Abstract
Smoking is a major cause for premature death. Work aimed at identifying genetic factors that contribute to nicotine addiction has revealed several single nucleotide polymorphisms (SNPs) that are linked to smoking-related behaviors such as nicotine dependence and level of smoking. One of these SNPs leads to an aspartic acid-to-asparagine substitution in the nicotinic receptor α5 subunit at amino acid position 398 [rs16969968; α5(Asn398)]. The α5 subunit is expressed both in the brain and in the periphery. In the brain, it associates with the α4 and β2 subunits to form α4β2α5 receptors. In the periphery, the α5 subunit combines with the α3 and β4 subunits to form the major ganglionic postsynaptic nicotinic receptor subtype. The α3β4α5 receptor regulates a variety of autonomic responses such as control of cardiac rate, blood pressure, and perfusion. In this paradigm, the α5(Asn398) variant may act by regulating autonomic responses that may affect nicotine intake by humans. Here, we have investigated the effect of the α5(Asn398) variant on the function of the α3β4α5 receptor. The wild-type or variant α5 subunits were coexpressed with the α3 and β4 subunits in human embryonic kidney 293 cells. The properties of the receptors were studied using whole-cell and single-channel electrophysiology. The data indicate that the introduction of the α5(Asn398) mutation has little effect on the pharmacology of receptor activation, receptor desensitization, or single-channel properties. We propose that the effect of the α5(Asn398) variant on nicotine use is not mediated by an action on the physiological or pharmacological properties of the α3β4α5 subtype.
Introduction
Smoking leads to over 400,000 premature deaths in the United States annually. More than 45 million people in the United States smoke; however, only about one third of those who initially experiment with tobacco go on to become regular smokers (McNeill, 1991). This indicates that there is significant individual variability in the progression to regular use of tobacco. Recent work attempting to determine genetic factors that contribute to nicotine addiction has identified several single-nucleotide polymorphisms (SNPs) that are associated with smoking-related behaviors such as nicotine dependence, level of smoking and age of initiation (Bierut, 2007; Bierut et al., 2007; Wang et al., 2009; Grucza et al., 2010; Saccone et al., 2010). One of these SNPs leads to an asparagine-to-aspartic acid substitution in the nicotinic receptor α5 subunit at the amino acid position 398 (Saccone et al., 2007; Bierut et al., 2008). The α5(Asp398) is the major allele in all populations studied, and the presence of the Asn398 variant is associated with a significantly elevated risk for increased nicotine use (Saccone et al., 2007; Bierut et al., 2008). It should be noted that the aspartic acid residue at position 398 is highly conserved across species.
The α5 subunit is expressed both in the brain and in the periphery. In the brain, it associates with α4 and β2 subunits to form presynaptic α4β2α5 receptors. By some accounts, up to 40% of epibatidine-labeled nicotinic receptors in the brain contain the α5 subunit (Brown et al., 2007; Mao et al., 2008). The α5 subunit is also highly expressed in the periphery, where it combines with the α3 and β4 subunits to form the major postsynaptic nicotinic receptor subtype in the autonomic ganglion cells (Vernallis et al., 1993; Conroy and Berg, 1995). In the α3β4α5 combination, the α5(Asn398) variant may be involved in the regulation of autonomic responses, such as control of cardiac rate, blood pressure, and perfusion, which may affect nicotine intake in humans. In addition, the α3, β4, and α5 subunits are expressed in a number of non-neural cells, including bronchial and epithelial cells and lung cancer cell lines, where the activation of nicotinic receptors may play a role in tumor initiation or growth (Egleton et al., 2008).
Here, we have examined the functional effect of the α5(Asn398) variant on the nicotinic receptor function. The wild-type and variant α5 subunits were coexpressed alongside the peripheral α3 and β4 subunits in HEK 293 cells and subjected to a battery of tests to investigate and compare the biophysical and pharmacological properties of wild-type and variant receptors. Our data indicate that the introduction of the α5(Asn398) variant has little effect on the pharmacology of the receptor, desensitization, or the major single-channel properties.
Materials and Methods
cDNAs and Molecular Biology.
The experiments were conducted on human embryonic kidney (HEK) 293 cells, transiently or stably expressing combinations of human nicotinic α3, β4, and α5 subunits. We initially created a HEK line stably expressing α3 and β4 subunits. This cell line was used in transient transfections with wild-type and mutant α5 subunits. The cDNAs for the wild-type α3 (accession number NP_000734.2) and β4 (accession number NP_000741.1) subunits were kindly provided by Dr. J. Lindstrom (University of Pennsylvania, Philadelphia, PA). A codon optimized human nicotinic α5 subunit cDNA (accession number BC033639) in pUC57 was obtained from Genscript USA Inc. (Piscataway, NJ). The cDNA was amplified by polymerase chain reaction using primers complementary to the 5′ and 3′ termini, introducing NotI endonuclease sites at each end of the polymerase chain reaction product. All subunits were subcloned into the pcDNA3 expression vector (Invitrogen, San Diego, CA). The FLAG epitope [DYKDDDDK (Hopp et al., 1988)] was introduced into α5 between the 6 and 7 positions of the mature polypeptide using the QuikChange site-directed mutagenesis kit (Stratagene, San Diego, CA). The full insert was sequenced to verify sequence integrity. The α5FLAG cDNA was mutated with QuikChange to generate the α5(Asn398), α5(V9′S), and α5(Asn398+V9′S) mutant clones. The mutated subunits were fully sequenced to confirm that only the desired mutation(s) had been produced.
Generation of HEK 293 Cells Stably Expressing Human Nicotinic α3β4 Receptors.
HEK 293 cells (American Tissue Culture Collection, Gaithersburg, MD) were maintained in a mixture of Dulbecco's modified Eagle's medium and Ham's F12 (1:1, also containing l-glutamine and 15 mM HEPES), with 10% fetal bovine serum (Hyclone, Logan, UT), penicillin (100 units/ml) and streptomycin (100 μg/ml) in a humidified atmosphere containing 5% CO2 at 37°C. The day before transfection, the cells were plated onto two 100-mm dishes (106 cells per dish).
The transfection procedures have been described in detail previously (Akk, 2002). A total of 21 μg of expression constructs for the human α3 and β4 subunits in the ratio of 1:1 per 100-mm dish was used in a calcium phosphate precipitation-based transfection. The cells were incubated with the precipitate at 37°C in 5% CO2 for approximately 20 h. The transfected dishes were rinsed three times with HEK media and then returned to the incubator. Two days after transfection, transfected cells were initially selected by growth in medium containing the antibiotic G418 (1000 μg/ml; Invitrogen, Carlsbad, CA). The culture medium was changed every few days to ensure adequate cell growth.
