stimulation for wound healing requires thorough understanding of the underlying mechanisms before therapeutic applications may be more successful, reliable, and consistent. Because electric stimulation may have numerous signaling and cellular targets, appropriate tests are needed to ensure that any beneficial outcome of electric stimulation targeting one type of cell or some particular signaling pathway is not counteracted by other effects on different types of cells and signaling pathways. Understanding how EFs may affect cellular behaviors and signaling pathways in tissues and their functional consequences should bring a new tool to manage chronic wounds.
FUTURE DEVELOPMENT
Despite the remarkable guidance effects of EF on many types of cells, it is still not known how the cells sense the EFs. This type of basic scientific understanding is fundamental to develop effective therapies. It would be important to delineate the signaling pathways and mechanisms of EF-induced cellular responses and the effects on the biology of wound healing.
It is highly important that devices and practice be standardized for wound care clinical trials. From a therapeutic standpoint, the development of safe, effective, and reliable stimulators to specifically manipulate cellular and tissue response in vitro as well as in vivo could have tremendous impact in this field. We are developing pharmacological approaches to affect the endogenous wound EFs through manipulation of ion transport in epithelial cells. This may represent a practical and easier way to standardize for manipulation of local EFs rather than by application of physical electrodes.
TAKE-HOME MESSAGE.
Basic science advances
Injury induces naturally occurring, endogenous wound EFs, which measure ~100–200 mV/mm at skin and corneal wounds.
EFs redistribute and activate important membrane receptors for wound healing—EGFRs, VEGFRs, and integrins.
Importantly, EF-induced activation of the signaling pathways is directional. EFs activate mitogen-activated kinase ERK1/2, PI3 kinase/Akt at the cathodal side in which direction cells migrate.
EFs of physiological strength stimulate directional cell migration and cell proliferation.
EFs of physiological strength override other directional cues and are able to guide cell migration against other directional cues in wound healing.
EFs stimulate endothelial cells to produce VEGF, and direct alignment, migration, and elongation of endothelial cells.
EFs stimulate and direct nerve sprouting into the wounds and in culture.
Exogenously applied electrical stimulation mimics the effects of the endogenous wound EFs, and is likely to stimulate and guide cell migration, thus enhances wound healing.
Pharmacological manipulation of ion transportation at the wound may be an effective way of enhancing the endogenous EFs and achieve better wound healing.
Clinical sciences
EF-based therapies may represent a powerful approach and a direction of future wound management. With the convincing demonstration of the effect of EFs in directing cell migration, medical bioengineering companies are developing and improving devices for electrical stimulation of wound healing.
Alternatively, pharmacological manipulation of the endogenous wound EFs may offer novel and effective approaches to develop wound-healing therapies, circumventing concerns of exogenous EF application—variable tissue resistance resulting in uneven distribution of EFs and EF effects to nontargeted tissues.
Relevance to clinical care
The Center for Medicare and Medicaid Services as well as third-party payers have approved coverage for electric stimulation to treat stage III and stage IV pressure ulcers, arterial ulcers, diabetic ulcers, and venous stasis ulcers. Chronic ulcers are defined as ulcers that have not healed in 30 days.
Considerable off-label use of electrical stimulatory devices, approved by Food and Drug Administration (FDA) for indications such as bone healing and deep brain stimulation, has been the practice until now (HCPCS code G0281). Currently, several companies are developing devices or bandages using EF-based concept specifically for wound-healing indications. One such bandage has FDA 510(k) premarketing approval (Prosit adhesive bandage; Vomaris (Chandler, AZ); www.accessdatafda.gov=cdrh_docs=pdf8=K081977.pdf).
Numerous reports suggest efficacy of these devices, but a Cochrane Review protocol reviewing the evidence40 now underway has still not come to any conclusions. Randomized clinical trials will be needed.
Pharmacological manipulation of ion transport of the epithelial cells may represent a promising new approach to achieve better wound healing based on the concept of EF-directed cell and tissue growth.
Use of diuretics, such as furosemide, may interfere with ion transport mechanisms in epithelial cells. Because ion transport is the basis of the endogenous wound EFs, which plays a significant role in wound healing, attention should be paid to diabetic patients who use diuretics and have nonhealing wounds or refractory wounds. Alternative medicine may minimize possible detrimental side effects on wound healing.
Acknowledgments
Wound healing research in the authors’ laboratory is supported by the Wellcome Trust, UC Davis Dermatology Development Fund (to M.Z.) and NIH R01-AR44518 (to R.R.I.). The project described was also supported by Award Number R01EY019101 from the National Eye Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute or the National Institutes of Health.
