Abstract
Preclinical and some clinical studies suggest a relationship between perturbation in magnesium (Mg2+) homeostasis and pathological anxiety, although the underlying mechanisms remain largely unknown. Since there is evidence that Mg2+ modulates the hypothalamic-pituitary adrenal (HPA) axis, we tested whether enhanced anxiety-like behaviour can be reliably elicited by dietary Mg2+ deficiency and whether Mg2+ deficiency is associated with altered HPA axis function. Compared with controls, Mg2+ deficient mice did indeed display enhanced anxiety-related behaviour in a battery of established anxiety tests. The enhanced anxiety-related behaviour of Mg2+ deficient mice was sensitive to chronic desipramine treatment in the hyponeophagia test and to acute diazepam treatment in the open arm exposure test. Mg2+ deficiency caused an increase in the transcription of the corticotropin releasing hormone in the paraventricular hypothalamic nucleus (PVN), and elevated ACTH plasma levels, pointing to an enhanced set-point of the HPA axis. Chronic treatment with desipramine reversed the identified abnormalities of the stress axis. Functional mapping of neuronal activity using c-Fos revealed hyper-excitability in the PVN of anxious Mg2+ deficient mice and its normalisation through diazepam treatment. Overall, the present findings demonstrate the robustness and validity of the Mg2+ deficiency model as a mouse model of enhanced anxiety, showing sensitivity to treatment with anxiolytics and antidepressants. It is further suggested that dysregulations in the HPA axis may contribute to the hyper-emotionality in response to dietary induced hypomagnesaemia.
This article is part of a Special Issue entitled ‘Anxiety and Depression’.
Keywords: Magnesium, Anxiety, Stress axis, Selective serotonin re-uptake inhibitor, Diazepam, Corticotropin releasing hormone, ACTH, Desipramine
Highlights
► Robustness and validity of the Mg2+ deficiency model of enhanced anxiety is shown. ► The Mg2+ deficiency model is sensitive to anxiolytic/antidepressant treatments. ► We suggest an inverse relationship between Mg2+ and anxiety. ► An aberrant HPA axis may contribute to the hyper-emotionality of Mg2+ deficiency.
1. Introduction
Anxiety disorders are currently the most common neuropsychiatric disorders in the USA and Europe (Andlin-Sobocki et al., 2005; Kessler et al., 2005) and represent one of the major health problems in the western world (WHO, 2004). In the aetiology of an anxiety disorder, the simultaneous occurrence of various intrinsic and extrinsic factors, including those relating to genes and environment, seems to be important. Among these, there is increasing evidence of a role of “poor” nutrition (Popkin, 2006) in the development of some psychiatric diseases (Coppen and Bolander-Gouaille, 2005; Kroll, 2007), despite an apparent over-nutrition in our industrialised and globalised societies. Altered values of diverse micronutrients, including electrolytes, minerals and vitamins, can be linked to symptoms in psychiatric patients (Armstrong et al., 2007; Thys-Jacobs, 2000; Zender and Olshansky, 2009). In this respect, changes in magnesium (Mg2+) homeostasis have been shown to contribute to affective disorders although some inconsistencies exist between the studies (for review see Murck, 2002). Furthermore, a causal relationship between Mg2+ and anxiety is suggested in mice with low plasma Mg2+ levels which display increased anxiety- and/or depression-related behaviour, irrespective of whether these depleted levels are natural or experimentally induced (Laarakker et al., 2011; Singewald et al., 2004), although in humans equivalent evidence is scarce (Jacka et al., 2009; Seelig, 1994). In further support of such a causal relationship, Mg2+ supplementation reduces the anxiety-related behaviour of mice (see for example Poleszak et al., 2004). Stimulated by these findings, it has been recently suggested that hypomagnesaemia is a possible physiological indicator of anxiety (Laarakker et al., 2011).
In the search for neurobiological mechanisms underlying abnormal anxiety, it may be that Mg2+ is an interesting player as it is one of the essential ions in the brain affecting many intracellular and interneuronal processes. For example, Mg2+ has been shown to modulate both glutamatergic neurotransmission (via a voltage-dependent block of NMDA receptors) (Haddad, 2005) and GABAergic neurotransmission, and to affect numerous transduction pathways, including that of proteinkinase C (for review see Murck, 2002).
In addition, Mg2+ has been shown to control the activity of the hypothalamic-pituitary adrenocortical (HPA) axis (Murck and Steiger, 1998), which is considered to be the main stress response system (for review see e.g. Young et al., 2008). In the course of HPA axis activation by the corticotropin releasing hormone (CRH), synthesised in the paraventricular hypothalamic nucleus (PVN), adrenal corticotropin hormone (ACTH) is released from the anterior pituitary into the blood stream which, in turn, stimulates the secretion of corticosterone. HPA axis activation via CRH is involved in the generation of many different autonomic, hormonal and behavioural changes during stress and elicits anxiety symptoms (for review see Lowry and Moore, 2006; Young et al., 2008). It has been suggested that dysregulations in the CRH-HPA axis system may contribute to pathological anxiety (for review see Charney and Drevets, 2008; Millan, 2003; Young et al., 2008).
On the basis of these data, it may be hypothesised that an altered HPA axis function underlies the enhanced anxiety of Mg2+ deficient mice that is elicited by dietary Mg2+ restriction (when mice are limited to 10% of their daily requirement) which results in a 45% reduction of plasma Mg2+ levels (Singewald et al., 2004). To address this possibility, we initially explored the robustness and validity of the model by inducing hypomagnesaemia according to our previous protocol for C57Bl/6J mice (Singewald et al., 2004) in two additional mouse strains with divergent levels of inborn anxiety (C57Bl/6N and BALB/c mice) (for review see e.g. Belzung and Griebel, 2001; Sartori et al., 2011), and then tested the effects of a clinically established benzodiazepine and the two antidepressant drugs, paroxetine and desipramine, with established clinical efficacy in patients with anxiety disorder (for review see Plag et al., 2009) and/or with demonstrated sensitivity in Mg2+ deficient mice to reduce depression-like behaviour (Singewald et al., 2004; Whittle et al., 2011). The emotional behaviour of Mg2+ deficient mice was assessed in a battery of anxiety tests including the open field test, the light/dark test, the stress-induced hypothermia test, and the hyponeophagia test, each of which are thought to mimic different aspects of human anxiety and which differ in their sensitivity to detecting the anxiolytic properties of drugs (for review see Crawley, 2000; Cryan and Holmes, 2005; Dulawa and Hen, 2005; Vinkers et al., 2008). We then quantified markers of HPA axis function including CRH gene expression and plasma ACTH levels in experimentally naive or unstressed Mg2+ deficient mice. Finally, functional relevance of potentially altered transcriptional processes in the HPA system was investigated in Mg2+ deficient mice using mapping of the immediate-early gene c-Fos as a marker of neuronal activation in response to an anxiety-provoking situation. Rather than addressing every aim in each strain, we allocated specific experiments to either the C57Bl/6N or the Balb/c strain (see Methods). Thus, using a reduced number of animals following the principles of animal welfare, this experimental design allowed revealing most information complementary to the one gained in C57Bl/6N mice (e.g. CRH expression in the PVN) by adding further aspects (e.g. the influence of inborn anxiety levels by using the Balb/c strain).
2. Methods
2.1. Animals
Male C57Bl/6N and BALB/c mice were purchased from Charles River (Germany). In all experiments adult animals (8 weeks old at the onset of the experiment) were used. They were group-housed (up to 10 animals per cage) side-by-side in a temperature- (22 ± 2 °C) and humidity- (50–60%) controlled animal care facility unit under a 12 h light/dark cycle (lights on at 07:00 h), and provided with pelleted food and water ad libitum. All experimental procedures were designed to minimise animal suffering as well as the number of animals used and were approved by the national ethical committee on animal care and use (Bundesministerium für Wissenschaft und Forschung) in compliance with international laws and policies.
