Abstract
Polycystic kidney disease 1-like 3 (Pkd1l3) is expressed specifically in sour-sensing type III taste cells that have synaptic contacts with afferent nerve fibers in circumvallate and foliate papillae located in the posterior region of the tongue, though not in fungiform papillae or the palate. To visualize the gustatory neural pathways that originate from type III taste cells in circumvallate and foliate papillae, we established transgenic mouse lines that express the transneuronal tracer wheat germ agglutinin (WGA) under the control of the mouse Pkd1l3 gene promoter/enhancer. The WGA transgene was accurately expressed in Pkd1l3-expressing type III taste cells in circumvallate and foliate papillae. Punctate WGA protein signals appeared to be detected specifically in type III taste cells but not in other types of taste cells. WGA protein was transferred primarily to a subset of neurons located in close proximity to the glossopharyngeal nerve bundles in the nodose/petrosal ganglion. WGA signals were also observed in a small population of neurons in the geniculate ganglion. This result demonstrates the anatomical connection between taste receptor cells in the foliate papillae and the chorda tympani nerves. WGA protein was further conveyed to neurons in a rostro-central subdivision of the nucleus of the solitary tract. These findings demonstrate that the approximately 10 kb 5’-flanking region of the mouse Pkd1l3 gene functions as a type III taste cell-specific promoter/enhancer. In addition, experiments using the pkd1l3-WGA transgenic mice reveal a sour gustatory pathway that originates from taste receptor cells in the posterior region of the tongue.
Keywords: PKD1L3, wheat germ agglutinin, type III taste cells, sour, gustatory neural pathway
Introduction
Taste compounds taken into the oral cavity are first detected by taste receptors localized in the taste pore areas at the apical end of taste buds (Ishimaru 2009, Ishimaru & Matsunami 2009, Yarmolinsky et al. 2009). This information is transmitted to peripheral gustatory neurons in the geniculate, petrosal, and nodose ganglia and further conveyed to central gustatory neurons, including the nucleus of the solitary tract (NST), parabrachial nucleus (PbN), thalamus, and primary gustatory cortex in the insula (Norgren 1995, Yarmolinsky et al. 2009, Saper 2000). Taste receptors for the five basic taste modalities -- sweet, bitter, sour, salty, and umami (savory) -- are exclusively expressed in distinct populations of taste receptor cells (TRCs), which means that TRCs sensing different basic taste modalities are mutually segregated in the taste buds (Ishimaru 2009, Yarmolinsky et al. 2009, Chandrashekar et al. 2010). However, the mechanism involved in the peripheral and central gustatory neuronal coding of gustatory information about each basic taste modality remains to be elucidated.
In the oral cavity, there are four major gustatory regions where taste buds are abundantly distributed: the circumvallate papillae (CvP), foliate papillae (FoP), fungiform papillae (FuP), and the palate (Norgren 1995, Saper 2000, Yarmolinsky et al. 2009). Taste buds in the FuP are scattered on the anterior tongue. Taste buds in the FuP and the palate are innervated by the chorda tympani (CT) and the greater superficial petrosal (GSP) nerves, respectively. These nerves are branches of the VIIth cranial nerve, and their cell bodies are accumulated in the geniculate ganglion (GG). In contrast, taste buds in the CvP and FoP are located in the posterior region of the tongue and are mostly innervated by the IXth cranial glossopharyngeal (GL) nerve, which has cell bodies in the nodose/petrosal ganglion (NPG). Electrophysiological analysis has revealed that taste buds in the FoP are also innervated by the CT nerves in rats (Yamamoto & Kawamura 1975).
Polycystic kidney disease 1-like 3 (Pkd1l3) is robustly expressed in the CvP and FoP, but not in the FuP or the palate (Ishimaru et al. 2006, Huang et al. 2006). In the CvP and FoP, Pkd1l3 is co-expressed with polycystic kidney disease 2-like 1 (Pkd2l1) in a subset of TRCs (i.e., type III taste cells), which are distinct from sweet, umami, and bitter-sensing TRCs (i.e., type II taste cells) (Ishimaru et al. 2006, Kataoka et al. 2008, Huang et al. 2006, LopezJimenez et al. 2006). In a heterologous expression system using human embryonic kidney (HEK) 293T cells, PKD1L3 and PKD2L1 interact with each other through their transmembrane domains, and this interaction is required for their functional cell surface expression (Ishimaru et al. 2006, Ishimaru et al. 2010). HEK293T cells transiently expressing Pkd1l3 and Pkd2l1 show an “off-response” to various types of acid solutions (Fujimoto et al. 2011, Ishimaru et al. 2006, Inada et al. 2008, Ishii et al. 2009, Ishimaru et al. 2010). This phenomena means that the PKD1L3/PKD2L1 channel opens only after the removal of an acid stimulus, but that the initial acid exposure is essential for the channel activation. Type III taste cells isolated from the CvP and FuP respond to sour taste stimuli (Yoshida et al. 2009, Kawaguchi et al. 2010, Huang et al. 2008). Recently, the “off-responses” upon acid stimuli have also been reported in native Pkd2l1-expressing TRCs from the CvP but not from the FuP using Ca2+-imaging and patch-clamp analyses (Kawaguchi et al. 2010). These results imply that Pkd1l3-positive type III taste cells play a role in sensing sour taste. Analysis of Pkd1l3 mutant mice revealed that protein localization of PKD2L1 at taste pores is regulated by PKD1L3 in taste cells of CvP and FoP (Ishimaru et al. 2010). However, Pkd1l3 mutant mice showed no significant reduction in taste response in behavioral or electrophysiological tests when compared with wild-type controls (Nelson et al. 2010, Horio et al. 2011). Therefore, the function of PKD1L3 in taste sensation remains to be elucidated.
The wheat germ agglutinin (WGA) transgene has been used as a transneuronal tracer to label neural pathways that originate from cells genetically modified to express WGA in a variety of nervous systems including the gustatory system (Ohmoto et al. 2008, Ohmoto et al. 2010, Damak et al. 2008, Yoshihara et al. 1999, Yoshihara 2002). We have previously established two types of transgenic mice in which WGA is expressed in different subsets of type II taste cells under the control of the mouse Tas1r3 or Tas2r105 (initially called T2r5) gene promoter/enhancer (i.e., t1r3-WGA and t2r5-WGA transgenic mice, respectively) (Ohmoto et al. 2008, Ohmoto et al. 2010). Analysis of the t1r3-WGA mice revealed sweet/umami gustatory pathways from Tas1r3-positive sweet/umami TRCs to the central neurons in the NST as well as the trigeminal neural pathway from solitary chemoreceptor cells that express Tas1r3 to brainstem neurons (Ohmoto et al. 2008). Analysis of the t2r5-WGA mice revealed the bitter gustatory pathway from Tas2r105 (T2r5)-positive bitter TRCs to the peripheral gustatory neurons in the GG and NPG (Ohmoto et al. 2010).
