Abstract
The primary aim of this study was to quantify genetic and environmental influences on the frequency of spontaneously occurring micronuclei in children and adults. To meet this aim, a total of 63 male and female twin pairs and 19 singletons (145 individuals) were evaluated, ranging in age from 7 to 85 years. Micronuclei frequencies significantly increased with age for both genders (r = 0.49, P < 0.001), with the lowest and highest rates being seen in the 7- to 9 (mean = 0.56%, SD = .28) and 60- to 69-year-olds (mean = 2.12%, SD = 1.0), respectively. This age effect was significantly more pronounced in females than males (P = 0.017). In addition to the main effect of age, the completion of puberty in either gender (P = 0.036) and menopause in females (P = 0.024) was associated with a significant increase in micronuclei frequencies. Genetic model fitting indicated that influences from both additive genetic (65.2% of variance) and unique environmental (34.8% of variance) sources best explained the observed micronuclei frequencies in monozygotic and dizygotic twin pairs. Self-reported health conditions associated with an increased frequency of micronuclei included a history of allergies (P < 0.007) and migraines (P = 0.026). Multivitamin use was also associated with increased micronuclei frequencies (P = 0.004). In contrast, significantly lower micronuclei frequencies were associated with arthritis (P = 0.002), as well as consuming fruit (P = 0.014), green, leafy vegetables (P < 0.001) and/or folate-enriched bread (P = 0.035). A sex-specific effect, resulting in a significantly increased frequency of micronuclei with tobacco usage, was observed for females (but not males). Gender differences also moderated the impact of vitamin D and calcium consumption. In conclusion, the frequency of spontaneously arising micronuclei in humans is a complex trait, being influenced by both heritable genetic and environmental components. Recognition of factors contributing to baseline levels of micronuclei should provide guidance to researchers in designing studies to evaluate agents hypothesised to influence chromosomal instability.
Introduction
Acquired chromosomal abnormalities were first reported to occur in human somatic cells in 1961 (1). Since that initial discovery, the majority of investigations of acquired chromosomal changes have centered on their association with particular types of neoplasia. Sandberg reported that approximately 85% of the affected tissues from patients with cancer have acquired chromosomal changes (2). In addition to cancer, new evidence is rapidly accumulating to suggest that there may also be a link between acquired chromosomal changes and other age-related conditions, such as Alzheimer's disease (3), rheumatoid arthritis (4) and osteoarthritis (5–9).
A critical step in understanding the variation in acquired chromosomal changes observed in people having age-related diseases would be to quantify their frequency of occurrence in ‘healthy people’ of differing ages. The ‘gold standard’ for scoring acquired chromosomal abnormalities has been the evaluation of metaphase chromosomes. While this technique allows for the characterisation of all cytogenetic findings present, it is limited in that it is (i) labour intensive, thereby reducing the number of observations that can be collected, and (ii) at risk for producing ‘artifactual’ anomalies as a result of the necessary cell culture, harvesting and slide making procedures. An alternative approach that has been used to estimate the frequency of acquired chromosomal changes is the cytokinesis-block micronucleus (CBMN) assay, which provides information regarding the presence of chromosomal errors in somatic cells prior to the influence of selective growth pressures (10). Given that this methodology allows for an assessment of a large number of cells (1000 or more) and is less labour intensive than conventional cytogenetic studies, it has potential for use as a high-throughput assay.
Micronuclei, which are the primary cytological structures scored in the CBMN assay, are thought to contain chromatin (from one or more chromosomes) that was not incorporated (‘lagging’ or ‘lost’) into the daughter binucleates following nuclear division (10). Micronuclei frequencies have been shown to increase with both age and DNA damage, providing data that closely parallels that of metaphase chromosomal analyses (11,12). Thus, the assessment of micronuclei frequencies has become a very attractive biosurveillance tool for quantifying genomic damage associated with environmental insults and occupational exposures, as well dietary and lifestyle practices (13). As anticipated from the above noted observations regarding chromosomal changes and age-related conditions, researchers have also reported micronuclei frequencies to be increased in individuals with different health problems, especially age-related conditions such as cancer, Alzheimer's disease and Parkinson's disease (14–20). Given the potential use of this assay to track a wide variety of health-related measures and outcomes, it is important to understand the factors that influence alterations in micronuclei frequencies. In particular, it is not known if most of the variation observed for micronuclei frequencies between individuals reflects environmental exposures or a predisposition based on one's genetic make-up.
One of the most robust methods for identifying sources of variation in humans is to analyse that trait in twins. Thus, the primary aim of this study was to measure the frequency of spontaneously occurring micronuclei in healthy twins of differing ages, including younger individuals for whom there is a paucity of data, and to determine the extent to which the variation in the frequency of micronuclei was determined by genetic and/or environmental factors. A secondary aim of this study was to determine if the environmental contribution to the observed variance on micronuclei frequencies was attributable, in part, to dietary, lifestyle and/or health influences.
Materials and methods
Sample ascertainment
A total of 145 individuals were studied, including 63 twin pairs and 19 singletons, whose co-twin did not complete the sample collection process. The twins were ascertained through the Mid-Atlantic Twin Registry. The only study inclusion criterion was that the twins be of the same gender to eliminate confounding effects in data interpretation that might arise from potential gender differences in the complete twin pairs sampled. After providing their informed consent (Virginia Commonwealth University IRB protocol #179), the twins participated in this study by completing a health history/diet/lifestyle questionnaire and submitting blood samples, the latter of which were obtained by a health care provider of the participants’ choosing. Following their collection, the blood specimens were shipped to our cytogenetics laboratory (Jackson-Cook) via an overnight delivery carrier.
DNA isolation and zygosity determination
Genomic DNA was isolated from whole blood using the Puregene DNA Isolation Kit (Qiagen). Zygosity was determined using 13 highly polymorphic short tandem repeat sequences (AmpFlSTR® Profiler Plus® and Cofiler® kits; Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol.
