Abstract
Objectives. To explore whether helplessness, internality and depression would mediate the relationship between disease activity and functional limitations in patients with AS in a 12-month longitudinal study.
Methods. A total of 294 participants with AS meeting modified New York criteria completed clinical and psychological assessments at 6-month intervals. Psychological measures evaluated helplessness, depression and internality. Path analysis evaluated the direct and indirect effects of baseline disease activity on 12-month functional limitations via the psychological measures of helplessness, internality and depression at 6 months.
Results. Baseline disease activity demonstrated direct and indirect effects on 12-month functional limitations. Helplessness and depression, but not internality, served as mediators of the relationship between disease activity and functional limitations.
Conclusion. Higher baseline disease activity predicted greater functional limitations at 12 months through helplessness and depression. Our findings suggest that helplessness and depression may constitute future treatment targets in reducing functional limitations in patients with AS.
Keywords: Ankylosing spondylitis, Disease activity, Functional limitations, Depression, Internality, Helplessness
Introduction
AS is a chronic inflammatory disease affecting the axial spine and the SI joints [1]. Common symptoms include pain and stiffness of joints with subsequent negative impact on patients’ quality of life, comprised of physical, psychological and social well-being [2]. Psychological distress, reported by as many as 20% of patients with AS, may contribute to functional limitations in this condition [3]. Self-reported questionnaires for disease activity, functional limitations and psychological functioning, identified by standardized assessment tools, increasingly guide therapeutic efforts [2].
Evidence suggesting an association between psychological factors and disease status originates from patients suffering from RA, with symptoms of depression occurring after the onset of pain and disability [4, 5]. Evidence exists for helplessness as a mediator between disease severity and depression in RA [6, 7]. Depression has demonstrated an association with functional limitations in RA [8]. Depression has proved to be a better predictor of functional limitations in RA than disease activity [9].
Psychological aspects of AS have received little attention until recently. A study of 89 patients with AS conducted by Martindale et al. [10] demonstrated that disease activity and functional impairment are correlated with anxiety, depression, internality and health status. However, the relatively small sample size limited the conclusions derived from this study. In a cross-sectional analysis of 294 patients, Brionez et al. [11, 12] reported that arthritis helplessness, depression and passive coping accounted for substantial variability in self-reported functional limitations. Further, arthritis helplessness and depression accounted for variability in self-reported disease activity beyond clinical and demographic variables in patients with AS [11, 12]. However, the cross-sectional nature of these findings precluded an interpretation of the direction of effects among these variables.
The current article explores whether helplessness, internality (beliefs in control over AS) and depression plausibly mediate the relationship between disease activity and functional limitations in a 12-month longitudinal study. If these variables mediate the relationship between disease activity and functional limitations, increased disease activity in AS should lead to greater helplessness, greater depression and lower internality, which in turn, should contribute to increased functional limitations.
Materials and methods
Study participants
The Prospective Study of Outcome in Ankylosing Spondylitis (PSOAS) recruited participants from four institutions: Cedars-Sinai Medical Center, University of Texas Health Science Center at Houston, National Institutes of Health and University of California, San Francisco (UCSF). Recruitment sources included the clinics of the investigators, local rheumatologists, patient support and advocacy groups and community advertisements [13–15]. All participants were ≥18 years old and met modified New York criteria for AS. Modified New York criteria consist of radiographic presence of sacroiliitis grade ≥2 bilaterally or ≥3 unilaterally in the presence of only one clinical criterion [16]. Clinical criteria include low back pain and stiffness for >3 months that improves with exercise but not with rest, limitation of the lumbar spine in sagittal and frontal planes, and limitation of chest expansion relative to normal values corrected for age and sex [17]. The current study includes longitudinal follow-up on all 294 participants evaluated for Brionez et al.’s cross-sectional study [11, 12]. This current study received approval from the institutional review boards of each institution [Cedars-Sinai IRB; Gloucestershire Research Ethics Committee at the University of Bath; Committee on Human Research at UCSF; Princess Alexandra Hospital (PAH) Human Research Ethics Committee and the University of Queensland at Brisbane; Committee for the Protection of Human Subjects at the University of Texas at Houston and National Institutes of Health (NIH)], and all participants provided written informed consent.