The initially selected cells were maintained in G418, and then repeatedly immunoselected (Chen et al., 1995) with monoclonal antibody 35 (Sigma-Aldrich, St. Louis, MO), which binds to an epitope on the extracellular surface of the α3 subunit. We call this procedure “panning.” The day before panning, 60-mm Petri dishes were coated with 3 ml of monoclonal antibody 35 at 25 μg/ml concentration in phosphate-buffered saline (140 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, and 1.8 mM KH2PO4, pH 7.4). The dishes were incubated at 37°C in 5% CO2. On the day of panning, the cells from the 100-mm dishes were released by washing with PBS and trypsin. Five milliliters of HEK media were added to the trypsinized dish, and the cells were collected into a 15-ml centrifuge tube. The cells were then centrifuged and resuspended with 5 ml of Hanks' balanced salt solution (Mediatech, Manassas, VA) containing 5% fetal bovine serum. The entire contents of the 15-ml centrifuge tube were dispensed into the precoated Petri dish and set at room temperature for 30 min, allowing the cells to settle down and adhere to the antibody. The Petri dish was then rinsed three times with Hanks' balanced salt solution, and unattached cells were aspirated and discarded. After that, 4 ml of HEK medium containing G418 were added to the dish and the dish was returned to the incubator. Typically, it took 2 days for the immunoselected and drug-resistant cells to grow to near 100% confluence. Panning was repeated three times to establish the stable α3β4 cell line used in this study. The cell line was then maintained in HEK growth media with 400 μg/ml G418.
The stable α3β4 cell line was used for transient transfections with wild-type or mutant α5 subunits to express α3β4α5 receptors. Transient transfections were done using Effectene (QIAGEN, Valencia, CA) according to manufacturer's instructions. In brief, 0.4 μg of cDNA per 35-mm dish was mixed with the Enhancer and the Effectene Transfection Reagent. The cells were incubated with the mix for 6 to 18 h. Electrophysiological experiments commenced the following day.
On the day of experiments, we employed a bead-binding technique to select α5 expressing cells for electrophysiology (Ueno et al., 1996). The α5 subunits incorporated a FLAG epitope tag in the aminoterminal region. We used a mouse monoclonal antibody to the FLAG epitope (M2; Sigma-Aldrich, St. Louis, MO), which had been adsorbed to immunobeads with a covalently attached goat anti-mouse IgG antibody (Invitrogen). The cells were incubated with the beads for 5 to 10 min with gentle shaking, and cells expressing the α5 subunit were identified from the presence of beads bound to the cell. Expression of the subunit combinations was similar, based on the average response to 1 mM ACh: −984 ± 689 pA (α3β4, 28 cells), −1560 ± 767 pA (α3β4α5, 20 cells), −1981 ± 1031 pA [α3β4α5(Asn398), 20 cells], −1502 ± 713 pA [α3β4α5(V9′S), 20 cells], and −1431 ± 661 pA [α3β4α5(Asn398 + V9′S), 19 cells] (mean ± S.D.). We note that all cells studied were selected because the cells bound beads.
Whole-Cell Recordings and Analyses.
Macroscopic currents were recorded using whole-cell voltage clamp. The bath solution contained 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4. The pipet (intracellular) solution contained 140 mM CsCl, 4 mM NaCl, 4 mM MgCl2, 0.5 mM CaCl2, 5 mM EGTA, and 10 mM HEPES, pH 7.4. The drugs were applied through the bath using an SF-77B fast perfusion stepper system (Warner Instruments, Hamden, CT).
The recording and analysis of whole-cell currents have been described in detail in previous publications (Li et al., 2006). The cells were clamped at −60 mV. All experiments were carried out at room temperature (19–22°C). The current traces were low-pass filtered at 2 kHz and digitized at 10 kHz. The analysis of whole-cell currents was carried out using the pClamp 9.0 software package (Molecular Devices, Union City, CA).
Single-Channel Recordings and Analyses.
Single-channel patch-clamp recordings were conducted in the cell-attached configuration. The bath solution contained 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4. The pipet solution contained 142 mM KCl, 5.4 mM NaCl, 1.7 mM MgCl2, 1.8 mM CaCl2, and 10 mM HEPES, pH 7.4. The agonist (ACh) was added to the pipet solution.
The recording and analysis of single-channel currents have been described in detail previously (Akk, 2001; Akk and Steinbach, 2003). The patch voltage was determined based on the combination of cell membrane potential and the applied potential. The cell membrane potential was estimated from the reversal potential of nicotinic receptor currents, assuming that the currents reverse at 0 mV. The majority of cells had a membrane potential of −30 to −20 mV in the bath solution used. The channel activity was recorded using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA), low-pass filtered at 10 kHz, and acquired with a Digidata 1320 series interface at 50 kHz using pClamp software (Molecular Devices). Before kinetic analysis, the currents were low-pass filtered at 3 to 5 kHz, and the data were idealized using the segmented-k-means algorithm (Qin et al., 1996).
Segments of high-frequency channel openings (i.e., bursts) selected by eye were isolated for further analysis. The open and closed interval durations were estimated from the idealized currents using a maximum likelihood method, which incorporates a correction for missed events (QuB Suite; http://www.qub.buffalo.edu). The records were initially analyzed by fitting a simple C ↔ O model. The number of closed states (or open states if estimating open-time durations) was increased as long as the increase in the log-likelihood justified the addition of extra free parameters. An increase of >25 units was considered significant. The newly added states were connected to the central open state and not connected to each other. The mean interval duration was calculated as the inverse of the fitted rate constant governing the transition. The single-channel amplitudes were estimated using the Amps module in the QuB Suite, and the single-channel conductance was calculated as slope conductance using event amplitudes estimated at membrane potentials between −100 and −25 mV.
Reagents and Statistical Tests.
The reagents were purchased from Sigma-Aldrich (St. Louis, MO) or Tocris Bioscience (Ellisville, MO). Stock solutions (100–500 mM) of ACh, nicotine, cytisine, or 1,1-dimethyl-4-phenylpiperazinium (DMPP) were made in distilled water. Final dilutions to the bath or pipet solutions were made as needed on the day of the experiment.
Statistical analyses were carried out using a paired t test (Excel, Microsoft, Richmond, WA). Data are reported as mean ± S.D. The fits of averaged concentration-effect curves and exponential curves were conducted using NFIT (The University of Texas, Medical Branch at Galveston, Galveston, TX), and results are reported as best-fitting value ± estimated 95% interval of the value.
Results
Macroscopic Pharmacological Profiles of α3β4 and α3β4α5* Receptors.
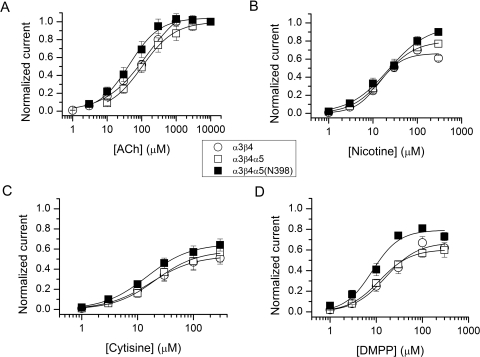
We determined the pharmacological profiles for the α3β4, α3β4α5, and α3β4α5(Asn398) receptors. The receptors were expressed in HEK 293 cells. The cells were exposed to 1 to 10,000 μM ACh, 1 to 300 μM nicotine, 1 to 300 μM cytisine, or 1 to 300 μM DMPP. In the cases of nicotine, cytisine, and DMPP, the responses were normalized to the peak response elicited by 1 mM ACh from the same cell. Our initial data suggested that there was relatively little difference in responses of receptors containing α3β4 and α3β4α5 subunits, which led to a concern that the α5 subunit was poorly incorporated into functional surface receptors. Accordingly, we inserted a FLAG epitope near the amino terminus of the α5 subunit (see Materials and Methods), and selected cells for study that bound small beads coated with antibody to the FLAG epitope. This approach meant that only cells expressing the α5 subunit were studied. To confirm that the α5 subunit was present in the receptors that responded to agonists, we also used a “reporter” mutation (a mutation of the 9′ position in the second transmembrane region), which produces a characteristic increase in the potency of agonists (i.e., a shift of concentration-response relationship to lower concentration of agonist) (Labarca et al., 1995; Krashia et al., 2010).