Cell culture
Lymphocytes, which were isolated using Histopaque-1077 (Sigma), were established in culture according to the protocol of Fenech (21). Briefly, cytochalasin B (3.0 μg/ml; Sigma, 14930-92-2) was added to the cells 44 h after culture initiation. At 72 h, binucleate interphase cells were harvested using standard techniques, which included a 10-min incubation in hypotonic solution (0.075 M KCl) and serial fixation (three times using a 3:1 methanol:acetic acid solution). Slides were made following standard procedures (22).
Micronuclei analysis
Micronuclei were visualised following Giemsa staining (4% Harleco Giemsa solution) and identified according to the criteria established by Fenech et al. (23). The frequencies of micronuclei observed in the cytochalasin-B-blocked binucleated cells of the twins were calculated by averaging the values obtained from two replicate scores (1000 binucleates were evaluated from two independent areas of the slide for a total of 2000 binucleates per study participant).
Dietary, lifestyle and health histories
Self-reported or parent-reported (for children) dietary, lifestyle and health history information was collected at the time of the twins’ sample collection using a questionnaire format (Virginia Commonwealth University IRB protocol #179). The items included in these questionnaires were adapted from previously reported tools (24). The questionnaire was originally designed to gain information about a small subset of factors that have been implicated to influence chromosomal aneuploidy and/or epigenetic patterns (25). Thus, this questionnaire was not designed to allow for a complete assessment of dietary intake. The information collected from both the children and adult twins included the number of days each week the individual ate green, leafy vegetables, fruit and/or fruit juices, soy products (including soy milk), vitamin-enriched bread and their use of vitamins (types used and how often consumed). Health information collected from all twins included their height; weight and whether they had experienced any of 28 common health conditions (including but not limited to heart disease, high blood pressure, arthritis, diabetes, cancer, hay fever or allergies, severe tension headaches, migraines, asthma, seizures, eating disorders and bowel disorders).
The adult twins also reported their current and past smoking behaviour (everyday, more than half the days, less than half the days, not at all), alcohol consumption (everyday, more than half the days, less than half the days, not at all), as well as prescription and non-prescription drug/herb usage (denoting the frequency and time interval of drug usage). In addition, the female adult twins provided information regarding when they started menstruating and, when applicable, their age at menopause.
Statistical analysis
The impact of additive genetic (A), common environmental (C) and unique environmental (E) factors on micronuclei frequencies was evaluated using a structural equation modelling approach that utilises monozygotic (MZ) and dizygotic (DZ) twin pairs, as described by Neale and Cardon (26). The modelling of the covariance structure within and between twin pairs was performed using the Mx statistical software program (27) and was preferred over other methods for this study since it allows for (i) a series of alternative models to be tested concerning the effects of genetic and environmental factors, (ii) confidence intervals to be calculated on all parameters, (iii) the inclusion of measured covariates on both the means model and variance components and (iv) relative ease of including replicate micronuclei scoring to remove potential bias in estimates of genetic and environmental parameters attributable to measurement error. Briefly, genetic and environment contributions to trait variance can be derived from the fact that MZ twins are genetically identical and, by definition, also share common environmental exposures (which are typically thought of as exposures that are shared as a result of being raised together but could extend to other shared sources). Therefore, any differences between MZ twins are ascribed to unique environmental influences, which also contain measurement error. In contrast, differences observed within DZ twin pairs can be attributed to, on average, one-half of their genes not shared, in addition to non-shared environmental influences and measurement error. Thus, phenotypic resemblance due to genetic factors will be greater in MZ versus DZ twin pairs. In order to limit type I errors, an alpha level of 5% was used for all statistical tests.
Given that unique environmental effects were shown to influence micronuclei frequencies (see Results), additional studies were done to assess the influence of the specific measured environmental influences of dietary, lifestyle and health conditions. Their influence was quantified using a mixed-effects modelling approach (28), including adjustments for the confounding effects of age, sex and zygosity status. Due to sample size limitations, these analyses were restricted to include only the health or dietary/lifestyle items that were reported by 10 or more twins.
Results
Study participant distribution and zygosity determination
The ages of the study participants ranged from 7 to 85 years and included 63 male and 82 female participants (Table I). The average age of the male and female participants was 49.8 (SD = 23.8) and 41.4 (SD = 21.7), respectively. The majority of samples were submitted from individuals living in the Mid-Atlantic region (Virginia and North Carolina were most common). However, specimens were collected from individuals residing in locales throughout the United States (including California, Colorado, Florida, Indiana, Michigan, Missouri, North Dakota, Pennsylvania, South Carolina, Texas, Tennessee and Washington).
Table I.
Sample distribution and micronuclei frequencies by gender and age group
| Age group | Gender | Total | MN frequency per 1000 binucleates (mean ± SD) | MN range (mean per 1000 binucleates) | MZ pairs | DZ pairs | Singletons |
| 7–9 | Males | 4 | 6.4 ± 3.2 | 3.5–11 | 2 | 0 | 0 |
| Females | 2 | 4.5 ± 0.7 | 4–5 | 1 | 0 | 0 | |
| 10–19 | Males | 6 | 11.4 ± 5.1 | 5–20 | 2 | 1 | 0 |
| Females | 20 | 6.0 ± 3.3 | 1–11 | 6 | 4 | 0 | |
| 20–29 | Males | 1 | 9.5 ± 0.7 | 9.5 | 0 | 0 | 1 |
| Females | 6 | 13.1 ± 10.0 | 2.5–29.5 | 3 | 0 | 0 | |
| 30–39 | Males | 8 | 12.3 ± 4.0 | 7–16 | 3 | 0 | 2 |
| Females | 8 | 13.4 ± 5.0 | 5.5–19 | 2 | 2 | 0 | |
| 40–49 | Males | 9 | 12.1 ± 5.1 | 4.5–22 | 3 | 1 | 1 |
| Females | 20 | 12.5 ± 5.9 | 5–23 | 5 | 3 | 4 | |
| 50–59 | Males | 7 | 10.3 ± 5.5 | 4–19.5 | 1 | 1 | 3 |
| Females | 7 | 14.6 ± 9.5 | 3–33.5 | 2 | 1 | 1 | |
| 60–69 | Males | 11 | 20.9 ± 10.7 | 11–45 | 3 | 2 | 1 |
| Females | 10 | 24.4 ± 10.2 | 13.5–44.5 | 2 | 1 | 5 | |
| 70–79 | Males | 15 | 12.3 ± 6.4 | 3–24 | 2 | 5 | 1 |
| Females | 6 | 11.6 ± 5.8 | 4–20 | 3 | 0 | 0 | |
| 80+ | Males | 2 | 16.0 ± 0.7 | 15.5–16.5 | 1 | 0 | 0 |
| Females | 3 | 16.2 ± 6.4 | 12–23.5 | 1 | 0 | 0 | |
| Total | Males | 63 | 13.2 ± 7.4 | 3–45 | 17 | 10 | 9 |
| Females | 82 | 12.4 ± 8.3 | 1–44.5 | 25 | 11 | 10 |
Of the 63 complete twin pairs collected, the zygosity testing showed 42 pairs to be MZ and 21 pairs to be DZ. The DZ twin pairs showed discordance at four or more markers, with no changes in marker patterns being detected in the MZ twin pairs.