Abbreviations and Acronyms
- AC
alternating current
- Akt
a downstream effecter of PI3 kinase. Akt is activated as a result of PI3 kinase activity
- cAMP
cyclic adenosine monophosphate (cyclic AMP or 3′-5′-cyclic adenosine monophosphate) is a second messenger that is important in many biological processes
- chemoattractant
inorganic or organic substances possessing chemotaxis inducer effect in motile cells
- chemotaxis
the phenomenon in which cells direct their movements according to certain chemicals in their environment
- contact inhibition
a natural process of arresting cell growth and movement when two or more cells come into contact with each other
- DC EFs
direct current electric fields
- ECM
extracellular matrix
- EF(s)
electric field(s)
- EGFR
epidermal growth factor receptor
- ERK1/2
extracellular-signaling regulated kinase 1/2
- FAD
Food and Drug Administration
- galvanotaxis/electrotaxis
directional cell migration in an electric field
- integrins
cell surface receptors that interact with the ECM and mediate various intracellular signals. They define cellular shape, mobility, and regulate the cell cycle
- pAkt
activated form of Akt
- PI3 kinase
phosphoinositide 3-kinases (PI 3-kinases or PI3Ks) are a family of related enzymes that are capable of phosphorylating the 3 position hydroxyl group of the inositol ring of phosphatidylinositol (PtdIns). They are also known as phosphatidylinositol-3-kinases
- PKC
protein kinase C
- population pressure
growth of adjacent cells form a pressure to push cells to move into a wound
- Pten
short of phosphatase and tensin homolog is a protein that acts as a tumor suppressor gene through the action of its phosphatase protein product. This phosphatase is involved in the regulation of the cell cycle, preventing cells from growing and dividing too rapidly
- Rac
subfamily of the Rho family of GTPases, small (~21 kDa) signaling G proteins (more specifically a GTPases)
- transepithelial potential difference
electric potential difference between the basal side and apical side of epithelial layers
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Footnotes
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
References
- 1.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 4.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 5.Stewart S, Rojas-Muñoz A, Belmonte JCI. Bioelectricity and epimorphic regeneration. Bio-essays. 2007;29:1133. doi: 10.1002/bies.20656. [DOI] [PubMed] [Google Scholar]
- 6.Olyaee Manesh A, Flemming K, Cullum NA, Ravaghi H. Electromagnetic therapy for treating pressure ulcers. Cochrane Database Syst Rev. 2006:CD002930. doi: 10.1002/14651858.CD002930.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Kloth LC. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds. 2005;4:23. doi: 10.1177/1534734605275733. [DOI] [PubMed] [Google Scholar]
- 8.Ojingwa JC, Isseroff RR. Electrical stimulation of wound healing. J Invest Dermatol. 2003;121:1. doi: 10.1046/j.1523-1747.2003.12454.x. [DOI] [PubMed] [Google Scholar]
- 9.Schaum KD. Decision on national coverage of electromagnetic therapy for wounds. Adv Skin Wound Care. 2004;17:316. doi: 10.1097/00129334-200407000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Huttenlocher A, Horwitz AR. Wound healing with electric potential. N Engl J Med. 2007;356:303. doi: 10.1056/NEJMcibr066496. [DOI] [PubMed] [Google Scholar]
- 11.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 12.McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85:943. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- 13.Pullar CE, Baier BS, Kariya Y, Russell AJ, Horst BA, Marinkovich MP, Isseroff RR. beta4 in-tegrin and epidermal growth factor coordinately regulate electric field-mediated directional migration via Rac1. Mol Biol Cell. 2006;17:4925. doi: 10.1091/mbc.E06-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzsimmons RJ, Gordon SL, Kronberg J, Ganey T, Pilla AA. A pulsing electric field (PEF) increases human chondrocyte proliferation through a transduction pathway involving nitric oxide signaling. J Orthop Res. 2008;26:854. doi: 10.1002/jor.20590. [DOI] [PubMed] [Google Scholar]
- 15.Gordon GA. Designed electromagnetic pulsed therapy: clinical applications. J Cell Physiol. 2007;212:579. doi: 10.1002/jcp.21025. [DOI] [PubMed] [Google Scholar]
- 16.Ramadan A, Elsaidy M, Zyada R. Effect of low-intensity direct current on the healing of chronic wounds: a literature review. J Wound Care. 2008;17:292. doi: 10.12968/jowc.2008.17.7.30520. [DOI] [PubMed] [Google Scholar]
- 17.Kloth LC, Zhao M. Endogenous and exogenous electrical fields for wound healing. In: McCulloch L, Kloth LC, editors. Wound Healing: Evidence-Based Management. 4. Brick: F.A. Davis; 2009. [Google Scholar]
