Abstract
Although inflammatory pathways have been linked with various chronic diseases including cancer, identification of an agent that can suppress these pathways has therapeutic potential. Herein we describe the identification of a novel compound bharangin, a diterpenoid quinonemethide that can suppress pro-inflammatory pathways specifically. We found that bharangin suppresses nuclear factor (NF)-κB activation induced by pro-inflammatory cytokine, tumor promoter, cigarette smoke, and endotoxin. Inhibition of NF-κB activation was mediated through the suppression of phosphorylation and degradation of inhibitor of nuclear factor-κB (IκBα); inhibition of IκBα kinase activation; and suppression of p65 nuclear translocation, and phosphorylation. The diterpenoid inhibited binding of p65 to DNA. A reducing agent reversed the inhibitory effect, and mutation of the Cys38 of p65 to serine abrogated the effect of bharangin on p65-DNA binding. Molecular docking revealed strong interaction of the ligand with the p65 via two hydrogen bonds one with Lys37 (2.204 Å) and another with Cys38 (2.023 Å). The inhibitory effect of bharangin on NF-κB activation was specific, inasmuch as binding of activator protein-1 and octameric transcription factor 1 to DNA was not affected. Suppression of NF-κB activation by this diterpenoid caused the down-regulation of the expression of proteins involved in tumor cell survival, proliferation, invasion, and angiogenesis, leading to potentiation of apoptosis, suppression of proliferation, and invasion of tumor cells. Furthermore, the genetic deletion of p65 and mutation of p65Cys38 residue to Ser abolished the affect of bharangin. Overall, our results demonstrate that bharangin specifically inhibits the NF-κB activation pathway by modifying p65 and inhibiting IκBα kinase activation and potentiates apoptosis in tumor cells.
Introduction
Despite great strides in understanding cancer biology, oncologists have made very little progress in cancer prevention and treatment. On the contrary, World Health Organization projections indicate that cancer incidence will double over the next 20 years. Therefore, cancer research today is at a crossroads and requires thinking outside the box. Monotargeted drugs, once called smart drugs and magic bullets, have not had a great impact on cancer treatment. Use of multitargeted promiscuous drugs or a polypharmacological approach in cancer treatment has become increasingly accepted, because cancer is caused by dysregulation of multiple pathways. Most natural products used to treat cancer for centuries are known to have multiple targets and thus are preferred over monotargeted drugs (Gupta et al., 2010a). Although medicinal plants have a long history of use in the treatment of cancer, what these plants are composed of and how their effects are mediated were unknown in ancient times. Premna herbacea, also known as Clerodendrum indicum (English: Tuber-flower, Turk's turban, and skyrocket; Hindi: bharangi; Sanskrit: bhangi; Ayurvedic: nirgundi kul), is one such plant. Different parts of the plant have been used either alone or as an ingredient in preparations for the treatment of bronchitis, asthma, blood pressure, epilepsy, fever, inflammation, rheumatism, respiratory disorders, and helminthiasis and as an antinociceptive (Kirtikar and Basu, 1918; Nayar et al., 1976; Narayanan et al., 2000). Furthermore, P. herbacea extract has exhibited anticancer activity in vitro and in tumor-bearing mouse models (Boonyaratanakornkit and Chantaptavan, 1993; Itharat et al., 1998; Dhamija et al., 2008; Kumar et al., 2008; Pai et al., 2008). Investigators have isolated a number of diterpenoids from the roots of this plant, one of which is bharangin (Sankaram and Rao, 1978; Sankaram et al., 1988; Sathish et al., 2009). Bharangin, a diterpenoid quinonemethide, chiefly present in P. herbacea, exhibited strong antimicrobial activity (Murthy et al., 2006) and reversed a drug-resistant phenotype of Escherichia coli cells carrying multidrug-resistant plasmids (Lakshmi et al., 1989). In addition, the monoacetylated derivative of bharangin showed greater antimicrobial activity than that of bharangin (Murthy et al., 2006). Whether bharangin has anticancer activity has yet to be studied, however. Because extracts from the root nodules of P. herbacea exhibit anticancer and anti-inflammatory effects, we hypothesized that bharangin may be responsible for these effects.
Extensive research over the past 3 decades has revealed that most pro-inflammatory and carcinogenic effects of carcinogens are linked with activation of the nuclear factor (NF)-κB pathway. NF-κB is a pleiotropic transcription factor found in nearly all animal cell types. In resting cells, NF-κB stays in an inactive state in the cytoplasm as a heterotrimer consisting of the subunits p50, p65, and the inhibitory subunit IκBα. In response to a variety of inducers such as cytokines and growth factors, IκB kinase (IKK) is rapidly activated, which in turn phosphorylates IκBα at Ser32 and Ser36. Phosphorylated IκBα then undergoes polyubiquitination and subsequent proteolytic degradation. After phosphorylation, p65 is released from the cytoplasm and translocated to the nucleus, where it binds to a specific DNA sequence and activates the transcription of more than 500 genes (Gupta et al., 2010a,b). Aberrant regulation of NF-κB and the signaling pathways that control its activity is involved in inflammation, drug resistance, radioresistance, tumor survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells (Aggarwal, 2004). Therefore, agents that can down-modulate this pathway have potential in cancer therapy. We postulated that bharangin is one such agent.
In the present study, we investigated whether treatment with bharangin would modulate the survival and proliferation of tumor cells alone and in combination with currently used therapeutics. If so, we sought to investigate the mechanism by which this agent manifests these effects. We observed that this diterpenoid 1) inhibited constitutive NF-κB activation; 2) inhibited NF-κB activation induced by carcinogens, tumor promoters, cigarette smoke, and endotoxin; 3) inhibited binding of p65 with DNA; 4) inhibited IKK activation; 5) decreased survival; and 6) enhanced the effects of currently used therapeutics in cancer cells.
Materials and Methods
Reagents.
Bharangin (Fig. 1A) was isolated from the acetone extract of the root nodules of P. herbacea using column chromatography and characterized by infrared, 1H NMR, 13C NMR, and mass spectroscopy as well as melting point matching with that reported in literature (Sankaram et al., 1988). Human recombinant tumor necrosis factor (TNF)-α provided by Genentech was purified from bacterial cells to homogeneity with a specific activity of 5 × 107 U/mg. Cigarette smoke condensate (CSC) was provided by Dr. C. Gary Gairola (University of Kentucky, Lexington, KY). Penicillin, streptomycin, RPMI 1640 medium, Iscove's modified Dulbecco's medium, Dulbecco's modified Eagle's medium, and fetal bovine serum (FBS) were obtained from Invitrogen. Phorbol myristate acetate (PMA), lipopolysaccharide (LPS), okadaic acid (OA), and an antibody against β-actin were obtained from Sigma. Antibodies against p50, cyclin D1, cyclooxygenase (COX)-2, matrix mellatoproteinase (MMP)-9, poly (ADP-ribose) polymerase (PARP), inhibitor of apoptosis protein (IAP)-1, IAP-2, Bcl-2, Bcl-xL, myeloid cell leukemia-1, intercellular adhesion molecule (ICAM)-1, Bcl-2–associated X protein (Bax), cytochrome-c, c-Myc, AKT, caspases, mTOR, PI3K, MSK1, TAK1 and an annexin V staining kit were obtained from Santa Cruz Biotechnology. An anti-X-linked IAP (XIAP) was purchased from BD Biosciences. The PDK1 and phospho-specific anti-IκBα (Ser32/36), p65 (Ser276 and Ser536), PDK1 (Ser241), PI3K [p85 (Tyr458) p55 (Tyr199)], mTOR (Ser2448), MSK1 (Ser376) antibodies were purchased from Cell Signaling Technology. The antibody against survivin, p65, and vascular endothelial growth factor (VEGF) was purchased from R and D Systems, Abcam, and NeoMarkers, respectively. The IκBα, IKK-α, IKK-β, phospho-AKT (Ser473), and cellular FLICE-inhibitory protein (c-FLIP) antibodies were obtained from Imgenex. The pcDNA3.1 and pcDNA expression vectors for mouse p65 and mouse p65C38S were kindly provided by Dr. T. D. Gilmore from Boston University (Boston, MA). The FuGENE 6 transfection reagent was obtained from Roche Applied Science (Indianapolis, IN).
Fig. 1.
