Abstract
Researchers have identified the association between the use of cocaine and sexual behavior as an important risk factor for HIV infection and have attempted to elucidate the nature of this association. Several lines of research have suggested that facilitation of sexual behavior during intoxication with cocaine may be due to the direct pharmacological effects of the drug (e.g., increase in sexual desire), whereas others have pointed to the importance of factors related to the context of drug use (e.g., opportunities for sexual behavior, expectations about the effects of the drug, social norms). The present study explored the perceived effects of cocaine and heroin on sexual behavior, as well as the social context of drug use as a function of drug type (cocaine versus heroin), among 46 inner-city drug users who reported a history of regular use of both crack cocaine and heroin. Results indicated that compared to heroin, cocaine had deleterious effects on participants’ perceived sexual desire and performance. Despite such deleterious effects on sexual behavior, cocaine was more frequently used with an intimate partner than heroin. Furthermore, participants did not differ in the extent to which they used the two drugs in other social contexts (e.g. with friends, family or neighbors). These preliminary results suggest that the relationship between cocaine and sexual behavior, especially among long-term cocaine users, may be facilitated by opportunities for sex that exist in the context of cocaine use, rather than by the pharmacological effects of the drug.
Keywords: sexual behavior, cocaine, social context
Substance use has been often associated with increased sexual activity (Rhodes, 1996). The pervasiveness of health related risks, such as HIV infection and other sexually transmitted diseases within the substance abusing population, has created an interest in assessing and understanding this relationship. An increasing body of empirical research suggests that individuals who use alcohol and other illicit drugs are more likely to have multiple sex partners, more casual sex partners, more unprotected sex, and higher rates of HIV and sexually transmitted diseases (Booth, Waters, & Chitwood, 1993; Chitwood & Comerford, 1990; Leigh, 1990; Logan, Cole, & Leukefeld, 2003; Maranda, Han, & Rainone, 2004; Rhodes, 1996; Stall & Leigh, 1994; Taylor, Fulop, & Green, 1999) than non substance users. Compared to alcohol and other drugs, the use of cocaine has been particularly associated with the spread of HIV and other sexually transmitted diseases (Bux, Lamb, & Iguchi, 1995; Kral, Bluthenthal, Booth, & Watters, 1998; Joe & Simpson, 1994; Wingood & DiClemente, 1998). Although there is substantial empirical evidence showing a link between cocaine and sexual behavior, these studies have typically focused on characterizing the pattern of sexual behaviors (e.g. type and frequency of risky sexual behaviors) associated with the use of the drug. However, the factors that may facilitate engagement in sexual behavior and may therefore increase the potential for health-compromising sexual behavior in association with the use of cocaine compared to other drugs, have not been systematically studied and remain poorly understood (Leigh & Stall, 1993; Pfaus et al., 2009; Stall & Leigh, 1994; Volkow et al., 2007).
A prominent hypothesis addressing this issue refers to the direct pharmacological effects of cocaine. As a psychomotor stimulant, cocaine is believed to increase sexual arousal or desire, stamina, performance and/or enjoyment by enhancing the activation of excitatory systems for sexual behavior (e.g., dopamine, norepinephrine, melanocortins, and oxytocin (e.g., Buffum, 1982; Califano, 1999; Crenshaw & Goldberg, 1996; Kall & Nilsonne, 1995; Melis & Argiolas, 1995; Pfaus et al., 2009; Rawson, Washton, Domier, & Reiber, 2002; Volkow, et al., 2007). From this perspective, cocaine facilitates sexual behavior directly though its acute pharmacological effects. However, these results are not unequivocal, as burgeoning research also demonstrates that cocaine use may disrupt sexual activity, especially among chronic cocaine users. As repeated stimulation generates deficiencies in the dopamine system, the impact of cocaine on dopamine may eventually inhibit sexual arousal and may create such adverse conditions as difficulty in maintaining an erection, delayed ejaculation and/or difficulty in achieving orgasm, and diminished sexual desire (Cocores, Miller, Pottash, & Gold, 1988; Gold & Miller, 1997; Gold, 1997; Brown, Domier, & Rawson, 2005; Henderson, Boyd, & Whitmarsh, 1995; Inciardi, Lockwood, & Pottiger, 1993; Peugh & Belenko, 2001; Weatherby et al., 1992).