Association of micronuclei frequencies with age differs by gender
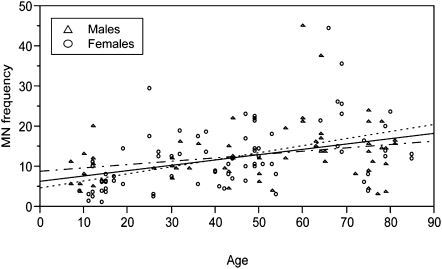
The highest frequency of micronuclei was observed in the twins who were 60–69 years of age (Table I and Figure 1). As anticipated, the lowest frequencies were noted for the twins who were 9 years of age or younger (Table I and Figure 1). A significant positive Spearman correlation between micronuclei frequency and age was computed using a randomly selected twin from each pair (r = 0.49, P < 0.001). We showed [by fitting a mixed-effect model (28) that included an interaction term to estimate the joint effect of age and sex, as well as a random effects term to account for the covariance within twin pairs (28)] that this relationship was gender dependent, with females having higher micronuclei frequencies with increasing age than males (P = 0.017).
Fig. 1.
Correlation of micronuclei frequencies with age in males and females. Age (x-axis) was significantly correlated with average micronuclei count (y-axis) when evaluated for all study participants (r = 0.388). However, these effects were significantly greater in the females (r = 0.482) compared to males (r = 0.257; P = 0.021). The best fitting lines, which were calculated from linear regression as a visual aid, are shown for the overall data [both males (triangles) and females (circles)] (solid black line), only females (small dashed line) and only males (hatched line). Age influences were also evaluated using a randomly selected twin from each pair and by fitting a mixed-effects model (see text).
Estimation of genetic and environmental influences on micronuclei frequencies
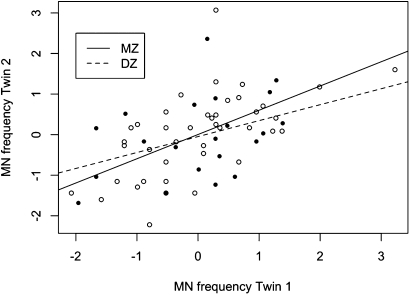
The correlation between replicate micronuclei scoring (1000 binucleates were evaluated from two independent areas of the slide for a total of 2000 binucleates per study participant) was calculated and found to be significantly positively correlated (Pearson correlation = 0.81, P < 0.001). The Spearman correlation coefficient for micronuclei frequencies for MZ twins was 0.63 (P < 0.001), compared to a value of 0.39 (P = 0.079) for the DZ twins and suggests familial factors responsible for twin resemblance (Figure 2). A series of biometrical models were fit to the data to obtain the maximum likelihood estimates of the genetic (A) and environmental (C and E) parameters while adjusting for the effects of age to remove confounding with C since twins of the same pair share birth dates (Table IIA). A common factor model was used to account for replicate micronuclei scoring and separation of scoring variance from E. Likelihood ratio tests of nested submodels revealed that either A (P = 0.551) or C (P = 0.585) could be removed from the model without a significant deterioration in fit but not both terms (P = 0.002). The best-fitting model, as judged by the Akaike information content (AIC) fit statistic (29), which balances goodness of fit with model complexity, was the AE model (AIC = 81.712). However, this fit was only marginally better than the CE model (AIC = 81.770) or the full genetic (ACE) model (AIC = 83.414). Additional models that allowed for the contributions of additive genetic and environmental influences to vary as a function of age (30) provided no significant improvement in model fit (P = 0.167).
Fig. 2.
Scatterplot of intra-pair MZ and DZ micronuclei frequencies. Micronuclei frequencies from twin 1 (x-axis) and twin 2 (y-axis) are shown for both MZ twins (solid circles and solid regression line) and DZ twins (open circles with dashed regression line). The data, expressed here on a standard normal scale (mean = 0; SD = 1), showed a significant correlation between MZ co-twins (r = 0.63; P < 0.001), but not DZ co-twins (r = 0.39, P = 0.079).
Table II.
Estimated genetic and environmental contributions to micronuclei frequencies from full and best-fitting reduced genetic models
| Source | Unstandardised estimate | Standardised estimatea | 95% confidence intervals |
| A | |||
| Additive genetic variance (A) | 0.375 | 0.600 | (0.283, 0.815) |
| Unique environment variance (E) | 0.251 | 0.400 | (0.185, 0.717) |
| Micronuclei scoring variance | 0.255 | — | — |
| Total variance | 0.881 | — | — |
| B | |||
| Additive genetic variance (A) | 0.346 | 0.652 | (0.326, 0.872) |
| Unique environment variance (E) | 0.184 | 0.348 | (0.128, 0.674) |
| Micronuclei scoring variance | 0.256 | — | — |
| Total variance | 0.786 | — | — |
‘A’, adjusted for age; ‘B’, adjusted for age and weekly intake of green leafy vegetables.
Standardised estimates were restricted to genetic and unique environmental variance components.