Bharangin down-regulates constitutive and inducible NF-κB activation in cancer cells. A, the chemical structure of bharangin. Dose- (B) and time- (C) dependent effects of bharangin on TNF-α-induced NF-κB activation. KBM-5 cells were incubated with bharangin at the indicated concentrations for 6 h (B) or 5 μM bharangin for the indicated times (C) and then treated with 0.1 nM TNF-α for 30 min. Nuclear extracts of the cells were prepared and assayed for NF-κB activation using EMSA. The fold activation of NF-κB and percentage of viable cells (%CV) are shown at the bottom. D, TNF-α-induced NF-κB is composed of p65 and p50 subunits. Nuclear extracts of untreated cells or cells treated with 0.1 nM TNF-α were incubated with the indicated antibodies, preimmune serum, an unlabeled NF-κB oligoprobe, or a mutant oligoprobe and then assayed for NF-κB activation using EMSA. E, bharangin inhibits NF-κB activation induced by CSC, OA, PMA, and LPS. KBM-5 cells were incubated with 2 and 5 μM bharangin for 6 h and then treated with 10 μg/ml CSC for 1 h, 500 nM OA for 4 h, 100 ng/ml PMA for 2 h, or 10 μg/ml LPS for 30 min. Nuclear extracts of the cells were analyzed for NF-κB activation using EMSA. The fold activation of NF-κB compared with that of control is shown at the bottom. F, bharangin suppresses TNF-α-induced NF-κB activation in different cancer cells. U937, HL-60, Jurkat, and K-562 cells were incubated with 2 and 5 μM bharangin for 6 h and then treated with 0.1 nM TNF-α for 30 min. Nuclear extracts of the cells were assayed for NF-κB activation using EMSA. G, bharangin suppresses constitutive NF-κB activation in U266 cells. Cells were incubated with bharangin at the indicated concentrations for 6 h, and nuclear extracts of the cells were analyzed for NF-κB activation using EMSA. H, left, bharangin has no effect on TNF-α-induced AP-1 activation. KBM-5 cells were incubated with different concentrations of bharangin for 6 h, treated with 0.1 nM TNF for 30 min, and then analyzed for AP-1 activation by EMSA. Right, bharangin does not affect the binding of AP-1 to DNA. Nuclear extracts prepared from cells treated with 0.1 nM TNF-α were incubated for 30 min with the indicated concentrations of bharangin in vitro. The effect of bharangin on AP-1-DNA binding was then analyzed by EMSA. I, bharangin does not affect Oct-1 activity. KBM-5 cells were treated with indicated concentrations of bharangin for 6 h; nuclear extract was prepared and analyzed by EMSA. Results of one of three independent experiments are shown. BG, bharangin.
Cell lines.
The human cell lines U937 (leukemic monocyte lymphoma), HL-60 (promyelocytic leukemia), Jurkat (T-cell leukemia), A293 (embryonic kidney), H1299 (lung adenocarcinoma), U266, RPMI-8226, and MM.1S (multiple myeloma) were obtained from the American Type Culture Collection. The human cell line KBM-5 (chronic myeloid leukemia) was provided by Dr. Nicholas J. Donato (University of Michigan Comprehensive Cancer Center, Ann Arbor, MI) and K-562 (chronic myeloid leukemia) was obtained from Dr. Hesham Amin (The University of Texas MD Anderson Cancer Center). A mouse embryonic fibroblast (MEF) from p65(−/−) C57BL/6J mice and its p65(+/+) counterpart were provided by Dr. David Baltimore (California Institute of Technology). KBM-5 cells were cultured in Iscove's modified Dulbecco's medium with 15% FBS; K-562, HL-60, Jurkat, H1299, U937, RPMI-8226, MM.1S, and U266 cells were cultured in RPMI 1640 with 10% FBS; and A293 cells, p65(+/+) MEFs, and p65(−/−) MEFs were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS. All culture media were supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin.
Electrophoretic Mobility Shift Assay.
To examine NF-κB activation in leukemia and myeloma cells, nuclear extracts were prepared and an EMSA was performed using these extracts (Gupta et al., 2010c). The EMSAs for AP-1 and Oct-1 were performed as described for NF-κB using 32P end-labeled double-stranded oligonucleotides.
Western Blot Analysis.
Cytoplasmic, nuclear, and whole-cell extracts of untreated and treated cells were used in Western blot analysis (Gupta et al., 2011).
Invasion Assay.
To determine the effect of bharangin on TNF-α-induced invasion, the BD BioCoat tumor invasion system (BD Biosciences, San Jose, CA) was used employing a method as described previously (Gupta et al., 2010c).
Kinase Assay.
To examine the effect of bharangin on IKK and TGF-β-activated kinase 1 (TAK1) activity, an immune complex kinase assay was performed using glutathione transferase-IκBα and His-mitogen-activated protein kinase kinase 6 as substrate, respectively (Bharti et al., 2003).
Immunocytochemistry for NF-κB p65 Localization.
To determine the effect of bharangin on nuclear translocation of p65, immunocytochemical analysis was performed (Pandey et al., 2007).
NF-κB-dependent Reporter Gene Expression.
The effect of bharangin on induction of NF-κB-dependent reporter gene transcription by TNF-α, TNF receptor (TNFR) 1, TNFR-associated death domain (TRADD), TNFR-associated factor 2 (TRAF2), NF-κB–inducing kinase (NIK), TAK1/TAB1, IKK-β, and p65 was analyzed using a secretory alkaline phosphatase (SEAP) assay (Darnay et al., 1999).
Assessment of Apoptosis Using a Live/Dead Assay.
To assess the plasma membrane integrity and intracellular esterase activity, a live/dead assay was performed (Takada et al., 2004).
Analysis of Apoptosis Using a Phosphatidylserine Externalization Assay.
The loss of membrane asymmetry, which occurs when phosphatidylserine (PS) moves to the extracellular surface of the cell membrane, was measured using an annexin V staining kit according to the manufacturer's instructions.
Measurement of Apoptosis and Cell Proliferation by Mitochondrial Dehydrogenase Activity.
To determine the effect of bharangin on the proliferation of cancer cells and the cytotoxic potential of TNF-α and the chemotherapeutic agents, the mitochondrial dehydrogenase activity was measured using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide as a substrate (Bharti et al., 2003).
Transfection.
A293 (5 × 105 cells/well) were plated in six-well plates and transfected by FuGENE 6 transfection reagent with pcDNA3.1 or pcDNA expression vectors for mouse p65 or mouse p65C38S for 48 h. Thereafter, nuclear extracts from transfected cells were prepared, incubated with bharangin without or with DTT and then measured for DNA binding by EMSA. The p65(−/−) cells were also transfected with p65C38S before treatment with bharangin and TNF-α to examine the sensitivity of these cells.
Molecular Docking of Bharangin into the NF-κB p65.
The energy-minimized three-dimensional structure of bharangin was generated using ChemBioDraw 12 (CambridgeSoft Corporation, Cambridge, MA) and SYBYL 8.0 (Tripos, St. Louis, MO). For the receptor template, the crystal structure of NF-κB p65 (Protein Data Bank code 2O61) was used. For docking of bharangin into NF-κB p65, a residue-based docking mode was used in the Surflex-Dock module of SYBYL 8.0. For protomol (three dimensional space into which the substrate docked) generation, the Cys38 was used with pocket radius set to 3.0 Å, bloat to 2, and threshold to 0.5. Ring flexibility, soft grid treatment, and post- and predock energy minimization were also applied in the docking procedure. The illustrations were made using UCSF Chimera (Pettersen et al., 2004).
Statistical Analysis.
Experiments were repeated a minimum of three times, with consistent results. The resulting data are presented as the mean ± S.D. Statistical analysis was carried out using a two-tailed unpaired Student's t test. P values less than 0.05 were considered statistically significant.
Results
The aim of the present study was to determine whether bharangin has anticancer potential and, if so, via what mechanism. We used TNF-α in most of our studies because activation of the NF-κB pathway by this cytokine is relatively well defined. We carried out most of the studies using KBM-5 chronic myeloid leukemia cells because 1) they express both types of TNF receptors and 2) the inflammatory pathway in these cells is better understood. However, we also used other cell types to determine the specificity of bharangin's effect.
Bharangin Suppresses NF-κB Activation in Tumor Cells.
We first investigated whether bharangin can modulate the NF-κB activation pathway. Our results indicated that TNF-α induced NF-κB activation, whereas bharangin alone did not (Fig. 1B). Furthermore, pretreatment of cells with this quinonemethide suppressed TNF-α-induced NF-κB activation in a dose- (Fig. 1B) and time-dependent (Fig. 1C) manner. Treatment with bharangin under these conditions had little effect on cell viability.
To confirm that the band visualized using EMSA was NF-κB, we incubated nuclear extracts of TNF-α stimulated cells with antibodies against p50 and p65. Incubation with the antibodies shifted the band to a higher molecular mass (Fig. 1D), suggesting that the TNF-α-activated NF-κB complex consisted of both p50 and p65. Addition of excess unlabeled NF-κB oligonucleotide (100-fold) caused a decrease in the band's intensity, whereas addition of a mutated oligonucleotide and preimmune serum did not affect DNA binding.
Bharangin Inhibits NF-κB Activation Induced by Carcinogens, Tumor Promoters, and Other Inflammatory Agents.
A variety of stimuli, including cigarette smoke condensate (CSC), OA, PMA, and endotoxin (LPS), can activate NF-κB via different mechanisms. We found that all four of these agents activated NF-κB in KBM-5 cells and that treatment with bharangin suppressed this activation in a dose-dependent manner (Fig. 1E). These results suggest that the quinonemethide acts at a step in the NF-κB activation pathway that is common to all five agents.
Bharangin-Induced Inhibition of NF-κB Activation Is Not Cell-Type–Specific.
Because induction of NF-κB activation is mediated by multiple pathways in different cell types, we investigated whether bharangin-mediated inhibition of TNF-α-induced NF-κB activation is cell-type–specific. We observed that the diterpenoid inhibited TNF-α-induced NF-κB activation in different tumor cells (U937, HL-60, Jurkat, and K-562) in a dose-dependent manner (Fig. 1F).
Bharangin Inhibits Constitutive NF-κB Activation in Myeloma Cells.
Because most tumor cells are addicted to NF-κB and those such as human multiple myeloma cells (U266) are known to express constitutive NF-κB, we investigated whether treatment with bharangin inhibits NF-κB activation in these cells. We found that bharangin inhibited constitutive NF-κB activation in a dose-dependent manner (Fig. 1G), indicating that bharangin can suppress not only inducible but also constitutive NF-κB in tumor cells.