To visualize the gustatory pathway that originates from sour-sensing TRCs in the posterior region of the tongue, we established transgenic mouse lines in which WGA was expressed in the type III taste cells of the CvP and FoP under the control of mouse Pkd1l3 gene promoter/enhancer. Pkd1l3 exhibits specific expression in the TRCs of the CvP and FoP (Ishimaru et al. 2006, Huang et al. 2006) but not in the solitary chemoreceptor cells of the nasal epithelium (Ohmoto et al. 2008). Among the three gustatory regions innervated by peripheral gustatory neurons in the GG, Pkd1l3 is expressed in the FoP but not in the FuP or the palate. The transgenic mice genetically revealed the sour gustatory pathway from the Pkd1l3-positive sour TRCs in the posterior region of the tongue primarily through the peripheral gustatory neurons in the NPG and GG to the central neurons in the NST. This pathway was labeled separately from the trigeminal neural pathway.
Materials and Methods
Generation of pkd1l3-WGA transgenic mice
All animal experiments were approved by the Animal Care and Use Committees at The University of Tokyo. The vector construct for the generation of pkd1l3-WGA transgenic mice is illustrated in Figure 1A. As a putative promoter/enhancer region of Pkd1l3, the 5’-upstream region (~10 kb) of Pkd1l3 gene was amplified by PCR using BAC clone RP23-178P21 (BACPAC Resources Center), which was derived from C57BL/6 mice as a template. The 5’-upstream region (~10 kb) of the Pkd1l3 gene was introduced into the Sac II and Not I sites of the plasmid, which contained a truncated form of wild-type WGA, rabbit β-globin gene introns, and an SV40 polyadenylation signal in pBstN (Yoshihara et al. 1999, Ohmoto et al. 2008). Transgenic vectors were injected into BDF1 mouse embryos, and four founder mice were generated. One founder mice (#2) was infertile and three lines of pkd1l3-WGA transgenic mice (#1, #3, and #4) were established and analyzed for expression of WGA transgene by in situ hybridization (ISH). Transgene integration was screened using PCR with specific primer pairs (forward primer: 5’-GTGGATCCTGAGAACTTCAGG-3’; reverse primer: 5’-GAGGAGACAATGGTTGTCAAC-3’). Dissections and fixations were performed essentially as described previously (Ohmoto et al. 2008).
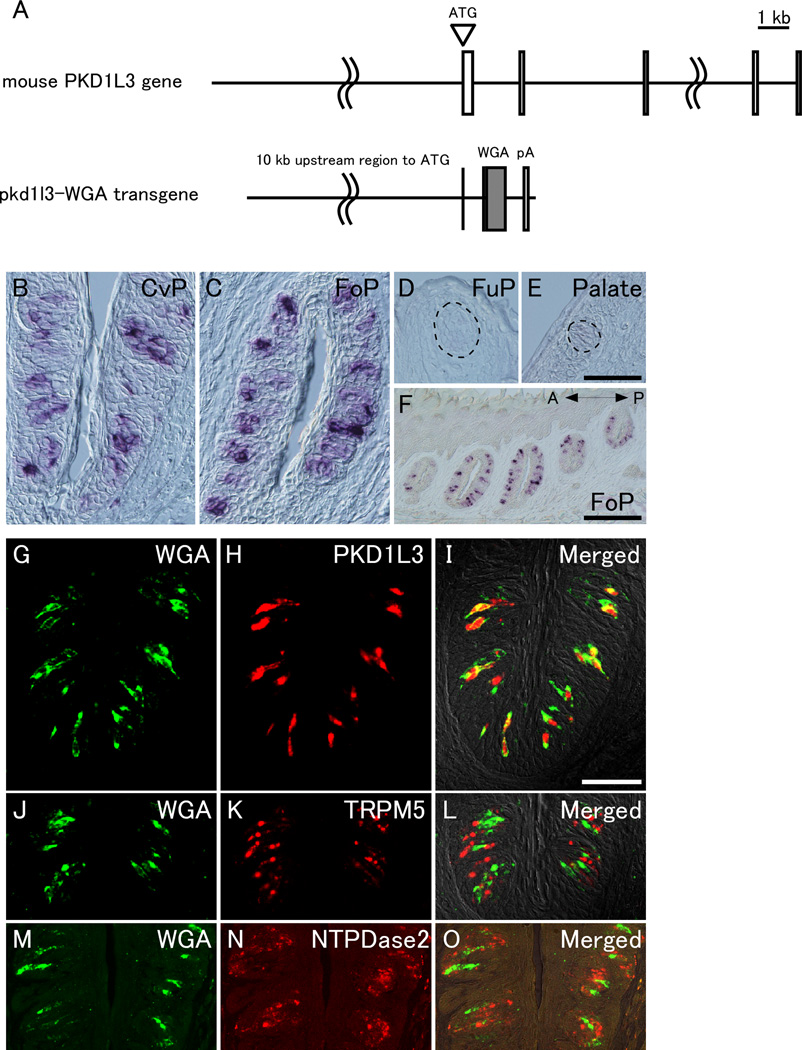
Figure 1. WGA mRNA is expressed in Pkd1l3-expressing type III taste cells in circumvallate and foliate papillae.
(A) Schematic representation showing pkd1l3-WGA transgene construct. The transgene consists of a 5’-upstream region (~10 kb) of the mouse Pkd1l3 gene, rabbit β-globin gene introns, truncated WGA cDNA, and SV40 polyadenylation signal. Boxes in mouse Pkd1l3 gene indicate the exons of Pkd1l3 gene. (B–F) In situ hybridization revealed that WGA mRNA was expressed in taste buds of the CvP (B) and the FoP (C and F) but not in the FuP (D) or palate (E). The dotted lines indicate the approximate area of taste buds (D and E). In the FoP, WGA mRNA was expressed in all of the taste buds along the anterior (A)-posterior (P) axis (F). (G–O) Double-label fluorescent in situ hybridization demonstrated that WGA mRNA signals (green) were completely overlapped with endogenous Pkd1l3 mRNA signals (red) (G–I) and segregated from those of Trpm5 (J–L) and Entpd2 which encodes NTPDase2 (M–O) (red) in the CvP. Scale bars: (in E) B–E, (in I) G–O, 50 µm; (in F) 200 µm.