The data demonstrate that ACh and nicotine have higher efficacy on any of the subunit combinations than cytisine or DMPP, for which the peak responses range from 50 to 80% of the ACh response. The EC50 for α3β4 receptors activated by ACh was 142 μM, similar to the values observed previously (Gerzanich et al., 1998; Krashia et al., 2010). The addition of the α5 subunit resulted in channels with a slightly reduced EC50 (119 μM). A similar leftward shift was previously observed for α3β4α5 receptors expressed in oocytes (Gerzanich et al., 1998). The incorporation of the α5(Asn398) variant further left-shifted the ACh concentration-response curve (EC50 = 57 μM). However, when the midpoints of concentration-response curves from individual cells were compared, the α5(Asn398) variant was not statistically different from the α3β4α5 (not shown). The introduction of the V9′S mutation to the second membrane-spanning domain strongly left-shifted the concentration-response curves. The ACh EC50 values were 16 or 19 μM when the V9′S mutation was introduced onto the α5(Asp398) or α5(Asn398) background, respectively. In each case the effect of the V9′S mutation was statistically significant (p < 0.03, two-tailed t test). The leftward shift in the concentration-response curves is expected given the nature and location of the mutation (Krashia et al., 2010) and serves as proof for the presence of the α5 subunit in the functional receptors.
The EC50 values for nicotine were tightly grouped (14–23 μM) for the α3β4, α3β4α5, and α3β4α5(Asn398) receptors. The introduction of the V9′S mutation to the α5 subunit left-shifted the midpoints of the concentration-response curves by 3- to 8-fold. Activation by cytisine resulted in peak currents from α3β4, α3β4α5, and α3β4α5(Asn398) receptors that were 53 to 65% of the response to 1 mM ACh. The estimated EC50 values were 15 to 21 μM. The presence of the gain-of-function V9′S mutation enhanced the relative cytisine response to 73 to 76% and shifted the concentration-response curves to lower cytisine concentrations. Receptors activated by DMPP showed peak currents of 60 to 80% of that in the presence of ACh. The midpoints of the concentration-response curves were 8 to 16 μM for the α3β4, α3β4α5, and α3β4α5(Asn398) receptors and 2 to 5 μM for the receptors containing the α5(V9′S) mutation. The DMPP concentration-response curves suggested that the presence of the α5(Asn398) variant may enhance the maximal current (Fig. 1D; Table 1) compared with α5(Asp398). To verify this, we separately compared responses to 1 mM ACh and 100 μM DMPP from the same set of cells. The relative response to DMPP was 71 ± 5% (n = 5 cells) and 67 ± 7% (n = 6 cells) in cells expressing α3β4α5 and α3β4α5(Asn398) receptors, respectively. We conclude that the α5(Asn398) variant does not modulate receptor sensitivity to DMPP.
Fig. 1.
Summary of activation concentration-response curves. The plots show concentration-response curves for ACh (A), nicotine (B), cytisine (C), and DMPP (D) for HEK cells stably expressing human α3β4 receptors or stable α3β4 cells transiently transfected with wild-type or variant (Asn398) α5 subunits. The α5 subunits contained a FLAG epitope inserted to the amino terminus of the subunit. Cells expressing the α5 subunit were selected with immunobeads coated with anti-FLAG antibody. Each data point represents mean ± S.E.M. from four to seven cells. The data were normalized to the highest ACh concentration used (A) or to the 1 mM ACh response obtained from the same cell (B–D). The curves were fitted with the Hill equation. The fitting results are given in Table 1.
TABLE 1.
Activation properties of the human α3β4, α3β4α5, and α3β4α5* receptors
The table gives the concentration-response data for the α3β4, α3β4α5, α3β4α5(N398), α3β4α5(V9′S), and α3β4α5(Asn398 + V9′S) receptors. The parameters are estimated by fitting data from n cells with the Hill equation, and the mean ± S.D. parameter values are given. All data were first normalized to the response to 1 mM ACh from the same cell. Imax gives the fit relative maximal response, EC50 is the concentration producing a half-maximal response, and nH is the Hill coefficient for the fit. Fig. 1 shows the averaged concentration response relationships for the cells.
| Agonist and Parameter | α3β4 | α3β4α5 | α3β4α5(Asn398) | α3β4α5(V9′S) | α3β4α5(Asn398 + V9′S) |
|---|---|---|---|---|---|
| ACh | |||||
| Imax | 1.17 ± 0.14 | 1.16 ± 0.15 | 1.06 ± 0.06 | 1.09 ± 0.02 | 1.08 ± 0.04 |
| EC50, μM | 142 ± 103 | 119 ± 68 | 57 ± 23 | 16 ± 10 | 19 ± 14 |
| nH | 0.98 ± 0.30 | 1.02 ± 0.30 | 0.95 ± 0.20 | 0.82 ± 0.31 | 0.77 ± 0.20 |
| n | 8 | 5 | 5 | 5 | 4 |
| Nicotine | |||||
| Imax | 0.67 ± 0.08 | 0.81 ± 0.09 | 0.95 ± 0.10 | 0.88 ± 0.07 | 0.91 ± 0.31 |
| EC50, μM | 14 ± 4 | 20 ± 10 | 23 ± 12 | 2 ± 1 | 9 ± 5 |
| nH | 1.66 ± 0.33 | 1.36 ± 0.26 | 1.12 ± 0.17 | 1.45 ± 0.75 | 1.15 ± 0.30 |
| n | 6 | 5 | 5 | 4 | 5 |
| Cytisine | |||||
| Imax | 0.54 ± 0.15 | 0.58 ± 0.20 | 0.65 ± 0.13 | 0.77 ± 0.06 | 0.82 ± 0.05 |
| EC50, μM | 21 ± 12 | 25 ± 14 | 15 ± 4 | 1 ± 1 | 4 ± 3 |
| nH | 1.17 ± 0.15 | 1.27 ± 0.34 | 1.16 ± 0.18 | 0.92 ± 0.34 | 1.03 ± 0.27 |
| n | 5 | 5 | 5 | 5 | 4 |
| DMPP | |||||
| Imax | 0.67 ± 0.13 | 0.61 ± 0.13 | 0.79 ± 0.05 | 0.81 ± 0.13 | 0.91 ± 0.17 |
| EC50, μM | 18 ± 8 | 12 ± 3 | 7 ± 1 | 6 ± 2 | 3 ± 2 |
| nH | 1.40 ± 0.17 | 1.35 ± 0.18 | 1.39 ± 0.18 | 1.03 ± 0.47 | 0.76 ± 0.08 |
| n | 5 | 5 | 3 | 5 | 4 |
The concentration-response data are summarized in Table 1 and the concentration-response curves for α3β4, α3β4α5, and α3β4α5(Asn398) receptors are shown in Fig. 1. Our overall conclusion is that the presence of the α5(Asn398) variant has little effect on the agonist concentration-response properties, compared with the major α5(Asp398) allele.