Bharangin Does Not Inhibit AP-1 and Oct-1 Activation in Tumor Cells.
We investigated whether bharangin inhibits activation of AP-1 and Oct-1 under the conditions in which it suppresses NF-κB. The results show that although TNF-α induced AP-1 in KBM-5 cells, pretreatment with bharangin had no affect on activity (Fig. 1H, left).
We next examined whether bharangin has ability to affect binding of AP-1 to DNA directly. We found that bharangin has no direct effect on binding of AP-1 to DNA (Fig. 1H, right). We further tested the affect of bharangin on constitutive Oct-1 in KBM-5 cells. We found that bharangin has no affect on constitutive Oct-1 (Fig. 1I).
Bharangin Inhibits IκBα Degradation.
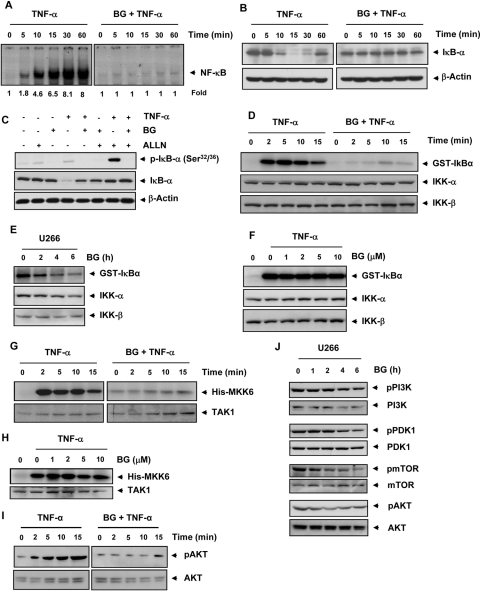
IκBα is the inhibitory subunit of the NF-κB complex that is proteolytically degraded before translocation of p65 from the cytoplasm to the nucleus. We therefore investigated whether inhibition of TNF-α-induced NF-κB activation results from suppression of IκBα degradation. The results showed that TNF-α induced NF-κB activation in a time-dependent manner, whereas bharangin completely suppressed NF-κB activation (Fig. 2A). In addition, TNF-α induced IκBα degradation in KBM-5 cells as early as 10 min and IκBα resynthesized at 60 min, whereas bharangin suppressed TNF-α-induced IκBα degradation (Fig. 2B).
Fig. 2.
Bharangin suppresses TNF-α-induced NF-κB activation via inhibition of IKK activation. A, KBM-5 cells were preincubated with 5 μM bharangin for 6 h and treated with 0.1 nM TNF-α for the indicated times. Nuclear extracts of the cells were analyzed for NF-κB activation using EMSA. The fold activation of NF-κB is indicated at the bottom. B, Western blot analysis of cytoplasmic extracts of KBM-5 cells for IκBα. C, bharangin inhibits TNF-α-induced IκBα phosphorylation. KBM-5 cells were treated with 5 μM bharangin for 6 h, incubated with 50 μg/ml N-acetyl-leucyl-leucyl-norleucinal (ALLN) for 30 min and treated with 0.1 nM TNF-α for 10 min. Cytoplasmic extracts of the cells were analyzed using Western blotting with a phosphorylated IκBα antibody (Ser32/Ser36). The same membrane was reprobed with IκBα. D, bharangin inhibits TNF-α-induced IKK activation. KBM-5 cells were pretreated with 5 μM bharangin for 6 h and then treated with 1 nM TNF-α for the indicated times. Whole-cell extracts of the cells were immunoprecipitated with an antibody against IKK-α and analyzed using an immune complex kinase assay. The effect of treatment with bharangin on IKK protein expression was determined using Western blot analysis with anti-IKK-α and anti-IKK-β antibodies. E, effect of bharangin on constitutive IKK activation in U266 cells. Cells were treated with 5 μM bharangin for the indicated times, and whole-cell extracts of the cells were used in an IKK assay. F, direct effect of treatment with bharangin on TNF-α-induced IKK activation. Whole-cell extracts of KBM-5 cells treated with 1 nM TNF-α were immunoprecipitated with an IKK-α antibody. The immunoprecipitated complex was incubated with bharangin at the indicated concentrations, and an immunocomplex kinase assay was performed. G, effect of bharangin on TNF-α-induced TAK1 activation. KBM-5 cells were preincubated with bharangin (5 μM) for 6 h and then treated with TNF-α (1 nM) for the indicated times. The protein extracts were immunoprecipitated with an antibody against TAK1 and analyzed by an immune complex kinase assay. H, bharangin does not directly affect TAK1 activity. The protein extract prepared from TNF-α treated KBM-5 cells were immunoprecipitated with an anti-TAK1 and the immunocomplex kinase assay was performed in the absence or presence of the indicated concentrations of bharangin. I, effect of bharangin on TNF-α-induced AKT activation. KBM-5 cells were incubated with 5 μM bharangin for 6 h and then treated with 1 nM TNF-α for the indicated times. Whole-cell extracts of the cells were prepared and analyzed using Western blotting with a phospho-specific AKT antibody. AKT was used as an internal control. J, bharangin inhibits constitutive activation of PI3K, PDK1, mTOR, and AKT in U266 cells. Cells were treated with bharangin (5 μM) for the indicated time. The whole-cell extracts of control and treated cells were analyzed by Western blotting using antibodies against pPI3K, pPDK1, pmTOR, and pAKT. The unphosphorylated protein was used as an internal control. BG, bharangin; GST, glutathione transferase.
Bharangin Inhibits TNF-α-Induced IκBα Phosphorylation.
Before proteolytic degradation, IκBα undergoes phosphorylation and ubiquitination. Therefore, we investigated whether inhibition of TNF-α-induced IκBα degradation is caused by inhibition of IκBα phosphorylation. Western blotting with an antibody that recognized the phosphorylated form of IκBα (Ser32 and Ser36) revealed that TNF-α induced phosphorylation of IκBα, whereas bharangin completely suppressed this phosphorylation (Fig. 2C).
Bharangin Inhibits TNF-α-Induced IKK Activation.
Because the presence of IKK is essential for TNF-α-induced IκBα phosphorylation and bharangin inhibits IκBα phosphorylation, we investigated the effect of bharangin on TNF-α-induced IKK activation. The results revealed that TNF-α activated IKK in KBM-5 cells in a time-dependent manner and that bharangin completely suppressed this activation (Fig. 2D). Bharangin also inhibited constitutive IKK activation in U266 cells (Fig. 2E).
We also sought to determine whether bharangin inhibits IKK activation by direct binding. We incubated an immunoprecipitated IKK complex with the diterpenoid at different concentrations and performed an IKK assay. We found that bharangin did not directly affect the activity of IKK (Fig. 2F).
Bharangin Inhibits TNF-α-Induced TAK1 Activation.
Because TAK1 plays an essential role in the TNF-α-induced IKK and NF-κB activation, and bharangin failed to inhibit IKK activity directly, we sought to determine whether the inhibitory effect of bharangin on IKK is mediated through TAK1. Results of the TAK1 kinase assay revealed that bharangin suppressed TNF-α-induced TAK1 activation (Fig. 2G). However, bharangin was unable to inhibit TNF-α-induced TAK1 activity directly (Fig. 2H).
Bharangin Inhibits TNF-α-Induced AKT Activation.
IKK activation has been linked with activation of AKT (Ozes et al., 1999). Therefore, we investigated the affect of this diterpenoid on TNF-α-induced AKT activity. The results indicated that TNF-α-induced AKT activation in a time-dependent manner and that bharangin inhibited this activation (Fig. 2I).
Bharangin Inhibits Constitutive Activation of PI3K, PDK1, mTOR, and AKT.
Next, we sought to determine whether bharangin has potential to inhibit constitutive AKT activation. If so, how does bharangin inhibit AKT activation was also investigated. We treated U266 cells with bharangin (5 μM) for 0 to 6 h and investigated the effect of this quinonemethide on AKT activation and on kinases upstream to AKT. We found that bharangin inhibited AKT activation in U266 cells. The inhibition in AKT activation was associated with decreased phosphorylation of PI3K, PDK1, and mTOR. However, the inhibitory affect of bharangin on mTOR phosphorylation was more prominent (Fig. 2J).
Bharangin Inhibits Constitutive and TNF-α-Induced Nuclear Translocation of p65.