Participants completed extensive assessments at baseline, including socio-demographic information, psychological status, medical history and radiographs of the pelvis, lumbar spine and cervical spine. Participants underwent a clinical evaluation, including a standardized physical examination at each site by a study rheumatologist. Clinical data collected at enrolment included age, gender, ethnicity, education level, marital status, employment, smoking history and comorbid medical conditions. Collection of clinical and psychological assessments, described below, occurred at 6-month intervals.
Clinical and psychological assessments
BASDAI and BASFI provided measures of disease activity and functional limitations, respectively. The BASDAI is a self-report, 6-item questionnaire measuring the severity of fatigue, spinal and peripheral joint pain, localized tenderness and morning stiffness over the past week using a 10 cm visual analogue scale (VAS), from none (0 mm) to very severe (100 mm). The final question quantifies the amount of morning stiffness, from 0 to ≥2 h, over the past week. The mean score of the last two questions is averaged with the remaining questions. The final BASDAI score has a range of 0–10, with lower scores indicating less disease activity [18]. BASFI is a self-report,10-item questionnaire evaluating the ability to function and cope with activities of daily living over the past week using a 10 cm VAS, from none (0 mm) to very severe (100 mm). The final BASFI score has a range of 0–10, with lower scores indicating a better functional status [19].
Psychological variables self-report questionnaires included depression, arthritis helplessness and arthritis internality measured at 6-month intervals. Indices of psychological status used the patient health questionnaire (PHQ-9) and the arthritis helplessness index (AHI). The PHQ-9 is a self-administered 9-item depression module that is a well-validated and widely used diagnostic and severity measure for depression. Each item is scored from 0 (not at all) to 3 (nearly every day), with a total score ranging from 0 to 27. The scale focuses exclusively on the nine diagnostic criteria for Diagnostic and Statistical Manual of Mental Disorders-IV depressive disorders and does not overlap with medical symptoms as extensively as many other depression measures [20–22]. The AHI is a 15-item self-report questionnaire designed to measure patients’ perceptions of loss of control with their chronic arthritis [23]. Two subscales, internality (seven items) and helplessness (five items), were adopted because each subscale has been shown to have higher reliability and validity than the total AHI score [24]. Arthritis internality assesses patients’ beliefs that their own behaviour can control their arthritis, while arthritis helplessness assesses patients’ perceptions of helplessness in controlling pain and disease course. These two subscales are negatively correlated, but reflect largely independent beliefs about the controllability of arthritis [24].
Statistical analysis
A 12-month, longitudinal, prospective design allowed examination of relationships among disease activity, psychological variables and functional limitations. The arthritis helplessness and internality subscales and depression were selected as psychological variables for this analysis since these variables accounted for significant variability in both disease activity and functional limitations in previous studies [11, 12]. Our findings have been computed based on non-imputed data. Mplus provides maximum-likelihood estimation, which is robust under the missing at random (MAR) assumption [25].