We also tested receptor activation by epibatidine. It is an extremely potent agonist, producing responses at concentrations as low as 10 nM on some types of nicotinic receptors. In our hands, exposure to epibatidine resulted in a long-lasting loss of response, especially at high concentrations. However, there seemed to be no major effects on receptors including either variant of the α5 subunit. We compared the responses of cells to 1 mM ACh and a single application of 1 μM epibatidine. The relative responses were 1.2 ± 0.2 (n = 7 cells), 1.1 ± 0.1 (5 cells), and 1.3 ± 0.3 (5 cells) for responses from α3β4, α3β4α5, and α3β4α5(Asn398) receptors, respectively. Accordingly, it does not seem that inclusion of the α5(Asn398) variant had a significant effect on responses to epibatidine.
We also examined receptor activation by cotinine, N-nitrosonornicotine (NNN), and 4-(methylnitrosamino)-1-(3-pyrydyl)-1-butanone (NNK). Cotinine is a major metabolite of nicotine, which has a long half-life in the body, whereas its nominal concentration can be much higher than that of nicotine. Its nicotine-mimicking effects are likely mediated by its ability to activate the nicotinic receptor, albeit with potency that is less than nicotine's by 2 orders of magnitude (O'Leary et al., 2008). NNN and NNK are potent carcinogens found in tobacco products, which can interact with the nicotinic receptor (Schuller, 2007).
All three drugs were ineffective at direct activation, with peak currents <1% of the response to saturating ACh. No differences were observed for the α3β4, α3β4α5, or α3β4α5(Asn398) receptors. We examined the modulatory effects of the drugs by coapplying NNN or NNK at concentrations up to 100 μM, or cotinine at concentrations up to 1 mM with 30 μM ACh (∼EC20). Overall, the data indicate that the drugs are weak inhibitors of the nicotinic receptor. The effects in the presence of NNN or NNK were minor, although in some cases statistically significant. For cotinine, we observed a strong and significant effect at 1 mM, whereas lower concentrations were largely without effect. The data are summarized in Table 2. As a positive control, we tested the effect of 5 mM tetraethylammonium, a low-efficacy agonist of the nicotinic receptor (Akk and Steinbach, 2003). Coapplication of tetraethylammonium with 30 μM ACh reduced the peak response from cells expressing α3β4 receptors to 3 ± 4% of control (n = 4 cells; p < 0.001).
TABLE 2.
Inhibition by NNN, NNK, and cotinine
The table summarizes the modulatory effects of nicotine metabolites NNN, NNK, and cotinine. NNN (100 μM), NNK (100 μM), or cotinine (1 mM) were coapplied with ACh (at EC20). The values are mean ± S.D. of control currents from five to eight cells for each condition. The statistical significance applies to comparison with control currents.
| Receptor | NNN | NNK | cotinine |
|---|---|---|---|
| % | |||
| α3β4 | 92 ± 10† | 92 ± 12† | 53 ± 9*** |
| α3β4α5 | 96 ± 11† | 96 ± 4† | 60 ± 10*** |
| α3β4α5(Asn398) | 88 ± 5** | 95 ± 2** | 52 ± 12*** |
Not significant.
P < 0.01.
P < 0.001.
Desensitization Properties of α3β4, α3β4α5, and α3β4α5(Asn398) Receptors.
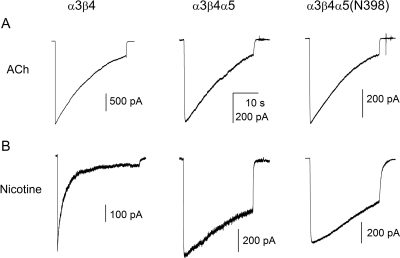
The desensitization properties of the receptors were examined by employing a 30-s exposure to 1 mM ACh or 100 μM nicotine (both are near-saturating concentrations). The extent of desensitization showed variability and the data traces themselves were in several cases not amenable to fits by exponential components. Accordingly, we analyzed the data with regard to the time for decay from peak to 50% of peak for ACh or 80% for nicotine and the extent of residual current at the end of the 30-s drug application. The time to 80% of peak was chosen for analysis of data with nicotine, because the decay was markedly slowed by the presence of either α5 subunit, and the current did not always decay to 50% of peak during the 30-s application.
A previous study (Ifune and Steinbach, 1993) examining the properties of ACh-activated currents in rat pheochromocytoma cells found that the rate of desensitization was highly dependent on recording time. As the time spent in whole-cell configuration increased the peak current and rate of desensitization were enhanced. Here, we did not see consistent changes in peak amplitude. However, the desensitization rate increased with recording time (data not shown). Accordingly, to minimize the effects from intrinsic changes in desensitization, the analysis was conducted on traces that were started within the first 30 s of entering the whole-cell mode.
The 100-to-50% decay time was 15 s in α3β4 receptors activated by 1 mM ACh (Table 3). The inclusion of the α5 or α5(Asn398) subunit had no significant effect on desensitization in the presence of ACh. The 100-to-50% decay times ranged from 13 to 17 s for the α3β4α5 and α3β4α5(Asn398) receptors. The residual current in the end of the 30-s drug application was similar at 25 to 29% of peak for the three-subunit combinations. We infer from the data that the presence of the α5 subunit, wild-type, or containing the α5(Asn398) variant does not influence receptor desensitization in the presence of ACh.
TABLE 3.
The effect of the α5 subunit on receptor desensitization
The receptors were activated by 1 mM ACh or 100 μM nicotine. The desensitization time courses were examined with respect to 100 to 50% of peak (or 80% for nicotine) decay time and the residual current levels at the end of the 30-s application. The data are mean ± S.D. from five to eight cells. All estimates are from traces started within 30 s of entering the whole-cell configuration. The statistical analysis applies to comparison with the data from the α3β4 and α3β4α5 receptors.
| Agonist and Receptor | Decay Time |
Residual Current | |
|---|---|---|---|
| 100-to-50% | 100-to-80% | ||
| s | % | ||
| ACh | |||
| α3β4 | 15 ± 12 | 29 ± 23 | |
| α3β4α5 | 13 ± 6† | 25 ± 14†,† | |
| α3β4α5(Asn398) | 17 ± 3†,† | 29 ± 7† | |
| Nicotine | |||
| α3β4 | 0.9 ± 0.6 | 9 ± 3 | |
| α3β4α5 | 8.6 ± 6.2* | 50 ± 25** | |
| α3β4α5(Asn398) | 9.6 ± 3.7***,† | 48 ± 15***,† | |
Not significant.
P < 0.05.
P < 0.01.
P < 0.001.
The rates of desensitization in the presence of 100 μM nicotine depended on the presence of the α5 subunit. Cells expressing α3β4 receptors had a 100-to-80% decay time of 0.9 s. When the α5 subunit was included in receptor complexes, the decay time increased to 8.6 s. The introduction of the α5(Asn398) variant did not further influence receptor desensitization (τ = 9.6 s). Sample current traces are shown in Fig. 2, and the summary of the analysis, including statistical significance data, are given in Table 3.