2.2. Diets and drug treatments
Mice assigned to Mg2+ deficient groups were allowed to freely access a 0.005% Mg2+ containing diet (Ssniff Spezialdiäten, Germany), which provided about 10% of the daily Mg2+ requirement (Kantak, 1988) as previously described (Singewald et al., 2004). Control mice were fed a normal, 0.2% Mg2+ containing diet (Ssniff Spezialdiäten, Germany) which offers four times more than the minimum Mg2+ requirement (Kantak, 1988), and hereafter are always referred to as “controls”. In Mg2+ deficient mice chronic treatment with either desipramine or paroxetine was given via the drinking water. A daily drug intake of approximately 30 mg/kg desipramine (Sigma Aldrich) and 5 mg/kg paroxetine (kindly provided by GlaxoSmithKline, UK), respectively, was achieved by adapting the concentrations of the drugs in the drinking solutions according to mean drinking volume and bodyweight per cage. However, the individual dose obtained may have varied due to variations in the mice in terms of water consumption and bodyweight. Mice were kept under the assigned experimental condition (diet/drug treatment) for at least three weeks until the completion of experiments. Diazepam (1 mg/kg, dissolved in purified water; Sigma, Germany) or vehicle were administered i.p. to mice in their home cages.
2.3. Behavioural experiments
Mice were left undisturbed in their home cages for three weeks from the start of diet and/or chronic drug treatment until the onset of behavioural experiments. After animals had been habituated to the testing room for at least 24 h, behavioural experiments were carried out between 09:00 h and 14:00 h. Testing chambers were carefully cleaned with tap water and a damp towel after each animal had been tested. In order to decrease the number of animals used, the behavioural experiments, with at least two days of rest between each, were conducted in the same animals starting with the stress-induced hyperthermia test (Lecci et al., 1990), the open field test (Broadhurst, 1961; DeFries et al., 1974), the light/dark test (Crawley, 1981), and the hyponeophagia test (Bodnoff et al., 1988) according to protocols used previously in our lab (Busquet et al., 2009; Singewald et al., 2004; Tschenett et al., 2003; Whittle et al., 2011).
2.3.1. Stress-induced hyperthermia
Mice were housed singly for at least 24 h prior to testing. With the mouse in its homecage, a glycerol lubricated thermistor probe connected to a digital thermometer (DM 852, Ellab, Denmark) was inserted into its rectum and left there until the temperature was stable for 20 s. The accuracy of the thermometer was 0.1 °C. Rectal temperature was measured in each mouse at T1, and then at T2 10 min later. As the intubation of the rectal probe for assessing the core temperature has been shown to sufficiently initiate a stress response in mice (Bouwknecht et al., 2007), the difference between temperatures T2 and T1 was taken as evidence of stress-induced hyperthermia. At the end of the stress-induced hyperthermia test, animals were group housed again for the following tests.
2.3.2. Open field test
The open field consisted of a plastic box (41 × 41 × 41 cm) equipped with an automated activity monitoring system (TruScan, Coulbourn Instruments, USA). The area of the open field, illuminated with 150 lux, was divided into a 28 × 28 cm central zone and a surrounding border zone. Mice were placed individually into the periphery of the open field and allowed to explore it for 10 min. The following anxiety-related parameters were recorded: entries into the central zone, time spent in it, number of rearings, and the overall distance travelled.
2.3.3. Light/dark test
The light/dark testing arena (41 × 41 × 41 cm; TruScan, Coulbourn Instruments, USA) was divided into two halves, comprising a white, aversive compartment illuminated with 400 lux at floor level and a dark, safe compartment covered by a black top illuminated with 10 lux. The compartments were connected by a small opening (7 × 7 cm) located in the centre of the partition at floor level. Animals were individually placed into the dark compartment facing away from the opening and allowed to freely explore the apparatus for 10 min. During the 10 min testing period the behaviour displayed by each mouse was automatically registered and the following anxiety-related parameters quantified: the latency to the first entry into the lit compartment, number of entries into the lit compartment, time spent in the lit compartment, number of rearings and overall distance travelled by the mice.
2.3.4. Hyponeophagia test
This paradigm was carried out in the open field arena (see above) with lighting set at 40 lux. Mice were food deprived, but allowed to freely drink water overnight. Animals were placed into a corner of the arena and the latency they took to start consuming oatflakes placed in the centre of the testing arena was registered, as well as the distance travelled.
2.4. Prepro-CRH mRNA expression
In a separate cohort of the C57Bl/6N strain, mice were fed either the control diet, the Mg2+ restricted diet, or the Mg2+ restricted diet and concomitant chronic desipramine treatment as described above and were left undisturbed in their home cages for three weeks. These experimentally naive mice were then sacrificed by carbon dioxide inhalation in a saturated chamber in accordance with established welfare guidelines (Hackbarth et al., 2000) and their brains were quickly removed. Consecutive, frozen sections (12 μm) at the level of the PVN, central amygdala and Barrington’s nucleus were prepared on slides (Paxinos and Franklin, 2001) and further processed for in situ hybridisation as previously described (Keck et al., 2005; Sartori et al., 2004). Briefly, a specific, [35S]-labelled oligonucleotide probe (Microsynth, Balgach, Switzerland) directed against bases 64–111 of the rat prepro-CRH clone (Jingami et al., 1985) showing high homology with the murine gene (accession number NM_205769.1) was hybridised to brain sections at 35 °C overnight. Subsequently, slides were washed in 0.5 saline sodium citrate three times at 55 °C followed by two washes at room temperature. Dry slides were exposed to BioMax MR film (Kodak, USA) for 7‑14 days. Quantitative analysis was performed on digitised autoradiograms using image analysis software (AnalySIS, Soft Imaging Systems, Germany). In two sections per animal, mean grey values from regions of interest were determined and converted into optical densities, and then further into nCi/g tissue equivalent using autoradiographic [14C]-microscales (Amersham Biosciences, UK) corrected for [35S].
2.5. Radioimmunoassays
At least five days after the last behavioural experiment, C57Bl/6N mice assigned to the control diet, the Mg2+ deficient diet, or the Mg2+ deficient diet and concomitant chronic treatment with desipramine were euthanised using carbon dioxide in a saturated chamber (Hackbarth et al., 2000). Trunc blood was collected from the sacrificed animals in EDTA-coated vials (Greiner Bio-one GmbH, Austria) and centrifuged (3000 rpm) for 10 min at 4 °C. Plasma samples were stored at −20 °C until measurement took place. Plasma ACTH and corticosterone concentrations were determined using commercially available kits (MP Biomedicals, USA), with an intra- and inter-assay variability of less than 10% and a lower detection limit of 6 pg/mL and 8 ng/mL, respectively.
2.6. c-Fos expression in response to emotional challenge
30 min after i.p. injection of either diazepam (1 mg/kg) or vehicle, BALB/c mice were subjected to the open arm exposure test as previously described (Muigg et al., 2009). During the 10-min testing period in which the animals were allowed to freely explore the open arm of an elevated plus maze, entries into and time spent in the proximal and distal compartments of the open arm as well as the total distance travelled were automatically quantified by the Videomot tracking system (TSE Systems, Germany). Two hours later mice were deeply anaesthetised (200 mg/kg sodium pentobarbital, i.p.) and transcardially perfused with saline followed by paraformaldehyde solution (4% in 0.1 M phosphate buffered saline, pH 7.4), and their brains were removed. Coronal brain sections (100 μm) at the level of the PVN were processed for c-Fos immunocytochemistry using a polyclonal rabbit anti-c-Fos primary antibody (1:20,000; Santa Cruz Biotechnology, USA), a biotinylated goat anti-rabbit secondary antibody (1:200; Vector Laboratories, USA) and an avidin-biotin-horseradish peroxidase procedure (Vectastain ABC kit, Vector laboratories, USA) with 3,3′-diaminobenzidine (Sigma, Germany) as chromogen according to previous protocols (Muigg et al., 2009; Singewald et al., 2003). The PVN cells containing a nuclear brown-black reaction product were considered to be c-Fos-positive cells and their number was counted bilaterally in a representative tissue area of 0.01 mm2 with the help of a light microscope (Olympus BX-40) equipped with an ocular grid.