In situ hybridization (ISH) and Immunohistochemistry
Flesh frozen sections of tongue, spinal cord, and brain and fixed sections of cranial sensory ganglion, 10 µm in thickness, on MAS coated glass slides (Matsunami Glass, Kishiwada, Japan) were used. For ISH, sections were fixed with 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) and treated with proteinase K followed by acetylation. Prehybridization, hybridization, washing, and development were performed using digoxigenin-labeled probes as described previously (Ishimaru et al. 2006). Double-label fluorescent ISH was performed with digoxigenin- and fluorescein-labeled RNA probes as described previously (Ishimaru et al. 2005). Probes were detected by incubation with peroxidase-conjugated anti-digoxigenin antibody and peroxidase-conjugated anti-fluorescein antibody (Roche, Indianapolis, IN), followed by incubation with TSA-AlexaFluor 555 and TSA-AlexaFluor 488 (Invitrogen, Carlsbad, CA) using the tyramide signal amplification method. For immunohistochemistry, PFA-fixed sections of tongue and ganglion were incubated with a goat anti-WGA antibody (1:3000 for tongue, 1:300 for ganglion, Vector Laboratories, Burlingame, CA) as described previously (Ohmoto et al. 2008). In brief, sections were blocked using PBS with 0.2% TritonX-100 (PBST) containing 5% normal rabbit serum, incubated with a goat anti-WGA antibody for 2 h, and then incubated with biotinylated rabbit anti-goat IgG antibody for 1 h followed by ABC solution (Vectastain ABC elite kit, Vector Laboratories). For brain, floating sections were stained with a goat anti-WGA antibody (1:3000) that had been preabsorbed with 1% acetone powder of mouse brains and biotinylated horse anti-goat IgG antibody using the tyramide signal amplification method as described previously (Ohmoto et al. 2008). For double immunofluorescent staining, PFA-fixed sections of tongue and ganglion were blocked with PBST containing 5% normal donkey serum (NDS) and incubated at 4°C overnight with a goat anti-WGA antibody (1:1000 for tongue, 1:300 for ganglion) together with one of the following primary antibodies: rabbit anti-AADC (1:2000, GeneTex, San Antonio, TX), rabbit anti-PLC-β2 (1:200, Santa Cruz biotechnology, Santa Cruz, CA), rabbit anti-P2X2 (1:600, Sigma, St Louis, MO), rabbit anti-P2X3 (1:3000, Chemicon, Temecula, CA), or rabbit anti-TRPV1 (1:300, Abcam, Cambridge, MA) antibody. For the double staining of WGA and AADC, sections were pretreated in Target Retrieval Solution (Dako Cytomation, Carpinteria, CA) for 20 min at 80°C before incubation with 5% NDS. Stained images were obtained using a fluorescence microscope (BX51; Olympus) equipped with a cooled CCD digital camera (DP71; Olympus), a confocal laser-scanning microscope (FV500; Olympus), or a Macro Zoom Microscope (MVX10; Olympus).
Results
Pkd1l3 is not expressed in neurons surrounding the central canal of the spinal cord
Pkd2l1 is expressed not only in a subset of TRCs in the CvP, FoP, FuP, and palate (Huang et al. 2006, Ishimaru et al. 2006) but also in neurons that surround the central canal of the spinal cord (Huang et al. 2006). To examine whether Pkd1l3 is also expressed in these neurons, we performed in situ hybridization (ISH) on sections of the spinal cord. Pkd2l1, but not Pkd1l3, was robustly expressed in specific neurons that surround the central canal of the spinal cord (Supplemental Figures 1A and B). Double-label fluorescent ISH confirmed the absence of Pkd1l3 mRNA expression in Pkd2l1-positive neurons (Supplemental Figures 1C–E). These results demonstrate that Pkd1l3, rather than Pkd2l1, is suitable for the induction of WGA expression to specifically label the neural pathway that originates from TRCs.
Generation of pkd1l3-WGA transgenic mice
To label the gustatory neural pathways that originate from type III taste cells in the CvP and FoP, we generated transgenic mouse lines in which the transneuronal tracer WGA was expressed specifically in Pkd1l3-positive TRCs. The 5’-flanking region (~10 kb) of the mouse Pkd1l3 gene was used as a putative Pkd1l3 gene promoter/enhancer to direct the expression of WGA in the type III taste cells of the CvP and FoP. The transgene consisted of the 5’-flanking region (~10 kb) of the mouse Pkd1l3 gene, rabbit β-globin gene introns, truncated WGA cDNA, and an SV40 polyadenylation signal (Figure 1A). Three pkd1l3-WGA transgenic mouse lines (#1, #3, and #4) were established; these mice were fertile and exhibited no growth defects or aberrant behavior. ISH analysis using a probe for WGA revealed that WGA mRNA was expressed in a subset of TRCs in the CvP and FoP but not in the FuP or the palate in two transgenic mouse lines (#3 and #4), whereas no signal of WGA mRNA was detected in all the four major gustatory regions in one transgenic mouse line (#1) (Figures 1B–F and data not shown). The expression pattern in the different gustatory regions was consistent with the expression of the endogenous Pkd1l3 gene (Ishimaru et al. 2006, Huang et al. 2006). In the FoP, WGA mRNA was expressed in all of the taste buds along the anterior-posterior axis (Figure 1F), which is consistent with endogenous PKD1L3 expression (Supplemental Figure 2). These two mouse lines (#3 and #4) were chosen for further analysis in this study, and there were no appreciable differences between the two lines.
To examine which types of cells expressed WGA mRNA, we performed double-label fluorescent ISH. In the CvP and FoP, almost all of the WGA-positive cells were also positive for Pkd1l3 but negative for Entpd2 encoding nucleoside triphosphate diphosphohydrolase-2 (NTPDase2), a marker for type I taste cells (Bartel et al. 2006), and Trpm5, which is a marker for type II taste cells and required for normal sweet, bitter, and umami sensations (Figures 1G–O and data not shown; Supplemental Table 1) (Perez et al. 2002, Zhang et al. 2003, Damak et al. 2006). Thus, these mouse lines exhibited the accurate cell type-specific expression of the transgene under the control of the Pkd1l3 gene promoter/enhancer.