Fig. 2.
Receptor desensitization in the presence of ACh or nicotine. The cells were exposed to 30 s pulses of 1 mM ACh (A) or 100 μM nicotine (B). The inclusion of the α5 subunit reduced the rate of desensitization in the presence of nicotine but was without effect when the receptors were activated by ACh. The summary of the analysis is given in Table 3.
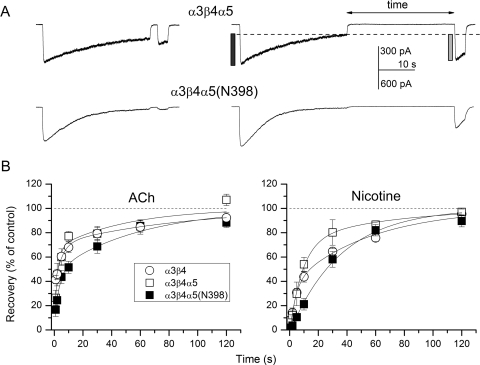
We next examined the time courses for recovery from desensitization. Receptor desensitization was induced by prolonged (30 s) applications of 1 mM ACh or 100 μM nicotine. The desensitizing pulse was followed (1–120 s later) with a brief pulse whose peak response was compared with that of the control application (Fig. 3). For the majority of receptor-agonist combinations, best fits for the time courses contained two exponential components. For the α3β4 receptor, the two components had mean durations of approximately 5 and 89 s in the presence of ACh. The components had roughly equal amplitudes. Receptors exposed to nicotine exhibited time courses with mean durations of the components at 4 and 55 s. The addition of the α5 subunit, and the introduction of the α5(Asn398) variant had a relatively small effect on the recovery time course for desensitization induced by ACh (Table 4). It is noteworthy that the recovery time course for α3β4α5(Asn398) receptors in the presence of nicotine contained a single component, which had a time constant that was intermediate to the fast and slow components seen with α3β4 and α3β4α5 receptors and similar to the weighted time constant from these receptors.
Fig. 3.
Recovery from desensitization. Desensitization was induced by 30-s exposure to 1 mM ACh or 100 μM nicotine. Recovery was measured with brief pulses of the same agonist after washout in bath solution for 1 to 120 s. A, sample recordings from cells expressing α3β4α5 (top) or α3β4α5(Asn398) (bottom) receptors. The two traces show recovery after washout for 2 s (left) or 30 s (right). Recovery was calculated as the ratio of second peak minus residual current (gray bar in the top right trace) over control peak minus residual current (black bar). Note that the extent of desensitization is greater than that shown in traces in Fig. 2. This is so because the rate and extent of desensitization correlated with recording time. B, recovery time courses were measured for α3β4, α3β4α5, and α3β4α5(Asn398) receptors. The curves were fitted to a single exponential [α3β4α5(Asn398) + nicotine] or the sums of two exponentials (everything else). The fitting results are given in Table 4.
TABLE 4.
The effect of the α5 subunit on recovery from desensitization
The receptors were exposed to 30-s pulses of 1 mM ACh or 100 μM nicotine, eliciting channel desensitization. Recovery from desensitization was tested with brief (3-s) pulses at 1- to 120-s time points. The table gives the fitting results (best fit estimates and 95% confidence limits) for curves describing the averaged recovery data points from four to eight cells.
| Receptor | τ1 (fraction) | τ2 |
|---|---|---|
| s (%) | s | |
| ACh | ||
| α3β4 | 4.8 ± 0.8 (55) | 89 ± 13 |
| α3β4α5 | 2.2 ± 1.8 (66) | 49 ± 32 |
| α3β4α5(Asn398) | 3.5 ± 2.0 (45) | 59 ± 16 |
| Nicotine | ||
| α3β4 | 3.9 ± 1.4 (40) | 55 ± 8 |
| α3β4α5 | 8.8 ± 1.9 (73) | 66 ± 31 |
| α3β4α5(Asn398) | 36 ± 3 (100) | N/A |
To gain more insight into the effect of the α5 subunit on recovery, we compared fractional recovery after a 30-s washout. After desensitization by 1 mM ACh, the mean fractional recovery was 79 ± 15% (n = 6 cells), 79 ± 11% (n = 5), and 69 ± 13% (n = 5) for cells expressing α3β4, α3β4α5, and α3β4α5(Asn398) receptors. The effect of the α5(Asn398) variant was statistically insignificant. After 100 μM nicotine, the α3β4 receptors recovered to 65 ± 6% (n = 6 cells) of control after a 30-s washout. Recovery was 80 ± 18% (n = 3) for α3β4α5 receptors and 58 ± 13% (n = 4) for α3β4α5(Asn398) receptors. The differences were not statistically significant, indicating that the presence of a single component in the recovery time course from α3β4α5(Asn398) receptors does not result in significant changes in recovery from desensitization.
Single-Channel Currents from α3β4 and α3β4α5* Receptors.
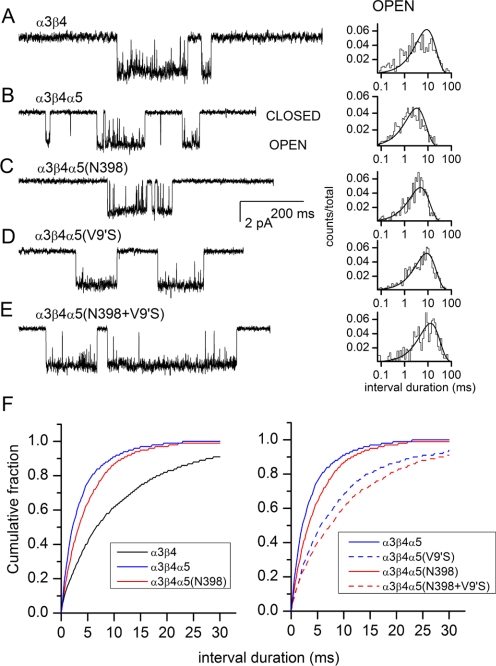
We investigated the effect of subunit composition and the α5(V9′S) mutation on single-channel parameters. The α3β4 stable cell line was supertransfected with the α5, α5(Asn398), α5(V9′S), or α5(Asn398+V9′S) subunit. The currents were recorded in the cell-attached configuration.
Single-channel activity elicited by 3 μM ACh consisted of a mixture of bursts of openings interlaced with single openings. The majority of single-channel data fell to a single conductance class (32–34 pS; see below), but in some cells, an additional, higher (> 50 pS) conductance class was observed. Because of its relative scarcity, the higher conductance class was not characterized.