2.7. Data presentation and statistics
Data represent mean ± standard error of the mean (SEM). Exact n-numbers are given in table and figure legends. Variations in n-numbers between tests and/or assays are explained by the combination of at least two separate sets of experiments using different n-numbers and the early withdrawal of some animals for other experiments, in part reported elsewhere (e.g. Whittle et al., 2011). Statistical analysis was performed using STATISTICA 7.1 (Stat Soft, Inc., USA). First, all experimental groups were tested for statistically significant outliers using the Grubb’s test. Data were then tested for homogeneity of variances using Levene’s test. If a parametric distribution was revealed, the data were further analysed using either a one- or two-way ANOVA followed by Fisher’s LSD test when allowed. Non-parametric data were statistically analysed using a Kruskal–Wallis ANOVA test followed by Mann–Whitney U tests. Statistical significance was set at P < 0.05 while non-significant P values were reported as n.s.
3. Results
At the beginning of the experiments, C57Bl/6N mice assigned to control diet, Mg2+ deficient diet or Mg2+ deficient diet chronically treated with either desipramine or paroxetine did not differ in bodyweight (mean bodyweight of 22.5 ± 0.1 g). After three weeks under the experimental conditions (diet/drug), bodyweight gain differed between experimental groups (F1,22 = 31.520, P < 0.001). Within this time period Mg2+ deficient animals (+5.1 ± 1.1%) put on more bodyweight than control animals (+2.4 ± 0.8%; P < 0.05). Compared with the Mg2+ deficient group, the gain in bodyweight was increased in Mg2+ deficient mice chronically treated with paroxetine (+10.9 ± 1.0%; P < 0.001) while the Mg2+ deficient mice chronically treated with desipramine even lost weight (−2.9 ± 1.1%; P < 0.001). Similar to C57Bl/6N mice, bodyweights did not differ between control mice and Mg2+ deficient mice (mean bodyweight of 20.0 ± 0.1 g) of the Balb/c strain at the beginning of the experiments. Three weeks of feeding a Mg2+ restricted diet caused a reduced increase in bodyweight in Balb/c mice (control: +11.8 ± 1.3%: Mg2+ deficient: +5.8 ± 1.5 g; P < 0.01).
3.1. Effect of Mg2+ deficiency and chronic antidepressant treatment on anxiety-related behaviour in C57Bl/6N mice
Using the stress-induced hyperthermia paradigm (for review see Vinkers et al., 2008), anxiety-related behaviour of C57Bl/6N mice assigned to either the control diet, the Mg2+ deficient, or the Mg2+ deficient diet and parallel chronic paroxetine treatment was tested (Table 1). Basal body temperature of control mice was low reaching 35.8 ± 0.2 °C, which indicates that animals were not pre-stressed under the given experimental conditions. There was a significant difference between mice in the body temperature T1 under unstressed conditions (F1,22 = 6.400, P < 0.01). Subsequent post-hoc analysis designated increased basal body temperature in Mg2+ deficient animals compared with controls and paroxetine-treated Mg2+ deficient mice. The mild stress of measuring T1 caused a rise in body temperature (T2) which by this stage no longer differed between the three experimental groups. The stress-induced rise in body temperature was smaller in Mg2+ deficient mice than it was in either control or paroxetine-treated Mg2+ deficient mice (F1,21 = 4.132, P < 0.05).
Table 1.
Effect of Mg2+ deficiency and chronic paroxetine treatment on behavioural measures assessed in diverse tests of anxiety in C57Bl/6N mice.
| Control | MgD | MgD + PAR | |
|---|---|---|---|
| Stress-induced hyperthermia | |||
| T1 (°C) | 35.8 ± 0.2 | 36.5 ± 0.1* | 35.6 ± 0.2## |
| T2 (°C) | 36.9 ± 0.1 | 37.2 ± 0.1 | 36.7 ± 0.2 |
| T2 − T1 (°C) | 1.1 ± 0.1 | 0.7 ± 0.1* | 1.2 ± 0.1# |
| Open field test | |||
| Centre entries (n) | 31.8 ± 3.0 | 23.3 ± 3.2a | 17.6 ± 3.3** |
| Centre time (s) | 87.8 ± 12 | 51.2 ± 8.4* | 45.7 ± 8.9** |
| Rearings (n) | 25.7 ± 3.2 | 18.4 ± 2.7a | 12.3 ± 2.0** |
| Distance travelled (cm) | 2399 ± 133 | 2060 ± 132 | 1892 ± 168 |
| Light/dark test | |||
| Latency (s) | 85.9 ± 14 | 229 ± 44** | 224 ± 25** |
| Entries into the lit arena (n) | 10.9 ± 1.0 | 8.8 ± 1.4 | 6.4 ± 1.2* |
| Time spent in lit arena (s) | 161 ± 22 | 155 ± 27 | 73.7 ± 15**# |
| Rearings (n) | 17.9 ± 1.9 | 17.9 ± 2.3 | 9.2 ± 1.2**## |
| Distance travelled (cm) | 2539 ± 120 | 2215 ± 127 | 2199 ± 87 |
Data are presented as means ± SEM. n = 10 for control and MgD groups, and n = 10 or 5 (in the stress-induced hyperthermia test) for MgD + PAR group.
P < 0.08, *P < 0.05, **P < 0.01 for MgD vs. control mice; #P < 0.05, ##P < 0.01 MgD + PAR vs. MgD mice. MgD: Mg2+ deficient mice; MgD + PAR: Mg2+ deficient mice chronically treated with paroxetine.
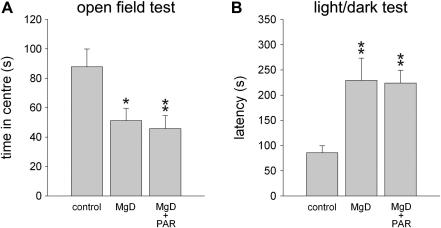
Animals of the C57Bl/6N strain were then tested in the open field test and light/dark test which are based on an exploration-avoidance conflict (for review see e.g. Cryan and Holmes, 2005). In the open field test (Table 1), experimental groups differed significantly in terms of number of entries into the centre of the testing arena (F2,27 = 5.021, P < 0.05), time spent there (F2,26 = 5.284, P < 0.05; Fig. 1) as well as number of rearings (F2,27 = 6.381, P < 0.01). Specifically, compared with mice fed the control diet, these measures were lower in Mg2+ deficient and paroxetine-treated Mg2+ deficient animals. In the light/dark test (Table 1; Fig. 1), we observed a significant difference in the latency to enter the brightly lit, aversive compartment of the light/dark chamber (F2,27 = 7.122, P < 0.01) as this latency was increased in both groups fed a Mg2+ restricted diet (Fig. 1). Furthermore, there was a significant group effect in terms of the number of entries into (F2,27 = 3.430, P < 0.05) and time spent (F2,27 = 5.045, P < 0.05) in the lit compartment of the light/dark test chamber, and in the rearing numbers (F2,27 = 7.361, P < 0.01). Paroxetine-treated Mg2+ deficient mice displayed reduced values in all three parameters compared with both control and Mg2+ deficient groups (Table 1).
Fig. 1.
Effect of Mg2+ deficiency and chronic paroxetine treatment on selected anxiety-related measures in C57Bl/6N mice. A. In mice fed a Mg2+ restricted diet (MgD) the time spent in the anxiogenic centre of an open field was decreased compared with mice fed the control diet (control) indicating enhanced anxiety-related behaviour. B. Anxiety-related behaviour was also elevated in the light/dark test as indicated by the increased latency to enter the (anxiogenic) brightly lit compartment. Chronic treatment with paroxetine (MgD + PAR) did not alter anxiety-related behaviour of Mg2+ deficient mice in these tests. n = 10 per experimental group. Data represent means ± SEM. *P < 0.05, **P < 0.01 for Mg2+ deficient vs. control mice.
