Abstract
The κ-opioid receptor is a widely expressed G-protein-coupled receptor that has been implicated in biological responses to pain, stress, anxiety, and depression, and its potential as a therapeutic target in these syndromes is becoming increasingly apparent. However, the prototypical selective κ-opioid antagonists have very long durations of action that have been attributed to c-Jun N-terminal kinase (JNK) 1 activation in vivo. To test generality of this proposed noncompetitive mechanism, we used C57BL/6 wild type mice to determine the durations of antagonist action of novel κ-opioid receptor ligands and examined their efficacies for JNK1 activation compared with conventional competitive antagonists. Of the 12 compounds tested, 5 had long durations of action that positively correlated with JNK activation: RTI-5989-97 [(3S)-7-hydroxy-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-(2-methylpropyl]-2-methyl-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide], RTI-5989-194 [(3R)-7-hydroxy-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-(2-methylbutyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide], RTI-5989-241 [(3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-methoxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide)], nor-binaltorphimine (nor-BNI); and (3R)-7-hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic). Seven had short durations of action and did not increase phospho-JNK-ir: RTI-5989-212[(3R)-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-(2-methylpropyl]-7-methoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxamide], RTI-5989-240 [(3R)-7-hydroxy-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]methyl}-(2-methylpropyl]-3-methyl-1,2,3,4-tetrahydroisoquinoline-3-carboxamide], JSPA0658 [(S)-3-fluoro-4-(4-((2-(3,5-dimethylphenyl)pyrrolidin-1-yl)methyl)phenoxy)benzamide], JSPA071B [(S)-3-fluoro-4-(4-((2-(3,5-bis(trifluoromethyl)phenyl)pyrrolidin-1-yl)methyl)phenoxy)benzamide]. PF-4455242 [2-methyl-N-((2′-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine], PF-4455242 [2-methyl-N-((2′-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine], FP3FBZ [(S)-3-fluoro-4-(4-((2-(3-fluorophenyl)pyrrolidin-1-yl)methyl)phenoxy)benzamide], and naloxone. After long-acting antagonist treatment, pJNK-ir did not increase in mice lacking the κ-opioid receptor; increased pJNK-ir returned to baseline by 48 h after treatment; and a second challenge with nor-BNI 72 h after the first did not increase pJNK-ir. Long-lasting antagonism and increased phospho-JNK-ir were not seen in animals lacking the JNK1 isoform. These results support the hypothesis that the duration of action of small molecule κ-opioid receptor antagonists in vivo is determined by their efficacy in activating JNK1 and that persistent inactivation of the κ-receptor does not require sustained JNK activation.
Introduction
The κ-opioid peptide receptor (KOPr) is a Gi/o-coupled receptor activated by the endogenous dynorphin opioid peptides (Chavkin et al., 1982; Bruchas et al., 2010). Dynorphins are released in the brain during painful and stressful stimuli and have been shown to mediate the dysphoric components of the stress-response (Land et al., 2008; Carlezon et al., 2009). κ-Opioid antagonists promote stress resilience in animal models and might have therapeutic utility in the treatment of stress-induced mood disorders (e.g., anxiety and depression) and stress-induced increase in drug addiction risk in humans (McLaughlin et al., 2003a; Carlezon et al., 2009; Land et al., 2009). However, development of clinically useful κ-antagonists has been slow, partly because the initially synthesized κ-opioid antagonists that demonstrated a high degree of selectivity [e.g., norbinaltorphimine (nor-BNI) and JDTic] showed remarkably long durations of action in vivo (lasting more than 21 day after a single administration), despite having receptor interactions that are freely reversible in vitro (Portoghese et al., 1987; Horan et al., 1992; Butelman et al., 1993; Broadbear et al., 1994; Negus et al., 2002; Carroll et al., 2004). The basis for this long in vivo duration of action was not initially clear and might have been a consequence of slow clearance from the brain (pharmacokinetic), of metabolic conversion in vivo to a product that covalently bound to the receptor (metabolic), or through a long-lasting inactivation of the receptor signaling complex through an undefined mechanism.
In a prior study, we reported that some of the long-lasting κ-opioid antagonists were actually “collateral agonists” able to activate c-Jun N terminal kinase (JNK) through a κ-opioid receptor-dependent mechanism (Bruchas et al., 2007). Inhibition of JNK by 1,9-pyrazoloanthrone (SP600125) blocked the long-lasting antagonism by nor-BNI on the analgesic effects of the κ-opioid agonist trans-3,4-dichloro-N-methyl-N-(2-(1-pyrrolidinyl)-cyclohexyl)-benzeneacetamide (U50,488) without blocking the acute κ antagonism by nor-BNI (Bruchas et al., 2007; Melief et al., 2010). This finding was extended by subsequently showing that nor-BNI was a short-acting competitive κ-antagonist in mice genetically lacking the JNK-1 isoform (Melief et al., 2010). These results suggested that the long-duration of κ-opioid antagonists in vivo might be a consequence of JNK activation, rather than a pharmacokinetic or metabolic effect. Furthermore, results showing that nor-BNI was able to activate a signaling pathway through the κ-receptor without producing analgesia typical of κ-opioid agonists provided evidence supporting the novel concept of ligand-directed signaling (also known as “functional selectivity” and “biased agonism”) that may be a general property of G-protein-coupled receptors (Kenakin, 2007; Urban et al., 2007).
In addition to the original selective κ-antagonists nor-BNI and JDTic, several other compounds have recently been synthesized (Carroll et al., 2004; Buezo et al., 2010; Mitch et al., 2010; Grimwood et al., 2011). Comparison of these compounds shows that the κ-antagonists have significant structural differences, considerable structural flexibility, and few overall common features (Fig. 1). JDTic and its analogs have a phenol group that was found to be crucial for KOPr selectivity (Thomas et al., 2003; Carroll et al., 2006), but the other compounds lack this chemical feature. Thus, the substantial structural differences among the compounds having κ-receptor antagonist activity obscure any underlying pattern to explain or predict their activity. It would seem that differences in the receptor conformation induced by ligand binding can control JNK activation efficacies, but in the absence of crystal structural information for the receptor-ligand complex, JNK activation needs to be determined empirically.
Fig. 1.
Compound names and chemical structures of κ-opioid antagonists. The labels A to L are also used to designate the compounds presented in Fig. 5.
To assess the validity and generality of the hypothesis that long duration of κ antagonism was a consequence of JNK activation, we examined the properties of this wide range of available ligands reported to produce selective κ-opioid antagonism (Portoghese et al., 1987; Carroll et al., 2004, 2006; Cueva et al., 2009; Grimwood et al., 2011). In this analysis, we find that for 12 structurally different κ-antagonists, stimulation of phospho-JNK-immunoreactivity (ir), in spinal cords of antagonist-injected mice and in KOPr-transfected HEK293 cells treated with antagonist in vitro, is strongly correlated with in vivo duration of antagonism of U50,488-induced analgesia.