These controversies regarding the direct effects of cocaine on sexual behavior have left open questions about the means by which cocaine use is related to sexual behavior. That is, if increased sexual activity is not solely due to the direct pharmacological effects of the drug, what other factors may account for the observed increases associated with cocaine use? One possibility that has often been emphasized in both human and animal behavior research refers to the context of drug use (Amaro, 1995; El-Bassel, Gilbert, & Rajah, 2003; Leigh, 1990; Leigh & Stall, 1993; Stall & Leigh, 1994; Pfaus, 2009). As Stall and Leigh (1994; Leigh & Stall, 1993) aptly noted, both sexual behavior and substance use are complex and sensitive behaviors that are almost certainly confounded with other personality, social, and contextual variables. It is thus possible that the precise way in which the use of cocaine is related to sexual behavior is determined not only by the drug pharmacology, but also by other factors inextricably linked to the social and cultural contexts in which the drug is used (Morningstar & Chitwood, 1987; Carlson & Siegal, 1991; Rhodes, 1996). Therefore, despite the negative pharmacological effects of chronic cocaine use, widespread beliefs about the positive effects of the drug on sexual performance may continue to heighten expectations and stimulate sexual activity in the context of drug use. Indeed, the use of cocaine has often been associated with social contexts and has been believed to function as a social lubricant due to its disinhibitory properties (Palamar, Mukherjee, & Halkitis, 2008; Rhodes, 1996). Therefore, in order to better understand the association between cocaine use and sexual behavior, it is important to consider the pharmacological effects of cocaine in light of cultural beliefs about the effects of the drug, individuals’ expectations and perceptions of its effects, and sex-roles and norms about drug obtainment (Leigh, 1990). All of these factors may facilitate the use of cocaine in contexts where sexual behavior is not only expected, but sought. No research has specifically investigated the perceived effects of cocaine on sexual behavior while also exploring whether the use of cocaine is more often associated with contexts that may facilitate sexual behavior. This type of research may offer important insights regarding the critical variables underling the association between cocaine use and sexual behavior.
In line with this objective, the present study represents a preliminary attempt to provide further clarification on the relationship between cocaine (as compared to heroin) and sexual behavior, with a specific focus on the perceived effects of cocaine on sexual desire and performance and the context of drug use. To this aim we examined 46 inner-city drug users who reported a history of regular use of both cocaine and heroin, two drugs with completely different pharmacological effects. Whereas, cocaine, as a CNS stimulant increases body temperature, blood pressure, and heart rate, heroin, as a CNS depressant, has sedative effects by lowering heart rate and breathing. In terms of sexual behavior, while cocaine is believed to increase libido, improve sexual performance and heighten sensation, heroin is believed to redirect blood away from the genitals and to suppress testosterone production which may result in decreased desire, response, and orgasm (Buffum, 1982, 1983, 1988; Crenshaw & Goldberg, 1996; Pfaus et al., 2009; Rawason et al., 2002). Using a within subjects design, the current study will allow us to compare perceived sexual desire and performance, as well as aspects of the social context surrounding drug use separately for the two drugs, while holding constant many potential confounds (e.g. personality, different social, economical and cultural backgrounds) that may be associated with drug choice in a between subject design. Thus, if drug use impacts sexual behavior solely through its direct or expected pharmacological effects, we should expect participants to report higher sexual desire and performance while using cocaine than while using heroin. However, if the association between drug use and sexual behavior is facilitated by other psychosocial factors related to the context of use, we should be able to see a difference between the contexts in which cocaine and heroin are used, regardless of the perceived pharmacological effects of the dugs. More specifically, we hypothesize that compared to heroin, cocaine should be used more often in social contexts that may facilitate sexual activity (i.e., with intimate partners).
Method
Overview
The current study sought to recruit a sample of individuals with a history of weekly cocaine and heroin use (not necessarily occurring at the same time). The overall design examined self-reported sexual desire and performance, as well as the context of drug use (the presence and type of a social context) as a function of drug type (cocaine vs. heroin) as a within subjects predictor variable.