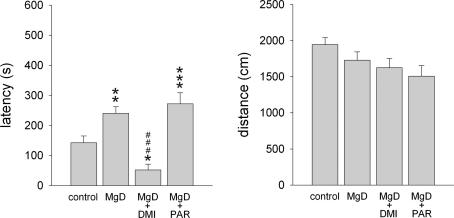
Next, we subjected all C57Bl/6N mice to the hyponeophagia paradigm, one of the few tests which are sensitive to the anxiolytic effects of chronic antidepressant treatment (Bodnoff et al., 1989; Dulawa and Hen, 2005; Gordon and Hen, 2004). There was a significant group effect in the latency to eat (F3,58 = 11.828, P < 0.001) the preferred food placed into the centre of the testing arena (Fig. 2). Mg2+ deficiency caused an increase in the latency to eat. In Mg2+ deficient mice chronic desipramine treatment reduced the latency to eat compared with untreated mice while long-term treatment with paroxetine did not affect this behavioural parameter (Fig. 2). Mg2+ deficiency and long-term antidepressant treatments did not alter general locomotor activity as indicated by the distances travelled in the open field test (n.s.; Table 1), the light/dark test (n.s.; Table 1), and the hyponeophagia test (n.s.; Fig. 2). All together these findings suggest that chronic Mg2+ deficiency was anxiogenic and that chronic desipramine, but not paroxetine treatment was effective in reducing anxiety in this model.
Fig. 2.
In the hyponeophagia test, the latency to eat a preferred food placed in the centre of the testing arena and the distance travelled is shown for C57Bl/6N mice fed either the control diet (control; n = 19), the Mg2+ deficient diet (MgD; n = 16), or the Mg2+ deficient diet and additional long-term treatment with either desipramine (MgD + DMI; n = 12) or paroxetine (MgD + PAR; n = 16). Data represent means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 for Mg2+ deficient vs. control mice, ###P < 0.001 for drug-treated Mg2+ deficient mice vs. Mg2+ deficient mice.
3.2. Effect of Mg2+ deficiency and desipramine treatment on prepro-CRH mRNA expression in the brain of C57Bl/6N mice
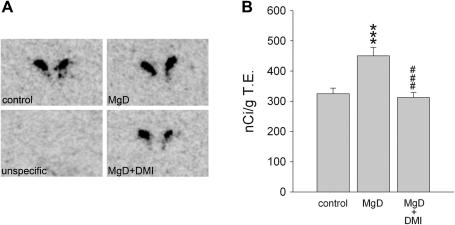
In C57Bl/6N mice prepro-CRH mRNA expression, as visualised by in situ hybridisation, was high in the PVN and moderate in the central amygdala and Barrington’s nucleus (Table 2), which is in good accordance with previous studies (e.g. Keegan et al., 1994). In the PVN a significant group effect was observed (F2,29 = 12.486, P < 0.001) as the abundance of prepro-CRH mRNA was increased in Mg2+ deficient mice compared with mice fed the control diet (P < 0.001), and chronic treatment with desipramine normalised this effect in Mg2+ deficient mice (P < 0.001) (Table 2, Fig. 3). In contrast to the PVN, prepro-CRH mRNA expression did not differ between experimental groups in the central amygdala (n.s.) or in the Barrington’s nucleus (n.s.) (Table 2).
Table 2.
Effect of Mg2+ deficiency and chronic desipramine treatment on prepro-CRH mRNA expression in C57Bl/6N mice.
| Control | MgD | MgD + DMI | |
|---|---|---|---|
| Paraventricular hypothalamic nucleus | 325 ± 18 | 450 ± 28*** | 313 ± 16### |
| Central amygdala | 140 ± 9.0 | 150 ± 13 | 145 ± 9.0 |
| Barrington’s nucleus | 164 ± 9.0 | 168 ± 16 | 162 ± 7.0 |
Data expressed in nCi/g T.E. are presented as means ± SEM. n = 10 per experimental group for the paraventricular hypothalamic nucleus and central amygdala, n = 9 per experimental group for the Barrington’s nucleus. ***P < 0.001 for MgD vs. control mice, ###P < 0.001 MgD + DMI vs. MgD mice. MgD: Mg2+ deficient mice; MgD + DMI: Mg2+ deficient mice chronically treated with desipramine.
Fig. 3.
This figure shows (A) representative autoradiograms and (B) quantification of prepro-CRH mRNA expression in the paraventricular hypothalamic nucleus for C57Bl/6N mice fed either the control diet (control; n = 10), the Mg2+ deficient diet (MgD; n = 10), or the Mg2+ deficient diet after long-term treatment with desipramine (MgD + DMI; n = 10); it also provides quantification of unspecific hybridisation. Data represent mean ± SEM. *P < 0.05, ***P < 0.001 for Mg2+ deficient vs. control mice and ###P < 0.001 for desipramine-treated Mg2+ deficient mice vs. Mg2+ deficient mice.
3.3. Effect of Mg2+ deficiency and desipramine treatment on plasma ACTH and corticosterone levels in C57Bl/6N mice
Under unstressed conditions plasma ACTH levels (H2,46 = 7.466, P < 0.05), but not corticosterone levels (n.s.), differed between experimental groups of the C57Bl/6N strain (Table 3). Mg2+ deficient animals showed increased plasma ACTH levels compared with controls fed a normal Mg2+ containing diet (P < 0.01). Chronic treatment with desipramine decreased the elevated ACTH plasma levels of Mg2+ deficient mice, which no longer differed in this respect from control mice (n.s.).
Table 3.
Effect of Mg2+ deficiency and chronic desipramine treatment on basal ACTH and corticosterone plasma levels in C57Bl/6N mice.
| Control | MgD | MgD + DMI | |
|---|---|---|---|
| ACTH (pg/ml) | 89.7 ± 14.7 (19) | 199 ± 30.4 (19)** | 136 ± 46.0 (8) |
| Corticosterone (ng/ml) | 74.9 ± 13.3 (30) | 105 ± 18.1 (30) | 83.8 ± 15.6 (21) |
Data represent means ± SEM. n – numbers per experimental group are given in parentheses behind values. **P < 0.01 for MgD vs. control mice. MgD: Mg2+ deficient mice; MgD + DMI: Mg2+ deficient mice chronically treated with desipramine.
3.4. Effect of Mg2+ deficiency and diazepam on anxiety-related behaviour and c-Fos expression in response to open arm exposure in Balb/c mice
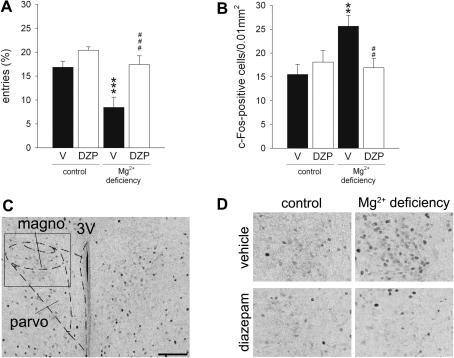
In an attempt to gain additional evidence for a possible functional relevance of increased PVN prepro-CRH mRNA expression in the Mg2+ deficiency-induced enhanced anxiety-related behaviour, c-Fos expression was used to map neuronal activation in the PVN in response to the mild emotional challenge of open arm exposure. As with the C57Bl/6N strain, Mg2+ deficiency increased anxiety-related behaviour in BALB/c mice which was reversed by diazepam treatment as indicated by significant dietary and drug effects in the measures “percentage of entries into the anxiogenic distal part of the open arm” (diet: F1,35 = 12.615, P < 0.01; drug: F1,35 = 15.359, P < 0.001, Table 4, Fig. 4A) and “total distance travelled” (diet: F1,35 = 6.120, P < 0.05; drug: F1,36 = 33.519, P < 0.001; Table 4). Furthermore, diazepam significantly affected the percentage of time spent in the distal compartment (F1,35 = 10.352, P < 0.01) (Table 4).
Table 4.
Effect of Mg2+ deficiency and diazepam (1 mg/kg) on anxiety-related behaviour of BALB/c mice in response to open arm exposure.
| Control |
Mg2+ deficiency |
|||
|---|---|---|---|---|
| Vehicle | diazepam | Vehicle | diazepam | |
| Time in distal compartment (%) | 11.3 ± 2.7 | 15.7 ± 1.3 | 2.2 ± 0.8* | 15.7 ± 4.6## |
| Distance travelled (cm) | 306 ± 25 | 676 ± 88### | 162 ± 39 | 512 ± 70### |
Data are presented as means ± SEM. n = 10 per experimental group with the exception of vehicle-treated control group where n = 9. *P < 0.05 for Mg2+ deficient vs. control mice, ##P < 0.01, ###P < 0.001 for diazepam vs. vehicle treatment.
Fig. 4.