Materials and Methods
Reagents.
nor-BNI and U50,488H, a selective κ-opioid agonist, were provided by the National Institute on Drug Abuse (Bethesda, MD). JDTic and the RTI-5989 compounds were synthesized at the Research Triangle Institute (Research Triangle Park, NC). FP3FBZ was synthesized at Lilly Research Laboratories (Indianapolis, IN). Structurally related analogs JSPA0658 [also known as LY2456302 (Buezo et al., 2010)] and JSPA071B were synthesized by McLean Hospital (Belmont, MA) and isolated as HCl salts. PF-4455242 was synthesized by Pfizer (Groton, CT). All compounds used in this study had confirmed purities >95%. FP3FBZ, JSPA0658, and JSPA071B were dissolved in 5% dimethyl sulfoxide and 20% polyethoxylated castor oil (Cremophor; BASF, Ludwigshafen, Germany), a vehicle that we found to have no effects in the analgesia or pJNK assays used (Bruchas et al., 2007; Melief et al., 2010). All other compounds were dissolved in 0.9% NaCl. All drugs were administered at a volume of 10 ml/kg. Other reagents were from Sigma-Aldrich (St Louis, MO).
κ-Opioids Used in This Study.
Naloxone, a nonselective opioid receptor antagonist, and nor-BNI (Portoghese et al., 1987) were used. The compounds provided by the Research Triangle Institute (RTI) include JDTic (Carroll et al., 2004) and methylated analogs of JDTic: RTI-5989-97, (3S)-7-hydroxy-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-(2-methylpropyl]-2-methyl-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide; RTI-5989-194, (3R)-7-hydroxy-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-(2-methylbutyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide;RTI-5989-212, (3R)-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-(2-methylpropyl]-7-methoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxamide; RTI-5989-240, (3R)-7-hydroxy-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]methyl}-(2-methylpropyl]-3-methyl-1,2,3,4-tetrahydroisoquinoline-3-carboxamide; and RTI-5989-241, (3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-methoxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide) (Thomas et al., 2004; Cueva et al., 2009; Beardsley et al., 2010). The diaryl ethers include FP3FBZ [(S)-3-fluoro-4-(4-((2-(3-fluorophenyl)pyrrolidin-1-yl)methyl)phenoxy)benzamide] (Mitch et al., 2010); JSPA0658 [(S)-3-fluoro-4-(4-((2-(3,5-dimethylphenyl)pyrrolidin-1-yl)methyl)phenoxy)benzamide] [previously referred to as LY-DMPF (Peters et al., 2011); also known as LY2456302 (Buezo et al., 2010)]; and JSPA071B, [(S)-3-fluoro-4-(4-((2-(3,5-bis(trifluoromethyl)phenyl)pyrrolidin-1-yl)methyl)phenoxy)benzamide]. PF-4455242 [2-methyl-N-((2′-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine] (Grimwood et al., 2011) was used.
Animals.
Male C57BL/6 mice (20–25g) were purchased from Charles River Laboratories (Wilmington, MA). κ-opioid receptor knockout mice [KOPr(−/−)] were generated by homologous recombination as described previously (Clarke et al., 2002). Breeding pairs of JNK1(−/−) mice were initially purchased from The Jackson Laboratory–West (Sacramento, CA) on a C57BL/6 background. KOPr(−/−) and JNK1(−/−) mice were bred by heterozygous crossing within the University of Washington vivarium under specific pathogen-free conditions. Mice were group-housed and kept on a 12-h light/dark cycle with food and water available ad libitum. Animal procedures were approved by the Animal Care and Use Committee of the University of Washington and conform to the guidelines on the care and use of animals promulgated by the National Institutes of Health (Institute of Laboratory Animal Resources, 1996).
Cell Culture.
HEK293 cells were grown as described previously (McLaughlin et al., 2003b; Bruchas et al., 2007) in Dulbecco's modified Eagle's medium/nutrient mixture F-12 with l-glutamine and 15 mM HEPES (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin at 37°C and 5% CO2. HEK293 cells transfected with green fluorescent protein tagged KOPr-GFP were maintained in the above media with an additional 200 μg/ml G418 to maintain selective pressure. Untransfected HEK293 cells for control experiments were grown in the absence of G418. Prior studies have established that the GFP tag on the C-terminal domain of the κ-opioid receptor does not block agonist-induced activation of G-protein signaling, ERK1/2 or p38 mitogen-activated protein kinase activation, agonist-induced receptor internalization, or JNK activation (McLaughlin et al., 2003b; Bruchas et al., 2007).
Analgesia.
Antinociceptive responses were measured using the warm-water tail-withdrawal assay as described previously (Vaught and Takemori, 1979; Melief et al., 2010). In brief, the latency to tail withdrawal after immersion in a 52.0°C water bath was measured before administration of 15 mg/kg i.p. U50,488H and 30 min after. Animals were tested for normal U50,488H-induced analgesic responses one day before administration of κ-opioid antagonists (10 mg/kg i.p., except diaryl esters, which were also given at 50 mg/kg i.p.) and were then retested for U50,488H-induced analgesia 60 min, 1 day, and 3, 7, 14, 21, and 28 days after administration of κ-opioid antagonists. Repeated administration of U50,488 by this schedule did not result in analgesic tolerance detected at time of assay. Animals administered naloxone (10 mg/kg i.p.) were injected with U50,488H 15 min after naloxone administration instead of 60 min because of the short duration of naloxone action.
Immunoblotting.
For spinal cord samples, mice were injected intraperitoneally with drug at the doses and times indicated, tissue was dissected 60 min after injection and was homogenized in lysis buffer (50 mM Tris-HCl, 300 mM NaCl, 1 mM EDTA, 1 m Na3VO4, 1 mM NaF, 10% glycerol, 1:100 phosphatase inhibitor mixture set 1 (Calbiochem, San Diego, CA), and 1:100 protease inhibitor mixture set 1 (Calbiochem) using a 2-ml Dounce homogenizer. For HEK293 lysate samples, KOPr-GFP expressing cells were treated with drug indicated for 5 min (pERK experiment), 15 min (naloxone samples), or 60 min and then homogenized in lysis buffer. Homogenates were sonicated for 5 s (setting 4, Fisher Sonic Dismembrator; Thermo Fisher Scientific, Waltham, MA). All lysates were centrifuged (14,000g, 20 min, 4°C) (model 5418; Eppendorf AG, Hamburg, Germany), and protein concentration was determined using bicinchoninic colorimetric assay (Thermo Fisher Scientific) with bovine serum albumin (BSA) standards. Totals of 15 μg (spinal cord samples) or 25 μg (cell lysates) protein for each sample were heated at 100°C for 5 min in Laemmli buffer before loading onto nondenaturing 10% bisacrylamide precast gels (Invitrogen) and running at 120V for 2 h. Blots were transferred to nitrocellulose (Whatman, Maidstone, Kent, UK) for 2 h at 30 V. The nitrocellulose was then blocked with 5% BSA/Tris-buffered saline (TBS) (60 min), and incubated overnight at 4°C in phospho-JNK (Thr-183/Tyr-185) rabbit antibody or phospho-ERK1/2 (Thr-202/Tyr-204) diluted 1:1000 in 5% BSA/TBS (Cell Signaling Technology, Danvers, MA). After overnight incubation, the blots were washed with TBS and 0.1% Triton X-100 (TBST) and incubated for 60 min at room temperature in anti-rabbit IRDye800 diluted 1:10,000 in a 1:1 mixture of 5% milk/TBST and Li-Cor blocking buffer (Li-Cor Biosciences, Lincoln, NE). The blots were washed with TBST and then scanned, and relative intensities of fluorescent bands were quantified using the Odyssey Infrared Imaging System (Li-Cor Biosciences).