Participants
Participants were recruited from a residential substance use treatment facility in the Washington D.C. metropolitan area over a period of one year. As part of the treatment, individuals admitted into the program participated in a structured interview aimed at establishing the existence of any psychopathology as well as the history and pattern of drug use. Through this initial interview, we screened a total of 142 individuals and selected those individuals who reported using both heroin and cocaine weekly (not necessarily at the same time) at any point in their life, but used alcohol and other drugs monthly or less (the measure is described in the next section). Recruitment resulted in 46 participants of whom 80.4% were males and 93.5% were African American, with a mean age of 47.93 (SD = 6.15). In terms of education level, 13% of the participants did not complete any high school education, 54% completed some high school or had graduated from high school, and 30% had some college, technical/business education, or had graduated from college.
Patients entered the treatment center either voluntarily or under a pretrial-release-to-treatment program through the District of Columbia Pretrial Services Agency (53.3% of our participants). This program offers drug offenders who are awaiting trial the option to receive substance abuse treatment as a way to ensure appearance in court, provide community safety, and address an underlying cause of recidivism. Patients were contracted to a specific length of stay upon entry into the treatment center. For the current sample, contract lengths included 30 days (41.4%), 60 days (29.7%), 90 days (6.3%), or 180 days (22.6%).
Procedure and Measures
Residents at the treatment center who, based on the initial screening, qualified for the study were invited to participate in a study examining sexual behavior among substance users in exchange for a $25 grocery store gift card. Only two residents refused to participate. Interested participants were given a more detailed explanation of the procedure and asked to provide written informed consent.
To ensure that withdrawal symptoms did not interfere with individuals’ ability to complete the study, as well as to control for the effects of time in treatment, participants were assessed no sooner than 48 hours and no later than 7 days after they entered the facility. It should be noted that individuals must have passed through detoxification and were free of drugs at intake, thereby limiting the likelihood of extreme withdrawal effects even at the 48-hour period. The center requires complete abstinence from drugs and alcohol (including the prohibition of any form of agonist treatment such as methadone) with the exception of nicotine and caffeine; patients are tested weekly and any evidence for drug use during treatment is grounds for dismissal from the center.
The procedure involved an initial assessment of the frequency of drug use through a standard drug use questionnaire modeled after the Alcohol Use Disorders Identification Test (AUDIT; Saunders, et al., 1993) specifically targeting heaviest lifetime use. Response options were: 0 (never), 1 (one time), 2 (monthly or less), 3 (2–4 times a month), 4 (2–3 times a week), and 5 (4 or more times a week).
Next, participants completed an interview aimed at differentiating between the effects of cocaine and heroin associated with a) sexual desire and performance while intoxicated with the drug in question, b) the context of drug use (alone vs. in the presence of other people), and c) the type of social context in which each drug was typically used (i.e., family, friends, romantic partner, neighborhood). For sexual desire and performance (ability to achieve/maintain an erection/orgasm), participants rated their desire and sexual ability when sober, as well as when using only cocaine, and only heroin, on a 3-point scale ranging from 1 (low desire/ability) to 3 (high desire/ability). The questions were based on previous measures of sexual desire and performance (Brown et al., 2005; Rawson, et al., 2002; Rohsenow, Sirota, Martin, & Monti, 2004). Our choice to use a 3-point scale rather than a larger range was motivated by the existing empirical debate regarding the sensitivity of various response formats in the assessment of substance effects. Although endorsement formats (true-false) have been shown to be as sensitive, or more sensitive that using ratings of strength of belief (Collins, Lapp, Emmons, & Isaac, 1990; Rohsenow et al., 2004), there are instances when giving participants rating options may result in a more sensitive assessment (Rohsenow, et al., 2004). Our objective in using a 3-point rating scale was to capture whether the drugs were perceived to have decreased or increased participants’ sexual desire and performance, with a medium point suggesting no change.
The context of drug use was assessed using several questions that focused on drug use with people in one’s social network. The questions were based on the Important People and Activities Instrument(Clifford & Longabaugh, 1991) and the Oregon Public School Drug Use Survey from the Social Development Research Group (Arthur et al., 1998). They assessed the use of each dug (i.e. cocaine and heroin) alone vs. in a social context. Response options included 1 (usually alone), 2 (with one other person), 3 (with a small group of people), 4 (with a large group of people). We also assessed the frequency of use within different social networks (i.e. romantic partner, close group of friends, immediate family, and neighborhood) on a scale from 1 (never) to 7 (more than 4 times per week). Again, the questions were asked for each drug separately and were anchored to the period of heaviest use. We chose to frame our assessments within the period of heaviest use in order to capture the time of greatest focus on the drug, while limiting the constraints on current use including recent incarceration and drug treatment. The three measures were counterbalanced to prevent order effects.