Micronuclei frequencies and health history questionnaire information
Given that unique environmental effects were shown to contribute to observed differences in an individual's micronuclei frequencies, additional studies were completed to identify specific factors that might exert an environmental influence (Tables III and IV). Furthermore, since age and gender were shown to be positively correlated with micronuclei frequencies (in the section “Association of micronuclei frequencies with age differs by gender”), all subsequent analyses were adjusted for the influences of these variables, as well as allowing for differences in covariance patterns by zygosity status.
Table III.
Age- and gender-corrected univariate regression analysis of the influence of dietary/lifestyle factors on lymphocyte micronuclei frequencies
| Diet/lifestyle factor | N | Coefficienta | SDa | P valuea |
| Tobaccob | ||||
| Current users | 16 | −0.127 | 0.944 | 0.174b |
| Non-users | 87 | |||
| Alcoholb | ||||
| Alcoholic | 2 | −0.024 | 0.436 | 0.590b |
| Everyday | 6 | |||
| Two to six days per week | 14 | |||
| One per week or less | 34 | |||
| Never | 38 | |||
| Fruit | ||||
| 5 days per week or more | 84 | −0.201 | 0.917 | 0.014 |
| <5 days per week | 41 | |||
| Folate-enriched bread | ||||
| 5 days per week or more | 62 | −0.141 | 0.749 | 0.035 |
| <5 days per week | 63 | |||
| Leafy green vegetables | ||||
| 5 days per week or more | 50 | −0.333 | 0.883 | <0.001* |
| <5 days per week | 75 | |||
| Vitamin C supplement | ||||
| 5 days per week or more | 12 | 0.170 | 1.111 | 0.134 |
| <5 days per week | 83 | |||
| Calcium supplementa | ||||
| 5 days per week or more | 24 | 0.081 | 0.897 | 0.383b |
| <5 days per week | 71 | |||
| Vitamin E supplement | ||||
| 5 days per week or more | 10 | 0.303 | 1.150 | 0.010 |
| <5 days per week | 85 | |||
| Vitamin D supplementb | ||||
| 5 days per week or more | 17 | 0.156 | 1.014 | 0.133b |
| <5 days per week | 78 | |||
| Fish oil supplement | ||||
| 5 days per week or more | 17 | 0.104 | 0.975 | 0.301 |
| <5 days per week | 78 | |||
| Multivitamin | ||||
| 5 days per week or more | 44 | 0.280 | 0.955 | 0.041 |
| <5 days per week | 51 | |||
Values in bold are significant (α < 0.05).
Total data (males and females).
Association varies with age and/or gender, see text.
Remains statistically significant after Bonferroni correction for multiple comparisons listed in this table and in Table IV.
Table IV.
Age- and gender-corrected univariate regression analysis of the influence of health-related conditions on lymphocyte micronuclei frequencies
| Trait | N | Coefficienta | SDa | P valuea |
| Allergies | ||||
| Yes | 56 | 0.107 | 0.436 | 0.007 |
| No | 69 | |||
| Arthritis | ||||
| Yes | 22 | -0.335 | 1.023 | 0.002 |
| No | 73 | |||
| BMI | ||||
| Obese | 27 | 0.029 | 0.577 | 0.578 |
| Overweight | 28 | |||
| Normal weight | 53 | |||
| Underweight | 15 | |||
| Menopause | ||||
| Yes | 20 | 0.360 | 1.109 | 0.024 |
| No | 28 | |||
| Migraines | ||||
| Yes | 17 | 0.243 | 1.062 | 0.026 |
| No | 78 | |||
| Puberty | ||||
| Yes | 111 | 0.427 | 2.393 | 0.037 |
| No | 28 |
Values in bold are significant (α < 0.05).
Total data (males and females).
The dietary items that were observed to have a significant influence on micronuclei frequencies included the twins’ consumption of: (i) fruit (P = 0.014); (ii) folate-supplemented bread (P = 0.035); (iii) green, leafy vegetables (P < 0.001); (iv) vitamin E supplements (P = 0.010) and (v) multivitamins (P = 0.041) (Table III). A significantly decreased frequency of micronuclei was associated with eating fruit (at least 5 days per week), leafy, green vegetables (at least 5 days per week) or folate-supplemented bread (at least 5 days per week; Table III). Surprisingly, a significantly increased frequency of micronuclei was seen in the twins who reported frequently (5–7 days each week) taking vitamin E supplements or multivitamins.
A subset of dietary/lifestyle practices was found to co-vary with age or gender (Table III). Variables that were shown to have a significant age- or gender-specific influence on micronuclei frequencies included: (i) tobacco usage, which resulted in an increased frequency of micronuclei in females (P < 0.001), but not males (P = 0.384); (ii) the use of vitamin D supplements, which was associated with a modestly decreased frequency of micronuclei in males (P = 0.044), but not females (P = 0.392); and (iii) the ingestion of calcium supplements, which were associated with an increased frequency of micronuclei in males (P = 0.001), but not females (P = 0.203). No clear association between micronuclei frequencies and alcohol consumption was detected.
Developmental and health-related conditions were also observed to have a differential influence on the frequency of micronuclei in the twins (Table IV). Interestingly, after controlling for chronological age effects, a significant increase in micronuclei frequencies was observed in both males and females following their completion of puberty (P = 0.037) [females aged 16 years or older and males aged 17 years or older were assumed to have completed puberty (31)]. Similarly, women who were post-menopausal (as determined by self-report in the health history questionnaire) had significantly higher frequencies of micronuclei (after correction for age effects) when compared to females who had not completed menopause (P = 0.024) (Table IV).
Other health-related factors that were reported by 10 or more individuals (but did not co-vary with age or sex) included body mass index, allergies, migraines and arthritis. No significant effect of body mass index on micronuclei frequency was observed (after adjusting for age and gender). A history of allergies (P = 0.007) and migraines (P = 0.026) was associated with a significantly increased frequency of micronuclei, while a history of arthritis (P = 0.002) was associated with a significantly decreased micronuclei frequency.