Sample recordings are shown in Fig. 4. The intraburst open time histograms were adequately fitted to a single exponential. The α3β4 receptors had a mean open duration of 10.7 ± 4.0 ms (n = 4 patches). In α3β4α5 receptors, the mean open duration was 3.5 ± 1.0 ms (n = 3 patches). As expected, the introduction of the α5(V9′S) mutation prolonged channel openings, increasing the mean open duration to 9.7 ± 1.8 ms (n = 3 patches). The presence of the α5(Asn398) variant had no significant effect on the mean open time (4.7 ± 0.9 ms; n = 3 patches) compared with α3β4α5. In the receptor containing the α5(Asn398+V9′S) double mutation, the mean open time was 9.7 ± 1.4 ms (n = 4 patches). We conclude that the α5(Asn398) variant is without effect on the open-time durations, regardless of the presence of the background α5(V9′S) mutation. Conversely, the data also show that the V9′S mutation similarly affects the open durations from receptors containing both variants of the α5 subunit. Cumulative plots of the open durations are shown in Fig. 4F. The reduction in open time after introduction of α5 or α5(Asn398) is highly significant (p < 0.001 for comparison to α3β4; Kolmogorov-Smirnov 2-tailed test). The differences between α5 and α5(V9′S) and between α5(Asn398) and α3β4α5(Asn398+V9′S) are also highly significant (p < 0.001 for each comparison).
Fig. 4.
Single-channel currents from the wild-type and mutant receptors activated by 3 μM ACh. A, the currents were recorded from a HEK cell stably expressing the human α3β4 receptors. The stably expressing α3β4 cells were used in transient transfections with wild-type (B) and mutant (C–E) α5 subunits. The wild-type and mutant α5 subunits contained a FLAG epitope inserted at the amino terminus of the subunit. Cells expressing the α5 subunit were selected with immunobeads coated with anti-FLAG antibody. The currents were recorded in the cell-attached configuration. Channel openings are shown as downward deflections. The intraburst open times were fitted with a single exponential. The mean open times were 13.3 ms (A), 2.7 ms (B), 4.3 ms (C), 7.1 ms (D), and 9.7 ms (E). The average open times from multiple patches are given in the text. The intraburst closed-time durations are given in Table 3. F, cumulative open-time distribution histograms. The addition of the α5 subunit results in briefer open-time durations (left). The introduction of the V9′S mutation to the α5 subunit results in longer open time durations (right).
The intraburst closed times contained two components. The briefer component had a mean duration of approximately 0.1 ms, making up the majority (90%) of all intraburst closed intervals. The longer-lived component had a mean duration of 1 to 3 ms. The presence of the α5 subunit did not affect the closed time distributions. The closed time data are summarized in Table 5.
TABLE 5.
The effect of the α5* subunit on the intraburst closed-time properties
The intraburst activity was fitted to the sums of two exponentials. The data are mean ± S.D. from three to four patches. The columns give receptor subunit composition and the mean durations and fractions of the two closed-time components.
| Receptor | CT1 | Fraction CT1 | CT2 | Fraction CT2 |
|---|---|---|---|---|
| ms | ms | |||
| α3β4 | 0.11 ± 0.05 | 0.88 ± 0.05 | 1.6 ± 0.5 | 0.12 ± 0.05 |
| α3β4α5 | 0.11 ± 0.03 | 0.91 ± 0.06 | 0.8 ± 0.3 | 0.09 ± 0.06 |
| α3β4α5(Asn398) | 0.12 ± 0.04 | 0.80 ± 0.18 | 0.8 ± 0.4 | 0.20 ± 0.18 |
| α3β4α5(V9′S) | 0.10 ± 0.02 | 0.88 ± 0.07 | 2.6 ± 1.7 | 0.12 ± 0.07 |
| α3β4α5(Asn398 + V9′S) | 0.10 ± 0.01 | 0.96 ± 0.001 | 1.2 ± 0.6 | 0.04 ± 0.001 |
We determined the slope conductance of single-channel activity by measuring the current-voltage relationship at membrane potentials of −100 to −25 mV. The single-channel conductances were indistinguishable: α3β4 receptors, 34.0 ± 2.1 pS (n = 4 patches); α4β4α5, 33.7 ± 3.6 pS (n = 3 cells); and α3β4α5(Asn398), 32.8 ± 1.9 pS (n = 4 cells).
A previous study employing outside-out patches from Xenopus laevis oocytes expressing α3β4 or α3β4α5 receptors found a single-channel conductance of 31 and 36 pS, respectively (Nelson and Lindstrom, 1999). Nicotinic currents from rat superior cervical ganglion cells, where α3β4 receptors form a major nicotinic receptor component, show a single-channel conductance of 35 to 37 pS (Sivilotti et al., 1997). Rat α3β4 receptors expressed in BOSC 23 cells predominantly shows a 34-pS conductance class (Ragozzino et al., 1997). Overall, our findings are similar to the previously published single-channel data on wild-type α3β4 and α3β4α5 receptors (e.g.,(Lewis et al., 1997; Nelson and Lindstrom, 1999; Boorman et al., 2003). Our single-channel data indicate that the α5(Asp398) and α5(Asn398) variants behave similarly in single-channel recordings.
Discussion
The neuronal nicotinic α5 subunit does not express as a homo-oligomer, and it cannot substitute for other neuronal α subunits that combine with a β subunit to form functional channels. Rather, the α5 subunit acts as an accessory subunit combining with, and in some cases modulating, other αβ hetero-oligomeric receptors. The α5 subunit is expressed in the brain, where it contributes to the α4β2α5 receptor, and in the periphery, where it combines with α3 and β4 subunits to form the major ganglionic postsynaptic receptor (Vernallis et al., 1993; Conroy and Berg, 1995). In addition, the nicotinic α5 subunit is expressed in lung epithelial cells with α3 and β4 and in small-cell and non–small-cell lung cancer cell lines (Maus et al., 1998; Egleton et al., 2008; Song and Spindel, 2008). We examined the effects of including two sequence variants of the α5 subunit on the physiological and pharmacological properties of α3β4 receptors. The minor allele [α5(Asn398)] is associated with increased risk of developing nicotine dependence (Saccone et al., 2007; Bierut et al., 2008). We considered the possibility that an effect on the function of ganglionic neuronal nicotinic receptors might significantly alter the actions of nicotine to produce aversive, or preferred, peripheral responses, and so modify behavioral responses.
We employed whole-cell and single-channel patch clamp to investigate the physiological and pharmacological properties of α3β4, α3β4α5, and α3β4α5(Asn398) receptors. We also generated and studied receptors containing a valine-to-serine mutation in the second membrane-spanning domain (V9′S) of the α5 subunit. This gain-of-function mutation acted as a functional reporter for the expression and incorporation of the α5 subunit.
Our findings indicate that the α5(Asn398) variant has no functional effect in the α3β4α5 receptor compared with the major allele, insofar as covered by the tests that we employed. Specifically, the variant was without effect on channel activation by ACh, nicotine, cytisine or DMPP, or the nicotine metabolites cotinine, NNN, or NNK. The variant was also without effect on channel desensitization properties, or single-channel parameters such as intracluster open and closed times and single-channel conductance.