Data Analysis.
Statistical significance for behavioral assays was determined by two-way ANOVA (time × treatment) followed by Bonferroni multiple-comparison post hoc tests. Statistical significance for immunoblot assays was determined by one-way ANOVA with Bonferroni post hoc tests.
Results
Activation of JNK by κ-Opioid Antagonists.
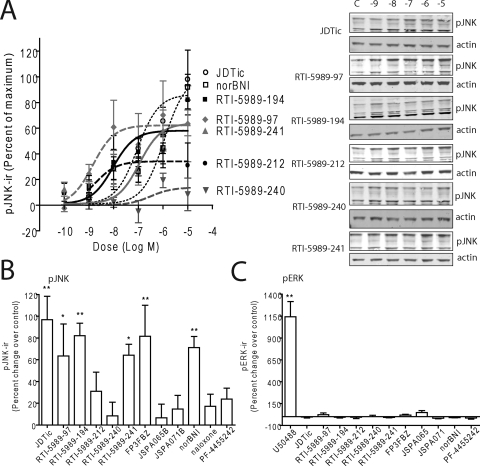
To determine the efficacy of these compounds in increasing pJNK-ir in vitro, we applied compounds at different concentrations to KOPr-GFP expressing HEK293 cells (McLaughlin et al., 2003b; Bruchas et al., 2007). Cells were harvested and lysed after 60 min, and pJNK-ir intensities were examined by Western blot analysis. Dose response curves were generated for the RTI compounds using JDTic and nor-BNI as positive controls (Fig. 2, A and B). RTI-5989-97, RTI-5989-194, and RTI-5989-241 significantly increased phospho-JNK-ir at 10 μM (one-way ANOVA with Bonferroni post hoc comparisons), whereas RTI-5989-212 and RTI-5989-240 did not (Fig. 2, A and B). Treatment with JSPA0658, JSPA071B, and PF-4455242 at 10 μM also did not significantly increase pJNK-ir, whereas FP3FBZ (at 10 μM) produced robust JNK activation under these conditions (Fig. 2B). In comparison, none of the antagonist compounds studied effectively increased pERK1/2-ir in KOPr-GFP expressing HEK293 cells, whereas U50,488H-produced robust ERK activation (Fig. 2C). This finding suggested that these antagonist compounds did not have partial agonist activities in this system.
Fig. 2.
Activation of JNK in HEK293 cells by κ-opioid antagonists. A, dose response curves generated from pJNK-ir quantification of western blots for JDTic, RTI-5989-97, RTI-5989-1 RTI-5989-94, RTI-5989-212, RTI-5989-240, RTI-5989-241, and nor-BNI (Western blot not shown). All compounds were administered for 60 min before lysing the cells. B, quantification of pJNK-ir from Western blots of cell lysates from cells treated with 10 μM concentrations of each compound for 60 min. C, quantification of pERK-ir from Western blots of cell lysates from cells treated with 10 μM concentrations of each compound or U50,488H for 5 min. Results were compared with vehicle controls and analyzed by one-way ANOVA with Bonferroni post hoc comparisons (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Long-Lasting Effects of κ-Opioid Antagonists.
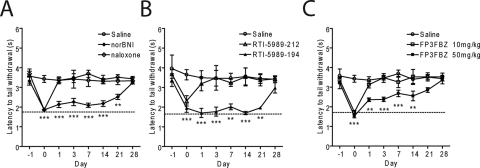
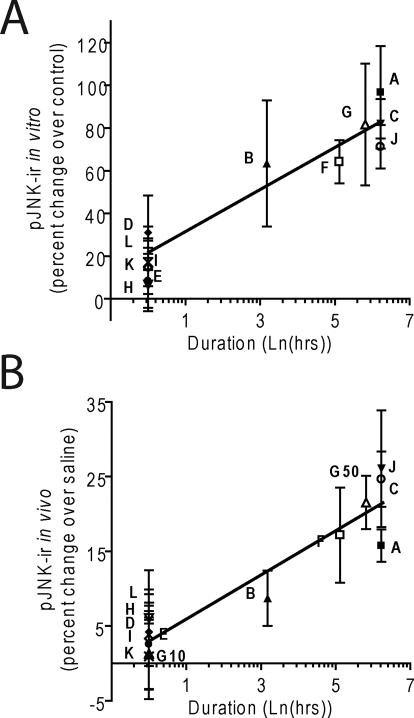
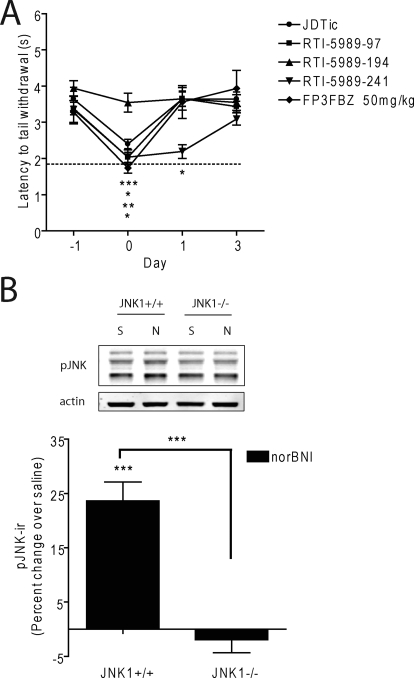
We showed previously that κ-opioid antagonists nor-BNI and JDTic increase pJNK-ir and produce persistent antagonist effects for weeks after a single in vivo administration (Bruchas et al., 2007). In this study, we next examined the duration of action for each compound using the warm water tail-withdrawal assay (Vaught and Takemori, 1979) (Fig. 3; Table 1). Animals were initially challenged with U50,488H (15 mg/kg i.p.) 24 h before antagonist (day −1), and latency of tail withdrawal from a 52°C water bath was measured. On day 0, animals received the indicated κ-opioid antagonist (10 mg/kg i.p.; for FP3FBZ, 10 and 50 mg/kg i.p.) 60 min (15 min for naloxone) before the second U50,488H administration and tail-withdrawal testing. The efficacy of U50,488H to induce analgesia was then repeatedly measured up to 28 days afterward. All of the antagonist compounds significantly reduced the U50,488H-induced analgesia nearly to baseline responses (dashed line) when challenged the same day (one-way ANOVA with Bonferroni post hoc comparisons) (Fig. 3; Table 1). As documented previously (Bruchas et al., 2007), nor-BNI blocked U50,488H-induced analgesia for 21 days compared with saline controls, whereas naloxone was effective only on day 0 (Fig. 3A). The effects of RTI-5989-97 persisted for 1 day before returning to control levels (Table 1). RTI-5989-194 and RTI-5989-241 showed long-lasting antagonistic effects at 21 and 7 days' duration, respectively, whereas RTI-5989-212 and RTI-5989-240 were only effective on day 0 (Fig. 3B; Table 1). FP3FBZ administered at 10 mg/kg i.p. was only effective acutely but showed long-lasting effects persisting up to 14 days when administered at 50 mg/kg i.p. (Fig. 3C). Because JSPA0658 and JSPA071B are analogs of FP3FBZ, we also tested these compounds at both doses (10 and 50 mg/kg i.p.). Consistent with the absence of JNK activation induced by these compounds in HEK293 cells and with previous results (Peters et al., 2011), both JSPA0658 and JSPA071B were short-acting at both doses tested (Table 1). PF-4455242 was also short-acting (Table 1). These results correlate well with the efficacies of each compound at activating JNK in vitro (slope, 9.84 ± 1.64; p = 0.0093; r2 = 0.92) (Fig. 5A).