Effect of Mg2+ deficiency and diazepam treatment (1 mg/kg, i.p.) on behaviour and c-Fos expression in the paraventricular hypothalamic nucleus (PVN) of BALB/c mice following exposure to the open arm of an elevated plus maze. A. In mice fed a Mg2+ restricted diet the anxiety-related behaviour was elevated as indicated by the number of entries into the (anxiogenic) distal compartment of the open arm. Treatment with diazepam normalised this anxious phenotype. B. Compared with vehicle-treated controls, c-Fos induction was increased in the magnocellular portion of the PVN in Mg2+ deficient mice and was reversed by diazepam treatment. C. Representative photograph (scale bar = 100 μm) showing delineation of the PVN regions analysed with the help of a mouse brain atlas (Paxinos and Franklin, 2001). D. High magnification photograph showing c-Fos-positive cells in the magnocellular portion of the PVN in all experimental groups. Data represent mean ± SEM. n = 10 per experimental group. *P < 0.05, ***P < 0.001 for Mg2+ deficient vs. control mice, ###P < 0.001 for diazepam vs. vehicle. 3V: third ventricle; DZP: diazepam; magno: magnocellular portion of the PVN; parvo: parvocellular portion of the PVN; V: vehicle.
Exposure to the open arm of an elevated plus maze induced c-Fos expression in the PVN (Fig. 4B and C). Two-way ANOVA revealed a significant diet × drug interaction in terms of the number of c-Fos-positive cells in the magnocellular portion of the PVN (F1,36 = 20.8301, P < 0.001) while there was a dietary effect in its parvocellular portion (F1,36 = 4.187, P < 0.05; data not shown). Specifically, Mg2+ deficiency increased emotional challenge-induced c-Fos expression and diazepam normalised it in BALB/c mice (Fig. 4B and C).
4. Discussion
In the present study we have shown that dietary Mg2+ restriction reproducibly enhanced anxiety-related behaviour in mice and that this effect was robust in terms of different strains and paradigms used. Mg2+ deficiency was associated with an increased transcription of prepro-CRH in the PVN, the main output region of the HPA axis, and elevated plasma ACTH levels pointed to an up-regulated stress system. Indeed, in Mg2+ deficient mice expression of the immediate-early gene c-Fos was increased in the PVN in response to a mildly anxiety-provoking situation indicating functional over-reactivity of this brain area. In parallel with a reversal of the behavioural changes brought about by Mg2+ deficiency, the observed abnormalities in the HPA axis system were restored by anxiolytic and antidepressant drug treatments. These data support a relationship between low Mg2+ levels and both anxiety-related behaviour and a modulated stress axis.
4.1. Effect of Mg2+ deficiency and chronic antidepressant treatment on mice
In the present study chronic feeding of C57Bl/6N mice with a low Mg2+ containing diet enhanced anxiety-like behaviour in the open field and light/dark test compared with controls. These findings are in line with our previous results using the C57Bl/6J substrain (Singewald et al., 2004) which were independently confirmed by Muroyama et al. (2009). In addition, we included another anxiety test, the hyponeophagia test, which refers to the inhibition of feeding in rodents upon exposure to novelty and which is one of the limited number of tests available in terms of its sensitivity to the anxiolytic effects of chronic, but not acute antidepressant treatment (for review see Dulawa and Hen, 2005). In this test the measures of anxiety-related behaviour were also enhanced in Mg2+ deficient mice compared with mice fed the control diet. In contrast to our previous study (Singewald et al., 2004), bodyweight gain was enhanced in Mg2+ deficient compared with control mice excluding the argument that reduced appetite may have contributed to the enhanced latency to feed. This, however, seems unlikely since the enhanced latency to feed was fully reversed by desipramine which is reported to exert anorexic effects or no change in bodyweight gain, rather than orexigenic effects at the beginning of treatments (Gobshtis et al., 2007; Sartori et al., 2004; Yalcin et al., 2005).
In the open field test, light/dark test and hyponeophagia test there was a trend towards a reduced anxiety-induced locomotion in Mg2+ deficient mice compared to mice fed the control diet. However, it is unlikely that the enhanced anxiety-related behaviour induced by Mg2+ deficiency is influenced by unspecific effects on locomotion or due to a general motor impairment as Mg2+ deficient and control groups do not differ in locomotor activity in their home cages as well as in the rotarod test (Singewald et al., 2004). Nevertheless, we examined the anxiogenic effects of Mg2+ deficiency in a test that is entirely independent of locomotion, the stress-induced hyperthermia test. We found that even under unstressed conditions, body temperature was increased in Mg2+ deficient mice compared with control mice which seemed to limit their hyperthermic response to exposure to the mild stress. The increased basal body temperature may therefore point towards a kind of chronically-stressed state induced by Mg2+ deficiency which caused a new set-point for body temperature (Keeney et al., 2001). Indeed, elevated basal body temperature is reported in chronically-stressed animals (Hayashida et al., 2010; Keeney et al., 2001) as well as in mutant mice (Guilloux et al., 2011) displaying signs of enhanced anxiety- and/or depression-related behaviour. In further support of this idea, the HPA-stress axis seems to be up-regulated in Mg2+ deficient compared with control mice (see below).
During the open arm exposure test Mg2+ deficiency also increased anxiety-related behaviour in BALB/c mice, a mouse strain with emotionality reported to be different to or even opposite that of C57Bl/6 mice (Griebel et al., 2000). This effect coincided with an attenuated distance travelled which is thought to be a sign of increased neophobia reflecting “trait” anxiety, observed particularly in BALB/c mice (for review see Belzung and Griebel, 2001). Thus, it seems that dietary Mg2+ deficiency further increased the innate anxiety of this strain. In addition to experimentally reducing plasma Mg2+ levels, natural low Mg2+ levels across mouse strains have also been associated with enhanced anxiety-like behaviour. For example, A/J mice characterised by enhanced anxiety-related behaviour compared with C57Bl/6 mice have lower plasma Mg2+ levels (Laarakker et al., 2011).
We next tested whether it was possible to attenuate the increased anxiety-related behaviour of Mg2+ deficient mice by the application of clinically effective pharmacotherapies. Application of the experimentally and clinically established anxiolytic diazepam (Aerni et al., 2004; Bentz et al., 2010; Soravia et al., 2006; for review see Millan, 2003) reversed the enhanced anxiety-related behaviour of Mg2+ deficient BALB/c mice, while it was ineffective in control mice suggesting efficacy of diazepam particularly in subjects with high emotionality. Likewise, specificity of action of diazepam is described in the HAB mouse model (Kromer et al., 2005) and various psychopathological rat models of enhanced anxiety including the HAB rats (Liebsch et al., 1998). In addition to the classical anxiolytic drug diazepam, chronic desipramine treatment also reduced anxiety-related behaviour of Mg2+ deficient C57BL/6N mice in the hyponeophagia test (the present study), while it was not effective in altering anxiety-like behaviour in the open field test and light/dark test (Singewald et al., 2004) whose limited sensitivity to the anxiolytic effects of chronic antidepressant treatments is well known (for review see Belzung, 2001). Anxiolytic effects of chronic desipramine treatment have also been reported in zinc-deficient mice displaying signs of enhanced anxiety-related behaviour (Whittle et al., 2009) as well as in rodents with normal levels of anxiety (Bodnoff et al., 1989, 1988; Merali et al., 2003; Santarelli et al., 2003). Hyponeophagia-based models in mice and rats predict the anxiolytic effects of antidepressants in a manner that is consistent with the time-course of their effects in humans (for review see Dulawa and Hen, 2005). In the clinical setting imipramine, the prodrug of desipramine, is used in the treatment of anxiety disorders that include generalised anxiety disorder, panic disorder and post-traumatic stress disorder (for review see Plag et al., 2009). The findings of anxiolytic effects resulting from diazepam and chronic desipramine treatment in Mg2+ deficient mice further underlines the validity of the Mg2+ deficiency model of enhanced anxiety-related behaviour in mice.