Given its passive nature, the expression and incorporation of the α5 subunit cannot be determined simply from the observation of a functional response. Nor are we aware of any specific antibodies to the α5 subunit or a pharmacological approach to eliminate non-α5 containing receptors. Accordingly, we engineered a FLAG epitope tag at the amino terminus of the subunit. Cells expressing the α5 subunit on the surface were selected by beadbinding. Although this approach does not guarantee the presence of the α5 subunit in each receptor-complex, bead binding indicates the incorporation of the α5 subunit in, at least, some surface receptors. The presence of the α5 subunit in the functional receptors we studied is confirmed by the leftward shift on the agonist concentration-response curves and the related prolongation of open durations observed when the α5 subunit contained the V9′S mutation in the second membrane-spanning domain. Original studies on the related muscle-type nicotinic receptor have shown that mutations that enhance the polarity of the 9′ residue (a leucine in most subunits) facilitate channel opening by reducing the channel-closing rate constant (Filatov and White, 1995; Labarca et al., 1995; Chen and Auerbach, 1998). This results in a leftward shift in the agonist concentration-response curve. Accordingly, we used this approach to probe for the presence of the α5 subunit. Our data indicate that the introduction of the V9′S mutation in the α5 subunit prolongs the mean open duration in single-channel recordings (Fig. 4) and shifts the macroscopic concentration-response curves to lower agonist concentrations (Table 1). It is noteworthy that the presence of the Asn398 variant on the α5(V9′S) background did not influence macroscopic parameters or the properties of single-channel currents, compared with the major allele. These findings are important, because in these experiments, the presence of the α5 subunit in receptor-complexes is unequivocally shown by V9′S-specific changes in channel properties.
It should be mentioned that a previous study examining currents from α3β4α5 receptors expressed in X. laevis oocytes found that a valine-to-threonine mutation at the 9′ position was without effect on the ACh concentration-response curve. The only reported effect of the mutation was a reduction in the apparent rate of desensitization (Groot-Kormelink et al., 2001).
A recent study demonstrated that α3β4 receptors exist in two stoichiometries: one with two α3 subunits and three β4 subunits, the other containing three α3s and two β4s (Krashia et al., 2010). The two stoichiometric isoforms differed in their relative sensitivity to DMPP (the three α isoform was more sensitive) and single-channel properties (the three α isoform had a larger single-channel conductance). Examination of our data suggests that the stable α3β4 cell line used in this study expresses receptors in the 3:2 (α3:β4) stoichiometry. The ratio of EC50 values for ACh versus DMPP is 7.7 (Table 1), similar to the 10-fold difference found by Krashia et al. (2010) for the three α isoform. However, our single-channel conductance estimate for α3β4 receptors (34 pS) is intermediate to the values for the two isoforms (26 and 39 pS for 2:3 and 3:2, respectively). It is not clear whether the discrepancy results from differences in recording conditions (cell-attached versus outside-out) or indicates that our cells express both isoforms.
Our estimates for activation properties of the α3β4 receptor show similarites as well as differences with previous studies. For example, our estimate for the EC50 for DMPP (16 μM) is close to the values obtained previously (10–18 μM; (Gerzanich et al., 1998; Stauderman et al., 1998)). Likewise, our estimate for the EC50 for ACh (142 μM) agrees with Gerzanich et al. (1998) (163 μM) and Papke et al. (2010) (79 μM). On the other hand, the estimates for nicotine potency vary considerably, with EC50 values at 14 μM (Table 1), 40 μM (Stauderman et al., 1998), or 106 μM (Gerzanich et al., 1998). A study examining stimulation of 86Rb+ flux yielded EC50 values of 28 and 114 μM for nicotine and ACh, respectively (Xiao et al., 1998). Our EC50 estimate for cytisine (21 μM) is similar to that obtained by Stauderman et al., 1998 (26 μM), but not that of Gerzanich et al. (1998) (76 μM). It is noteworthy that the relative efficacy of cytisine is similar in all cases.
The pharmacology of macroscopic currents from cells expressing α3β4α5 receptors are generally in agreement with previous studies (Gerzanich et al., 1998; Papke et al., 2010). The major discrepancy is in the estimates of the relative efficacy of DMPP. Our experiments (Table 1) demonstrate that the peak current in the presence of DMPP is 61% of that for ACh, whereas Gerzanich et al. (1998) found that the relative efficacy of DMPP was only 13%. In addition, we find that the whole-cell responses from α3β4 and α3β4α5 cells show little difference in the rate and extent of desensitization in the presence of ACh. In contrast, Gerzanich et al. (1998) found that the rate of desensitization in the presence of ACh was significantly faster in oocytes expressing α3β4α5 receptors.
It is not immediately clear what causes the discrepancies, but the difference in expression systems and its effect on subunit stoichiometry may have influenced the results. In general, our data more closely resemble the results by Stauderman et al. (1998), who like us expressed the receptors in HEK cells, whereas Gerzanich et al. (1998) used X. laevis oocytes. It remains to be determined which stoichiometric type is more prevalent in vivo, although for the α3β4 receptor, Lewis et al. (1997) demonstrated that receptors expressed in a mammalian cell line resembled activity from superior cervical ganglion neurons more closely than oocyte-expressed receptors. Other possible reasons for the discrepancies are post-translational modifications such as phosphorylation and glycosylation. These could also depend on the nature of the expression system.
The introduction of the α5(Asn398) variant had no significant effect on the pharmacological or biophysical properties of the α3β4α5 receptor. A recent study found that the α5(Asn398) does not affect sensitivity to ACh, the rate of desensitization or Ca2+ permeability of α3β4α5 receptors (Kuryatov et al., 2011). Our study extends these findings by additionally examining the effect of the α5(Asn398) variant on channel activation by nicotine, cytisine, DMPP, and epibatidine, by nicotine metabolites NNN, NNK, and cotinine, recovery from desensitization, and single-channel properties (single-channel conductance, intraburst open and closed times). The data indicate that the α5(Asn398) variant, previously shown to be linked to individual variability in nicotine use, does not modify the major physiological or pharmacological characteristics of the ganglionic α3β4α5 receptor expressed in HEK cells. However, we note that the variant may act by influencing receptor expression and hence nicotinic current amplitude (Frahm et al., 2011). It is also possible that the α5(Asn398) variant influences nicotine use by acting on the centrally expressed α4β2α5 receptor. This suggestion is supported by previous work in which it was found that the Ca2+ influx was lower for cells expressing α4β2α5(Asn398) compared with α4β2(Asp398) (Bierut et al., 2008). A possible explanation for this lies in the finding that the α5(Asn398) variant both reduces the Ca2+ permeability and increases the desensitization of α4β2α5 receptors (Kuryatov et al., 2011).
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA26918] and the National Institutes of Health National Cancer Institute [Grant CA89392]. J.H.S. is the Russell and Mary Shelden Professor of Anesthesiology.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.073841.
- SNP
- single-nucleotide polymorphism
- HEK
- human embryonic kidney
- DMPP
- 1,1-dimethyl-4-phenylpiperazinium
- NNN
- N-nitrosonornicotine
- NNK
- 4-(methylnitrosamino)-1-(3-pyrydyl)-1-butanone
- ACh
- acetylcholine.
Authorship Contributions
Participated in research design: Steinbach and Akk.
Conducted experiments: Li, McCollum, Bracamontes, and Akk.
Performed data analysis: Li, Steinbach, and Akk.
Wrote or contributed to the writing of the manuscript: Steinbach and Akk.