WGA protein localization in the taste buds of the circumvallate and foliate papillae
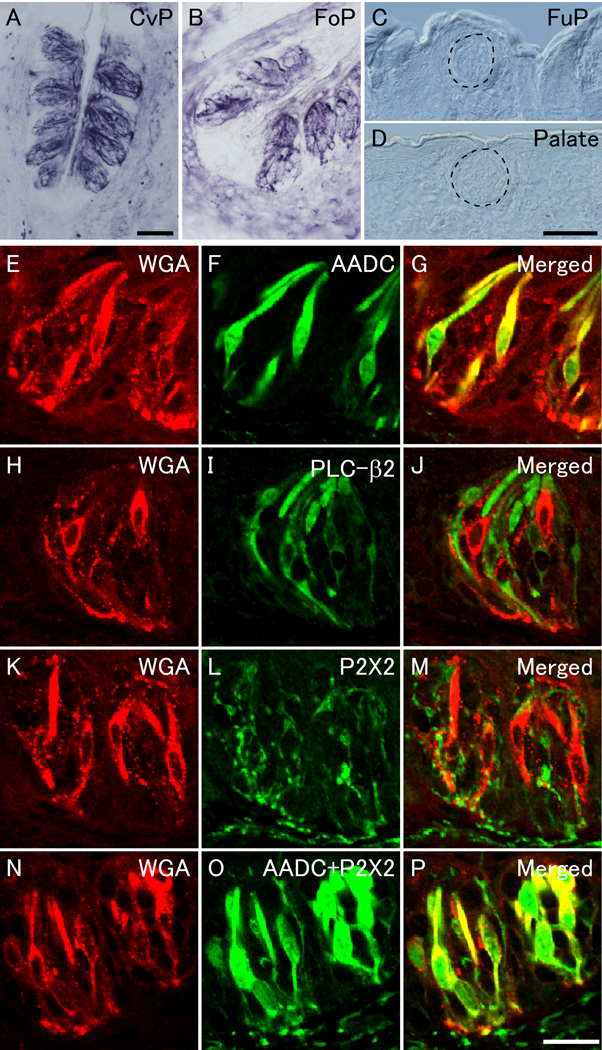
To examine the expression of WGA protein in the taste buds of pkd1l3-WGA transgenic mice, we performed immunostaining using an anti-WGA antibody on sections of the CvP, FoP, FuP, and palate. Strong punctate immunoreactivity for WGA was detected in the taste buds of the CvP and FoP but not in the FuP or the palate (Figures 2A–D), but no WGA signal was observed in non-transgenic mice (data not shown) as demonstrated previously (Ohmoto et al. 2008). Aromatic L-amino acid decarboxylase (AADC), an enzyme of serotonin synthesis, is used as a marker for type III taste cells, and phospholipase C-β2 (PLC-β2) is used as a marker for sweet-, bitter-, and umami-sensing type II taste cells (Clapp et al. 2004, Asano-Miyoshi et al. 2000, Ohmoto et al. 2008, Zhang et al. 2003). Double fluorescent immunostaining using antibodies against WGA together with antibodies against either AADC or PLC-β2 revealed that most of WGA-positive cells (approximately 95%) were also positive for AADC but negative for PLC-β2 in the CvP (Figures 2E–J and Supplemental Table 1), which agrees with the mRNA expression patterns. These results show that the WGA protein expressed in the type III taste cells was not laterally transferred to the type II taste cells in the taste buds. WGA protein signals were also detected in the nerve fibers that project toward the taste buds (Figure 2A) and the intragemmal fibers surrounding the TRCs (Figures 2K–M); these fibers were positive for the P2X2 purinergic receptor for ATP (Figures 2K–M), which is a candidate transmitter released from TRCs (Finger et al. 2005). Finally, double fluorescent immunostaining using antibodies against WGA and mixed antibodies against AADC and P2X2 revealed that the sum of AADC and P2X2 signals appeared to contain the signals of WGA protein (Figures 2N–P).
Figure 2. WGA protein expression in the circumvallate and foliate papillae of pkd1l3-WGA transgenic mice.
(A–D) Immunostaining revealed robust expression of WGA protein in the CvP (A) and FoP (B) but not in the FuP (C) or palate (D). The dotted lines indicate the approximate area of taste buds (C and D). (E–J) Double fluorescent immunostaining showed that WGA-positive cells (red) were also positive for AADC (green) (E–G) but negative for PLC-β2 (green) (H–J) in the CvP. (K–P) Double fluorescent immunostaining revealed that the signals of WGA protein (red) were detected in the fiber-like structures that were positive for P2X2 (green) (K–M) and that the sum of AADC and P2X2 signals (green) appeared to contain the signals of WGA protein (red) (N–P) in the CvP. Scale bars: (in A), (in D) B–D, 50 µm; (in P) E–P, 20 µm.
WGA transfer to gustatory neurons in the nodose/petrosal and geniculate ganglia
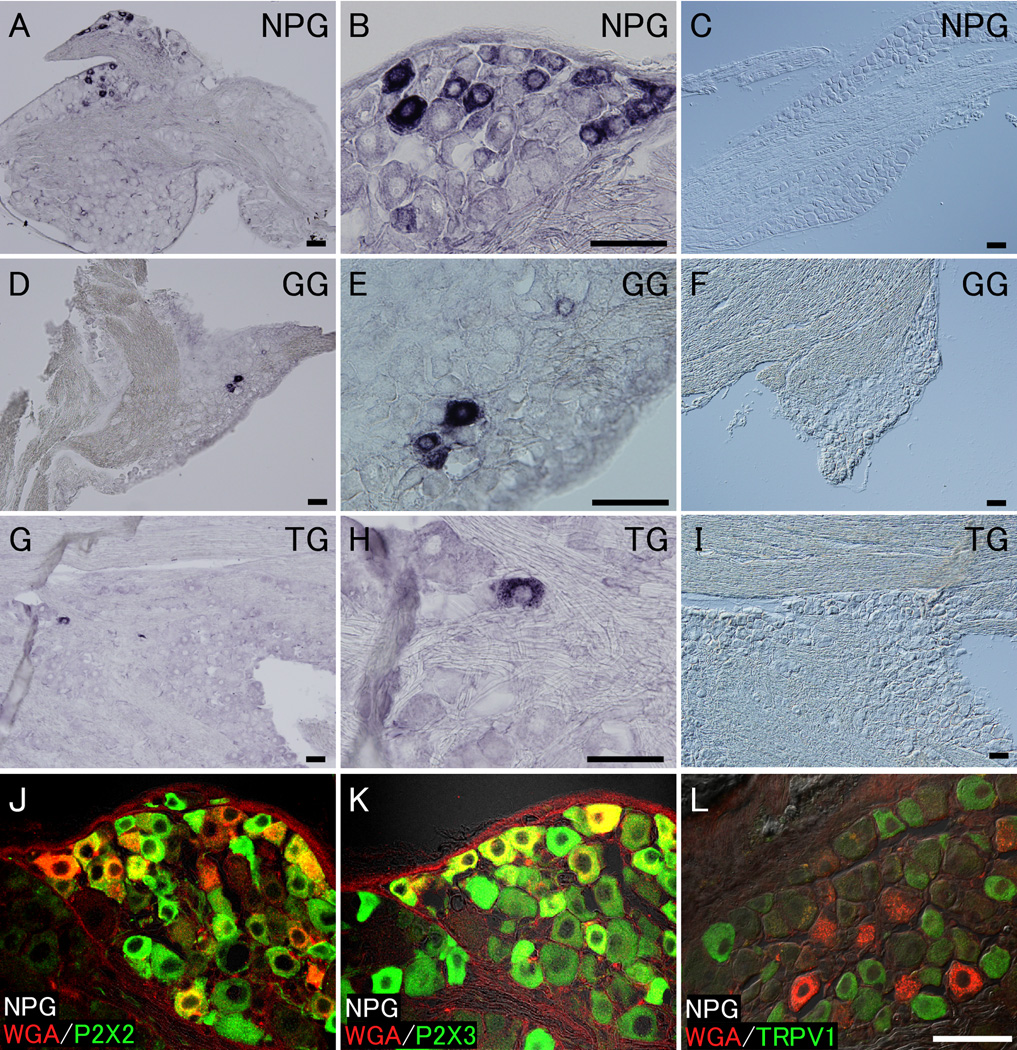
To investigate whether WGA protein was transferred from Pkd1l3-positive TRCs to gustatory neurons, we performed immunostaining using an anti-WGA antibody on sections of cranial sensory ganglia, including the NPG, GG, and trigeminal ganglion (TG), where the cell bodies of the peripheral sensory neurons are accumulated. WGA protein signals were primarily detected with a variety of intensity in approximately 5% of neurons (551 WGA-positive neurons in a total of 10,825 neurons from 42 sections) located in close proximity to the GL nerve bundles in the NPG (Figures 3A, B, and Supplemental Figure 3). In the GG, WGA signals were observed only in a small population of neurons (~1%; 20 WGA-positive neurons in a total of 2,294 neurons from 30 sections) (Figures 3D and E). These GG neurons appear to give rise to CT fibers that innervate Pkd1l3-positive TRCs in the FoP. Because ISH analysis demonstrated that WGA mRNA was absent in the NPG and GG (Figures 3C and F), it is likely that the WGA protein in the cranial sensory ganglia originated from Pkd1l3-positive TRCs. Interestingly, some WGA signals were occasionally detected in a much smaller population of neurons in the TG (~0.1%; 6 WGA-positive neurons in a total of approximately 6,000 neurons from 10 sections) (Figures 3G and H). However, the origin of the WGA signals remains unclear because Pkd1l3 mRNA was not expressed in the TG (Figure 3I) or the solitary chemoreceptor cells of the nasal epithelium (Ohmoto et al. 2008). To characterize the WGA-positive cells in the NPG, double fluorescent immunostaining was performed using antibodies against WGA together with antibodies against P2X2 or P2X3 or the transient receptor potential vanilloid 1 (TRPV1). P2X2 and P2X3, as receptors for ATP, are expressed in a large population of neurons in the NPG, and TRPV1 is a marker of nociceptor neurons. Double fluorescent immunostaining revealed that WGA signals in the NPG were present in subsets of P2X2- or P2X3-positive neurons (Figures 3J and K) but not in TRPV1-positive nociceptor neurons (Figure 3L).