In contrast to desipramine, chronic treatment with paroxetine did not affect anxiety-related behaviour in the hyponeophagia test. This finding is not due to possible underdosing as a dose of 5 mg/kg paroxetine has been recently shown to be effective in reducing depression-like behaviour in Mg2+ deficient mice (Whittle et al., 2011). Although, to our knowledge, paroxetine has not so far been tested in the hyponeophagia test, another SSRI, fluoxetine, has proven to exert anxiolysis following chronic, but not acute, application in rodents (Bodnoff et al., 1989; Dulawa et al., 2004; Santarelli et al., 2003), suggesting that Mg2+ deficient mice are insensitive to the anxiolytic effects of chronic SSRI (paroxetine) treatment. This finding is of potential research interest, as Mg2+ deficient mice may model a considerable proportion of patients with an anxiety disorder that does not respond to SSRI treatment (Liebowitz, 1997; for review see Zamorski and Albucher, 2002).
4.2. Mg2+ deficiency is associated with HPA axis dysregulation which is modulated by therapeutic drug treatment
In an attempt to gain insight into neurobiological mechanism(s) underlying the enhanced anxiety-related behaviour of Mg2+ deficient mice, we followed a hypothesis-driven approach rather than using an unbiased technique (see also German-Fattal et al., 2008) that was previously used in our lab, where brain protein changes were identified that were correlated with the altered depression-related behaviour of Mg2+ deficient mice (Whittle et al., 2011). Mg2+ modulates various neurobiological mechanisms, including neurotransmitter systems and the HPA axis (for review see Murck, 2002). The HPA axis is an interesting substrate as it is known to play a role in stress processing and in normal and pathological anxiety (for review see Charney and Drevets, 2008; Millan, 2003; Young et al., 2008). Specifically, as Mg2+ reduces HPA axis activity (Held et al., 2002; Murck and Steiger, 1998), a disinhibition of this system during Mg2+ deficiency may be postulated. In order to address this hypothesis, we investigated markers of HPA axis activity, including prepro-CRH transcription and ACTH plasma levels, in experimentally naive or unstressed control mice, Mg2+ deficient mice and Mg2+ deficient mice chronically treated with desipramine, the drug that was shown to be behaviourally active.
The abundance of prepro-CRH mRNA was enhanced in the PVN, the main output region of the HPA axis, of Mg2+ deficient mice compared with control mice. In addition, ACTH plasma levels were elevated in Mg2+ deficient mice, indicating that the increase in prepro-CRH transcription translated into increased ACTH release from the pituitary, while corticosterone plasma levels did not differ between experimental groups. A similar divergence between baseline plasma ACTH and corticosterone levels has been previously reported in low aggressive mice and may be explained by altered adrenocortical sensitivity to ACTH (Veenema et al., 2003). In Mg2+ deficient mice the findings of elevated abundance of prepro-CRH mRNA and ACTH release point towards an up-regulated set-point of the HPA axis during Mg2+ deficiency which is also observed in some, but not all patients with an anxiety disorder (for review see Charney and Drevets, 2008; Young et al., 2008), and this is therefore suggested to contribute to the enhanced anxiety-related behaviour of Mg2+ deficient mice. In further support of this, we observed increased neuronal activity in the PVN of Mg2+ deficient BALB/c mice in response to the open arm of an elevated plus maze which, though considered as being mildly anxiogenic, is able to cause the release of stress hormones and to induce neuronal activation in rodents compared with an unstressed condition (Muigg et al., 2009; Nguyen et al., 2009; Salome et al., 2004). Interestingly, while stress-induced neuronal activation has been shown to be blunted in cortical areas of Balb/c mice compared with C57Bl/6 mice, PVN activation is similar between the two strains (O’Mahony et al., 2010) supporting the use of Balb/c mice complementary to C57Bl/6N mice in the present study. The finding of an over-reactive PVN in Mg2+ deficient animals, thus, demonstrates functional relevance of the PVN in mediating hyper-anxiety. This over-reactivity of the PVN may be triggered by the enhanced transcription rate of prepro-CRH. Like Mg2+ deficient mice, HAB mice and rats with high trait anxiety (Kromer et al., 2005; Liebsch et al., 1998), also show increased c-Fos expression in the PVN compared with their low anxiety (LAB) counterparts in response to open arm exposure (Muigg et al., 2009; Salome et al., 2004). In the PVN, CRH levels have been shown to be up-regulated by a number of different stressors (for review see e.g. Holsboer, 2000), including neonatal maternal separation, a proposed animal model of anxiety and depression (for review see Sartori et al., 2011), and in rats with high trait anxiety (Bosch et al., 2006). In line with the present findings, CRH concentrations in the cerebrospinal fluid are elevated in chronic, combat-related post-traumatic stress disorder (Baker et al., 1999; Bremner et al., 1997). However, such reported abnormalities of the HPA axis are inconsistent between studies as, for example, basal plasma or urine cortisol concentrations have been shown to be increased, unaltered or decreased in patients with anxiety disorder (for review see Charney and Drevets, 2008; Young et al., 2008).
In Mg2+ deficient mice, application of the benzodiazepine diazepam reduced the hyper-reactivity of the PVN in response to a mild emotional challenge. The ability of benzodiazepines to decrease stress-induced neuronal activation in the PVN has been previously shown in mice (Imaki et al., 1995) and rats (Beck and Fibiger, 1995; de Medeiros et al., 2005). Furthermore, chronic desipramine treatment via the drinking water normalised CRH overexpression in the PVN and elevated ACTH plasma levels. Tricyclic antidepressants have been shown to attenuate HPA axis activity which is thought to contribute to their anxiolytic and antidepressant actions (for review see Holsboer, 2000). Both findings further support the idea of a critical involvement of the HPA axis, and in particular of the PVN, in terms of contributing to the anxiogenic effects of Mg2+ deficiency. However, it should be noted that due to an existing comorbid link between anxiety and depression, it is not possible to make a clear distinction between mechanisms underlying anxiety alone. Indeed, Mg2+ deficient mice are also characterised by a pro-depressive phenotype which can be reversed by chronic antidepressant treatments including desipramine and paroxetine (Muroyama et al., 2009; Singewald et al., 2004; Whittle et al., 2011). Thus, it may be that the up-regulated HPA axis in Mg2+ deficient mice also is of relevance to the enhanced depression-like behaviour. In humans too, depression has been shown to be associated with an abnormally elevated HPA axis which is restored after clinical improvement (for review see e.g. Holsboer, 2000).
Taken together, the present findings demonstrate the robustness and validity of the Mg2+ deficiency model as model of enhanced anxiety-related behaviour and further supports emerging evidence in humans that reduced Mg2+ levels are associated with different facets of anxiety behaviour (Jacka et al., 2009; Seelig, 1994). Hence, an inverse relationship between Mg2+ and anxiety is suggested by these data. In terms of the induced anxiogenesis, the Mg2+ deficiency model appears to be reproducible across mouse strains, regardless of the level of inborn anxiety displayed. Finally, it is suggested that dysregulations in the HPA axis may contribute to the hyper-emotionality induced by dietary induced hypomagnesaemia.
Acknowledgements
This work was funded by the Austrian Science Fund (FWF): P22931-B18 to NS.