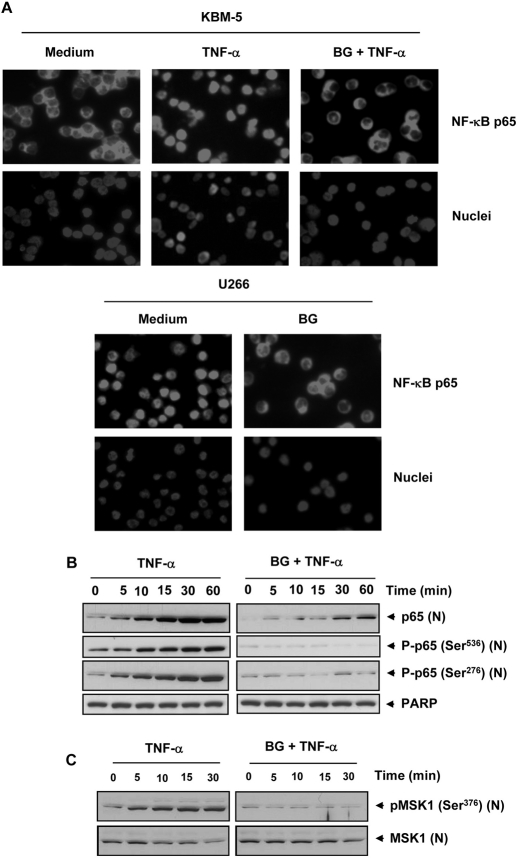
In addition, we investigated whether inhibition of TNF-α-induced IKK activity and IκBα degradation by bharangin is associated with inhibition of nuclear translocation of p65. An immunocytochemical analysis revealed that TNF-α induced translocation of p65 from the cytoplasm to the nucleus in KBM-5 cells. Pretreatment of the cells with bharangin suppressed this translocation, however (Fig. 3A, top). We also observed that the quinonemethide induced redistribution of p65 from the nucleus to the cytoplasm in U266 cells (Fig. 3A, bottom).
Fig. 3.
Bharangin inhibits TNF-α-induced phosphorylation, and nuclear translocation of p65. A, top, KBM-5 cells were treated with 5 μM bharangin for 6 h, exposed to 1 nM TNF-α for 15 min, and analyzed for p65 localization. Bottom, bharangin induced redistribution of p65 in U266 cells. The cells were incubated with 5 μM bharangin for 6 h and analyzed for p65 localization. B, KBM-5 cells were left untreated or pretreated with 5 μM bharangin for 6 h and then treated with 0.1 nM TNF-α for the indicated times. Nuclear extracts of the cells were analyzed using Western blotting with antibodies against p65 and phosphorylated p65 (Ser536 and Ser276). PARP was used as an internal control. The data are from one of three independent experiments. C, bharangin inhibits TNF-α-induced MSK1 phosphorylation. KBM-5 cells were first treated with bharangin for 6 h and then with TNF-α for the indicated time. The nuclear extract prepared from treated and untreated cells was analyzed by Western blotting using indicated antibodies. BG, bharangin.
To confirm the results of immunocytochemical analysis, we performed Western blot analysis using nuclear extracts and an antibody against p65. The results indicated that TNF-α increased nuclear level of p65 in a time-dependent manner, whereas pretreatment of the cells with bharangin abolished this level (Fig. 3B).
Bharangin Inhibits Phosphorylation of p65.
TNF-α has been shown to induce phosphorylation of p65 at multiple sites, including Ser276 and Ser536, which is required for p65's transcriptional activity (Viatour et al., 2005). Therefore, we sought to determine the effect of bharangin on p65 phosphorylation. As shown in Fig. 3B, TNF-α induced phosphorylation of p65 at Ser276 and Ser536 in KBM-5 cells, whereas pretreatment of the cells with the diterpenoid completely suppressed this phosphorylation.
Bharangin Inhibits Phosphorylation of MSK-1.
The phosphorylation of p65 at Ser276 is carried out by mitogen- and stress activated protein kinase 1 (MSK-1). Because bharangin inhibited p65 phosphorylation, we sought to determine the effect of this diterpenoid on MSK-1 activity. We found that the TNF-α induced MSK-1 phosphorylation in KBM-5 cells in a time-dependent manner, whereas pretreatment with bharangin almost completely suppressed MSK-1 phosphorylation (Fig. 3C).
Bharangin Suppresses NF-κB-Dependent Reporter Gene Expression.
Using EMSA, we showed that bharangin inhibited NF-κB activation. Because DNA binding alone does not always correlate with NF-κB-dependent gene transcription, we sought to determine whether treatment with bharangin affects TNF-α-induced reporter gene transcription. To do so, we transiently transfected A293 cells with an NF-κB-regulated SEAP reporter construct, treated them with bharangin, and then stimulated with TNF-α. We observed 8.8-fold greater induction of SEAP activity by TNF-α than by a vector control. The induction was completely suppressed by a dominant-negative IκBα, indicating the specificity of the reporter gene assay. When we pretreated the cells with diterpenoid, TNF-α-induced, NF-κB-dependent SEAP expression was inhibited in a dose-dependent manner (Fig. 4A). These results indicated that bharangin inhibits NF-κB-dependent reporter gene expression induced by TNF-α.
Fig. 4.
Bharangin inhibits NF-κB-dependent reporter gene expression induced by TNF-α and different molecules in the NF-κB signaling pathway and inhibits direct binding of p65 to DNA. A, bharangin inhibits TNF-α-induced NF-κB-dependent SEAP expression. A293 cells were transiently transfected with a plasmid containing a NF-κB–SEAP gene. Cells were then treated with bharangin at the indicated concentrations for 6 h followed by 1 nM TNF-α for 24 h. Cell supernatants were collected and assayed for SEAP activity. The results are expressed as the change in activity relative to that of the vector control. DN, dominant-negative. B, bharangin inhibits NF-κB-dependent reporter gene expression induced by TNF-α, TNFR1, TRADD, TRAF2, NIK, TAK1/TAB1, IKK-β, and p65. A293 cells were transfected with a NF-B -SEAP plasmid, expression plasmid, and control plasmid for 24 h and then treated with bharangin. Cell supernatants were then assayed for SEAP activity. Where indicated, the cells were exposed to 1 nM TNF-α for 12 h. The results are expressed as the change in activity relative to that of the vector control. The values are the means ± the SDs for three independent replicates. * and # indicate the significance of difference compared with control and TNF-α/plasmid alone groups, respectively; p < 0.05. C, bharangin inhibits of direct binding of NF-κB to DNA. Nuclear extracts of KBM-5 cells from untreated or cells treated with 0.1 nM TNF-α for 30 min were incubated with bharangin at the indicated concentrations for 30 min and then assayed for NF-κB activation using EMSA. D, DTT reverses direct binding of NF-κB to DNA. Nuclear extracts of TNF-α-treated KBM-5 cells were incubated with 5 μM bharangin for 30 min in the absence or presence of 100 μM DTT and then assayed for NF-κB activation using EMSA. E, DTT reverses bharangin-induced suppression of DNA binding of recombinant p65 in vitro. A293 cells were transfected with a p65 plasmid, and nuclear extracts of the cells were prepared and incubated with 5 μM bharangin in the absence or presence of DTT for 30 min and assayed for NF-κB activation using EMSA. F, lack of an effect of bharangin on DNA binding of mutated p65 (p65-C38S). A293 cells were transfected with p65 wild-type and mutated p65 plasmids. Nuclear extracts of transfected cells were incubated with 5 μM bharangin for 30 min and then assayed for NF-κB activation using EMSA. BG, bharangin.
Bharangin Inhibits NF-κB Activation Stimulated by TNFR1, TRADD, TRAF2, NIK, TAK1/TAB1, IKK-β, and p65.
Because TNF-α-induced NF-κB activation is mediated through the sequential interaction of TNFR1 with TRADD, TRAF2, TAK1, and IKK-β, we sought to determine the site of action for bharangin in the TNF-α signaling pathway. A SEAP assay revealed that the plasmids TNFR1, TRADD, TRAF2, NIK, TAK1/TAB1, IKK-β, and p65 significantly induced reporter gene expression and that treatment with bharangin significantly suppressed this plasmid-induced expression (Fig. 4B).
Bharangin Interferes with the Binding of p65 to DNA.
We examined whether bharangin interferes with the binding of p65 to DNA. The results showed that the quinonemethide inhibited this binding, with maximum inhibition occurring at a concentration of 5 μM (Fig. 4C). These results suggested that bharangin inhibits binding of p65 to DNA.
Bharangin-Induced Inhibition of p65 Binding to DNA Is Reversed by a Reducing Agent.
A previous study showed that cysteine residues in p65 protein are crucial for DNA binding (Chen et al., 1998). Therefore, we investigated whether the inhibitory effect of bharangin on binding of NF-κB to DNA can be reversed by treatment with the reducing agent dithiothreitol (DTT). We observed that DTT reversed the inhibitory effect of bharangin on DNA binding (Fig. 4D).
Bharangin Inhibits the Binding of Recombinant p65 to DNA, and DTT Reverses This Inhibition.
To determine whether bharangin targets to the p65 subunit of NF-κB, we transfected A293 cells with a p65-containing plasmid. EMSA results indicated that p65 bound to the DNA in these extracts and that bharangin suppressed this binding. In addition, DTT completely reversed bharangin-mediated inhibition of p65 binding to DNA (Fig. 4E).
Mutation of Cys38 in p65 to A Serine Abolishes the Inhibitory Effect of Bharangin.
Because Cys38 in p65 is susceptible to modification, we investigated whether this residue is involved in the inhibitory effect of bharangin. We transfected A293 cells with a pcDNA3.1 or pcDNA expression vector containing p65(+/+) or p65-C38S for 48 h. We then incubated nuclear extracts of transfected cells with bharangin. EMSA results showed that bharangin inhibited the binding of p65(+/+) but not mutated p65 to DNA (Fig. 4F). These results indicated that Cys38 in p65 is a target for bharangin.
Bharangin Down-Regulates the Expression of Inducible and Constitutive NF-κB-Regulated Cell Survival Proteins.