Lists of medication usage were tabulated to determine if the observed differences in micronuclei frequencies of individuals with these conditions might be confounded by treatment medications (e.g. anti-inflammatory drugs used by people having arthritis, etc.). However, the sample sizes of individuals taking medication for these conditions were too small to allow for an unbiased statistical assessment.
Integration of influences from specific environmental effects for modelling sources of variation contributing to micronuclei frequencies
Using the conservative Bonferroni correction for multiple comparisons, the only dietary or lifestyle influence that remained significant after adjustment was the consumption of leafy green vegetables (P < 0.001). Thus, we re-evaluated the biometrical models (described in the section Statistical Analysis) while regressing micronuclei frequency on both age and leafy green vegetables (Table IIB). Inclusion of leafy green vegetables explained an additional 10.8% of the total variance in micronuclei frequency (0.881 in Table IIA versus 0.786 in Table IIB). As before, both A (P = 0.151) and C (P = 0.998) could be removed from the model but not both (P = 0.002) and the AE model fit the data best as indicated by the AIC (AICAE = 67.291 versus AICACE = 69.291). The unstandardised estimates showed that inclusion of leafy green vegetables primarily resulted in a reduction of E (0.251 versus 0.184). As expected, the variance attributed to micronuclei scoring remained constant for both versions of the model. The standardised estimates showed that, after accounting for the overall reduction in total variance, the inclusion of leafy green vegetables, as expected, was attributable to unique environmental sources as seen in the proportional reduction of E between model versions (40.0% in Table IIA to 34.8% in Table IIB).
Discussion
A strong positive correlation was observed between micronuclei frequencies and age in the twins evaluated in this study, which is a finding that is consistent with the results of previous investigators who have studied singletons (12,32). Similar to the findings of Fenech et al. (33) and Bonassi et al. (34), we found micronuclei frequencies in women to increase at a greater rate with age compared to males. Interestingly, our observation of a peak in micronuclei frequencies in the members of our 60- to 69-year-old age group has also been seen by other investigators, who have shown that the frequency of micronuclei appears to peak between ages 50–69, remaining either unchanged or slightly lower in the individuals who live to be 80 or older (35,36). Bonassi et al. (34) proposed that the observed plateau or decrease in micronuclei frequencies seen in females later in life might result from a decrease in hormones after menopause. As they conjectured, we did see an effect of menopause on micronuclei frequencies in our female twins. However, this trend, which was not seen by Landi and Barale (37), was towards an increase rather than a decrease in micronuclei frequencies in women who were post-menopausal.
Little information is available about micronuclei frequencies in children. However, in their meta- and pooled analyses of baseline levels of micronuclei frequencies, Neri et al. (38) reported the mean number of micronuclei per 1000 binucleated cells from children aged 0–18 years to be 5.2 ± 5.1 (SD). The mean number of micronuclei per 1000 binucleated cells for the children participating in our study was 6.7 ± 4.0 (SD). However, our study included children of ages 7–18 years, while ∼30% of the children in the Neri et al. (38) study were younger than 7 years. Despite the variation in the ages of participants between studies, the results appear to be in good agreement (39). Our study design, in which we evaluated children and young adults, allowed for the detection of a significant increase in micronuclei following the completion of puberty. The causes of this increase are not known, but could include biological influences (hormonal changes), as well as environmental exposures associated with late adolescence.
Since micronuclei frequencies are increasingly being used as a biomarker, it is important to know what factors influence their frequency in healthy people. By studying twins, we were able to provide the first estimate of the proportion of variation in spontaneous micronuclei frequencies attributable to unique environmental, common environmental and/or additive genetic effects. The model fitting analyses that were completed on age-adjusted micronuclei frequencies were best explained by a model in which both additive genetic and unique environmental factors (AE) contributed to the observed variation. While, the nested models did not allow for a clear distinction between influences attributable to additive genetic/unique environment (AE), common/unique environment (CE), or additive genetic/common/unique environment (ACE) influences, they did allow for the rejection of models that attributed the variation to be solely influenced by unique environmental exposures (E). Our observation of a significantly high correlation between the replicate scoring measures of micronuclei frequencies provides strong validity for the reproducibility of these measures. However, the impact of scoring variation on genetic and environmental parameter estimates would be to inflate environmental influences, thereby reducing genetic influences. If micronuclei scoring variance was a strong contributing factor to the observed E effects, one would not expect to see a significant intraclass twin correlation (MZ correlation of 0.63; P < 0.001). Nonetheless, to correct for this bias, the variability in replicate scores of micronuclei levels was modelled, using a common factor design, to allow for the separation of this scoring variance from the estimation of unique environmental variance. Using this biometrical approach the age-adjusted models showed that micronuclei frequency appeared to be best explained by both genetic (60.0% of trait variance) and unique environmental (40.0% of trait variance) influences.
Given that a significant proportion of variation in micronuclei frequencies was attributable to unique environmental influences, we evaluated the association of several dietary/lifestyle or health influences on micronuclei frequencies to determine if a specific environmental association could be identified. One such association was a significant reduction in micronuclei frequencies with the study participants’ regular ingestion (at least 5 days per week) of fruit, leafy green vegetables and folate-enriched bread. Collectively, these observations suggest that folate may be important for regulating micronuclei frequencies, providing a ‘protective’ effect that is associated with a decrease in frequency. This finding confirms the results of several other researchers, who have also reported folate usage to be associated with decreased micronuclei frequencies (33,40–45). Given that folate is required for DNA synthesis and repair, with it acting as a methyl donor for thymidylate synthesis, it seems logical that increased folate ingestion would be associated with decreases in micronuclei frequencies (40).
The influence of ingesting supplemental vitamins was also evaluated. Of the vitamins assessed (C, E, D, calcium, fish oil/omega 3 fatty acids and multivitamins), only vitamins D and E, as well as multivitamin ingestion, were found to significantly influence micronuclei frequencies. However, unexpectedly, the use of each of these supplements was associated with an increased frequency of micronuclei. Other investigators who have studied this effect have reported varied results, with increases, decreases and no effect being seen for vitamin E and C ingestions and micronuclei frequencies (13). Interestingly, Fenech et al. (42) observed a decreased frequency of micronuclei with vitamin E intake but did report an increased frequency of micronuclei with biotin, riboflavin and pantothenic acid ingestion. It is also interesting to note that vitamin E is found in many fruits and leafy green vegetables (along with folate, iron, calcium and vitamins A, K and C), yet our sample showed a decreased frequency of micronuclei associated with their consumption. Thus, the protective influence conferred from the ingestion of these fruits/vegetables may arise primarily from the other components (such as folate), or the effect of vitamins may be varied depending on whether they are ingested as a supplement (perhaps over-use) versus ingested as a component of natural foods.