Fig. 3.
Duration of action of κ-opioid antagonists. Duration of action was measured by antagonism of U50,488H induced analgesia at different times after a single antagonist injection on day 0 (10 mg/kg i.p.; FP3FBZ was also given at 50 mg/kg i.p.). The dashed line represents baseline (pre-U50,488) tail withdrawal latency responses. Control mice received saline (10 ml/kg i.p.). Animals were challenged by 15 mg/kg U50,488 on days 1, 3, 7, 14, 21, and 28 after injection. Compounds shown are naloxone (NLX) and nor-BNI (A), RTI-5989-212 and RTI-5989-194 (B), and FP3FBZ (10 mg/kg) and FP3FBZ (50 mg/kg) (C). All compounds were statistically compared with saline controls. Data analyzed by two-way ANOVA with Bonferroni post hoc comparisons (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
TABLE 1.
Duration of action of kappa opioid antagonists
Data were measured as in Fig. 3 and are presented as latency to tail withdrawal (seconds ± S.E.M.) for each time point measured. Animals were given each compound at 10 mg/kg i.p. (and 50 mg/kg i.p. as indicated) 60 min before U50,488H challenge on day 0 (15 min for naloxone). Duration is defined as the last time point at which treated animals differed significantly from saline controls.
| Compound | Latency to Tail Withdrawal |
Duration | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day −1 | Day 0 | Day 1 | Day 3 | Day 7 | Day 14 | Day 21 | Day 28 | ||
| s | day | ||||||||
| Saline | 3.58 ± 0.36 | 3.43 ± 0.18 | 3.36 ± 0.18 | 3.47 ± 0.23 | 3.69 ± 0.18 | 3.40 ± 0.21 | 3.52 ± 0.13 | 3.44 ± 0.12 | N.A. |
| JDTic | 3.47 ± 0.46 | 2.19 ± 0.14*** | 2.34 ± 0.06*** | 2.38 ± 0.23*** | 2.32 ± 0.15*** | 2.35 ± 0.13*** | 2.55 ± 0.16** | 3.27 ± 0.09 | 21 |
| RTI-5989-97 | 3.74 ± 0.27 | 2.17 ± 0.13*** | 2.38 ± 0.27* | 3.33 ± 0.36 | 3.19 ± 0.23 | 3.39 ± 0.39 | 3.79 ± 0.23 | 3.68 ± 0.32 | 1 |
| RTI-5989-194 | 3.38 ± 0.33 | 1.95 ± 0.21*** | 1.71 ± 0.23*** | 1.78 ± 0.23*** | 2.02 ± 0.15** | 1.71 ± 0.08*** | 1.97 ± 0.03** | 2.98 ± 0.27 | 21 |
| RTI-5989-212 | 3.68 ± 0.22 | 2.88 ± 0.58** | 3.19 ± 0.36 | 3.75 ± 0.52 | 3.15 ± 0.16 | 3.84 ± 0.44 | 3.37 ± 0.28 | 3.44 ± 0.11 | <1 |
| RTI-5989-240 | 4.11 ± 0.78 | 2.49 ± 0.27** | 3.58 ± 0.17 | 3.51 ± 0.42 | 3.51 ± 0.34 | 3.42 ± 0.27 | 3.25 ± 0.06 | 3.42 ± 0.24 | <1 |
| RTI-5989-241 | 3.40 ± 0.30 | 1.74 ± 0.16*** | 2.06 ± 0.20** | 1.74 ± 0.19*** | 1.83 ± 0.16*** | 2.59 ± 0.20 | 3.40 ± 0.38 | 3.07 ± 0.06 | 7 |
| FP3FBZ | |||||||||
| 10 mg/kg | 3.37 ± 0.16 | 1.73 ± 0.09*** | 3.13 ± 0.16 | 3.49 ± 0.12 | 3.26 ± 0.29 | 3.53 ± 0.38 | 3.53 ± 0.21 | 3.53 ± 0.20 | <1 |
| 50 mg/kg | 3.21 ± 0.29 | 1.53 ± 0.09*** | 2.36 ± 0.09** | 2.38 ± 0.11*** | 2.69 ± 0.17*** | 2.55 ± 0.25** | 2.86 ± 0.12 | 3.37 ± 0.19 | 14 |
| JSPA0658 | |||||||||
| 10 mg/kg | 3.37 ± 0.03 | 1.60 ± 0.13*** | 3.47 ± 0.17 | 3.05 ± 0.03 | N.A. | N.A. | N.A. | N.A. | <1 |
| 50 mg/kg | 3.67 ± 0.29 | 1.38 ± 0.10*** | 3.28 ± 0.47 | 3.33 ± 0.36 | N.A. | N.A. | N.A. | N.A. | <1 |
| JSPA071B | |||||||||
| 10 mg/kg | 3.40 ± 0.21 | 2.21 ± 0.17** | 2.83 ± 0.15 | 3.28 ± 0.11 | N.A. | N.A. | N.A. | N.A. | <1 |
| 50 mg/kg | 3.95 ± 0.31 | 1.67 ± 0.09*** | 3.04 ± 0.16 | 3.40 ± 0.47 | N.A. | N.A. | N.A. | N.A. | <1 |
| Nor-BNI | 3.32 ± 0.21 | 1.86 ± 0.06*** | 2.13 ± 0.16*** | 2.26 ± 0.19*** | 2.09 ± 0.11*** | 2.18 ± 0.15*** | 2.52 ± 0.13** | 3.24 ± 0.07 | 21 |
| Naloxone | 3.69 ± 0.13 | 1.86 ± 0.09*** | 3.36 ± 0.12 | 3.42 ± 0.45 | 3.37 ± 0.32 | 3.32 ± 0.13 | 3.33 ± 0.19 | 3.33 ± 0.16 | <1 |
| PF-4455242 | 3.63 ± 0.36 | 1.91 ± 0.24*** | 3.11 ± 0.07 | 3.30 ± 0.22 | N.A. | N.A. | N.A. | N.A. | <1 |
N.A., not applicable.
p < 0.05.
p < 0.01.
p < 0.001.
Fig. 5.