A path-analysis approach evaluated plausible causal models using the BASDAI assessed at baseline; arthritis helplessness, arthritis internality and PHQ-9 assessed at 6 months; and BASFI assessed at 12 months. Path analysis confers benefits over and above those provided by standard generalized linear model approaches (e.g. linear regression, logistic regression, etc.). While inclusion of the predictors (i.e. BASDAI, AHI helplessness, AHI internality and PHQ-9) in a regression model would explain the same proportion of variance in the criterion (i.e. BASFI) as a path model, the regression approach does not permit specification of the relationships among the predictors. This capability of path analysis allows specification of models representing hypothesized causal chains in which some variables predict other variables, which, in turn, predict subsequent variables. As with all arguments for causality, the internal validity of the conclusions cannot rely purely on statistical evidence. In the current context of a prospective observational study, models must be evaluated in terms of criteria such as those delineated by Hill (e.g. temporal precedence of cause and effect, theoretical plausibility, etc.) [26]. We hypothesized putative causal relations and then tested these specifications against the data. Indices of model fit include standard measures (e.g. χ2, comparative fit index, root mean square error of approximation, etc.) that evaluate the discrepancy between the observed and model-implied covariance matrices. In addition, R2 provides an index of the predictive power of the model. Identification of intermediate variables that demonstrate evidence of transmitting the effect of an initial variable to one more distal provides a potential target(s) for treatment insofar as it is modifiable. West et al. [27] described a similar use of meditational analyses in the context of clinical trials.
Investigation of mediators utilized Mplus v. 5.2 [25]. Calculation of indirect effects used the product limit method with bootstrapped CIs [28–30]. It is the case that causal inference is made most strongly in the context of specific study designs [e.g. randomized controlled trials (RCTs)]. The current analysis seeks to identify plausible causal mechanisms that might ultimately be targeted for treatment in an RCT.
Results
Participant characteristics
At the time of study entry, 294 participants with AS were enrolled in the study, of whom 68.2% were male and 82% were white (Table 1). The mean (s.d.) age of the participants was 45.1 (14.1) years and the mean (s.d.) duration of AS was 21.2 (13.8) years. Respectively, 65.6 and 55.8% of the participants with AS were employed and married. The majority of participants completed high school. A total of 209 participants at 6 months and 211 participants at 12 months completed follow-up assessments. Reasons for non-completion included non-attendance, closure of one of the study sites and incomplete data recording.
Table 1.
Participant characteristics at study entry (n = 294)
| Characteristic | Value |
|---|---|
| Age, mean (s.d.), years | 45.1 (14.4) |
| Duration of AS, mean (s.d.), years | 21.2 (13.8) |
| Education level, mean (s.d.), yearsa | 3.7 (1.3) |
| Male, % | 68.2 |
| White, % | 82 |
| Smoking status, % | 11 |
| Employed, % | 65.5 |
| Married, % | 55.8 |
aEducation level labelled as 1–4 (1: ≤12 years, 2: 13–15 years, 3: 16 years and 4: >16 years).
Measurement
Mean (s.d.) BASDAI score at entry was 3.53 (2.3). Mean (s.d.) scores for PHQ-9, arthritis internality and arthritis helplessness at 6 months were 5.5 (5.5), 25.9 (5.7) and 13.1 (5.3), respectively. Mean (s.d.) score for BASFI at 12 months was 30.7 (25.3) (Table 2). Internal consistency of the measures (i.e. Cronbach’s alpha) was good for BASDAI, BASFI and PHQ-9: 0.89, 0.92, and 0.89, respectively. Cronbach’s alpha was lower for arthritis internality and arthritis helplessness (0.78 and 0.70, respectively).
Table 2.