References
- Aerni A., Traber R., Hock C., Roozendaal B., Schelling G., Papassotiropoulos A., Nitsch R.M., Schnyder U., de Quervain D.J. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am. J. Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- Andlin-Sobocki P., Jonsson B., Wittchen H.U., Olesen J. Cost of disorders of the brain in Europe. Eur. J. Neurol. 2005;12(Suppl. 1):1–27. doi: 10.1111/j.1468-1331.2005.01202.x. [DOI] [PubMed] [Google Scholar]
- Armstrong D.J., Meenagh G.K., Bickle I., Lee A.S., Curran E.S., Finch M.B. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin. Rheumatol. 2007;26:551–554. doi: 10.1007/s10067-006-0348-5. [DOI] [PubMed] [Google Scholar]
- Baker D.G., West S.A., Nicholson W.E., Ekhator N.N., Kasckow J.W., Hill K.K., Bruce A.B., Orth D.N., Geracioti T.D., Jr. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Beck C.H., Fibiger H.C. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J. Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C. Rodent models of anxiety-like behaviors: are they predictive for compounds acting via non-benzodiazepine mechanisms? Curr. Opin. Investig. Drugs. 2001;2:1108–1111. [PubMed] [Google Scholar]
- Belzung C., Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav. Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Bentz D., Michael T., de Quervain D.J., Wilhelm F.H. Enhancing exposure therapy for anxiety disorders with glucocorticoids: from basic mechanisms of emotional learning to clinical applications. J. Anxiety Disord. 2010;24:223–230. doi: 10.1016/j.janxdis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Bodnoff S.R., Suranyi-Cadotte B., Aitken D.H., Quirion R., Meaney M.J. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl.) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Bodnoff S.R., Suranyi-Cadotte B., Quirion R., Meaney M.J. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology (Berl.) 1989;97:277–279. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- Bosch O.J., Kromer S.A., Neumann I.D. Prenatal stress: opposite effects on anxiety and hypothalamic expression of vasopressin and corticotropin-releasing hormone in rats selectively bred for high and low anxiety. Eur. J. Neurosci. 2006;23:541–551. doi: 10.1111/j.1460-9568.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- Bouwknecht A., Olivier B., Paylor R.E. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci. Biobehav. Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Licinio J., Darnell A., Krystal J.H., Owens M.J., Southwick S.M., Nemeroff C.B., Charney D.S. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhurst P.L. Analysis of maternal effects in the inheritance of behavior. Anim. Behav. 1961;9:129–141. [Google Scholar]
- Busquet P., Khoi Nguyen N., Schmid E., Tanimoto N., Seeliger M.W., Ben-Yosef T., Mizuno F., Akopian A., Striessnig J., Singewald N. CaV1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int. J. Neuropsychopharmacol. 2009:1–15. doi: 10.1017/S1461145709990368. [DOI] [PubMed] [Google Scholar]
- Charney D.S., Drevets W.C. Neurobiological basis of anxiety disorders. In: Davis K.L., Charney D., Coyle J.T., Nemeroff C., editors. Neuropsychopharmacology – 5th Generation of Progress. American College of Neuropsychopharmacology; Nashville: 2008. [Google Scholar]
- Coppen A., Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J. Psychopharmacol. 2005;19:59–65. doi: 10.1177/0269881105048899. [DOI] [PubMed] [Google Scholar]
- Crawley J.N. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol. Biochem. Behav. 1981;15:695–699. doi: 10.1016/0091-3057(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Crawley J.N. Wiley-Liss; 2000. What’s Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. [Google Scholar]
- Cryan J.F., Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- de Medeiros M.A., Carlos Reis L., Eugenio Mello L. Stress-induced c-Fos expression is differentially modulated by dexamethasone, diazepam and imipramine. Neuropsychopharmacology. 2005;30:1246–1256. doi: 10.1038/sj.npp.1300694. [DOI] [PubMed] [Google Scholar]
- DeFries J.C., Hegmann J.P., Halcomb R.A. Response to 20 generations of selection for open-field activity in mice. Behav. Biol. 1974;11:481–495. doi: 10.1016/s0091-6773(74)90800-1. [DOI] [PubMed] [Google Scholar]
- Dulawa S.C., Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci. Biobehav. Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Dulawa S.C., Holick K.A., Gundersen B., Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- German-Fattal M., Lecerf F., Sabbagh F., Maurois P., Durlach J., Bac P. Neuroprotective gene profile in the brain of magnesium-deficient mice. Biomed. Pharmacother. 2008;62:264–272. doi: 10.1016/j.biopha.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Gobshtis N., Ben-Shabat S., Fride E. Antidepressant-induced undesirable weight gain: prevention with rimonabant without interference with behavioral effectiveness. Eur. J. Pharmacol. 2007;554:155–163. doi: 10.1016/j.ejphar.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Gordon J.A., Hen R. Genetic approaches to the study of anxiety. Annu. Rev. Neurosci. 2004;27:193–222. doi: 10.1146/annurev.neuro.27.070203.144212. [DOI] [PubMed] [Google Scholar]
- Griebel G., Belzung C., Perrault G., Sanger D.J. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl.) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Guilloux J.P., David D.J., Xia L., Nguyen H.T., Rainer Q., Guiard B.P., Reperant C., Deltheil T., Toth M., Hen R., Gardier A.M. Characterization of 5-HT(1A/1B)−/− mice: an animal model sensitive to anxiolytic treatments. Neuropharmacology. 2011;61:478–488. doi: 10.1016/j.neuropharm.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Hackbarth H., Kuppers N., Bohnet W. Euthanasia of rats with carbon dioxide – animal welfare aspects. Lab. Anim. 2000;34:91–96. doi: 10.1258/002367700780578055. [DOI] [PubMed] [Google Scholar]
- Haddad J.J. N-methyl-d-aspartate (NMDA) and the regulation of mitogen-activated protein kinase (MAPK) signaling pathways: a revolving neurochemical axis for therapeutic intervention? Prog. Neurobiol. 2005;77:252–282. doi: 10.1016/j.pneurobio.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Hayashida S., Oka T., Mera T., Tsuji S. Repeated social defeat stress induces chronic hyperthermia in rats. Physiol. Behav. 2010;101:124–131. doi: 10.1016/j.physbeh.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Held K., Antonijevic I.A., Kunzel H., Uhr M., Wetter T.C., Golly I.C., Steiger A., Murck H. Oral Mg(2+) supplementation reverses age-related neuroendocrine and sleep EEG changes in humans. Pharmacopsychiatry. 2002;35:135–143. doi: 10.1055/s-2002-33195. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Imaki T., Wang X.Q., Shibasaki T., Harada S., Chikada N., Takahashi C., Naruse M., Demura H. Chlordiazepoxide attenuates stress-induced activation of neurons, corticotropin-releasing factor (CRF) gene transcription and CRF biosynthesis in the paraventricular nucleus (PVN) Brain Res. Mol. Brain Res. 1995;32:261–270. doi: 10.1016/0169-328x(95)00086-8. [DOI] [PubMed] [Google Scholar]
- Jacka F.N., Overland S., Stewart R., Tell G.S., Bjelland I., Mykletun A. Association between magnesium intake and depression and anxiety in community-dwelling adults: the Hordaland health study. Aust. N. Z. J. Psychiatry. 2009;43:45–52. doi: 10.1080/00048670802534408. [DOI] [PubMed] [Google Scholar]
- Jingami H., Mizuno N., Takahashi H., Shibahara S., Furutani Y., Imura H., Numa S. Cloning and sequence analysis of cDNA for rat corticotropin-releasing factor precursor. FEBS Lett. 1985;191:63–66. doi: 10.1016/0014-5793(85)80994-7. [DOI] [PubMed] [Google Scholar]
- Kantak K.M. Magnesium deficiency alters aggressive behavior and catecholamine function. Behav. Neurosci. 1988;102:304–311. doi: 10.1037//0735-7044.102.2.304. [DOI] [PubMed] [Google Scholar]
- Keck M.E., Sartori S.B., Welt T., Muller M.B., Ohl F., Holsboer F., Landgraf R., Singewald N. Differences in serotonergic neurotransmission between rats displaying high or low anxiety/depression-like behaviour: effects of chronic paroxetine treatment. J. Neurochem. 2005;92:1170–1179. doi: 10.1111/j.1471-4159.2004.02953.x. [DOI] [PubMed] [Google Scholar]
- Keegan C.E., Herman J.P., Karolyi I.J., O’Shea K.S., Camper S.A., Seasholtz A.F. Differential expression of corticotropin-releasing hormone in developing mouse embryos and adult brain. Endocrinology. 1994;134:2547–2555. doi: 10.1210/endo.134.6.8194481. [DOI] [PubMed] [Google Scholar]