Results
Participants’ gender had no effect on the outcome variables of interest and therefore it was excluded from any further analysis.
Sexual Desire and Performance
To explore the effect of each drug on perceived sexual desire and functioning we conducted three separate within subjects contingency analyses on participants’ scores corresponding to each variable as a function of drug type. The results revealed that a significantly higher percentage of participants reported decreased sexual desire when using cocaine only (41.3%) than when using heroin only (21.7), McNemar χ2 (1, N = 46) = 7.44, p = .05, or when sober (13%), McNemar χ2 (1, N = 46) = 15.1, p < .05. Participants’ sexual desire when sober did not differ significantly from their desire when using only heroin.
A similar analysis was performed to compare self-reported sexual ability to achieve/maintain an erection/orgasm when sober versus when using each drug. As in the case of sexual desire, a larger number of participants reported decreased sexual ability when only using cocaine (47.8%) compared to when only using heroin (21.7%), McNemar χ2 (1, N = 46) = 13.24, p < .05., or when they were sober (21.7%), McNemar χ2 (1, N = 46) = 10.34, p < .05. Again, there was no difference in sexual functioning when sober compared to when only using heroin.
The Context of Drug Use
A second objective of this study was to compare the context of drug use. To this aim we first performed a within subject contingency analysis on the frequency with which participants use each drug alone vs. in the presence of others. We first collapsed the four response options in two categories, alone vs. social context (including in the presence of one other person, in the presence of a small group and in the presence of a large group) and analyzed it as a function of drug type (crack/cocaine vs. heroin). The analysis showed that cocaine was more frequently used in a social context (89.1%) than was heroin (69%), McNemar χ2 (1, N = 46) = 4.9, p < .05.
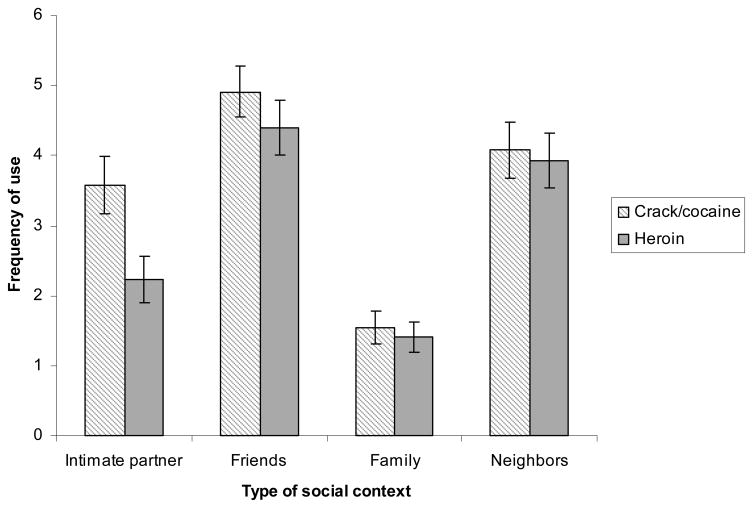
We further explored the type of social context in which participants used each drug through a 4 × 2 repeated measure ANOVA where both the social context (drug use with an intimate partner, with a group of friends, with the close family and with people in the neighborhood) and drug type (cocaine vs. heroin) were entered as within subject variables. A significant interaction between the two variables emerged, F(3, 135) = 5.16, p < .05, η2 = .10. As shown in Figure 1, participants did not differ in the extent to which they used each drug with their group of friends, family or neighbors (p > .05). However, they used cocaine more frequently with an intimate partner (M = 3.58, SD = .41) than heroin (M = 2.23, SD = .33), F(1, 45) = 14.44, p < .01, η2 = .24.
Figure 11.
Frequency of drug use in a social context as a function of drug type.
1 Frequency of use was measured as follows: 0 (never), 1 (one time), 2 (monthly or less), 3 (2–4 times a month), 4 (2–3 times a week), and 5 (4 or more times a week).