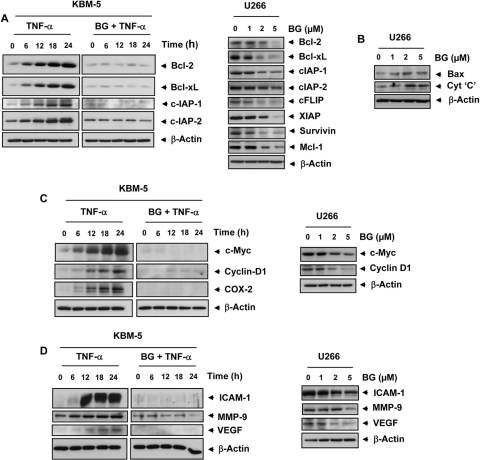
Because NF-κB is involved in the regulation of cell survival proteins and bharangin inhibits NF-κB activation, we sought to determine whether this diterpenoid has potential to modulate expression of these proteins. We found that TNF-α induced expression of antiapoptotic proteins such as Bcl-2, Bcl-xL, cIAP-1, and c-IAP-2, whereas pretreatment with bharangin down-regulated the expression of these proteins (Fig. 5A, left). Bharangin also inhibited the constitutive expression of cell survival proteins in a concentration-dependent manner (Fig. 5A, right).
Fig. 5.
Bharangin inhibits TNF-α-induced and constitutive expression of NF-κB-dependent antiapoptotic, proliferative, and metastatic proteins. KBM-5 cells were incubated with 2 μM bharangin for 6 h and then treated with 1 nM TNF-α for the indicated times. In addition, U266 cells were treated with bharangin at the indicated concentrations for 24 h. Whole-cell extracts of control and treated cells were analyzed using Western blotting with antibodies against (A) antiapoptotic, (B) proapoptotic, (C) proliferative, and (D) metastatic and angiogenic proteins. The data are from one of three independent experiments. BG, bharangin.
Bharangin Up-Regulates the Expression of Proapoptotic Proteins.
We also examined whether bharangin modulates the expression of proapoptotic proteins. We observed that this diterpenoid up-regulated the expression of Bax and cytochrome c in U266 cells in a dose-dependent manner (Fig. 5B).
Bharangin Suppresses the Expression of Proteins Involved in Tumor Cell Proliferation, Invasion, and Angiogenesis.
Furthermore, we investigated whether bharangin affects the expression of proteins involved in tumor cell proliferation, such as cyclin D1, c-Myc, and COX-2. The results indicated that the diterpenoid inhibited both inducible (Fig. 5C, left) and constitutive (Fig. 5C, right) expression of these proliferative proteins. In addition, we found that the quinonemethide inhibited both inducible and constitutive expression of ICAM-1, MMP-9, and VEGF (Fig. 5D).
Bharangin Suppresses the Proliferation of Tumor Cells.
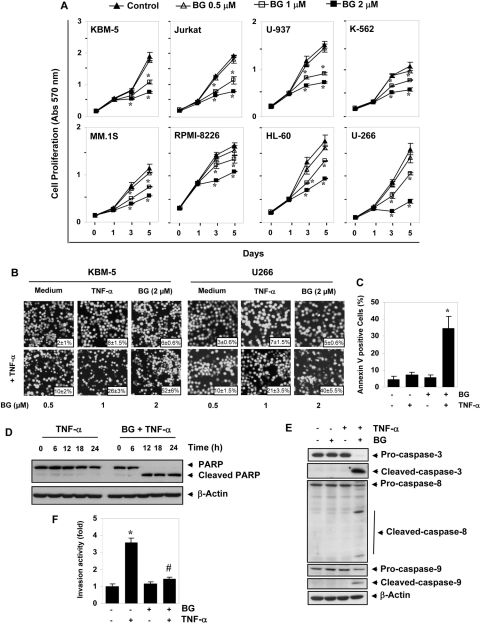
Next, we examined the effect of bharangin on the proliferation of tumor cells. We found that this diterpenoid suppressed the proliferation of myeloid leukemia (KBM-5, HL-60, U937, and K-562), T-cell lymphoma (Jurkat), and myeloma (U266, MM.1S, and RPMI-8226) cells in a dose- and time-dependent manner. Furthermore, at concentrations as low as 1 μM, bharangin inhibited the proliferation of cancer cells after 3 days of treatment (Fig. 6A).
Fig. 6.
Bharangin suppresses proliferation and potentiates the TNF-α-induced apoptosis in tumor cells. A, the indicated cells (3000/100 μl) were seeded in quadruplicate treated with bharangin at the indicated concentrations, and analyzed for mitochondrial dehydrogenase activity by measuring the absorbance at 570 nm on days 0, 1, 3, and 5. The values are the means ± the S.D. for the four replicates. B, KBM-5 and U266 cells were pretreated with bharangin at the indicated concentrations for 6 h and then incubated with 1 nM TNF-α for 24 h. The cells were stained with a live/dead assay reagent for 30 min and analyzed under a fluorescence microscope. Magnification, 100×. Values across each photomicrograph represent mean ± S.D. of apoptotic cells. C, bharangin potentiate TNF-α-induced early apoptosis in KBM-5 cells. Cells were pretreated with 2 μM bharangin for 6 h and then incubated with 1 nM TNF-α for 24 h. Apoptosis was then assessed using a fluorescein isothiocyanate-conjugated annexin V antibody with flow cytometry. D, treatment of cells with bharangin before TNF-α induces PARP cleavage. KBM-5 cells were pretreated with 2 μM bharangin for 6 h and then incubated with 1 nM TNF-α for the indicated times. Whole-cell extracts were prepared and analyzed using Western blotting with a PARP antibody. E, bharangin potentiates TNF-α-induced caspase activation. KBM-5 cells were incubated with 2 μM bharangin for 6 h and then treated with 1 nM TNF-α for 24 h. Whole-cell extracts were prepared and analyzed using Western blotting with the indicated antibodies. F, bharangin suppresses TNF-α-induced cell invasion. H1299 cells (2.5 × 104) were seeded into the top chamber of a Matrigel invasion chamber system overnight in the absence of serum and then treated with 2 μM bharangin for 6 h. Cells were then treated with TNF-α in the presence of 1% serum and assayed for invasion as described under Materials and Methods. The value for control was set to 1.0. The values are the means ± the SDs of three replicates. * and # indicate significance of the difference compared with the control and the TNF-α group, respectively; p < 0.05. BG, bharangin.
Bharangin Potentiates Apoptosis Induced by TNF-α and Chemotherapeutic Agents in Tumor Cells.
TNF-α is one of the most effective apoptosis-inducing cytokines (Aggarwal, 2003). We examined whether bharangin potentiates TNF-α-induced apoptosis. As determined by examining intracellular esterase activity, we found that bharangin enhanced TNF-α-induced apoptosis in KBM-5 and U266 cells in a dose-dependent manner. Specifically, TNF-α-induced apoptosis was increased from 8 to 52% in KBM-5 cells and from 7 to 40% in U266 cells (Fig. 6B).
To confirm the results of intracellular esterase activity, we assessed the mitochondrial dehydrogenase activity. The results indicated that bharangin potentiated TNF-α-induced apoptosis not only in KBM-5 and U266 cells but also in other cancer cells (Table 1). We next examined whether treatment with bharangin can enhance apoptosis induced by chemotherapeutic agents. We found that this diterpenoid potentiated the apoptotic effects of both 5-fluorouracil and thalidomide in various tumor cells (Table 1).
TABLE 1.
Effect of bharangin on apoptosis induced by TNF-α and chemotherapeutic agents in tumor cells
Cells were pretreated with the indicated concentrations of bharangin, and then incubated with chemotherapeutic agents (1 nM TNF-α, 0.1 μM 5-FU, and 10 μg/ml thalidomide) for 24 h. Cell viability was analyzed by measuring mitochondrial dehydrogenase activity. Values given are mean ± S.D. of four replicates.