Figure 3. WGA protein is transferred to the gustatory neurons in the nodose/petrosal, geniculate, and trigeminal ganglia.
(A and B) WGA protein signals were detected with different intensities in a subset of neurons located close to the GL nerve bundles in the NPG. (D and E) WGA signals were observed only in a small population of neurons in the GG. (G and H) WGA signals were occasionally detected in a much smaller population of neurons in the TG. (C, F, and I) In situ hybridization analysis demonstrated that WGA mRNA was absent in the NPG, GG, and TG. (J–L) Double fluorescent immunostaining revealed that WGA signals (red) close to the GL nerve bundles in the NPG were detected in a subpopulation of P2X2- or P2X3-expressing neurons (green) (J and K) but not in TRPV1-positive nociceptor neurons (green) (L). Scale bars: (in A–I), (in L) J–L, 50 µm.
WGA transfer to central neurons in the nucleus of the solitary tract
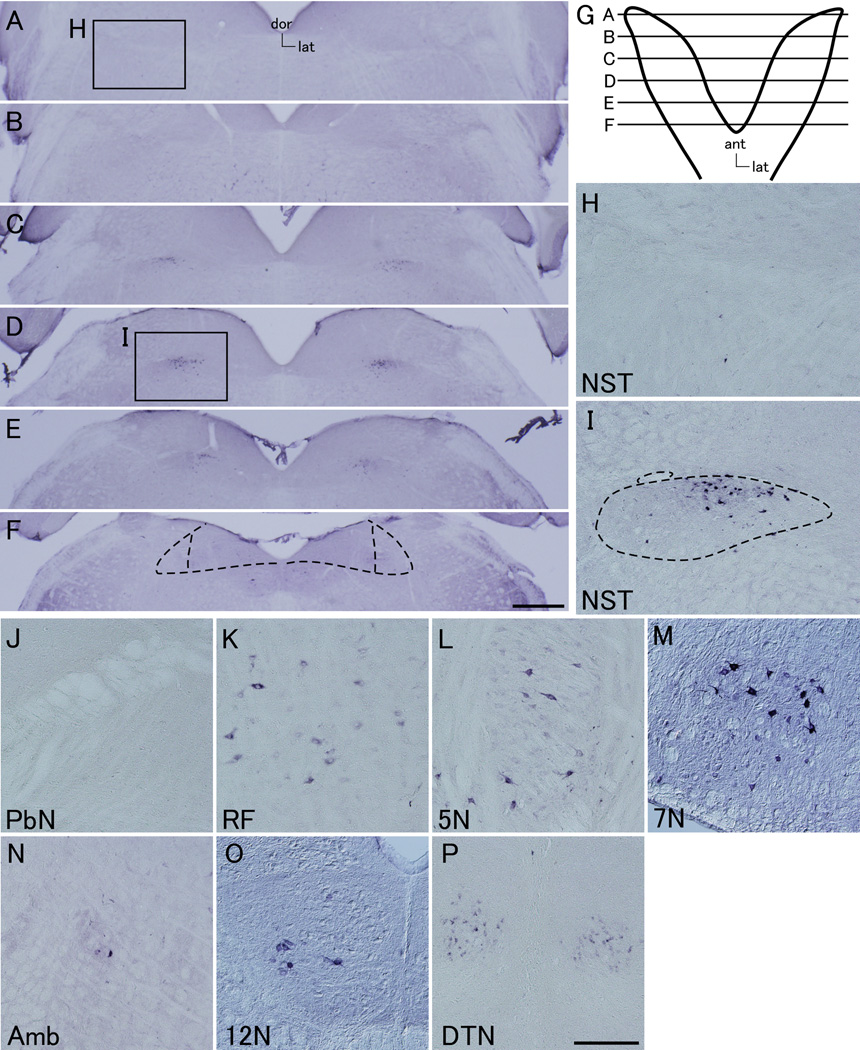
The gustatory neurons in the NPG and GG afferently innervate neurons in the NST of the brainstem (Buck 2000, Lundy Jr. & Norgren 2004). To investigate whether the WGA protein was further transferred from the peripheral gustatory neurons in the NPG and GG to the central neurons in the NST, we performed immunostaining using an anti-WGA antibody in brain sections that include the NST. Strong and weak WGA signals were observed in the rostral (Figures 4C–E) and intermediate (Figure 4F) portions, respectively, of the NST along the rostro-caudal axis but not in the caudal portion of the NST (data not shown). It should be noted that only weak WGA signals, if any, were detected in a few neurons in the most rostral portion of the NST, where most of the CT and GSP nerve afferents terminate (Figures 4A and H) (May & Hill 2006, Hamilton & Norgren 1984, Whitehead 1988). In the rostral NST, WGA-positive neurons were localized in the central subdivision (Figure 4I), which contains the majority of the GL nerve afferents (Renehan et al. 1994, Whitehead 1988, Hamilton & Norgren 1984, May & Hill 2006, Lasiter 1992, Harrer & Travers 1996, Di Lorenzo & Lemon 2000). Unlike the t1r3-WGA mice, no WGA signals were detected in the lateral region of the intermediate NST (Figure 4F) (Ohmoto et al. 2008), which is the central target of the trigeminal neurons (Marfurt & Rajchert 1991, Menetrey & Basbaum 1987). Little WGA immunoreactivity was detected in the neurons in the PbN, which were afferently innervated by the NST neurons (Figure 4J) (Norgren 1995, Saper 2000). Signals of WGA protein were also detected in other brain areas, such as the reticular formation, trigeminal motor nucleus, facial motor nucleus, ambiguous nucleus, hypoglossal nucleus, and the dorsal tegmental nucleus (Figures 4K–P). However, no signal of WGA mRNA was detected throughout the brain including these nuclei (Supplemental Figure 4 and data not shown). These findings indicate that WGA protein was likely transferred to these areas from WGA-expressing taste cells via gustatory nerves.