Efficacy of JNK activation correlates with duration of action. Quantification of pJNK-ir from Western blots was plotted as a function of the natural log of duration of antagonism for each compound. Natural log was used to transform the x values because the recovery of response after receptor inactivation follows the asymptotic kinetics of receptor expression approach to equilibrium. Letters correspond to labels in Fig. 1 (G10, 10 mg/kg FP3FBZ; G50, 50 mg/kg FP3FBZ). Results were analyzed by linear regression. Duration of action was correlated with JNK activation as determined in HEK293 cells (slope, 9.84 ± 1.64; p = 0.009; r2 = 0.92) (A) and spinal cord homogenates (slope, 2.94 ± 0.44; p = 0.0069; r2 = 0.94) (B).
Activation of JNK by Long-Lasting κ-Opioid Antagonists.
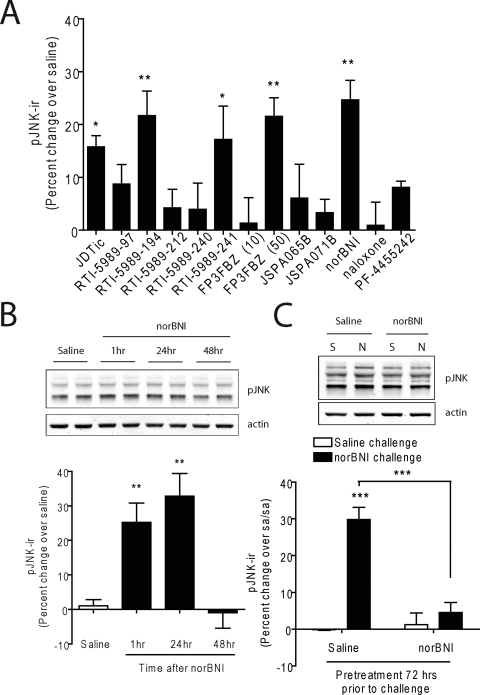
To determine whether pJNK-ir responses observed in vitro were also evident in the animal model, we examined the efficacy of each compound in activating JNK in C57BL/6 mice by isolating the spinal cords of mice 60 min (15 min for naloxone) after drug administration. nor-BNI, FP3FBZ (50 mg/kg), RTI-5989-194, RTI-5989-241, and JDTic each significantly increased pJNK-ir compared with saline-injected controls (one-way ANOVA with Bonferroni post hoc comparisons) (Fig. 4A). In contrast, RTI-5989-97, RTI-5989-212, RTI-5989-240, FP3FBZ (10 mg/kg), JSPA0658, JSPA071B, and PF-4455242 did not significantly increase pJNK-ir in spinal cord. Comparing the relative activities of the 12 κ compounds, we observed a highly significant correlation between the duration of antagonist action in the analgesia assay and the increase in pJNK-ir induced in the mouse spinal cord (slope, 2.94 ± 0.44; p = 0.0069; r2 = 0.94) (Fig. 5B).
Fig. 4.
Activation of JNK in spinal cord by κ-opioid antagonists. A, quantification of pJNK-ir from Western blots of spinal cord homogenates taken from animals treated with each compound for 60 min (15 min for NLX). Each compound was administered at 10 mg/kg i.p., except that FP3FBZ was administered at 10 mg/kg i.p. (FP3FBZ 10) and 50 mg/kg i.p. (FP3FBZ 50). B, quantification of pJNK from spinal cords of animals treated with a nor-BNI time course. Animals were administered saline or nor-BNI and tissue was harvested at 1, 24, or 48 h. Data were compared with saline controls and analyzed by one-way ANOVA with Bonferroni post hoc comparisons (*, p < 0.05; **, p < 0.01). C, quantification of pJNK from spinal cords of animals pretreated with saline or nor-BNI (10 mg/kg i.p.) 72 h before challenge with saline (S) or nor-BNI (10 mg/kg i.p.) (N) 1 h before dissection. Data are presented as percentage change over the saline/saline group and is analyzed by two-way ANOVA with Bonferroni post hoc comparisons (***, p < 0.001).
We have previously shown that the long-lasting effects of nor-BNI are not due to sequestration of the compound in the lipid bilayer and sustained receptor occupancy in vivo (Bruchas et al., 2007). To determine whether these effects were caused by persistent activation of JNK in the absence of continued receptor occupancy, we measured pJNK-ir in spinal cord homogenates 1, 24, and 48 h after treatment with nor-BNI (10 mg/kg i.p.). Expression of pJNK-ir was significantly increased over saline controls 1 and 24 h after administration but returned to baseline by 48 h (one-way ANOVA with Bonferroni post hoc comparisons) (Fig. 4B). These data indicate that persistent JNK activation is not responsible for long durations of action of these compounds (which can persist for 21 days).
After long-acting antagonist treatment, κ-receptor agonists did not evoke a behavioral or [35S]GTPγS response (Melief et al., 2010), but whether the JNK activation by subsequent antagonist treatment was also blocked was not clear. To determine whether receptor inactivation by nor-BNI precludes further antagonist-stimulated JNK responses, animals were pretreated with saline or nor-BNI (10 mg/kg i.p.) 72 h before a second challenge with saline or nor-BNI (10 mg/kg i.p.). Spinal cord homogenates dissected 1 h after the second challenge showed that nor-BNI significantly increased pJNK-ir in saline pretreated animals, but it had no effect in nor-BNI–pretreated animals (two-way ANOVA with Bonferroni post hoc comparisons) (Fig. 4C). These data indicate that the persistent inactivation of the κ-receptor does not require sustained JNK activation and that both agonist and collateral agonist responses were persistently blocked.
Long Duration of Action Requires the JNK1 Isoform.
We have previously shown that nor-BNI does not have long-lasting effects in mice lacking the JNK1 isoform [JNK1(−/−)] (Melief et al., 2010). To determine whether the other long-acting antagonists also required activation of the JNK1 isoform, RTI-5989-97, RTI-5989-194, RTI-5989-241, FP3FBZ (50 mg/kg i.p.), and JDTic were administered to JNK1(−/−) mice as described for previous experiments. RTI-5989-194 was ineffective as an antagonist in mice lacking JNK1 (Fig. 6A). The other compounds remained effective competitive antagonists (one-way ANOVA with Bonferroni post hoc comparisons), but their effects did not persist longer than 24 h. We measured pJNK-ir in response to nor-BNI in the spinal cords of JNK1(−/−) mice and found that knockout of the JNK1 isoform completely prevented the increase of pJNK-ir induced by nor-BNI compared with wild-type littermate controls (Fig. 6B). Together these results indicate that JNK1 activation has a critical role in the persistent inactivation of the KOPr produced by long-lasting antagonists.
Fig. 6.
Long duration of action is dependent on the JNK1 isoform. A, long-lasting κ-opioid antagonists [JDTic, RTI-5989-97, RTI-5989-194, RTI-5989-241, and FP3FBZ (50 mg/kg)] were administered to JNK1(−/−) mice as in Fig. 3. Duration of antagonism was determined by blockade of U50-488H induced analgesia. Results were analyzed by two-way ANOVA with Bonferroni post hoc comparisons (*, p < 0.05; **, p < 0.01; ***, p < 0.001). B, quantification of pJNK-ir from spinal cord homogenates of JNK1(+/+) and JNK1(−/−) mice treated with nor-BNI for 60 min compared with saline controls. Data analyzed by one-way ANOVA with Bonferroni post hoc comparisons (*, p < 0.05).