The effect of tobacco and alcohol usage on micronuclei frequencies of the adults who participated in this study was also evaluated. Prior studies of the impact of tobacco on micronuclei frequencies have led to varied conclusions. Specifically, in the Human Micronucleus project, in which over 5000 individuals were evaluated (12), no clear influence of tobacco was observed, but other investigators have shown an increased frequency of micronuclei with tobacco usage (21,46). The results of the current study indicated that micronuclei frequencies and tobacco usage co-varied with age and gender. Specifically, older females who smoke tended to have higher micronuclei frequencies than younger females or males (regardless of age). This observation is consistent with the variable results that have been observed in previous studies.
No clear association between alcohol consumption and micronuclei frequencies was observed in this study. However, a factor confounding the interpretation of this data, as well as the data from studies completed by other investigators, is the potential variation in the effect of moderate to low alcohol use (which may have no effect) compared to excessive alcohol usage, the latter of which has been associated with the presence of increased frequencies of micronuclei (47,48). While the effect of alcohol on micronuclei frequencies has been noted to provide varied results in studies reported by other investigators (13), researchers have consistently found an increased frequency of micronuclei with alcohol use in individuals having genetic variants in the alcohol metabolising enzyme, alcohol dehydrogenase (49,50). Interestingly, Teo and Fenech (51) suggested that there may be an interaction between folic acid levels and alcohol use, with increased folic acid values providing a protective influence against potential DNA damage caused by increased ethanol ingestion.
Additional findings of this study were the significant associations of micronuclei frequencies with a history of allergic disease (an increased frequency) or arthritis (a decreased frequency). In their previous study, Herrstrom et al. (52) also saw an increase of micronuclei in lymphocytes of children with allergic disease. However, unlike our study, they reported an increase in B-cells, but not T-cells. Arthritis has previously been associated with an increase in various chromosomal aberrations locally at the site of arthritis, but not in the peripheral blood (7). Thus, given the paucity of information available, these observations warrant further evaluation to determine if these findings are reproducible and if their influence might be confounded by the effects of medications used for treatment.
To optimise our ability to model the sources of variation influencing micronuclei frequencies, we used the results of the measured environmental covariate analyses to inform the ACE model. For this analysis, we included only effects that were significantly different using the very conservative Bonferroni correction for multiple comparisons. Thus, we recalculated our ACE model adjusting for age and consumption of green leafy vegetables. The overall conclusions of the models were unchanged, with the best fitting model remaining the reduced AE model and, as expected, the contribution of E was reduced with the inclusion of green leafy vegetables. Based on this adjusted model, the frequency with which an individual forms micronuclei appears to be a complex trait that is best described as reflecting influences from both unique environmental influences (34.8% of variation), as well as additive genetic (65.2% of variation) effects.
In summary, the frequency of spontaneously arising micronuclei in humans is familial and best viewed as a complex trait influenced by unique environmental factors, as well as additive genetic (and/or possibly common environmental) effects. Recognition of the factors contributing to the baseline level of micronuclei is important for helping to better design studies that include the assessment of micronuclei frequency data for evaluating agents hypothesised to influence genomic instability.
Funding
National Institute of Environmental Health (R01 ES12074 to C.J.-C.).
Acknowledgments
This study was done through the cooperation of many generous individuals, most of whom are members of the Mid-Atlantic Twin Registry. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences, National Institutes of Health.
Conflict of interest statement: None of the authors has a conflict of interest (financial or otherwise) regarding this research study.
References
- 1.Jacobs PA, Court Brown WM, Doll R. Distribution of human chromosome counts in relation to age. Nature. 1961;191:1178–1180. doi: 10.1038/1911178a0. [DOI] [PubMed] [Google Scholar]
- 2.Sandberg AA. Chromosomes in Human Cancer and Leukemia. New York, NY, USA: Elsevier Press; 1990. Chromosome lesions and solid tumors: an overview; pp. 35–60. [Google Scholar]
- 3.Zekanowski C, Wojda U. Aneuploidy, chromosomal missegregation, and cell cycle reentry in Alzheimer's disease. Acta Neurobiol. Exp. 2009;69:232–253. doi: 10.55782/ane-2009-1748. [DOI] [PubMed] [Google Scholar]
- 4.Tascioglu F, Durak B, Oner C, Artan S. Trisomy 7 in synovial fluid cells of patients with rheumatoid arthritis. Rheumatol. Int. 2005;25:571–575. doi: 10.1007/s00296-004-0477-6. [DOI] [PubMed] [Google Scholar]