Figure 4. WGA protein is further transferred to the rostro-central subdivision in the nucleus of the solitary tract and other brain areas.
(A–F) Serial sections of the NST appear along the rostro-caudal axis in the pkd1l3-WGA mice; the rostral (A–E) and intermediate (F) portions of NST. Strong WGA signals were observed in the rostral NST (C–E). In the lateral region of the intermediate NST, which is the central target of the trigeminal neurons, no WGA signals were detected (F). The dotted lines indicate the approximate area of the NST, and two vertical dotted lines within the NST indicate the border between the medial and lateral regions of the intermediate NST (F). dor, dorsal; lat, lateral. (G) Schematic representation of the dorsal view of the NST is presented. Positions of the coronal sections for immunostaining of WGA in A–F are indicated. ant, anterial; lat, lateral. (H) Only weak WGA signals, if any, were detected in a few neurons in the most rostral portion of the NST. (I) In the rostral NST, WGA-positive neurons were localized in the central subdivision. The dotted lines indicate the approximate area of the NST. (J) Little WGA immunoreactivity was detected in the central neurons in the PbN. (K–P) WGA protein signals were also detected in other brain areas, such as the reticular formation (RF; K), trigeminal motor nucleus (5N; L), facial motor nucleus (7N; M), ambiguous nucleus (Amb; N), hypoglossal nucleus (12N; O), and the dorsal tegmental nucleus (DTN; P). Scale bars: (in F) A–F, 500 µm, (in P) H–P, 200 µm.
Discussion
We have previously established two types of transgenic mice that express WGA in different subsets of type II taste cells that lack conventional synaptic structures: the t1r3-WGA and t2r5-WGA transgenic mice (Ohmoto et al. 2008, Ohmoto et al. 2010). In this study, we generated the pkd1l3-WGA transgenic mice in which WGA was expressed in type III taste cells that form synaptic contacts with afferent nerve fibers in the posterior region of the tongue. An approximately 10 kb 5’-flanking region of the mouse Pkd1l3 gene was sufficient to confer type III taste cell-specific expression of the WGA transgene. The pkd1l3-WGA transgenic mice genetically revealed gustatory neural pathways that originate from sour-sensing type III taste cells in the CvP and FoP via the cranial sensory ganglia, including the NPG and GG, toward the rostro-central subdivision in the NST; this pathway is separate from the trigeminal neural pathway.
WGA signals detected in taste receptor cells and neurons in cranial sensory ganglia and brainstem
The number of WGA-traceable cells that are sequentially connected in a neural pathway depends on the amount of WGA present in the original cells, because WGA proteins are passively transferred between cells (Yoshihara et al. 1999, Yoshihara 2002). WGA signals were detected in the central gustatory neurons in the brainstem of pkd1l3-WGA mice and t1r3-WGA mice, but WGA signals were detected only in the peripheral gustatory neurons and not in the brainstem of t2r5-WGA mice (Ohmoto et al. 2008, Ohmoto et al. 2010). These differences were probably due to the distinct promoter/enhancer activity among these genes because ISH analysis revealed that both Pkd1l3 and Tas1r3 mRNA are much more abundantly expressed in the TRCs than is Tas2r105 (T2r5) (Ishimaru et al. 2006, Huang et al. 2006, LopezJimenez et al. 2006). In addition, the synaptic structures present in the type III taste cells might contribute to the high transfer efficiency of WGA protein to the gustatory afferent nerves (Murray 1973, Kataoka et al. 2008).
A large population of WGA mRNA and protein were present exclusively in type III taste cells of the CvP and FoP, and they did not appear in other types of taste cells, such as type II taste cells in the pkd1l3-WGA mice (Figures 1, 2 and Supplemental Table 1). Similarly, WGA protein was present in subsets of type II taste cells but was absent in type III taste cells in the t1r3-WGA and t2r5-WGA mice (Ohmoto et al. 2008, Damak et al. 2008, Ohmoto et al. 2010). These results demonstrate that the WGA protein was not transferred laterally between the type II and type III taste cells in all three types of transgenic mice that we generated. Accordingly, gustatory information appears to be primarily transmitted from TRCs directly to the gustatory afferent nerves and not through other types of taste cells. However, we cannot exclude the possibility that gustatory information is processed between taste cells within the taste buds (Chaudhari & Roper 2010).
WGA transfer from the Pkd1l3-positive type III taste cells to the cranial sensory ganglia
Our immunohistochemistry in the cranial sensory ganglia revealed that WGA protein was transferred from type III taste cells in the CvP and FoP to neurons not only in the NPG but also in the GG (Figure 3). Electrophysiological analysis has shown that taste buds in the FoP are innervated by both the CT and GL nerves in rats (Yamamoto & Kawamura 1975). We provided the first anatomical evidence that shows the connection between TRCs in the FoP and CT nerves in mice. It remains unclear which taste buds in the FoP are innervated by the CT nerve because both Pkd1l3 and WGA mRNA were expressed in all taste buds of the FoP along the anterior-posterior axis in the pkd1l3-WGA mice (Figure 1F and Supplemental Figure 2). In the NPG of the pkd1l3-WGA mice, most of WGA-positive neurons were localized in the narrow region, which is close to the GL nerve bundles (Figure 3 and Supplemental Figure 3). In contrast, WGA signals are detected throughout the NPG in t1r3-WGA mice (Ohmoto et al. 2008). Possibly, the cell bodies of peripheral neurons that convey sour taste information were localized in the narrow region of the NPG. Alternatively, it is possible that the different distribution patterns of WGA signals within the NPG reflected the different anatomical locations of cells that expressed Pkd1l3 and cells that expressed Tas1r3. WGA-positive neurons that innervated type III taste cells were subsets of P2X2- or P2X3-positive neurons but were distinct from TRPV1-positive nociceptor neurons in the NPG of pkd1l3-WGA mice (Figure 3), which is similar to t1r3-WGA and t2r5-WGA mice (Ohmoto et al. 2008, Ohmoto et al. 2010). Further study will be needed to define whether the WGA-positive neurons observed in the three types of WGA transgenic mice are identical, partially overlapping, or completely distinct.