JNK Activation Requires KOPr.
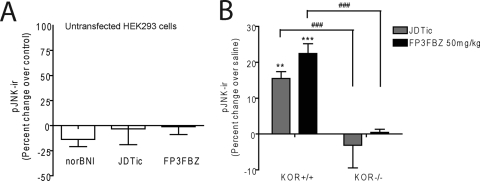
To ensure that the JNK activation we observed was dependent on KOPr-mediated effects, we determined the efficacy of JNK activation in untransfected HEK293 cells. JDTic, FP3FBZ, and nor-BNI, examples from each family of compounds that elicited robust JNK activation in KOPr-GFP–expressing cells, were applied to untransfected HEK293 cells (10 μM) for 60 min. All were unable to significantly activate JNK in the untransfected cells (Fig. 7A). In addition, to determine the necessity of KOPr in JNK activation in vivo, mice lacking functional KOPr [KOPr(−/−)] were administered saline, JDTic (10 mg/kg i.p.), or FP3FBZ (50 mg/kg i.p.), and spinal cords were dissected 60 min later. As has been previously seen with nor-BNI (Bruchas et al., 2007), neither JDTic nor FP3FBZ significantly increased pJNK-ir in KOPr(−/−) mice (Fig. 7B).
Fig. 7.
JNK activation requires functional KOPr. A, untransfected HEK293 cells were treated with nor-BNI, JDTic, or FP3FBZ (10 μM) for 60 min. pJNK-ir was determined by Western blot of cell lysates. Results were analyzed by one-way ANOVA and were insignificant. B, KOPr(+/+) and KOPr(−/−) mice were administered saline, JDTic (10 mg/kg i.p.), or FP3FBZ (50 mg/kg i.p.) and spinal cord homogenates were prepared 60 min later. pJNK-ir was determined by Western blot. Results were analyzed by two-way ANOVA with Bonferroni post hoc comparisons. [**, p < 0.01; ***, p < 0.001 compared with saline controls; ###, p < 0.001 compared with KOPr(+/+) controls].
Discussion
In this study, we determined the durations of action of several novel κ-opioid antagonists and compared them with the efficacy of each compound at activating JNK. We found 1) that κ-opioid antagonists that effectively activate JNK in KOPr-GFP–transfected HEK293 cells and mouse spinal cord also have longer durations of analgesic antagonist action than those that are classic competitive antagonists and 2) that JNK1 is the isoform specifically required for these long-lasting effects. The in vitro data suggest that activation of JNK is a direct consequence of KOPr activation by long-acting antagonists in vivo, but how JNK activation results in κ-receptor inactivation is not yet clear. The long duration was not caused by persistent effect, because JNK activation in spinal cord was not evident 48 h after nor-BNI treatment. In addition, residual antagonist would not be able to produce sustained JNK activation, because a second challenge with nor-BNI 72 h after the first did not increase pJNK-ir.
Although this will require further molecular characterization, the presumed actions of long-acting κ-antagonists are better described as “collateral” agonism than as “inverse” agonism (Bruchas et al., 2007). Although both collateral and inverse agonists would produce effects that oppose the actions of the conventional agonist, they would do this by distinctly different molecular mechanisms. Unlike neutral antagonists that competitively block the agonist binding site, collateral agonists are thought to activate an alternative signaling pathway, resulting in a distinctly different molecular action (e.g., JNK-dependent receptor inactivation) than conventional agonists (e.g., Gβγ-dependent effector activation), whereas inverse agonists are thought to act by reducing constitutive receptor activity (e.g., inhibition of basal G-protein activation in the absence of agonist) (Kenakin, 2004; Cotecchia, 2007). In addition, like neutral antagonists, inverse agonist effects would presumably be terminated by drug dissociation from the receptor, whereas JNK-dependent receptor inactivation would presumably persist after ligand dissociation. Although these proposed distinctions require additional experimental definition, based on this conceptual scheme, the actions of long-duration κ-antagonists are more consistent with the emerging concepts of “ligand-directed signaling” as reviewed by Urban et al., (2007).
Unexpectedly, no obvious structural homology seems to be required for JNK activation by κ-opioid antagonists, because JDTic, RTI-5989-194, and RTI-5989-241 were long-lasting, whereas their closely related analogs RTI-5989-212 and RTI-5989-240 were not. Likewise, nor-BNI, which is structurally unrelated to the RTI compounds, also has a long duration of action. Long duration of action is also apparently unrelated to receptor binding affinity, potency, or clearance of the compounds, because the Kd values for long and short-acting compounds are similar (Peters et al., 2011), and the persistence of compounds in the brain is not correlated with duration of antagonist action (Beardsley et al., 2010).
It is noteworthy that although some compounds are incapable of activating JNK at the doses tested, for some ligands, the activation of JNK and subsequent inactivation of the receptor seem to be dose-dependent. Our in vitro data indicate that the novel κ-opioid antagonist FP3FBZ is capable of activating JNK at a very high concentration, as evidenced by a robust increase in pJNK-ir in HEK293 cells after a 10 μM dose for 60 min. In vivo, however, we found that a moderate dose of FP3FBZ (10 mg/kg) functionally blocks KOPr-induced analgesia without activating JNK or showing persistent effects (Fig. 3C), which is consistent with previous studies (Peters et al., 2011). Based on our in vitro data, we examined the effects of this compound at a much higher dose. When administered at 50 mg/kg, FP3FBZ does activate JNK and is long-lasting, indicating a threshold or low efficacy for JNK activation. This property was unique for FP3FBZ, because the structurally related compounds JSPA0658 and JSPA071B do not have the same dose-dependent effects. It is possible that FP3FBZ is no longer selective for KOPr at the high dose, but as duration of action was measured as blockade of U50,488H-induced analgesia (which requires KOPr), we consider the FP3FBZ effects to be KOPr-mediated. The tight correlation between pJNK-ir in spinal cord homogenates and duration of antagonistic effects that we observed indicates that the relative efficacy of each compound at activating JNK determines how long the antagonist effect persists. For compounds that activate this system, these data point to a receptor occupancy model, in which compounds producing robust activation of JNK inactivate the KOPr system entirely. In this case receptor synthesis may be required for reinstatement of KOPr function (Bruchas et al., 2007). In comparison, compounds that weakly or moderately activate JNK, such as RTI-5989-97, impair only a subset of the available KOPrs, and recovery of the KOPr system is faster. This model of receptor occupancy in determining receptor effects and MAPK activation by different ligands has been shown before in different systems (Nandagopal et al., 2001; Huwiler et al., 2009).