- 5.Castellanos M, Hernandez J, Ramos L, Belen M, Gutierrez N, Leone P, Lumbreras E, Robledo C, Garcia J. Chromosomal abnormalities are related to the location and grade of osteoarthritis. Osteoarthr. Cartil. 2004;12:982–985. doi: 10.1016/j.joca.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Dahlen A, Broberg K, Domanski H, Toksvig-Larsen S, Lindstrand A, Mandahl N, Mertens F. Analysis of the distribution and frequency of trisomy 7 in vivo in synovia from patients with osteoarthritis and pigmented villonodular synovitis. Cancer Genet. Cytogenet. 2001;131:19–24. doi: 10.1016/s0165-4608(01)00488-5. [DOI] [PubMed] [Google Scholar]
- 7.Kinne RW, Liehr T, Beensen V, et al. Mosaic chromosomal aberrations in synovial fibroblasts of patients with rheumatoid arthritis, osteoarthritis, and other inflammatory joint diseases. Arthr. Res. 2001;3:319–330. doi: 10.1186/ar322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mertens F, Pålsson E, Lindstrand A, et al. Evidence of somatic mutations in osteoarthritis. Hum. Genet. 1996;98:651–656. doi: 10.1007/s004390050278. [DOI] [PubMed] [Google Scholar]
- 9.Broberg K, Hoglund M, Lindstrand A, Toksvig-Larsen S, Mandahl N, Mertens F. Polyclonal expansion of cells with trisomy 7 in synovia from with osteoarthritis. Cytogenet. Cell Genet. 1998;83:30–34. doi: 10.1159/000015160. [DOI] [PubMed] [Google Scholar]
- 10.Fenech M, Morley A. The effect of donor age on spontaneous and induced micronuclei. Mutat. Res. 1985;148:99–105. doi: 10.1016/0027-5107(85)90212-x. [DOI] [PubMed] [Google Scholar]
- 11.Miller B, Pötter-Locher F, Seelbach A, Stopper H, Utesch D, Madle S. Evaluation of the in vitro micronucleus test as an alternative to the in vitro chromosomal aberration assay. Mutat. Res. 1998;410:81–116. doi: 10.1016/s1383-5742(97)00030-6. [DOI] [PubMed] [Google Scholar]
- 12.Bonassi S, Fenech M, Lando C, et al. Human Micronucleus project: international database comparison for the results with the cytokinesis-block micronucleus assay in human lymphocytes: i. effect of laboratory protocol, scoring criteria, and host factors on the frequency of micronuclei. Environ. Mol. Mutagen. 2001;37:31–45. [PubMed] [Google Scholar]
- 13.Battershill J, Burnett K, Bull S. Factors affecting the incidence of genotoxicity biomarkers in peripheral blood lymphocytes: impact on design of biomonitoring studies. Mutagenesis. 2008;23:423–437. doi: 10.1093/mutage/gen040. [DOI] [PubMed] [Google Scholar]
- 14.Tucker JD, Nath J, Hando JC. Activation status of the X chromosome in human micronucleated lymphocytes. Hum. Genet. 1996;97:471–475. doi: 10.1007/BF02267069. [DOI] [PubMed] [Google Scholar]
- 15.Duffaud F, Orsière T, Villani P, Pelissier AL, Volot F, Favre R, Botta A. Comparison between micronucleated lymphocyte rates observed in healthy subjects and cancer patients. Mutagenesis. 1997;12:227–231. doi: 10.1093/mutage/12.4.227. [DOI] [PubMed] [Google Scholar]
- 16.Venkatachalam P, Paul SF, Mohankumar MN, Prabhu BK, Gajendiran N, Kathiresan A, Jeevanram RK. Higher frequency of dicentrics and micronuclei in peripheral blood lymphocytes of cancer patients. Mutat. Res. 1999;425:1–8. doi: 10.1016/s0027-5107(98)00238-3. [DOI] [PubMed] [Google Scholar]
- 17.Znaor A, Fucić A, Strnad M, Barković D, Skara M, Hozo I. Micronuclei in peripheral blood lymphocytes as a possible cancer risk biomarker: a cohort study of occupationally exposed workers in Croatia. Croatian Med. J. 2003;444:441–446. [PubMed] [Google Scholar]
- 18.Olaharski AJ, Sotelo R, Solorza-Luna G, Gonsebatt ME, Guzman P, Mohar A, Eastmond DA. Tetraploidy and chromosomal instability are early events during cervical carcinogenesis. Carcinogenesis. 2006;27:337–343. doi: 10.1093/carcin/bgi218. [DOI] [PubMed] [Google Scholar]
- 19.Migliore L, Testa A, Scarpato R, Pavese N, Petrozzi L, Bonuccelli U. Spontaneous and induced aneuploidy in peripheral blood lymphocytes of patients with Alzheimer's disease. Hum. Genet. 1997;101:299–305. doi: 10.1007/s004390050632. [DOI] [PubMed] [Google Scholar]
- 20.Migliore L, Coppede F. Genetic and environmental factors in cancer and neurodegenerative diseases. Mutat. Res. 2002;512:135–153. doi: 10.1016/s1383-5742(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 21.Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat. Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-l. [DOI] [PubMed] [Google Scholar]
- 22.Leach N, Jackson-Cook C. The application of spectral kayotyping (SKY and fluorescent in situ hybridization (FISH) technology to determine the chromosomal content(s) of micronuclei. Mutat. Res. 2001;495:11–19. doi: 10.1016/s1383-5718(01)00194-2. [DOI] [PubMed] [Google Scholar]
- 23.Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E. HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. 2003;534:65–75. doi: 10.1016/s1383-5718(02)00249-8. [DOI] [PubMed] [Google Scholar]
- 24.Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women. Arch. Intern. Med. 2010;170:711–718. doi: 10.1001/archinternmed.2010.76. [DOI] [PubMed] [Google Scholar]
- 25.Kim K, Friso S, Cho S-W. DNA methylation, an epigenetic mechanism connecting folate to healthy embryonic development and aging. J. Nutr. Biochem. 2009;20:917–926. doi: 10.1016/j.jnutbio.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 27.Neale MC, Boker SM, Xie G, Maes HM. Mx: Statistical Modeling. Richmond, VA, USA: Department of Psychiatry, Virginia Commonwealth University; 1999. [Google Scholar]
- 28.Faraway JJ. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models. New York, NY, USA: Chapman & Hall/CRC; 2006. [Google Scholar]
- 29.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- 30.Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- 31.Eaves L, Silberg J, Foley D, Bulik C, Maes H, Erkanli A, Angold A, Costello EJ, Worthman C. Genetic and environmental influences on the relative timing of pubertal change. Twin Res. 2004;7:471–481. doi: 10.1375/1369052042335278. [DOI] [PubMed] [Google Scholar]