- 18.Junger M, Arnold A, Zuder D, Stahl HW, Heising S. Local therapy and treatment costs of chronic, venous leg ulcers with electrical stimulation (Dermapulse): a prospective, placebo controlled, double blind trial. Wound Repair Regen. 2008;16:480. doi: 10.1111/j.1524-475X.2008.00393.x. [DOI] [PubMed] [Google Scholar]
- 19.Brighton CT, Wang W, Clark CC. The effect of electrical fields on gene and protein expression in human osteoarthritic cartilage explants. J Bone Joint Surg. 2008;90:833. doi: 10.2106/JBJS.F.01437. [DOI] [PubMed] [Google Scholar]
- 20.Mathew NT. Dynamic optimization of chronic migraine treatment: current and future options. Neurology. 2009;72(5 Suppl):S14. doi: 10.1212/WNL.0b013e3181974821. [DOI] [PubMed] [Google Scholar]
- 21.Reinold MM, Macrina LC, Wilk KE, Dugas JR, Cain EL, Andrews JR. The effect of neuromuscu-lar electrical stimulation of the infraspinatus on shoulder external rotation force production after rotator cuff repair surgery. Am J Sports Med. 2008;36:2317. doi: 10.1177/0363546508322479. [DOI] [PubMed] [Google Scholar]
- 22.Griffin XL, Warner F, Costa M. The role of electromagnetic stimulation in the management of established non-union of long bone fractures: what is the evidence? Injury. 2008;39:419. doi: 10.1016/j.injury.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Investig. 2007;117:1219. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eaglstein WH, Falanga V. Chronic wounds. Surg Clin North Am. 1997;77:689. doi: 10.1016/s0039-6109(05)70575-2. [DOI] [PubMed] [Google Scholar]
- 25.Kantor J, Margolis DJ. Expected healing rates for chronic wounds. Wounds. 2000;12:155. [Google Scholar]
- 26.Du Bois-Reymond E. Untersuchungen uber thier-ische Elektricitat, Zweiter Band, Zweite Abtheilung (Erste Lieferung) Berlin: Georg Reimer; [Google Scholar]
- 27.Mukerjee EV, Isseroff RR, Nuccitelli R, Collins SD, Smith RL. Microneedle array for measuring wound generated electric fields. Conf Proc IEEE Eng Med Biol Soc. 2006;1:4326. doi: 10.1109/IEMBS.2006.260205. [DOI] [PubMed] [Google Scholar]
- 28.Nuccitelli R, Nuccitelli P, Ramlatchan S, Sanger R, Smith PJ. Imaging the electric field associated with mouse and human skin wounds. Wound Repair Regen. 2008;16:432. doi: 10.1111/j.1524-475X.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid B, Nuccitelli R, Zhao M. Non-invasive measurement of bioelectric currents with a vibrating probe. Nat Protoc. 2007;2:661. doi: 10.1038/nprot.2007.91. [DOI] [PubMed] [Google Scholar]
- 30.Reid B, Song B, McCaig CD, Zhao M. Wound healing in rat cornea: the role of electric currents. FASEB J. 2005;19:379. doi: 10.1096/fj.04-2325com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramamurthi S, Rahman MQ, Dutton GN, Ramaesh K. Pathogenesis, clinical features and management of recurrent corneal erosions. Eye. 2006;20:635. doi: 10.1038/sj.eye.6702005. [DOI] [PubMed] [Google Scholar]
- 32.Robinson KR. The responses of cells to electrical fields: a review. J Cell Biol. 1985;101:2023. doi: 10.1083/jcb.101.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003;58:1. doi: 10.1016/s0070-2153(03)58001-2. [DOI] [PubMed] [Google Scholar]
- 34.Farahani RM. Biological mediators of wound healing: the importance of the big picture. Int Wound J. 2008;5:414. doi: 10.1111/j.1742-481X.2008.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaffe LF, Vanable JW., Jr Electric fields and wound healing. Clin Dermatol. 1984;2:34. doi: 10.1016/0738-081x(84)90025-7. [DOI] [PubMed] [Google Scholar]
- 36.Zhao M, Dick A, Forrester JV, McCaig CD. Electric field-directed cell motility involves up-regulated expression and asymmetric redistribution of the epidermal growth factor receptors and is enhanced by fibronectin and laminin. Mol Biol Cell. 1999;10:1259. doi: 10.1091/mbc.10.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown MJ, Loew LM. Electric field-directed fibroblast locomotion involves cell surface molecular reorganization and is calcium independent. J Cell Biol. 1994;127:117. doi: 10.1083/jcb.127.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fenteany G, Janmey PA, Stossel TP. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr Biol. 2000;10:831. doi: 10.1016/s0960-9822(00)00579-0. [DOI] [PubMed] [Google Scholar]
- 39.Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117(Pt 3):397. doi: 10.1242/jcs.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez-Chimeno M, Houghton PE, Holey L. Electrical stimulation for chronic wounds [Protocol] Cochrane Database of Systematic Reviews. 2004;(1) doi: 10.1002/14651858.CD004550. Art. No.: CD004550. [DOI] [Google Scholar]
- 41.Fang KS, Ionides E, Oster G, Nuccitelli R, Isseroff RR. Epidermal growth factor receptor relocalization and kinase activity are necessary for directional migration of keratinocytes in DC electric fields. J Cell Sci. 1999;112(Pt 12):1967. doi: 10.1242/jcs.112.12.1967. [DOI] [PubMed] [Google Scholar]