We did note an unexpected dissociation between RTI-5989-97 induced JNK activation observed in vitro and the results we observed in vivo. This compound produced significant JNK phosphorylation in KOPr-GFP expressing HEK293 cells, and was more potent than the other RTI compounds in doing so (Fig. 2, A and B). In contrast, phosphorylation of JNK in the spinal cord was not as robust as that induced by JDTic or RTI-5989-194, and the antagonist effects did not persist as long (1 day versus 21 days). A possible explanation for this divergence is a difference in the cohort of signaling proteins anchored to the receptor in our cultured cells compared with spinal neuronal cells. As previously stated, JNK associates with a number of different anchoring proteins, which in turn couple with different signaling targets (Bogoyevitch and Kobe, 2006). A possible explanation would include more rapid degradation of the compound in vivo, or poor penetration of the drug across the blood-brain barrier, although its evident ability to antagonize KOPr effects in vivo would seem to exclude this latter explanation. Nevertheless, the lack of robust JNK activation in spinal cord is consistent with the short duration of RTI-5989-97 antagonism in vivo.
Another unexpected result was the effect of RTI-5989-194 in JNK1(−/−) animals. Contrary to results found with the other long-lasting compounds, RTI-5989-194 was not effective as an antagonist in mice lacking functional JNK1. This was surprising, because our data, as well as those from a previous study (Beardsley et al., 2010), clearly show that RTI-5989-194 is an antagonist of KOPr-induced behaviors in wild-type animals. That the knockout of JNK1 could prevent antagonist effects indicates that RTI-5989-194 is not a simple competitive antagonist but requires JNK activation to block activity at the KOPr.
Although this study does not address the mechanisms by which each of these compounds activate JNK or by which JNK inactivates the receptor, we have previously shown that the nor-BNI–induced increases in JNK phosphorylation required KOPr expression and were blocked by 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole (Gö6976; a PKC selective inhibitor), suggesting that nor-BNI binding to the κ-opioid receptor activates protein kinase C, which subsequently increases JNK phosphorylation (Melief et al., 2010). The details of the pathways have not yet been resolved, and how JNK activation by long-acting κ-antagonists results in prolonged receptor inactivation is not yet clear. The drug does not persist in the tissue (Bruchas et al., 2007), and JNK activation is not evident 48 h after nor-BNI treatment (current study). κ-Receptor binding sites are not reduced in number or agonist affinity by nor-BNI (Horan et al., 1992; Bruchas et al., 2007), yet JNK activation by nor-BNI blocks agonist stimulation of guanosine 5′-O-(3-[35S]thio)triphosphate binding to spinal cord membranes isolated from mice administered nor-BNI 7 days before tissue harvest (Melief et al., 2010). A parsimonious explanation is that JNK activation phosphorylates a substrate that tightly binds to the κ-receptor and sterically blocks G-protein association, although the hypothetical JNK substrate has not been identified, and alternative mechanisms have yet to be excluded. There are a large number of known JNK targets that couple to each of the individual anchoring proteins that bind JNK and have a variety of downstream effects (Davis, 2000; Bogoyevitch and Kobe, 2006; Engström et al., 2010). Alternatively, there are numerous regulatory proteins that are known to sequester or inactivate G-proteins, a number of which have been identified as regulating morphine signaling in the μ-opioid receptor system, in some cases in a protein kinase C-dependent manner (Garzón et al., 2005a,b; Rodríguez-Muñoz et al., 2006; Ajit et al., 2007).
κ-Opioid antagonists are becoming recognized as potentially important therapeutic tools in managing disorders of stress, anxiety, and depression (Land et al., 2008; Carlezon et al., 2009). In animal models, such compounds have been shown to be efficacious at preventing dysphoria and relapse to drug-seeking in animals exposed to drugs of abuse (Land et al., 2009; Beardsley et al., 2010). As the clinical development of these compounds advances, understanding the mechanism of action by which they exert their effects will become increasingly important. At present, only two κ-opioid antagonists, JDTic and RTI-5989-194, are known to be effective when administered orally (Beardsley et al., 2010), and both of these are long-lasting in our studies. Therapeutic agents that have long durations of action are often undesirable for use in humans because some patients may not tolerate the effects well. In such cases, a short-acting compound may be preferable, in that its use would allow quick substitution to a different therapeutic agent. On the other hand, in patients that do respond well to treatment, long duration of action may be desirable, especially for compounds that are not effective orally and may need to be parenterally administered. Of course, the effects of JNK activation on measures not tested in studies thus far may also have implications for the behavioral and clinical effects of long-lasting κ-opioid antagonists.
In conclusion, we have demonstrated that a subset of κ-opioid antagonists that are able to activate JNK1 also have long durations of action. The limited structure activity analysis described here did not reveal the key features distinguishing short from long-lasting ligands. Further characterization of the mechanisms of long duration κ-opioid antagonism is necessary to better understand the structure and regulation of the signaling complex.
Acknowledgments
We thank Daniel I. Messinger for help with mouse breeding and genotyping. We thank Dr. John Pintar for KOPr(−/−) mice.
This study was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA11672].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.074195.
- KOPr
- κ-opioid receptor
- nor-BNI
- norbinaltorphimine
- JDTic
- (3R)-7-hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide
- JNK
- c-Jun N terminal kinase
- U50,488
- trans-3,4-dichloro-N-methyl-N-(2-(1-pyrrolidinyl)-cyclohexyl)-benzeneacetamide
- ir
- immunoreactivity
- HEK
- human embryonic kidney
- RTI-5989-97
- (3S)-7-hydroxy-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-(2-methylpropyl]-2-methyl-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide
- RTI-5989-194
- (3R)-7-hydroxy-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-(2-methylbutyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide
- RTI-5989-212
- (3R)-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-(2-methylpropyl]-7-methoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxamide
- RTI-5989-240
- (3R)-7-hydroxy-N-[(1S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]methyl}-(2-methylpropyl]-3-methyl-1,2,3,4-tetrahydroisoquinoline-3-carboxamide
- RTI-5989-241
- (3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-methoxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide)
- FP3FBZ
- (S)-3-fluoro-4-(4-((2-(3-fluorophenyl)pyrrolidin-1-yl)methyl)phenoxy)benzamide
- JSPA0658
- (S)-3-fluoro-4-(4-((2-(3,5-dimethylphenyl)pyrrolidin-1-yl)methyl)phenoxy)benzamide
- JSPA071B
- (S)-3-fluoro-4-(4-((2-(3,5-bis(trifluoromethyl)phenyl)pyrrolidin-1-yl)methyl)phenoxy)benzamide]
- PF-4455242
- [2-methyl-N-((2′-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine
- GFP
- green fluorescent protein
- ERK
- extracellular signal-regulated kinase
- BSA
- bovine serum albumin
- TBS
- Tris-buffered saline
- TBST
- Tris-buffered saline/Triton X-100
- Gö6976
- 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole
- SP600125
- 1,9-pyrazoloanthrone
- PF-4455242
- 2-methyl-N-((2′-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine.
Authorship Contributions
Participated in research design: Melief, Miyatake, and Chavkin.
Conducted experiments: Melief and Miyatake.
Contributed new reagents or analytic tools: Carroll, Béguin, Carlezon, Cohen, Grimwood, Mitch, and Rorick-Kehn.
Performed data analysis: Melief, Miyatake, and Chavkin.
Wrote or contributed to the writing of the manuscript: Melief, Miyatake, and Chavkin.