Additional Analyses
Although we have argued for framing our assessments within the heaviest period of use, one may question if the results would change if the sample was limited only to those with current weekly use of both crack cocaine and heroin. To explore this possibility we repeated the above analyses on the sub-sample evidencing current use. Due to the reduction in sample size from 46 to 26, resulting analyses did not reach statistical significance at the .05 level. However, it is notable that for both sexual desire F(2, 52) = 1.68, p =.16, η2 = .06 and performance F(2, 52) = 2.32, p = .10, η2 = .14, as well as for context of use F(3, 78) = 2.11, p = .11, η2= .07, the effect sizes are largely identical to those observed for the full sample. These additional analyses indicate that the magnitude of the effect observed for our sample is unlikely to be due to temporal factors that may differentiate the heaviest use from current use.
Discussion
The current study represents an attempt to gain insight into potential factors underlying the relationship between cocaine use and sexual behavior. To this aim, we examined sexual desire and ability as well as relevant contextual factors associated with the use of two drugs with very different pharmacological effects (i.e., cocaine and heroin) in a sample of individuals who reported weekly use of both drugs. This within subjects design allowed for comparisons of our outcome variables for each drug separately while holding constant many potential confounds (e.g. personality) that may be associated with drug choice and would likely be evident in a between subjects design.
Regarding sexual desire and performance, a significantly higher percentage of individuals reported perceived impairment when using cocaine compared to when using heroin or when sober. Such results are consistent with previous findings in the literature and suggest that facilitations of sexual behavior via direct pharmacological effects of cocaine may be quite limited. Given this evidence, the question remains why and how the use of cocaine is associated with high rates of sexual behavior, despite its deleterious effects on sexual desire and performance.
One possibility suggested by our additional results is that cocaine is more likely to be used in a social setting than heroin, and may thus be associated with more opportunities for sexual encounters. This is consistent with research suggesting that cocaine is a social drug that is often used under social pressure (Palmar et al., 2008; Perry & Mandel, 1995) in social contexts (e.g. dance clubs), and as a social lubricant and glue (De Micheli & Formigoni, 2004; Riely et al., 2007; Waldrop, Back, Verduin, & Brady, 2007). Moreover, when comparing the use of each drug (heroin and cocaine) in specific social contexts, cocaine was more frequently used than heroin in the context of an intimate relationship. The use of cocaine with an intimate partner, despite the deleterious effects of the drug on sexual desire and performance, suggests that opportunities for sex associated with the use of cocaine (more so than with other drugs, in this case heroin) may play an important role in maintaining the association between cocaine and sexual behavior. Widely spread norms and expectations associated with the positive effects of the drug on sexual performance may prompt people to consume the drugs in contexts that may offer the opportunity for sexual experiences. In addition, the expectations regarding the effects of the drug on sexual desire may also offer justification and reasons for engaging in sexual behaviors in which people may want to engage, but perhaps know they should not (Rhodes, 1996). Finally, sex is also currency often exchanged for cocaine (Edlin, Irwin, Faruque, & McCoy, 1994; Hoffman, Klein, Eber, & Crosby, 2000; Logan & Leukefeld, 2000) which may be one of the important factors explaining why cocaine is often used with an intimate partner despite its deleterious effects on sexual desire and performance. All these factors should be explored more systematically in future research.
Limitations, future directions, and implications
Our study offered the unique opportunity to explore several outcomes related to sexual behavior as well as social context surrounding the use of two drugs with different pharmacological effects. Despite the advantages offered by the use of the within subject design (as discussed above) several limitations must be acknowledged. One set of limitations is related to our sample and measurements. First, our results may have limited generalizability due to the fact that the current sample consisted only of treatment seeking drug users. It is possible that being in treatment may have increased the saliency of the negative aspects of drug use, which may have in turn affected the perception of positive sexual benefits of drug use. However, although this limitation may affect our ability to compare sexual desire and performance when under the influence of the drugs versus when sober, it should not have affected the relative difference between the effects of cocaine and heroin. Secondly, our research relied on retrospective self reports of sexual behavior and drug use patterns. Although participants’ ability to accurately report their past drug use and sexual behaviors may be questionable, there is little reason to suspect that biases and inaccuracies in their self-report would systematically impact their reports about one drug and not the other. Future studies would nevertheless benefit from using prospective designs or laboratory based designs with drug administration. Finally, our assessment of sexual desire did not separate between the ability to achieve or maintain an erection and the occurrence of an orgasm. Clearly these are different physiological process and although both can be considered in terms of desire, greater separation will be important in future work.