Mean (s.d.) score for clinical and psychological status at each assessment
| Clinical/psychological assessment | Enrolment | 6 months | 12 months |
|---|---|---|---|
| BASDAI | 3.5 (2.3) | 3.5 (2.4) | 3.6 (2.4) |
| PHQ-9 | 5.2 (4.9) | 5.5 (5.5) | 4.9 (5.0) |
| AHI internality | 25.6 (6.0) | 25.9 (5.7) | 26.1 (6.0) |
| AHI helplessness | 13.1 (5.2) | 13.1 (5.3) | 12.4 (5.0) |
| BASFI | 31.4 (25) | 29.6 (23.1) | 30.7 (25.3) |
Path analysis
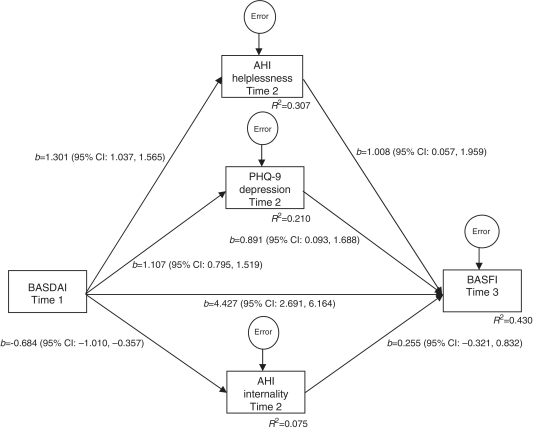
A path analysis evaluated the degree to which arthritis helplessness, arthritis internality and PHQ-9 at 6 months plausibly mediated the effect of BASDAI at baseline on BASFI at 12 months (Fig. 1). The proportion of variance in BASFI at 12 months that is attributable to the direct effect of BASDAI at baseline and the indirect effects of this variable through arthritis helplessness, arthritis internality and PHQ-9 at 6 months was R2 = 0.430. Evaluation of path coefficients clarifies the nature of the plausible causal relations. A direct effect of BASDAI on BASFI exists in the presence of the indirect effects (4.427, 95% CI 2.67, 6.185), indicating that a 1-point increase in disease activity at baseline predicts a 4.427-point increase in functional impairment at 12 months. Indirect effects of BASDAI on BASFI were also found. Inspection of path coefficients indicates that while arthritis helplessness (b = 1.312, 95% CI 0.024, 2.600) and PHQ-9 (b = 0.986, 95% CI 0.094, 1.879) are plausible mediators of the effect of BASDAI on BASFI, no such evidence emerges for arthritis internality (b = −0.174, 95% CI −0.579, 0.230). Over and above the direct effects of disease activity on functional limitations, this suggests that every 1-point increment in disease activity at baseline predicts a 1.312- and 0.986-point increase in functional limitations at 12 months that is plausibly transmitted via arthritis helplessness and depression, respectively. In summary, coefficient estimates suggest that partial mediation of the effect of disease activity on functional limitations via arthritis helplessness and depression is plausible. Inspection of these potential mediators of the effect of BASDAI on BASFI suggests that patients’ perceptions of loss of control with their pain and disease course, as well as depression, constitute treatment targets. Pilot estimates of the degree to which an effective treatment could potentially act to reduce functional limitations exist in the form of indirect effect estimates.
Fig. 1.
Effect of BASDAI at Time 1 (baseline) on BASFI at Time 3 (12 months) mediated by AHI helplessness, AHI internality and PHQ-9 at Time 2 (6 months).
Discussion
Psychological variables have received little attention in AS. While a recent cross-sectional analysis of 294 participants with AS by Brionez et al. [11, 12] demonstrated a strong correlation of psychological variables with self-reported AS functional limitations and disease activity, the nature of the design limited interpretation of the direction of these effects. This 12-month longitudinal analysis permitted examination of the effects of disease activity and psychological variables on functional limitations in a well-characterized cohort of 294 participants with AS.
Findings from our cohort indicated that previous disease activity affected functional limitations. Importantly, this effect appears to be partially mediated by psychological variables, particularly arthritis helplessness and depression. These data converge with results from an 18-month longitudinal study of 89 patients with AS reported by Martindale et al. [10], who demonstrated that disease status scores such as BASDAI, BASFI and BASMI in AS correlated significantly with anxiety, depression, internality and health status. A notable difference was that arthritis internality did not play a crucial role in functional disability in the present study.
A study of 236 RA patients by Escalante et al. [31] illustrated that 33% of the variability in the physical disability score was attributable to disease characteristics such as disease duration, articular signs and symptoms and performance-based functional limitations, while 20% was attributable to psychological status and depression. The current study advances and demonstrates a plausible mechanism by which these psychological variables interact and affect disability in AS. Our study suggests that interventions in AS patients that focus on ameliorating arthritis helplessness and managing depression may reduce functional limitations. While substantial research literature has documented the efficacy of psychological approaches for managing other conditions (e.g. OA and RA), such treatments have not been evaluated for AS at this juncture [32].