References
- Ajit SK, Ramineni S, Edris W, Hunt RA, Hum WT, Hepler JR, Young KH. (2007) RGSZ1 interacts with protein kinase C interacting protein PKCI-1 and modulates mu opioid receptor signaling. Cell Signal 19:723–730 [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Pollard GT, Howard JL, Carroll FI. (2010) Effectiveness of analogs of the kappa opioid receptor antagonist (3R)-7-hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic) to reduce U50,488-induced diuresis and stress-induced cocaine reinstatement in rats. Psychopharmacology (Berl) 210:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch MA, Kobe B. (2006) Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev 70:1061–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. (1994) Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on κ-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 115:311–319 [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. (2010) The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C. (2007) Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem 282:29803–29811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buezo ND, Pedregal-Tercero C, McKinzie DL, Mitch CH. (2010), inventors; Eli Lilly and Co., assignee. Kappa selective opioid receptor antagonist. U.S. patent 7,709,522 2010 May 10
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. (1993) Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther 267:1269–1276 [PubMed] [Google Scholar]
- Carlezon WA, Jr, Béguin C, Knoll AT, Cohen BM. (2009) Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther 123:334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Melvin MS, Nuckols MC, Mascarella SW, Navarro HA, Thomas JB. (2006) N-substituted 4β-methyl-5-(3-hydroxyphenyl)-7α-amidomorphans are potent, selective κ-opioid receptor antagonists. J Med Chem 49:1781–1791 [DOI] [PubMed] [Google Scholar]
- Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, Pollard GT, Aceto MD, Harris LS. (2004) Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur J Pharmacol 501:111–119 [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. (1982) Dynorphin is a specific endogenous ligand of the κ-opioid receptor. Science 215:413–415 [DOI] [PubMed] [Google Scholar]
- Clarke S, Czyzyk T, Ansonoff M, Nitsche JF, Hsu MS, Nilsson L, Larsson K, Borsodi A, Toth G, Hill R, et al. (2002) Autoradiography of opioid and ORL1 ligands in opioid receptor triple knockout mice. Eur J Neurosci 16:1705–1712 [DOI] [PubMed] [Google Scholar]
- Cotecchia S. (2007) Constitutive activity and inverse agonism at the alpha1 adrenoceptors. Biochem Pharmacol 73:1076–1083 [DOI] [PubMed] [Google Scholar]
- Cueva JP, Cai TB, Mascarella SW, Thomas JB, Navarro HA, Carroll FI. (2009) Synthesis and in vitro opioid receptor functional antagonism of methyl-substituted analogues of (3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic). J Med Chem 52:7463–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252 [DOI] [PubMed] [Google Scholar]
- Engström W, Ward A, Moorwood K. (2010) The role of scaffold proteins in JNK signalling. Cell Prolif 43:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzón J, Rodríguez-Muñoz M, de la Torre-Madrid E, Sánchez-Blázquez P. (2005a) Effector antagonism by the regulators of G protein signalling (RGS) proteins causes desensitization of mu-opioid receptors in the CNS. Psychopharmacology (Berl) 180:1–11 [DOI] [PubMed] [Google Scholar]
- Garzón J, Rodríguez-Muñoz M, López-Fando A, Sánchez-Blázquez P. (2005b) Activation of μ-opioid receptors transfers control of Gα subunits to the regulator of G-protein signaling RGS9–2. J Biol Chem 280:8951–8960 [DOI] [PubMed] [Google Scholar]
- Grimwood S, Lu Y, Schmidt AW, Vanase-Frawley MA, Sawant-Basak A, Miller E, McLean S, Freeman J, Wong S, McLaughlin JP, et al. (2011) Pharmacological characterization of 2-methyl-N-((2′-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine (PF-04455242), a high-affinity antagonist selective for kappa opioid receptors. J Pharmacol Exp Ther doi:10.1124/jpet.111.185108 [DOI] [PubMed] [Google Scholar]
- Horan P, Taylor J, Yamamura HI, Porreca F. (1992) Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther 260:1237–1243 [PubMed] [Google Scholar]
- Huwiler KG, Machleidt T, Chase L, Hanson B, Robers MB. (2009) Characterization of serotonin 5-hydroxytryptamine-1A receptor activation using a phospho-extracellular-signal regulated kinase 2 sensor. Anal Biochem 393:95–104 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Kenakin T. (2004) Principles: receptor theory in pharmacology. Trends Pharmacol Sci 25:186–192 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (2007) Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol 72:1393–1401 [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. (2008) The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci 28:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. (2009) Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA 106:19168–19173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. (2003a) Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 23:5674–5683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Xu M, Mackie K, Chavkin C. (2003b) Phosphorylation of a carboxyl-terminal serine within the kappa-opioid receptor produces desensitization and internalization. J Biol Chem 278:34631–34640 [DOI] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. (2010) Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci USA 107:11608–11613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch CH, Quimby S, Matt J, Diaz N, Pedregal C, De La Torre M, Shi Q, Canada E, McKinzie D, Statnick M. (2010) Discovery and characterization of a new kappa selective opioid receptor antagonist; 240th ACS National Meeting; 2010 Aug 22–26; Boston, MA American Chemical Society, Washington DC [Google Scholar]
- Nandagopal K, Popp DM, Niyogi SK. (2001) Utilization of a receptor reserve for effective amplification of mitogenic signaling by an epidermal growth factor mutant deficient in receptor activation. J Cell Biochem 83:326–341 [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Linsenmayer DC, Jones RM, Portoghese PS. (2002) Kappa opioid antagonist effects of the novel kappa antagonist 5′-guanidinonaltrindole (GNTI) in an assay of schedule-controlled behavior in rhesus monkeys. Psychopharmacology (Berl) 163:412–419 [DOI] [PubMed] [Google Scholar]
- Peters MF, Zacco A, Gordon J, Maciag CM, Litwin LC, Thompson C, Schroeder P, Sygowski LA, Piser TM, Brugel TA. (2011) Identification of short-acting kappa-opioid receptor antagonists with anxiolytic-like activity. Eur J Pharmacol 661:27–34 [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Lipkowski AW, Takemori AE. (1987) Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci 40:1287–1292 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M, Bermúdez D, Sánchez-Blázquez P, Garzón J. (2006) Sumoylated RGS-RZ proteins act as scaffolds for mu-opioid receptors and G-protein complexes in mouse brain. Neuropsychopharmacology 32:842–850 [DOI] [PubMed] [Google Scholar]
- Thomas JB, Atkinson RN, Vinson NA, Catanzaro JL, Perretta CL, Fix SE, Mascarella SW, Rothman RB, Xu H, Dersch CM, et al. (2003) Identification of (3R)-7-hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide as a novel potent and selective opioid kappa receptor antagonist. J Med Chem 46:3127–3137 [DOI] [PubMed] [Google Scholar]
- Thomas JB, Fix SE, Rothman RB, Mascarella SW, Dersch CM, Cantrell BE, Zimmerman DM, Carroll FI. (2004) Importance of phenolic address groups in opioid kappa receptor selective antagonists. J Med Chem 47:1070–1073 [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- Vaught JL, Takemori AE. (1979) A further characterization of the differential effects of leucine enkephalin, methionine enkephalin and their analogs on morphine-induced analgesia. J Pharmacol Exp Ther 211:280–283 [PubMed] [Google Scholar]