A second set of limitations is more theoretical in nature. Although our findings suggest that non-pharmacological factors may play an important role in the relationship between cocaine and sexual behavior, they remain limited in specifying the nature of such factors. Future work would greatly benefit from a more comprehensive approach specifying and integrating possible psychosocial and contextual factors (e. g. expectancies regarding the effects of the drug, social and cultural norms related to sexual behavior and cocaine use).
In closing, the current research on the effects of cocaine on sexual behavior remains largely limited due to small and nongeneralizable samples, lack of controlled research designs and/or adequate comparison groups, heavy reliance on self-report measures, and lack of systematic control of psychological and social variables. These limitations may perpetuate myths and beliefs about the drug, rather than promoting scientific knowledge that may inform both the public and the health prevention community. The current study is small in scope but provides an important contribution to the literature addressing the relationship between cocaine and sexual behavior. It clearly suggests that despite its deleterious effects on sexual desire and performance, cocaine may still facilitate sexual behavior due to its use in contexts that offer opportunities for sex. As this line of research moves forward with more sophisticated tools and in-depth batteries, it should provide important empirical support to inform prevention efforts aimed at undermining the popular myths that cocaine use enhances sexual performance.
Acknowledgments
Funding for this study was provided by NIDA grants (R01 DA19405, PI: Lejuez; F31 DA023302, PI: Reynolds); NIDA had no further role in study design, data collection, analysis and interpretation, in the writing of the report, or in the decision to submit the paper for publication. Authors Lejuez, C. and Reynolds, E. designed the study and wrote the protocol. Author Kopetz, C. undertook the statistical analysis, managed the literature search, summarized previous related work, and wrote the first draft of the manuscript.
Footnotes
Author disclosures
All authors contributed to and have approved the final manuscript. There is no conflict of interest.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pha
References
- Amaro H. Love, sex, and power: Considering women’s realities in HIV prevention. American Psychologist. 1995;50(6):437–447. doi: 10.1037//0003-066x.50.6.437. [DOI] [PubMed] [Google Scholar]
- Booth RE, Watters JK, Chitwood DD. HIV risk-related sex behaviors among injection drug users, crack smokers, and injection drug users who smoke crack. American Journal of Public Health. 1993;83:1144–1148. doi: 10.2105/ajph.83.8.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AH, Domier CP, Rawson RA. Stimulants, Sex, & Gender. Sexual Addiction and Compulsivity. 2005;12:169–180. [Google Scholar]
- Buffum J. Pharmacosexology: The effects of drugs on sexual function: A review. Journal of Psychoactive Drugs. 1982;14(1):5–44. doi: 10.1080/02791072.1982.10471907. [DOI] [PubMed] [Google Scholar]
- Buffum J. Substance abuse and high-risk sexual behavior: Drugs and sex: The dark side. Journal of Psychoactive Drugs. 1988;20(2):165–168. doi: 10.1080/02791072.1988.10524489. [DOI] [PubMed] [Google Scholar]
- Buffum JC. Pharmacosexology update: Heroin and sexual function. Journal of Psychoactive Drugs. 1983;15(4):317–318. doi: 10.1080/02791072.1983.10471969. [DOI] [PubMed] [Google Scholar]
- Bux DA, Lamb RJ, Iguchi MY. Cocaine use and HIV risk behavior in methadone maintenance patients. Drug and Alcohol Dependence. 1995;37(1):29–35. doi: 10.1016/0376-8716(94)01058-s. [DOI] [PubMed] [Google Scholar]
- Califano JA. Dangerous liaisons: substance abuse and sex. The National Center on Addiction and Substance Abuse at Columbia University (CASA); New York: 1999. [Google Scholar]
- Carlson RG, Siegal HA. The crack life: an ethnographic overview of crack use and sexual behavior among African Americans in a Midwest metropolitan city. Journal of Psychoactive Drugs. 1991;21:11–20. doi: 10.1080/02791072.1991.10472570. [DOI] [PubMed] [Google Scholar]