Brainstem target nuclei of the sour gustatory pathway from the posterior region of the tongue visualized with WGA transgene
The CT and GSP nerves terminate primarily in the rostral pole of the NST, but the GL nerves project to neurons in the rostral and intermediate NST in a manner that overlaps with the CT and GSP nerves (Hamilton & Norgren 1984, May & Hill 2006, Whitehead 1988). The trigeminal nerves project to the lateral regions of the NST throughout the rostro-caudal axis, especially in the intermediate NST, but the vagus nerve projects to the caudal NST (Marfurt & Rajchert 1991, Menetrey & Basbaum 1987, Hamilton & Norgren 1984, Finger 1987). In the NST of the pkd1l3-WGA mice, intense WGA signals were observed in the rostral portion, except for the most rostral pole (approximately 200 µm in length), and not in the caudal portion (Figures 4A–F); WGA signals appeared to be mainly detected in the region where the GL nerve afferents terminate. In the rostral NST, WGA-positive neurons were localized in the central subdivision (Figure 4I), which contains the majority of GL nerve afferents (Renehan et al. 1994, Whitehead 1988, Hamilton & Norgren 1984, May & Hill 2006, Lasiter 1992, Harrer & Travers 1996, Di Lorenzo & Lemon 2000). In contrast, WGA signals were broadly distributed in the rostral, intermediate, and caudal portions of the NST in the t1r3-WGA mice (Ohmoto et al. 2008). These results are in good agreement with the different distribution pattern of WGA signals in the cranial sensory ganglia between pkd1l3-WGA and t1r3-WGA mice. In the t1r3-WGA mice, WGA protein was transferred from Tas1r3-expressing TRCs and solitary chemoreceptor cells in the nasal epithelium to the peripheral gustatory neurons in GG and NPG and to the trigeminal neurons in TG (Ohmoto et al. 2008). The WGA protein was transferred primarily to neurons in the NPG, to a small population of neurons in GG, and to a much smaller population of neurons in TG in the pkd1l3-WGA mice (Figure 3). Accordingly, the gustatory neural pathway, but not the trigeminal neural pathway, was specifically visualized in the NST of the pkd1l3-WGA mice. In contrast, both the gustatory and trigeminal pathways are labeled in the NST of the t1r3-WGA mice (Ohmoto et al. 2008).
In addition to the NST, WGA signals were also detected in other brain areas, such as the reticular formation, trigeminal motor nucleus, facial motor nucleus, ambiguous nucleus, hypoglossal nucleus, and the dorsal tegmental nucleus (Figures 4K–P). The central subdivision of the rostral NST projects directly to the reticular formation that is located ventral to the rostral NST, which in turn projects to the trigeminal motor nucleus (Nasse et al. 2008, Beckman & Whitehead 1991, Travers 1988). However, because no WGA signal was detected in the central neurons in the PbN (Figure 4J), which is innervated by the NST neurons (Norgren 1995, Saper 2000), it is less likely that the WGA signals detected in these two brain areas were transferred from the central subdivision of the rostral NST. Although these two areas are the central targets of the trigeminal neurons (Marfurt & Rajchert 1991, Menetrey & Basbaum 1987), the number of WGA-positive neurons in the TG was so small that it is unlikely that all of the WGA signals observed in these areas were transferred from the neurons in the TG. In addition, no signal of WGA mRNA was detected throughout the brain (Supplemental Figure 4 and data not shown). These results show that WGA protein was neither produced in the reticular formation or the trigeminal motor, facial motor, ambiguous, hypoglossal or dorsal tegmental nuclei, nor transported there from other parts of the brain with projections to these areas. WGA protein was likely transferred to these structures from WGA-expressing taste cells via gustatory nerves. The presence of WGA signals in brain areas other than the NST may indicate that gustatory information is also conveyed to multiple nuclei in the brainstem including the motor nuclei.
In conclusion, in the pkd1l3-WGA transgenic mice, the transneuronal tracer WGA was specifically expressed in the type III taste cells of the CvP and FoP using an approximately 10 kb 5’-flanking region of the Pkd1l3 gene as a promoter/enhancer. These mice revealed a sour gustatory pathway from the posterior region of the tongue that is separate from the trigeminal neural pathway.
Supplementary Material
Acknowledgments
We are grateful to Dr. Yoshihiro Yoshihara (RIKEN Brain Science Institute) for providing us with the WGA cDNA. We also thank Taeko Fukuda for expert technical assistance and members of the Taste Science laboratory for critical readings of the manuscript. This work was supported in part by a Grant-in-Aid for Young Scientists (B) 20780089 and (A) 22688010 to Y.I. and Grants-in-Aid for Scientific Research 20380183 to K.A. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a grant from the Mishima Kaiun Memorial Foundation to Y.I.; NIH grant R03DC011143 to I. M.; and a Research and Development Program for New Bio-industry Initiatives to K.A.
Abbreviations
- AADC
aromatic L-amino acid decarboxylase
- CT
chorda tympani
- CvP
circumvallate papillae
- FoP
foliate papillae
- FuP
fungiform papillae
- GG
geniculate ganglion
- GL
glossopharyngeal
- GSP
greater superficial petrosal
- HEK
human embryonic kidney
- ISH
in situ hybridization
- NDS
normal donkey serum
- NPG
nodose/petrosal ganglion
- NST
nucleus of the solitary tract
- PbN
parabrachial nucleus
- PBS
phosphate buffered saline
- PFA
paraformaldehyde
- PKD1L3
polycystic kidney disease 1-like 3
- PKD2L1
polycystic kidney disease 2-like 1
- PLC
phospholipase C
- TG
trigeminal ganglion
- TRC
taste receptor cell
- TSA
tyramide signal amplification
- WGA
wheat germ agglutinin
References
- Asano-Miyoshi M, Abe K, Emori Y. Co-expression of calcium signaling components in vertebrate taste bud cells. Neurosci Lett. 2000;283:61–64. doi: 10.1016/s0304-3940(00)00911-3. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman ME, Whitehead MC. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res. 1991;557:265–279. doi: 10.1016/0006-8993(91)90143-j. [DOI] [PubMed] [Google Scholar]
- Buck LB. Smell and taste: the chemical senses. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York: McGraw-Hill; 2000. pp. 625–647. [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- Damak S, Mosinger B, Margolskee RF. Transsynaptic transport of wheat germ agglutinin expressed in a subset of type II taste cells of transgenic mice. BMC Neurosci. 2008;9:96. doi: 10.1186/1471-2202-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Lemon CH. The neural code for taste in the nucleus of the solitary tract of the rat: effects of adaptation. Brain Res. 2000;852:383–397. doi: 10.1016/s0006-8993(99)02187-3. [DOI] [PubMed] [Google Scholar]