| Cell Lines | Cell Viability |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medium | TNF-α |
5-FU |
Thalidomide |
||||||||||
| 2 μM BG | 0 μM BG | 0. μM BG5 | 1 μM BG | 2 μM BG | 0 μM BG | 0.5 μM BG | 1 μM BG | 2 μM BG | 0 μM BG | 0.5 μM BG | 1 μM BG | 2 μM BG | |
| % | |||||||||||||
| KBM-5 | 96 ± 2.5 | 95 ± 5.4 | 88 ± 6.6 | 64 ± 3.3* | 49 ± 3.4* | 96 ± 3.9 | 91 ± 3.7 | 71 ± 5.2* | 53 ± 5.5* | 94 ± 7.0 | 87 ± 3.4 | 70 ± 3.6* | 49 ± 4.7* |
| Jurkat | 94 ± 4.3 | 95 ± 3.5 | 90 ± 3.1 | 63 ± 4.1* | 40 ± 5.7* | 97 ± 3.1 | 90 ± 3.4 | 74 ± 4.1* | 49 ± 2.2* | 96 ± 2.9 | 88 ± 3.3 | 76 ± 4.3* | 47 ± 3.5* |
| U-937 | 93 ± 8.5 | 94 ± 2.9 | 84 ± 2.7* | 66 ± 2.2* | 43 ± 2.8* | 95 ± 2.5 | 89 ± 4.6 | 72 ± 4.1* | 54 ± 2.8* | 92 ± 4.9 | 83 ± 3.5* | 64 ± 5.9* | 42 ± 2.9* |
| K-562 | 92 ± 5.5 | 95 ± 2.4 | 91 ± 3.2 | 56 ± 2.5* | 43 ± 3.5* | 94 ± 6.2 | 87 ± 3.2* | 59 ± 5.4* | 49 ± 3.4* | 96 ± 3.0* | 80 ± 3.9 | 63 ± 2.9* | 50 ± 4.1* |
| U-266 | 95 ± 4.0 | 97 ± 3.2 | 86 ± 4.4* | 63 ± 2.9* | 47 ± 3.6* | 95 ± 1.9 | 88 ± 3.0 | 74 ± 4.7* | 59 ± 2.7* | 94 ± 5.1 | 89 ± 5.1 | 79 ± 5.8* | 56 ± 3.9* |
| MM0.1S | 94 ± 6.7 | 94 ± 4.7 | 89 ± 6.8 | 74 ± 5.6* | 53 ± 3.0* | 94 ± 3.1 | 86 ± 5.9* | 79 ± 3.3* | 54 ± 3.2* | 94 ± 4.8 | 87 ± 5.4* | 74 ± 3.0* | 51 ± 3.3* |
| RPMI-8226 | 92 ± 4.8 | 94 ± 4.9 | 85 ± 7.0* | 67 ± 2.0* | 44 ± 5.8* | 95 ± 2.1 | 83 ± 3.6* | 69 ± 3.0* | 51 ± 5.5* | 96 ± 3.3 | 87 ± 5.1* | 74 ± 3.0* | 52 ± 3.3* |
| HL-60 | 91 ± 7.3 | 96 ± 2.3 | 87 ± 2.3* | 73 ± 2.1* | 50 ± 2.8* | 95 ± 2.3 | 88 ± 2.8 | 74 ± 2.5* | 47 ± 2.6* | 94 ± 4.6* | 84 ± 2.9 | 72 ± 3.3* | 46 ± 2.9* |
P < 0.05, significance of difference compared with control.
BG, bharangin; 5-FU, 5-fluorouracil.
One of the very early events of apoptosis is externalization of the membrane PS on the cell surface. Because of annexin V's affinity for PS, staining for annexin V can be used to detect early apoptotic cells. Therefore, we examined the effect of treatment with bharangin on PS externalization induced by TNF-α. We found that the number of annexin V-positive cells increased significantly when we pretreated the cells with bharangin before TNF-α (Fig. 6C).
Bharangin in Combination with TNF-α Induces PARP Cleavage and Caspase Activation.
One of the hallmarks of apoptosis is the cleavage of PARP from the native 116 to 89 kDa protein that is mediated through activation of caspases. We therefore investigated whether treatment with quinonemethide enhances TNF-α-induced activation of caspase-3, caspase-8, and caspase-9 and cleavage of PARP. We found that TNF-α alone had little effect on PARP cleavage and caspase activation but that pretreatment of the cells with bharangin sensitized them to PARP cleavage (Fig. 6D) and caspase activation (Fig. 6E).
Bharangin Suppresses TNF-α-Induced Tumor-Cell Invasion.
We also investigated whether treatment with bharangin can modulate TNF-α-induced tumor-cell invasion. We found that TNF-α increased the tumor cell invasion almost 3.6-fold. Whereas treatment with bharangin alone had no effect, pretreatment significantly reduced TNF-α-induced tumor cell invasion (Fig. 6F).
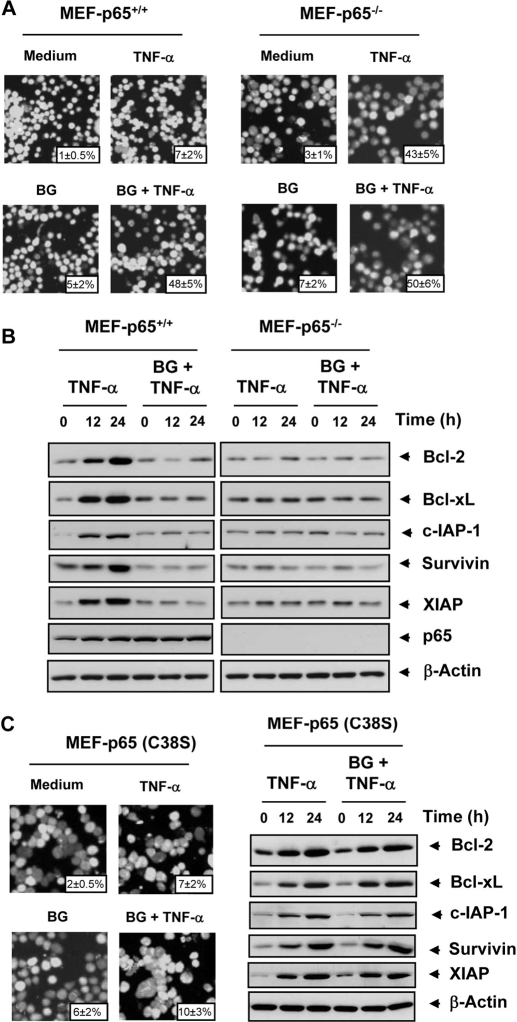
Deletion of p65 Abolishes the Effect of Bharangin on TNF-α-Induced Apoptosis.
p65 is known to be the active subunit of NF-κB, which regulates antiapoptotic gene products. Thus far, all of the results have indicated that bharangin interferes with the NF-κB pathway. We investigated whether deletion of p65 affects the potentiation of TNF-α-induced apoptosis by bharangin. We found that bharangin potentiated TNF-α-induced apoptosis from 7 to 48% in p65(+/+) MEFs. In p65(−/−) MEFs lacking functional NF-κB, TNF-α induced apoptosis by 43%. The diterpenoid neither had an effect alone nor significantly potentiated the apoptosis induced by TNF-α in p65(−/−) MEFs (Fig. 7A). Taken together, these results indicated that deletion of p65 abolished the potentiation of TNF-α-induced apoptosis by bharangin.
Fig. 7.
Potentiation of TNF-α-induced apoptosis and suppression of antiapoptotic protein expression by bharangin requires functional p65. A, the p65(+/+) and p65(−/−) cells were pretreated with 2 μM bharangin for 6 h and then incubated with 1 nM TNF-α for 24 h. Apoptosis in the cells was assessed using a live/dead assay. Magnification, 100×. Values across each photomicrograph represent mean ± S.D. of apoptotic cells. B, the p65(+/+) and p65(−/−) cells were pretreated with 2 μM bharangin for 6 h and then incubated with 1 nM TNF-α for the indicated times. Whole-cell extracts of cells were prepared and analyzed using Western blotting with the indicated antibodies. C, The p65(−/−) cells were transfected with p65 (C38S) plasmid before treatment with bharangin and TNF-α. The apoptosis was examined using live/dead assay (left) and antiapoptotic proteins were examined by Western blotting (right). The data are from one of three independent experiments. BG, bharangin.
Suppression of TNF-α-Induced Expression of Antiapoptotic Proteins by Bharangin Requires Functional p65.
We determined whether the presence of functional p65 is essential for the inhibitory effects of bharangin on TNF-α-induced expression of antiapoptotic gene products, we used both p65(+/+) and p65(−/−) MEFs. The results showed that TNF-α induced antiapoptotic proteins and that bharangin inhibited this induction in p65(+/+) MEFs. In p65(−/−) MEFs, however, TNF-α failed to induce expression of antiapoptotic proteins (Fig. 7B). These results suggested that the presence of functional p65 is essential for the induction and inhibition of antiapoptotic proteins.
Introduction of p65 C38S in MEF-p65(−/−) Cells Decreases Sensitivity to Bharangin.
Because p65 (C38S) mutant exhibited resistance to NF-κB inhibition by bharangin, we sought to determine the sensitivity of these mutants to apoptosis potentiation by bharangin. For this, we transfected p65(−/−) cells with p65 (C38S) plasmid, treated first with bharangin and then stimulated with TNF-α. We found that TNF-α did not induce apoptosis and bharangin potentiate the apoptosis (Fig. 7C, left). Finally, we examined the ability of bharangin to modulate antiapoptotic proteins in these cells. Although TNF-α induced antiapoptotic proteins in a time dependent manner, pretreatment with bharangin had an insignificant affect (Fig. 7C, right). Taken together, these results suggest the importance of p65 C38 in the determining sensitivity against bharangin.
Discussion
Up to 70% of anticancer drugs are derived from natural products. Traditional medicine using natural products has served mankind for thousands of years, but neither their active components nor their mechanisms of action are well understood. In the present study, we identified bharangin, a compound derived from a plant routinely used in traditional medicine and delineated the cell signaling pathway by which its anticancer effects are mediated. Our results demonstrated that this diterpenoid exhibits anticancer, anti-inflammatory, and immunomodulatory effects via modulation of pro-inflammatory pathways.
We found that bharangin inhibits NF-κB activation induced by proinflammatory cytokine, tumor promoter, cigarette smoke, and endotoxin. The inhibition of NF-κB activation by bharangin was specific in that it failed to inhibit AP-1 and Oct-1 activation. How bharangin inhibits TNF-α-induced NF-κB activation was investigated. The IKK complex is an important site of integration of signals that regulate the NF-κB pathway. We found that this quinonemethide inhibits IKK activity, leading to inhibition of phosphorylation and degradation of IκBα and nuclear translocation of p65. Our results indicated that this diterpenoid is not a direct inhibitor of IKK activation, suggesting that kinases upstream of IKK are involved. Several kinases, including AKT (Ozes et al., 1999) and TAK1, reportedly function upstream of IKK. Our results indicated that bharangin might inhibit IKK activation via inhibition of TAK1 and AKT activation. The ability of bharangin to inhibit PI3K, PDK1, and mTOR activation indicates that bharangin may mediate its inhibitory affect on AKT activation through PI3K/PDK1/mTOR pathway. However, how this diterpenoid inhibits TAK1 remains to be elucidated.