- Chitwood D, Comerford M. Drugs, sex and AIDS risk. American Behavioral Scientist. 1990;22:465–477. [Google Scholar]
- Clifford PR, Longabaugh R. Adapted for use by Project MATCH for NIAAA 5 R01AA06698-05 Environmental Treatment of Alcohol Abusers. Richard Longabaugh, Principal Investigator; 1991. Manual for the Administration of the Important People and Activities Instrument. [Google Scholar]
- Cocores JA, Miller NS, Pottash AC, Gold MS. Sexual dysfunction in abusers of cocaine and alcohol. American Journal of Drug and Alcohol Abuse. 1988;14(2):169–173. doi: 10.3109/00952999809001544. [DOI] [PubMed] [Google Scholar]
- Collins RL, Lapp WM, Emmons KM, Isaac LM. Endorsement and strength of alcohol expectancies. Journal of Studies on Alcohol. 1990;51:336–342. doi: 10.15288/jsa.1990.51.336. [DOI] [PubMed] [Google Scholar]
- Crenshaw TL, Goldberg JP. Sexual pharmacology: Drugs that affect sexual functioning. New York, NY US: W. W. Norton & Co; 1996. [Google Scholar]
- De Micheli D, Formigoni MLOS. Drug use by Brazilian students: Associations with family, psychosocial, health, demographic and behavioral characteristics. Addiction. 2004;99(5):570–578. doi: 10.1111/j.1360-0443.2003.00671.x. [DOI] [PubMed] [Google Scholar]
- Edlin BR, Irwin KL, Faruque S, McCoy CB. Intersecting epidemics: Crack cocaine use and HIV infection among inner-city young adults. The New England Journal of Medicine. 1994;331(21):1422–1427. doi: 10.1056/NEJM199411243312106. [DOI] [PubMed] [Google Scholar]
- El-Bassel N, Gilbert L, Rajah V. The relationship between drug abuse and sexual performance among women on methadone. Heightening the risk of sexual intimate violence and HIV. Addictive Behaviors. 2003;28(8):1385–1403. doi: 10.1016/s0306-4603(02)00266-6. [DOI] [PubMed] [Google Scholar]
- Gold MS. Cocaine (and crack): clinical aspects. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance abuse: a comprehensive textbook. 3. Baltimore, MD: Williams & Wilkins; 1997. pp. 181–199. [Google Scholar]
- Gold MS, Miller NS. Cocaine (and crack): Neurobiology. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance abuse: a comprehensive textbook. 3. Baltimore, MD: Williams & Wilkins; 1997. pp. 166–181. [Google Scholar]
- Henderson DJ, Boyd CJ, Whitmarsh J. Women and illicit drugs: Sexuality and crack cocaine. Health Care for Women International. 1995;16(2):113–124. doi: 10.1080/07399339509516163. [DOI] [PubMed] [Google Scholar]
- Hoffman JA, Klein H, Eber M, Crosby H. Frequency and intensity of crack use as predictors of women’s involvement in HIV-related sexual risk behaviors. Drug and Alcohol Dependence. 2000;58(3):227–236. doi: 10.1016/s0376-8716(99)00095-2. [DOI] [PubMed] [Google Scholar]
- Inciardi JA, Lockwood D, Pottiger AE. Women and crack cocaine. New York: Macmillan; 1993. [Google Scholar]
- Joe GW, Simpson DD. HIV risks, gender, and cocaine use among opiate users. Drug and Alcohol Dependence. 1995;37(1):23–28. doi: 10.1016/0376-8716(94)01030-o. [DOI] [PubMed] [Google Scholar]
- Kall K, Nilsonne A. Preference for sex on amphetamine: A marker for HIV risk behaviour among male intravenous amphetamine users in Stockholm. AIDS Care. 1995;7(2):171–188. doi: 10.1080/09540129550126696. [DOI] [PubMed] [Google Scholar]
- Kral AH, Bluthenthal RN, Booth RE, Watters JK. HIV seroprevalence among street-recruited injection drug and crack cocaine users in 16 US municipalities. American Journal of Public Health. 1998;88(1):108–113. doi: 10.2105/ajph.88.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh B. The relationship of substance use during sex to high-risk sexual behavior. Journal of Sex Research. 1990;27:199–213. [Google Scholar]
- Leigh BC, Stall R. Substance use and risky sexual behavior for exposure to HIV: Issues in methodology, interpretation, and prevention. American Psychologist. 1993;48(10):1035. doi: 10.1037//0003-066x.48.10.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan TK, Leukefeld C. HIV risk behavior among bisexual and heterosexual drug users. Journal of Psychoactive Drugs. 2000;32(3):239–248. doi: 10.1080/02791072.2000.10400446. [DOI] [PubMed] [Google Scholar]