- 32.Bolognessi C, Lando C, Forni A, Landini E, Scarpato R, Migliore L, Bonassi S. Chromosomal damage and ageing: effect on micronuclei frequency in peripheral blood lymphocytes. Age Ageing. 1999;28:393–397. doi: 10.1093/ageing/28.4.393. [DOI] [PubMed] [Google Scholar]
- 33.Fenech M, Neville S, Rinaldi J. Sex is an important variable affecting spontaneous micronucleus frequency in cytokinesis-blocked lympocytes. Mutat. Res. 1994;313:203–207. doi: 10.1016/0165-1161(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 34.Bonassi S, Bolognesi C, Abbondandolo A, et al. Influence of sex on cytogenetic end points: evidence from a large human sample and review of the literature. Cancer Epidemiol. Biomarkers Prev. 1995;4:671–679. [PubMed] [Google Scholar]
- 35.Wojda A, Witt M. Manifestation of ageing at the cytogenetic level. J. Appl. Genet. 2003;44:383–399. [PubMed] [Google Scholar]
- 36.Bolognesi C, Abbondandolo A, Barale R, et al. Age-related increase of baseline frequencies of sister chromatid exchanges, chromosome aberrations, and micronuclei in human lymphocytes. Cancer Epidemiol. Biomarkers Prev. 1997;6:249–256. [PubMed] [Google Scholar]
- 37.Landi S, Barale R. Sister chromatid exchanges, chromosome aberrations and micronuclei in female lymphocytes: correlations with biological rhythms, miscarriages and contraceptive pill use. Mutagenesis. 1999;14:581–585. doi: 10.1093/mutage/14.6.581. [DOI] [PubMed] [Google Scholar]
- 38.Neri M, Ceppi M, Knudsen LE, Merlo DF, Barale R, Puntoni R, Bonassi S. Baseline micronuclei frequency in children: estimates from meta- and pooled analyses. Environ. Health Perspect. 2005;113:1226–1229. doi: 10.1289/ehp.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neri M, Fucic A, Knudsen L, Lando C, Merlo F, Bonassi S. Micronuclei frequency in children exposed to environmental mutagens: a review. Mutat. Res. 2003;544:243–254. doi: 10.1016/j.mrrev.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Fenech M. Recommended dietary allowance (RDAs) for genomic stability. Mutat. Res. 2001;480–481:51–54. doi: 10.1016/s0027-5107(01)00168-3. [DOI] [PubMed] [Google Scholar]
- 41.Fenech M. Micronutrients and genomic stability: a new paradigm for recommended dietary allowances (RDAs) Food Chem. Toxicol. 2002;40:1113–1117. doi: 10.1016/s0278-6915(02)00028-5. [DOI] [PubMed] [Google Scholar]
- 42.Fenech M, Baghurst P, Luderer W, Turner J, Record S, Ceppi M, Bonassi S. Low intake of calcium, folate, nicotinic acid, vitamin E, retinol, B-carotene and high intake pantothenic acid, biotin, and riboflavin are significantly associated with increased genome instability—results from dietary intake and micronucleus index survey in South Australia. Carcinogenesis. 2005;26:991–999. doi: 10.1093/carcin/bgi042. [DOI] [PubMed] [Google Scholar]
- 43.Abramsson-Zetterberg DL, Yang-Wallentin F, Rytter E, Vessby B. The impact of folate status and folic acid supplementation on the micronucleus frequency in human erythrocytes. Mutat. Res. 2006;603:33–40. doi: 10.1016/j.mrgentox.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Fenech M. Important variables that influence base-line micronucleus frequency in cytokinesis-blocked lymphocytes—a biomarker for DNA damage in human populations. Mutat. Res. 1998;404:155–165. doi: 10.1016/s0027-5107(98)00109-2. [DOI] [PubMed] [Google Scholar]
- 45.Titenko-Holland N, Jacob R, Shang N, Balaraman A, Smith M. Micronuclei in lymphocytes and exfoliated buccal cells of postmenopausal women with dietary changes in folate. Mutat. Res. 1998;417:101–114. doi: 10.1016/s1383-5718(98)00104-1. [DOI] [PubMed] [Google Scholar]
- 46.Schneider M, Diemer K, Engelhart K, Zankl H, Tromme W, Biesalski H. Protective effects of vitamins C and E on the number of micronuclei in lymphocytes in smokers and their role in ascorbate free radical formation in plasma. Free Radic. Res. 2001;34:209–219. doi: 10.1080/10715760100300201. [DOI] [PubMed] [Google Scholar]
- 47.Castelli E, Hrelia P, Maffei F, Fimognari C, Foschi FG, Caputo F, Cantelli-Forti G, Stefanini GF, Gasbarrini G. Indicators of genetic damage in alcoholics: reversibility after alcohol abstinence. Hepatogastroenterology. 1999;46:1664–1668. [PubMed] [Google Scholar]
- 48.Maffei F, Forti G, Castelli E, Stefanini G, Mattioli S, Hrelia P. Biomarkers to assess the genetic damage induced by alcohol abuse in human lymphocytes. Mutat. Res. 2002;514:49–58. doi: 10.1016/s1383-5718(01)00318-7. [DOI] [PubMed] [Google Scholar]
- 49.Ishikawa H, Miyatsu Y, Kurihara K, Yokoyama K. Gene-environmental interactions between alcohol-drinking behavior and ALDH2 and CYP2E1 polymorphisms and their impact on micronuclei frequency in human lymphocytes. Mutat. Res. 2006;594:1–9. doi: 10.1016/j.mrfmmm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Kimv J, Kim Y, Kim T, Song J, Cho Y, Park Y, Chung H. Association of ALDH2 polymorphism with sensitivity to acetaldehyde-induced micronuclei and facial flushing after alcohol intake. Toxicology. 2005;210:169–174. doi: 10.1016/j.tox.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Teo T, Fenech M. The interactive effect of alcohol and folic acid on genome stability in human WIL2-NS cells measured using the cytokinesis-block micronucleus cytome assay. Mutat. Res. 2008;657:32–38. doi: 10.1016/j.mrgentox.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Herrström P, Bratt I, Holmén A, Högsted B. Micronuclei in lymphocyte subsets in relation to immune proteins and allergic disease. Mutat. Res. 1998;405:35–40. doi: 10.1016/s0027-5107(98)00125-0. [DOI] [PubMed] [Google Scholar]