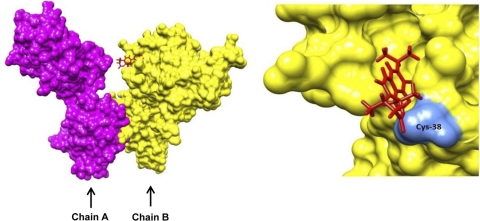
We also found that bharangin inhibits NF-κB activation by modifying p65 subunit of NF-κB. These results are consistent with our findings that bharangin suppressed p65-induced NF-κB reporter activity. Previous studies have shown that a Cys38 residue in p65 is crucial for DNA binding (Chen et al., 1998). This residue contacts a phosphate in the DNA backbone, is responsible for redox regulation of NF-κB (Ghosh et al., 1995; Müller et al., 1995), and has to be in reduced state to bind DNA (Matthews et al., 1993). We observed that bharangin inhibited the DNA binding of recombinant p65(+/+). However, a p65 mutant (C38S) was resistant to this inhibition, suggesting that bharangin can modify Cys38. There are two possible ways by which bharangin could modify p65 at Cys38: 1) redox cycling and 2) direct interaction. Redox cycling results in the generation of reactive oxygen species (ROS) and depletes cellular glutathione level. A previous study demonstrated that the depletion of cellular GSH by diethyl maleate prevented NF-κB induction in rat hepatocytes (Vos et al., 1999). Although our unpublished observations indicated bharangin's potential to generate ROS in cells, its effect is unlikely to be mediated through ROS generation because p65-DNA binding was inhibited in vitro. This eliminates the first possibility. We found that bharangin has potential to dock into the NF-κB p65. The docking produced a positive total score value of 4.31 indicating strong interaction of the ligand with the protein. Furthermore, the docked structure showed that the bharangin docked in a groove on the receptor surface adjacent to Cys38. The diterpenoid was found to sit in the groove by forming two hydrogen bonds, one with Lys37 (2.204 Å) and another with Cys38 (2.023 Å) (Fig. 8). Although these observations suggest bharangin's potential to modify p65 at Cys38 by direct interaction, involvement of indirect intermediates cannot be completely ruled out. Our observations thus indicate that bharangin can inhibit NF-κB activation pathway by inhibiting IKK activation and modifying NF-κB p65. Because IKK activation precedes p65 nuclear translocation, we speculate that inhibition of IKK activation by bharangin is a primary step, whereas p65 modification is a secondary step.
Fig. 8.
Putative bharangin binding site in NF-κB p65. Left, the surface diagram of p65 (chain A, magenta; chain B, yellow) docked with bharangin (red). Right, expanded view of the region in chain B where bharangin is docked. The groove adjacent to Cys38 (cornflower blue) is the possible binding site of the ligand.
In addition, we found that treatment with bharangin inhibited constitutive NF-κB activation in myeloma cells. Researchers have reported that constitutive NF-κB is essential for the proliferation of a number of tumor cells (Bharti et al., 2003; Shishodia et al., 2005; Jackson-Bernitsas et al., 2007). It is not fully understood why tumor cells express constitutively active NF-κB, but IKK has been implicated (Bharti et al., 2003; Shishodia et al., 2005). In our observations, bharangin inhibited constitutive IKK activation, which may have accounted for bharangin's inhibition of constitutive NF-κB activation and suppression of tumor-cell proliferation. TNF-α activates NF-κB via sequential recruitment of TNFR1, TRADD, TRAF2, NIK, TAK1/TAB1, and IKK. Bharangin suppressed the activation of NF-κB induced by overexpression of these intermediates in our study.
Drug resistance manifested by failure to induce apoptosis is one of the major reasons for failure of cancer therapy. We found that bharangin potentiated apoptosis induced by TNF-α and chemotherapeutic agents in tumor cells. Thus, our results provide an opportunity to use this diterpenoid in combination with existing drugs to induce apoptosis in cancer cells. In this study, we investigated how bharangin potentiates apoptosis in detail. We found that this quinonemethide enhanced cleavage of caspase-3, caspase-8, and caspase-9, suggesting that it can potentiate apoptosis via both extrinsic and intrinsic pathways. We also found that bharangin suppressed the expression of cell-survival proteins. Overexpression of these proteins has been associated with survival and development of chemoresistance and radioresistance in numerous tumor types (Hanahan and Weinberg, 2000). Therefore, inhibition of the expression of these gene products may account for potentiation of apoptosis by bharangin. We also found that bharangin up-regulated expression of the proapoptotic protein Bax (Bcl-2-associated X protein), suggesting another mechanism by which bharangin enhances TNF-α-induced apoptosis.
Furthermore, we found that treatment with bharangin inhibited cancer-cell proliferation. Down-regulation of expression of gene products involved in cell proliferation (c-Myc, cyclin D1, and COX-2) may account for observed inhibition of cancer-cell proliferation. In agreement with these observations, previous reports showed that diterpenoids such as oridonin (Ikezoe et al., 2005), ponicidin (Hsieh et al., 2005), and eriocalyxin B (Zhang et al., 2010) can suppress proliferation of a wide variety of cancer cells. In addition, we found that this diterpenoid suppressed the expression of MMP-9 and ICAM-1, two major mediators of tumor-cell invasion (Watanabe et al., 1993; Sun et al., 1999). The down-regulation of expression of these proteins was concomitant with suppression of tumor-cell invasion, suggesting that bharangin has a role in prevention of tumor cell invasion and metastasis. In addition, VEGF is a major signaling mediator involved in tumor angiogenesis (Berse et al., 1992). Bharangin's potential to inhibit constitutive and TNF-α-induced VEGF expression suggests that this diterpenoid is an antiangiogenic agent, as well. The genes involved in tumor cell survival, proliferation, invasion, and angiogenesis are all regulated by NF-κB (Aggarwal, 2004). The observed inhibition of NF-κB activation may have accounted for down-regulation of the expression of these gene products by bharangin. In fact, we found that bharangin was unable to potentiate TNF-α-induced apoptosis in p65(−/−) cells. That the MEF p65(−/−)cells harboring C38S mutation were insensitive to the bharangin suggests the importance of Cys38 in determining sensitivity against bharangin. We found that bharangin also inhibited proliferation of cancer cells (KBM-5, U-937, HL-60, Jurkat, and K-562) that do not express constitutive NF-κB. Therefore, it is likely that bharangin is modulating pathways other than NF-κB to suppress tumor cell proliferation.
Overall, our results indicate that this diterpenoid exhibits anticancer potential through inhibition of NF-κB activation pathway. However, further studies in various animal models are required before its clinical potential is fully realized in cancer prevention and/or treatment.
Acknowledgments
We thank Don Norwood from the Department of Scientific Publications for carefully proofreading the manuscript and Dr. Bryant Darnay for supplying the His-mitogen-activated protein kinase kinase 6 protein.
This research was supported by the National Institutes of Health National Cancer Institute [Grant P01-CA124787-01A2]; and a grant from the M.D. Anderson Cancer Center for Targeted Therapy. B.B.A. is the Ransom Horne, Jr., Professor of Cancer Research.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.073122.
- NF-κB
- nuclear factor-κB
- IκBα
- inhibitor of nuclear factor-κBα
- IKK
- IκB kinase
- TNF
- tumor necrosis factor
- FBS
- fetal bovine serum
- MEF
- mouse embryonic fibroblast
- EMSA
- electrophoretic mobility shift assay
- AP-1
- activator protein-1
- Oct-1
- octameric transcription factor 1
- TAK1
- TGF-β-activated kinase 1
- TNFR
- tumor necrosis factor receptor
- TRADD
- TNFR-associated death domain
- TRAF
- TNFR-associated factor
- NIK
- NF-κB-inducing kinase
- TAB1
- TAK-1 binding protein-1
- SEAP
- secretory alkaline phosphatase
- PS
- phosphatidylserine
- DTT
- dithiothreitol
- CSC
- cigarette smoke condensate
- OA
- okadaic acid
- PMA
- phorbol 12-myristate 13-acetate
- LPS
- lipopolysaccharide
- PI3K
- phosphatidylinositol 3-kinase
- PDK1
- phosphoinositide-dependent protein kinase-1
- mTOR
- mammalian target of rapamycin
- AKT
- AKT8 virus oncogene cellular homolog
- MSK
- mitogen- and stress activated protein kinase
- ICAM
- intercellular adhesion molecule
- COX
- cyclooxygenase
- MMP
- matrix metalloproteinase
- VEGF
- vascular endothelial growth factor
- PARP
- poly (ADP-ribose) polymerase
- ROS
- reactive oxygen species
- Bcl-2
- B-cell lymphoma-2
- Bcl-xL
- B cell lymphoma extra large
- c-Myc
- cellular-myelocytomatosis.
Authorship Contributions
Participated in research design: Gupta and Aggarwal.
Conducted experiments: Gupta, Kannapan, Rahman, and Das.
Contributed new reagents or analytical reagents: Francis, Raveendran, and Nair.