The strength of our study lies in its large well-characterized cohort, allowing us to observe clinical and psychological variables over a period of time and determine directional relationships. Although our hypothesized model identified possible therapeutic targets for clinical management, it should be noted that our approach may represent only one possible causal model of complex clinical and psychosocial interactions in AS. We selected to test two subscales of the AHI, internality (seven items) and helplessness (five items), which are more reliable and valid than the total AHI score [24]. Since the design of the study was observational, any plausible, causal relationships require evaluation in an RCT. Our study may have been limited by the loss of follow-up data due to the closure of one of the sites. However, the reason for the closure of that site was related to personnel changes and not to the subject population at that particular site. Generalizability may have been somewhat limited by a slightly lower rate of males in our study than the prevalence estimated for AS in the general population.
In summary, this study illustrated that AS disease activity contributed to functional limitations over time, and that psychological factors such as arthritis helplessness and depression also served as mediators of this relationship. Our data imply that psychological factors may be plausible targets for treatment to achieve better health outcomes in patients with AS. RCTs are necessary to further evaluate the clinical significance in treatment of these psychological factors and subsequent changes in health outcomes in patients with AS.

Acknowledgements
Funding: This work was supported by grants from the Australo-Anglo-American Spondylitis Consortium (TASC), US Department of Health and Human Services, NIH, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (P01-AR-052915-01) and the National Center for Research Resources (NCRR) General Clinical Research Centers (GCRC) (M01-RR00425) and the Intramural Research Program, NIAMS/NIH.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Khan M. Ankylosing spondylitis: clinical aspects. In: Calin A, Taurog J, editors. The spondyloarthritides. Oxford: Oxford University Press; 1998. pp. 27–40. [Google Scholar]
- 2.Ward MM. Quality of life in patients with ankylosing spondylitis. Rheum Dis Clin North Am. 1998;24:815–27. doi: 10.1016/s0889-857x(05)70043-0. x. [DOI] [PubMed] [Google Scholar]
- 3.Bakker C, van der Linden S, van Santen-Hoeufft M, Bolwijn P, Hidding A. Problem elicitation to assess patient priorities in ankylosing spondylitis and fibromyalgia. J Rheumatol. 1995;22:1304–10. [PubMed] [Google Scholar]
- 4.Anderson KO, Bradley LA, Young LD, McDaniel LK, Wise CM. Rheumatoid arthritis: review of psychological factors related to etiology, effects, and treatment. Psychol Bull. 1985;98:358–87. [PubMed] [Google Scholar]
- 5.Blalock SJ, DeVellis BM, DeVellis RF, Sauter SVH. Self-evaluation processes and adjustment to rheumatoid arthritis. Arthritis Rheum. 1988;31:1245–51. doi: 10.1002/art.1780311005. [DOI] [PubMed] [Google Scholar]
- 6.Schoenfeld-Smith K, Petroski GF, Hewett JE, et al. A biopsychosocial model of disability in rheumatoid arthritis. Arthritis Care Res. 1996;9:368–75. doi: 10.1002/1529-0131(199610)9:5<368::aid-anr1790090505>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Smith TW, Peck JR, Ward JR. Helplessness and depression in rheumatoid arthritis. Health Psychol. 1990;9:377–89. doi: 10.1037//0278-6133.9.4.377. [DOI] [PubMed] [Google Scholar]
- 8.Peck JR, Smith TW, Ward JR, Milano R. Disability and depression in rheumatoid arthritis. A multi-trait, multi-method investigation. Arthritis Rheum. 1989;32:1100–6. doi: 10.1002/anr.1780320908. [DOI] [PubMed] [Google Scholar]
- 9.McFarlane AC, Brooks PM. Determinants of disability in rheumatoid arthritis. Br J Rheumatol. 1988;27:7–14. doi: 10.1093/rheumatology/27.1.7. [DOI] [PubMed] [Google Scholar]