- Keeney A.J., Hogg S., Marsden C.A. Alterations in core body temperature, locomotor activity, and corticosterone following acute and repeated social defeat of male NMRI mice. Physiol. Behav. 2001;74:177–184. doi: 10.1016/s0031-9384(01)00541-8. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kroll J.L. New directions in the conceptualization of psychotic disorders. Curr. Opin. Psychiatry. 2007;20:573–577. doi: 10.1097/YCO.0b013e3282f08759. [DOI] [PubMed] [Google Scholar]
- Kromer S.A., Kessler M.S., Milfay D., Birg I.N., Bunck M., Czibere L., Panhuysen M., Putz B., Deussing J.M., Holsboer F., Landgraf R., Turck C.W. Identification of glyoxalase-I as a protein marker in a mouse model of extremes in trait anxiety. J. Neurosci. 2005;25:4375–4384. doi: 10.1523/JNEUROSCI.0115-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laarakker M.C., van Lith H.A., Ohl F. Behavioral characterization of A/J and C57BL/6J mice using a multidimensional test: association between blood plasma and brain magnesium-ion concentration with anxiety. Physiol. Behav. 2011;102:205–219. doi: 10.1016/j.physbeh.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Lecci A., Borsini F., Volterra G., Meli A. Pharmacological validation of a novel animal model of anticipatory anxiety in mice. Psychopharmacology (Berl.) 1990;101:255–261. doi: 10.1007/BF02244136. [DOI] [PubMed] [Google Scholar]
- Liebowitz M.R. Panic disorder as a chronic illness. J. Clin. Psychiatry. 1997;58(Suppl. 13):5–8. [PubMed] [Google Scholar]
- Liebsch G., Linthorst A.C., Neumann I.D., Reul J.M., Holsboer F., Landgraf R. Behavioral, physiological, and neuroendocrine stress responses and differential sensitivity to diazepam in two Wistar rat lines selectively bred for high- and low-anxiety-related behavior. Neuropsychopharmacology. 1998;19:381–396. doi: 10.1016/S0893-133X(98)00042-6. [DOI] [PubMed] [Google Scholar]
- Lowry C.A., Moore F.L. Regulation of behavioral responses by corticotropin-releasing factor. Gen. Comp. Endocrinol. 2006;146:19–27. doi: 10.1016/j.ygcen.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Merali Z., Levac C., Anisman H. Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol. Psychiatry. 2003;54:552–565. doi: 10.1016/s0006-3223(02)01827-9. [DOI] [PubMed] [Google Scholar]
- Millan M.J. The neurobiology and control of anxious states. Prog. Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Muigg P., Scheiber S., Salchner P., Bunck M., Landgraf R., Singewald N. Differential stress-induced neuronal activation patterns in mouse lines selectively bred for high, normal or low anxiety. PLoS One. 2009;4:e5346. doi: 10.1371/journal.pone.0005346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murck H. Magnesium and affective disorders. Nutr. Neurosci. 2002;5:375–389. doi: 10.1080/1028415021000039194. [DOI] [PubMed] [Google Scholar]
- Murck H., Steiger A. Mg2+ reduces ACTH secretion and enhances spindle power without changing delta power during sleep in men – possible therapeutic implications. Psychopharmacology (Berl.) 1998;137:247–252. doi: 10.1007/s002130050617. [DOI] [PubMed] [Google Scholar]
- Muroyama A., Inaka M., Matsushima H., Sugino H., Marunaka Y., Mitsumoto Y. Enhanced susceptibility to MPTP neurotoxicity in magnesium-deficient C57BL/6N mice. Neurosci. Res. 2009;63:72–75. doi: 10.1016/j.neures.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Nguyen N.K., Sartori S.B., Herzog H., Tasan R., Sperk G., Singewald N. Effect of neuropeptide Y Y2 receptor deletion on emotional stress-induced neuronal activation in mice. Synapse. 2009;63:236–246. doi: 10.1002/syn.20597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony C.M., Sweeney F.F., Daly E., Dinan T.G., Cryan J.F. Restraint stress-induced brain activation patterns in two strains of mice differing in their anxiety behaviour. Behav. Brain Res. 2010;213:148–154. doi: 10.1016/j.bbr.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Paxinos K.B.L., Franklin G. second ed. Academic Press; London: 2001. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Plag J., Siegmund A., Strohle A. Pharmakotherapie bei Angsterkrankungen. Z. Psychiatr. Psychol. Psychother. 2009;57:185–194. [Google Scholar]
- Poleszak E., Szewczyk B., Kedzierska E., Wlaz P., Pilc A., Nowak G. Antidepressant- and anxiolytic-like activity of magnesium in mice. Pharmacol. Biochem. Behav. 2004;78:7–12. doi: 10.1016/j.pbb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Popkin B.M. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006;84:289–298. doi: 10.1093/ajcn/84.1.289. [DOI] [PubMed] [Google Scholar]
- Salome N., Salchner P., Viltart O., Sequeira H., Wigger A., Landgraf R., Singewald N. Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): differential Fos expression in HAB and LAB rats. Biol. Psychiatry. 2004;55:715–723. doi: 10.1016/j.biopsych.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sartori S.B., Burnet P.W., Sharp T., Singewald N. Evaluation of the effect of chronic antidepressant treatment on neurokinin-1 receptor expression in the rat brain. Neuropharmacology. 2004;46:1177–1183. doi: 10.1016/j.neuropharm.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Sartori S.B., Landgraf R., Singewald N. The clinical implications of mouse models of enhanced anxiety. Future Neurol. 2011;6:531–571. doi: 10.2217/fnl.11.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig M.S. Consequences of magnesium deficiency on the enhancement of stress reactions; preventive and therapeutic implications (a review) J. Am. Coll. Nutr. 1994;13:429–446. doi: 10.1080/07315724.1994.10718432. [DOI] [PubMed] [Google Scholar]
- Singewald N., Salchner P., Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol. Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Singewald N., Sinner C., Hetzenauer A., Sartori S.B., Murck H. Magnesium-deficient diet alters depression- and anxiety-related behavior in mice – influence of desipramine and Hypericum perforatum extract. Neuropharmacology. 2004;47:1189–1197. doi: 10.1016/j.neuropharm.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Soravia L.M., Heinrichs M., Aerni A., Maroni C., Schelling G., Ehlert U., Roozendaal B., de Quervain D.J. Glucocorticoids reduce phobic fear in humans. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thys-Jacobs S. Micronutrients and the premenstrual syndrome: the case for calcium. J. Am. Coll. Nutr. 2000;19:220–227. doi: 10.1080/07315724.2000.10718920. [DOI] [PubMed] [Google Scholar]
- Tschenett A., Singewald N., Carli M., Balducci C., Salchner P., Vezzani A., Herzog H., Sperk G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur. J. Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Veenema A.H., Meijer O.C., de Kloet E.R., Koolhaas J.M., Bohus B.G. Differences in basal and stress-induced HPA regulation of wild house mice selected for high and low aggression. Horm. Behav. 2003;43:197–204. doi: 10.1016/s0018-506x(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Vinkers C.H., van Bogaert M.J., Klanker M., Korte S.M., Oosting R., Hanania T., Hopkins S.C., Olivier B., Groenink L. Translational aspects of pharmacological research into anxiety disorders: the stress-induced hyperthermia (SIH) paradigm. Eur. J. Pharmacol. 2008;585:407–425. doi: 10.1016/j.ejphar.2008.02.097. [DOI] [PubMed] [Google Scholar]
- Whittle N., Li L., Chen W.Q., Yang J.W., Sartori S.B., Lubec G., Singewald N. Changes in brain protein expression are linked to magnesium restriction-induced depression-like behavior. Amino Acids. 2011;40:1231–1248. doi: 10.1007/s00726-010-0758-1. [DOI] [PubMed] [Google Scholar]
- Whittle N., Lubec G., Singewald N. Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids. 2009;36:147–158. doi: 10.1007/s00726-008-0195-6. [DOI] [PubMed] [Google Scholar]
- WHO, 2004. Global burden of disease report.
- Yalcin I., Aksu F., Belzung C. Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur. J. Pharmacol. 2005;514:165–174. doi: 10.1016/j.ejphar.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Young E.A., Abelson J.L., Liberzon I. Stress hormones and anxiety disorders. In: Blanchard R.J., Blanchard D.C., Griebel G., Nutt D., editors. Handbook of Anxiety and Fear. Academic Press; Oxford: 2008. pp. 455–473. [Google Scholar]
- Zamorski M.A., Albucher R.C. What to do when SSRIs fail: eight strategies for optimizing treatment of panic disorder. Am. Fam. Physician. 2002;66:1477–1484. [PubMed] [Google Scholar]
- Zender R., Olshansky E. Women’s mental health: depression and anxiety. Nurs. Clin. North Am. 2009;44:355–364. doi: 10.1016/j.cnur.2009.06.002. [DOI] [PubMed] [Google Scholar]