References
- Akk G. (2001) Aromatics at the murine nicotinic receptor agonist binding site: mutational analysis of the alphaY93 and alphaW149 residues. J Physiol 535:729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G. (2002) Contributions of the non-alpha subunit residues (loop D) to agonist binding and channel gating in the muscle nicotinic acetylcholine receptor. J Physiol 544:695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Steinbach JH. (2003) Activation and block of mouse muscle-type nicotinic receptors by tetraethylammonium. J Physiol 551:155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ. (2007) Genetic variation that contributes to nicotine dependence. Pharmacogenomics 8:881–883 [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, et al. (2007) Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet 16:24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, et al. (2008) Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165:1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman JP, Beato M, Groot-Kormelink PJ, Broadbent SD, Sivilotti LG. (2003) The effects of beta3 subunit incorporation on the pharmacology and single channel properties of oocyte-expressed human alpha3beta4 neuronal nicotinic receptors. J Biol Chem 278:44033–44040 [DOI] [PubMed] [Google Scholar]
- Brown RW, Collins AC, Lindstrom JM, Whiteaker P. (2007) Nicotinic alpha5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. J Neurochem 103:204–215 [DOI] [PubMed] [Google Scholar]
- Chen J, Auerbach A. (1998) A distinct contribution of the delta subunit to acetylcholine receptor channel activation revealed by mutations of the M2 segment. Biophys J 75:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Fletcher GH, Steinbach JH. (1995) Selection of stably transfected cells expressing a high level of fetal muscle nicotinic receptors. J Neurosci Res 40:606–612 [DOI] [PubMed] [Google Scholar]
- Conroy WG, Berg DK. (1995) Neurons can maintain multiple classes of nicotinic acetylcholine receptors distinguished by different subunit compositions. J Biol Chem 270:4424–4431 [DOI] [PubMed] [Google Scholar]
- Egleton RD, Brown KC, Dasgupta P. (2008) Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci 29:151–158 [DOI] [PubMed] [Google Scholar]
- Filatov GN, White MM. (1995) The role of conserved leucines in the M2 domain of the acetylcholine receptor in channel gating. Mol Pharmacol 48:379–384 [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, et al. (2011) Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron 70:522–535 [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. (1998) alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther 286:311–320 [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Boorman JP, Sivilotti LG. (2001) Formation of functional alpha3beta4alpha5 human neuronal nicotinic receptors in Xenopus oocytes: a reporter mutation approach. Br J Pharmacol 134:789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Johnson EO, Krueger RF, Breslau N, Saccone NL, Chen LS, Derringer J, Agrawal A, Lynskey M, Bierut LJ. (2010) Incorporating age at onset of smoking into genetic models for nicotine dependence: evidence for interaction with multiple genes. Addict Biol 15:346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp TP, Prickett KS, Price VL, Libby RT, March CJ, Cerretti DP, Urdal DL, Conlon PJ. (1988) A short polypeptide marker sequence useful for recombinant protein identification and purification. Bio-Technology 6:1204–1210 [Google Scholar]
- Ifune CK, Steinbach JH. (1993) Modulation of acetylcholine-elicited currents in clonal rat phaeochromocytoma (PC12) cells by internal polyphosphates. J Physiol 463:431–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashia P, Moroni M, Broadbent S, Hofmann G, Kracun S, Beato M, Groot-Kormelink PJ, Sivilotti LG. (2010) Human alpha3beta4 neuronal nicotinic receptors show different stoichiometry if they are expressed in Xenopus oocytes or mammalian HEK293 cells. PLoS One 5:e13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J. (2011) Acetylcholine receptor (AChR) α5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (α4β2)2α5 AChR function. Mol Pharmacol 79:119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, Lester HA. (1995) Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature 376:514–516 [DOI] [PubMed] [Google Scholar]
- Lewis TM, Harkness PC, Sivilotti LG, Colquhoun D, Millar NS. (1997) The ion channel properties of a rat recombinant neuronal nicotinic receptor are dependent on the host cell type. J Physiol 505:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Covey DF, Steinbach JH, Akk G. (2006) Dual potentiating and inhibitory actions of a benz[e]indene neurosteroid analog on recombinant alpha1beta2gamma2 GABAA receptors. Mol Pharmacol 69:2015–2026 [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. (2008) The alpha4beta2alpha5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem 104:446–456 [DOI] [PubMed] [Google Scholar]
- Maus AD, Pereira EF, Karachunski PI, Horton RM, Navaneetham D, Macklin K, Cortes WS, Albuquerque EX, Conti-Fine BM. (1998) Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol Pharmacol 54:779–788 [DOI] [PubMed] [Google Scholar]
- McNeill AD. (1991) The development of dependence on smoking in children. Br J Addict 86:589–592 [DOI] [PubMed] [Google Scholar]
- Nelson ME, Lindstrom J. (1999) Single channel properties of human alpha3 AChRs: impact of beta2, beta4 and alpha5 subunits. J Physiol 516:657–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary K, Parameswaran N, McIntosh JM, Quik M. (2008) Cotinine selectively activates a subpopulation of alpha3/alpha6beta2 nicotinic receptors in monkey striatum. J Pharmacol Exp Ther 325:646–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Wecker L, Stitzel JA. (2010) Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 333:501–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. (1996) Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J 70:264–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino D, Fucile S, Giovannelli A, Grassi F, Mileo AM, Ballivet M, Alemà S, Eusebi F. (1997) Functional properties of neuronal nicotinic acetylcholine receptor channels expressed in transfected human cells. Eur J Neurosci 9:480–488 [DOI] [PubMed] [Google Scholar]
- Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, et al. (2010) Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet 6:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, et al. (2007) Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet 16:36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller HM. (2007) Nitrosamines as nicotinic receptor ligands. Life Sci 80:2274–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti LG, McNeil DK, Lewis TM, Nassar MA, Schoepfer R, Colquhoun D. (1997) Recombinant nicotinic receptors, expressed in Xenopus oocytes, do not resemble native rat sympathetic ganglion receptors in single-channel behaviour. J Physiol 500:123–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Spindel ER. (2008) Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in lung cancer provides a new target for cancer therapy. J Pharmacol Sci 106:180–185 [DOI] [PubMed] [Google Scholar]
- Stauderman KA, Mahaffy LS, Akong M, Veliçelebi G, Chavez-Noriega LE, Crona JH, Johnson EC, Elliott KJ, Gillespie A, Reid RT, et al. (1998) Characterization of human recombinant neuronal nicotinic acetylcholine receptor subunit combinations alpha2beta4, alpha3beta4 and alpha4beta4 stably expressed in HEK293 cells. J Pharmacol Exp Ther 284:777–789 [PubMed] [Google Scholar]
- Ueno S, Zorumski C, Bracamontes J, Steinbach JH. (1996) Endogenous subunits can cause ambiguities in the pharmacology of exogenous gamma-aminobutyric acidA receptors expressed in human embryonic kidney 293 cells. Mol Pharmacol 50:931–938 [PubMed] [Google Scholar]
- Vernallis AB, Conroy WG, Berg DK. (1993) Neurons assemble acetylcholine receptors with as many as three kinds of subunits while maintaining subunit segregation among receptor subtypes. Neuron 10:451–464 [DOI] [PubMed] [Google Scholar]
- Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, Budde JP, Duan W, Fox L, Grucza RA, Kern J, et al. (2009) Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet 18:3125–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. (1998) Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol Pharmacol 54:322–333 [DOI] [PubMed] [Google Scholar]