- Logan TK, Cole J, Leukefeld C. Gender differences in the context of sex exchange among individuals with a history of crack use. AIDS Education and Prevention. 2003;15(5):448–464. doi: 10.1521/aeap.15.6.448.24041. [DOI] [PubMed] [Google Scholar]
- Maranda MJ, Han C, Rainone GA. Crack cocaine and sex. Journal of Psychoactive Drugs. 2004;36:315–322. doi: 10.1080/02791072.2004.10400032. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A. Dopamine and sexual behavior. Neuroscience & Biobehavioral Reviews. 1995;19(1):19–38. doi: 10.1016/0149-7634(94)00020-2. [DOI] [PubMed] [Google Scholar]
- Morningstar PJ, Chitwood DD. How women and men get cocaine: sex-role stereotypes and acquisition patters. Journal of Psychoactive Drugs. 1987;19:135–142. doi: 10.1080/02791072.1987.10472397. [DOI] [PubMed] [Google Scholar]
- Palamar JJ, Mukherjee PP, Halkitis PN. A longitudinal investigation of powder cocaine use among club-drug using gay and bisexual men. Journal of Studies on Alcohol and Drugs. 2008;69(6):806–813. doi: 10.15288/jsad.2008.69.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MJ, Mandell W. Psychosocial factors associated with the initiation of cocaine use among marijuana users. Psychology of Addictive Behaviors. 1995;9(2):91–100. [Google Scholar]
- Pfaus JG, Wilkins MF, DiPietro N, Benibgui M, Toledano R, Rowe A, et al. Inhibitory and disinhibitory effects of psychomotor stimulants and depressants on the sexual behavior of male and female rats. Hormones and Behavior. 2009 doi: 10.1016/j.yhbeh.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Washton A, Domier CP, Reiber C. Drugs and sexual effects: role of drug type and gender. Journal of Substance Abuse and Treatment. 2002;22:103–108. doi: 10.1016/s0740-5472(01)00215-x. [DOI] [PubMed] [Google Scholar]
- Rhodes T. Culture, drugs, and unsafe sex: confusion about causation. Addiction. 1996;91(6):753–758. doi: 10.1111/j.1360-0443.1996.tb03565.x. [DOI] [PubMed] [Google Scholar]
- Riley SCE, James C, Gregory D, Dingle H, Cadger M. Patterns of recreational drug use at dance events in Edinburgh, Scotland. Addiction. 2001;96(7):1035–1047. doi: 10.1046/j.1360-0443.2001.967103513.x. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Sirota AD, Martin RA, Monti PM. The cocaine effects questionnaire for patient populations: Development and psychometric properties. Addictive Behaviors. 2004;29:537–553. doi: 10.1016/j.addbeh.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Screening Test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption. II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Stall R, Leigh B. Understanding the relationship between drug and alcohol use and risk sexual activity for HIV transmission: where do we go from here. Addiction. 1994;89:131–134. doi: 10.1111/j.1360-0443.1994.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Taylor J, Fulop N, Green J. Drink, illicit drugs and unsafe sex in women. Addiction. 1999;94:1209–1218. doi: 10.1046/j.1360-0443.1999.948120911.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F, Jayne M, Wong C. Stimulant-induced enhanced sexual desire as a potential contributing factor in HIV transmission. American Journal of Psychiatry. 2007;164(1):157–160. doi: 10.1176/ajp.2007.164.1.157. [DOI] [PubMed] [Google Scholar]
- Waldrop AE, Back SE, Verduin ML, Brady KT. Triggers for cocaine and alcohol use in the presence and absence of posttraumatic stress disorder. Addictive Behaviors. 2007;32(3):634–639. doi: 10.1016/j.addbeh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Weatherby NL, Shultz JM, Chitwood DD, McCoy HV, McCoy CB, Ludwig DD, Edlin BR. Crack cocaine use and sexual activity in Miami, Florida. Journal of Psychoactive Drugs. 1992;24(4):373–380. doi: 10.1080/02791072.1992.10471661. [DOI] [PubMed] [Google Scholar]
- Wingood G, DiClemente R. The influence of psychosocial factors, alcohol and drug use on African American Women’s high risk sexual behavior. American Journal of Preventive Medicine. 1998;15(1):54–59. doi: 10.1016/s0749-3797(98)00027-0. [DOI] [PubMed] [Google Scholar]