Performed data analysis: Gupta, Kim, and Aggarwal.
Wrote or contributed to the writing of the manuscript: Gupta and Aggarwal.
References
- Aggarwal BB. (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3:745–756 [DOI] [PubMed] [Google Scholar]
- Aggarwal BB. (2004) Nuclear factor-kappaB: the enemy within. Cancer Cell 6:203–208 [DOI] [PubMed] [Google Scholar]
- Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. (1992) Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell 3:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti AC, Donato N, Singh S, Aggarwal BB. (2003) Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood 101:1053–1062 [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit L, Chantaptavan V. (1993) Identification and specification of khao-yen-neua and khao-yen-tai. Thai J Pharm Sci 1:79–90 [Google Scholar]
- Chen YQ, Ghosh S, Ghosh G. (1998) A novel DNA recognition mode by the NF-kappa B p65 homodimer. Nat Struct Biol 5:67–73 [DOI] [PubMed] [Google Scholar]
- Darnay BG, Ni J, Moore PA, Aggarwal BB. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J Biol Chem 274(12):7724–7731, 1999 [DOI] [PubMed] [Google Scholar]
- Dhamija I, Pai K, Setty M, Manjula S. (2008) Evaluation of in vitro and in vivo antitumour activity of alcoholic and ethyl acetate extracts of premna herbacea roxb against tumor models in mice. Ind J Pharmacol 40(Suppl 2):S80–S81 [Google Scholar]
- Ghosh G, van Duyne G, Ghosh S, Sigler PB. (1995) Structure of NF-kappa B p50 homodimer bound to a kappa B site. Nature 373:303–310 [DOI] [PubMed] [Google Scholar]
- Gupta SC, Kim JH, Prasad S, Aggarwal BB. (2010a) Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 29:405–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Prasad S, Reuter S, Kannappan R, Yadav VR, Ravindran J, Hema PS, Chaturvedi MM, Nair M, Aggarwal BB. (2010c) Modification of cysteine 179 of IkappaBalpha kinase by nimbolide leads to down-regulation of NF-kappaB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J Biol Chem 285:35406–35417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Reuter S, Phromnoi K, Park B, Hema PS, Nair M, Aggarwal BB. (2011) Nimbolide sensitizes human colon cancer cells to TRAIL through ROS- and ERK-dependent up-regulation of death receptors, p53, and Bax. J Biol Chem 286:1134–1146 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gupta SC, Sundaram C, Reuter S, Aggarwal BB. (2010b) Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta 1799:775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. (2000) The hallmarks of cancer. Cell 100:57–70 [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Wijeratne EK, Liang JY, Gunatilaka AL, Wu JM. (2005) Differential control of growth, cell cycle progression, and expression of NF-kappaB in human breast cancer cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin, diterpenoids from the chinese herb Rabdosia rubescens. Biochem Biophys Res Commun 337:224–231 [DOI] [PubMed] [Google Scholar]
- Ikezoe T, Yang Y, Bandobashi K, Saito T, Takemoto S, Machida H, Togitani K, Koeffler HP, Taguchi H. (2005) Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits the proliferation of cells from lymphoid malignancies in association with blockade of the NF-kappa B signal pathways. Mol Cancer Ther 4:578–586 [DOI] [PubMed] [Google Scholar]
- Itharat A, Singchangchai P, Ratanasuwan P. (1998) Wisdom of Southern Thai traditional doctors. pp. 1–6 Research Report of Prince of Songkla University, Songkla, Thailand [Google Scholar]
- Jackson-Bernitsas DG, Ichikawa H, Takada Y, Myers JN, Lin XL, Darnay BG, Chaturvedi MM, Aggarwal BB. Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-kappaB activation and proliferation in human head and neck squamous cell carcinoma. Oncogene 26(10):1385–1397, 2007 [DOI] [PubMed] [Google Scholar]
- Kirtikar KR, Basu BD. (1918) Indian Medicinal Plants, Vol. III, pp. 883–884 Lalit Mohan Basu, Allahabad, India [Google Scholar]
- Kumar R, Pai K, Dhamija I, Setty M, Manjula S, Ramalingayya G. (2008) An appraisal of the antitumor activity of alcoholic extract of Premna herbacea roxb in Ehrlich.s ascetic carcinoma model. Ind J Pharmacol 40 (Suppl 2):S67 [Google Scholar]
- Lakshmi VV, Sridhar P, Polasa H. (1989) Loss of plasmid linked antibiotic resistance in Escherichia coli on treatment with some phenolic compounds. FEMS Microbiol Lett 48:275–278 [DOI] [PubMed] [Google Scholar]
- Matthews JR, Kaszubska W, Turcatti G, Wells TN, Hay RT. (1993) Role of cysteine62 in DNA recognition by the P50 subunit of NF-kappa B. Nucleic Acids Res 21:1727–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CW, Rey FA, Sodeoka M, Verdine GL, Harrison SC. Structure of the NF-kappa B p50 homodimer bound to DNA. Nature 373(6512):311–317, 1995 [DOI] [PubMed] [Google Scholar]
- Murthy MM, Subramanyam M, Giridhar KV, Jetty A. (2006) Antimicrobial activities of bharangin from Premna herbaceae Roxb. and bharangin monoacetate. J Ethnopharmacol 104:290–292 [DOI] [PubMed] [Google Scholar]
- Narayanan N, Thirugnanasambantham P, Viswanathan S, Kannappa Reddy M, Vijayasekaran V, Sukumar E. (2000) Antipyretic, antinociceptive and anti-inflammatory activity of Premna herbacea roots. Fitoterapia 71:147–153 [DOI] [PubMed] [Google Scholar]
- Nayar R, Yoganarsimhan S, Subramanyam K. (1976) Pharmacognosy of a local market sample of bharangin: pygmacopremna herbaceae. Ind J Pharmacol 38:39–44 [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401(6748):82–85, 1999 [DOI] [PubMed] [Google Scholar]
- Pai K, Dhamija I, Setty M, Manjula S. (2008) Antitumor activity of ethyl acetate extracts of Premna herbacea roxb in ehrlich.s ascitic carcinoma model. Ind J Pharmacol 40 (Suppl 2):S67–S68 [Google Scholar]
- Pandey MK, Sandur SK, Sung B, Sethi G, Kunnumakkara AB, Aggarwal BB. (2007) Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-kappaB and NF-kappaB-regulated gene expression through direct inhibition of IkappaBalpha kinase beta on cysteine 179 residue. J Biol Chem 282:17340–17350 [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612 [DOI] [PubMed] [Google Scholar]
- Sankaram A, Marthanda Murthi M, Bhaskaraiah K, Narsimha Rao G, Subramanyam M, Shoolery J. (1988) Bharangin, a novel diterpenoid quinonemethide from Pigmacopremna herbaceae (Roxb.) Moldenke. Tetrahedron Lett 29:245–248 [Google Scholar]
- Sankaram A, Rao G. (1978) Bharangin, a novel diterpenoid quinonemethide from Pygmacopremna herbaceae, in Proceedings of IUPAC 11th International Symposium of Chemistry of Natural Products; 1989 Sep; Varna, Bulgaria 2:97–100 [Google Scholar]
- Sathish T, Brahmaiah P, Sathya K, Bhojaraju P, Naik NG, Kezia D, Prakasam RS. (2009) A novel RP-HPLC method for the determination of bharangin in Ghantu bharangi crude extracts. Pak J Pharm Sci 22:68–73 [PubMed] [Google Scholar]
- Shishodia S, Amin HM, Lai R, Aggarwal BB. (2005) Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol 70:700–713 [DOI] [PubMed] [Google Scholar]
- Sun JJ, Zhou XD, Liu YK, Tang ZY, Feng JX, Zhou G, Xue Q, Chen J. (1999) Invasion and metastasis of liver cancer: expression of intercellular adhesion molecule 1. J Cancer Res Clin Oncol 125:28–34 [DOI] [PubMed] [Google Scholar]
- Takada Y, Khuri FR, Aggarwal BB. (2004) Protein farnesyltransferase inhibitor (SCH 66336) abolishes NF-kappaB activation induced by various carcinogens and inflammatory stimuli leading to suppression of NF-kappaB-regulated gene expression and up-regulation of apoptosis. J Biol Chem 279:26287–26299 [DOI] [PubMed] [Google Scholar]
- Viatour P, Merville MP, Bours V, Chariot A. (2005) Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends in biochemical sciences 30:43–52 [DOI] [PubMed] [Google Scholar]
- Vos TA, Van Goor H, Tuyt L, De Jager-Krikken A, Leuvenink R, Kuipers F, Jansen PL, Moshage H. (1999) Expression of inducible nitric oxide synthase in endotoxemic rat hepatocytes is dependent on the cellular glutathione status. Hepatology 29:421–426 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Nakanishi I, Yamashita K, Hayakawa T, Okada Y. (1993) Matrix metalloproteinase-9 (92 kDa gelatinase/type IV collagenase) from U937 monoblastoid cells: correlation with cellular invasion. J Cell Sci 104:991–999 [DOI] [PubMed] [Google Scholar]
- Zhang YW, Jiang XX, Chen QS, Shi WY, Wang L, Sun HD, Shen ZX, Chen Z, Chen SJ, Zhao WL. (2010) Eriocalyxin B induces apoptosis in lymphoma cells through multiple cellular signaling pathways. Exp Hematol 38:191–201 [DOI] [PubMed] [Google Scholar]