- 10.Martindale J, Smith J, Sutton CJ, Grennan D, Goodacre L, Goodacre JA. Disease and psychological status in ankylosing spondylitis. Rheumatology. 2006;45:1288–93. doi: 10.1093/rheumatology/kel115. [DOI] [PubMed] [Google Scholar]
- 11.Brionez T, Assassi S, Reveille JD, et al. Psychological correlates of self-reported functional limitation in patients with ankylosing spondylitis. Arthritis Res Ther. 2009;11:R182. doi: 10.1186/ar2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brionez TF, Assassi S, Reveille JD, et al. Psychological correlates of self-reported disease activity in ankylosing spondylitis. J Rheumatol. 2010;37:829–34. doi: 10.3899/jrheum.090476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gensler LS, Ward MM, Reveille JD, Learch TJ, Weisman MH, Davis JC., Jr Clinical, radiographic and functional difference between juvenile-onset and adult-onset ankylosing spondylitis: results from the PSOAS cohort. Ann Rheum Dis. 2008;67:233–7. doi: 10.1136/ard.2007.072512. [DOI] [PubMed] [Google Scholar]
- 14.Ward MM, Hendrey MR, Malley JD, et al. Clinical and immunogenetic prognostic factors for radiographic severity in ankylosing spondylitis. Arthritis Rheum. 2009;61:859–66. doi: 10.1002/art.24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward MM, Weisman MH, Davis JC, Jr, Reveille JD. Risk factors for functional limitations in patients with long-standing ankylosing spondylitis. Arthritis Rheum. 2005;53:710–7. doi: 10.1002/art.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 17.Goie The HS, Steven MM, van der Linden SM, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a comparison of the Rome, New York and modified New York criteria in patients with a positive clinical history screening test for ankylosing spondylitis. Br J Rheumatol. 1985;24:242–9. doi: 10.1093/rheumatology/24.3.242. [DOI] [PubMed] [Google Scholar]
- 18.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–91. [PubMed] [Google Scholar]
- 19.Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21:2281–5. [PubMed] [Google Scholar]
- 20.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 21.Lowe B, Kroenke K, Herzog W, Grafe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9) J Affect Disord. 2004;81:61–6. doi: 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicassio PM, Wallston KA, Callahan LF, Herbert M, Pincus T. The measurement of helplessness in rheumatoid arthritis. The development of the arthritis helplessness index. J Rheumatol. 1985;12:462–7. [PubMed] [Google Scholar]
- 24.Stein MJ, Wallston KA, Nicassio PM. Factor structure of the Arthritis Helplessness Index. J Rheumatol. 1988;15:427–32. [PubMed] [Google Scholar]
- 25.Muthén B, Muthén L. Mplus (version 5.2) Los Angeles, CA: Statmodel; 2008. [Google Scholar]
- 26.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 27.West SG, Aiken LS, Todd M. Probing the effects of individual components in multiple component prevention programs. Am J Commun Psychol. 1993;21:571–605. doi: 10.1007/BF00942173. [DOI] [PubMed] [Google Scholar]
- 28.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- 29.MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivar Behav Res. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test the significance of the mediated effect. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escalante A, del Rincón I. How much disability in rheumatoid arthritis is explained by rheumatoid arthritis? Arthritis Rheum. 1999;42:1712–21. doi: 10.1002/1529-0131(199908)42:8<1712::AID-ANR21>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 32.Dixon KE, Keefe FJ, Scipio CD, Perri LM, Abernethy AP. Psychological interventions for arthritis pain management in adults: a meta-analysis. Health Psychol. 2007;26:657–9. doi: 10.1037/0278-6133.26.3.241. [DOI] [PubMed] [Google Scholar]