- Finger TE. Gustatory nuclei and pathways in the central nervous system. In: Finger TE, Silver WL, editors. Neurobiology of taste and smell. New York: Wiley; 1987. pp. 331–354. [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Fujimoto C, Ishimaru Y, Katano Y, Misaka T, Yamasoba T, Asakura T, Abe K. The single pore residue Asp(523) in PKD2L1 determines Ca(2+) permeation of the PKD1L3/PKD2L1 complex. Biochem Biophys Res Commun. 2011;404:946–951. doi: 10.1016/j.bbrc.2010.12.086. [DOI] [PubMed] [Google Scholar]
- Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222:560–577. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- Harrer MI, Travers SP. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res. 1996;711:125–137. doi: 10.1016/0006-8993(95)01410-1. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio N, Yoshida R, Yasumatsu K, Yanagawa Y, Ishimaru Y, Matsunami H, Ninomiya Y. Sour taste responses in mice lacking PKD channels. PLoS One. 2011;6:e20007. doi: 10.1371/journal.pone.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada H, Kawabata F, Ishimaru Y, Fushiki T, Matsunami H, Tominaga M. Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep. 2008;9:690–697. doi: 10.1038/embor.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Misaka T, Kishi M, Kaga T, Ishimaru Y, Abe K. Acetic acid activates PKD1L3-PKD2L1 channel--a candidate sour taste receptor. Biochem Biophys Res Commun. 2009;385:346–350. doi: 10.1016/j.bbrc.2009.05.069. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y. Molecular mechanisms of taste transduction in vertebrates. Odontology. 2009;97:1–7. doi: 10.1007/s10266-008-0095-y. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Katano Y, Yamamoto K, Akiba M, Misaka T, Roberts RW, Asakura T, Matsunami H, Abe K. Interaction between PKD1L3 and PKD2L1 through their transmembrane domains is required for localization of PKD2L1 at taste pores in taste cells of circumvallate and foliate papillae. FASEB J. 2010;24:4058–4067. doi: 10.1096/fj.10-162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Matsunami H. Transient receptor potential (TRP) channels and taste sensation. J Dent Res. 2009;88:212–218. doi: 10.1177/0022034508330212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Okada S, Naito H, Nagai T, Yasuoka A, Matsumoto I, Abe K. Two families of candidate taste receptors in fishes. Mech Dev. 2005;122:1310–1321. doi: 10.1016/j.mod.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sevigny J, Kinnamon JC, Finger TE. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 2008;33:243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi H, Yamanaka A, Uchida K, Shibasaki K, Sokabe T, Maruyama Y, Yanagawa Y, Murakami S, Tominaga M. Activation of polycystic kidney disease-2-like 1 (PKD2L1)-PKD1L3 complex by acid in mouse taste cells. J Biol Chem. 2010;285:17277–17281. doi: 10.1074/jbc.C110.132944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiter PS. Postnatal development of gustatory recipient zones within the nucleus of the solitary tract. Brain Res Bull. 1992;28:667–677. doi: 10.1016/0361-9230(92)90245-s. [DOI] [PubMed] [Google Scholar]
- LopezJimenez ND, Cavenagh MM, Sainz E, Cruz-Ithier MA, Battey JF, Sullivan SL. Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J Neurochem. 2006;98:68–77. doi: 10.1111/j.1471-4159.2006.03842.x. [DOI] [PubMed] [Google Scholar]
- Lundy RF, Jr, Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. San Diego: Elsevier Academis Press; 2004. pp. 891–921. [Google Scholar]
- Marfurt CF, Rajchert DM. Trigeminal primary afferent projections to "non-trigeminal" areas of the rat central nervous system. J Comp Neurol. 1991;303:489–511. doi: 10.1002/cne.903030313. [DOI] [PubMed] [Google Scholar]
- May OL, Hill DL. Gustatory terminal field organization and developmental plasticity in the nucleus of the solitary tract revealed through triple-fluorescence labeling. J Comp Neurol. 2006;497:658–669. doi: 10.1002/cne.21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol. 1987;255:439–450. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- Murray RG. The ultrastructure of taste buds. In: Friedmann II, editor. Ultrastructure of sensory organs I. New York: American Elsevier; 1973. pp. 1–81. [Google Scholar]
- Nasse J, Terman D, Venugopal S, Hermann G, Rogers R, Travers JB. Local circuit input to the medullary reticular formation from the rostral nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1391–R1408. doi: 10.1152/ajpregu.90457.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TM, Lopezjimenez ND, Tessarollo L, Inoue M, Bachmanov AA, Sullivan SL. Taste function in mice with a targeted mutation of the pkd1l3 gene. Chem Senses. 2010;35:565–577. doi: 10.1093/chemse/bjq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. San Diego: Academis Press; 1995. pp. 751–771. [Google Scholar]
- Ohmoto M, Maeda N, Abe K, Yoshihara Y, Matsumoto I. Genetic tracing of the neural pathway for bitter taste in t2r5-WGA transgenic mice. Biochem Biophys Res Commun. 2010;400:734–738. doi: 10.1016/j.bbrc.2010.08.139. [DOI] [PubMed] [Google Scholar]
- Ohmoto M, Matsumoto I, Yasuoka A, Yoshihara Y, Abe K. Genetic tracing of the gustatory and trigeminal neural pathways originating from T1R3-expressing taste receptor cells and solitary chemoreceptor cells. Mol Cell Neurosci. 2008;38:505–517. doi: 10.1016/j.mcn.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Renehan WE, Jin Z, Zhang X, Schweitzer L. Structure and function of gustatory neurons in the nucleus of the solitary tract. I. A classification of neurons based on morphological features. J Comp Neurol. 1994;347:531–544. doi: 10.1002/cne.903470405. [DOI] [PubMed] [Google Scholar]
- Saper CB. Brain stem, reflexive behavior, and the cranial nerves. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York: McGraw-Hill; 2000. pp. 873–888. [Google Scholar]
- Travers JB. Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res. 1988;457:1–11. doi: 10.1016/0006-8993(88)90051-0. [DOI] [PubMed] [Google Scholar]
- Whitehead MC. Neuronal architecture of the nucleus of the solitary tract in the hamster. J Comp Neurol. 1988;276:547–572. doi: 10.1002/cne.902760409. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kawamura Y. Dual innervation of the foliate papillae of the rat: an electrophysiological study. Chem Senses. 1975;1:241–244. [Google Scholar]
- Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Miyauchi A, Yasuo T, Jyotaki M, Murata Y, Yasumatsu K, Shigemura N, Yanagawa Y, Obata K, Ueno H, Margolskee RF, Ninomiya Y. Discrimination of taste qualities among mouse fungiform taste bud cells. J Physiol. 2009;587:4425–4439. doi: 10.1113/jphysiol.2009.175075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y. Visualizing selective neural pathways with WGA transgene: combination of neuroanatomy with gene technology. Neurosci Res. 2002;44:133–140. doi: 10.1016/s0168-0102(02)00130-x. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Mizuno T, Nakahira M, Kawasaki M, Watanabe Y, Kagamiyama H, Jishage K, Ueda O, Suzuki H, Tabuchi K, Sawamoto K, Okano H, Noda T, Mori K. A genetic approach to visualization of multisynaptic neural pathways using plant lectin transgene. Neuron. 1999;22:33–41. doi: 10.1016/s0896-6273(00)80676-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.