Abstract
Because constitutive activation of signal transducers and activators of transcription-3 (STAT3) has been linked with cellular transformation, survival, proliferation, chemoresistance, and angiogenesis of various tumor cells, agents that can suppress STAT3 activation have potential as cancer therapeutics. In the present report, we identified a flavone from the leaves of a Thai plant, Gardenia obtusifolia, 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone (PMF), that has the ability to inhibit STAT3 activation. PMF inhibited both constitutive and interleukin-6-inducible STAT3 activation in multiple myeloma (MM) cells, as indicated by suppression of STAT3 phosphorylation, nuclear translocation, DNA binding, and STAT3-regulated gene expression. The inhibition of STAT3 by PMF was reversible. We found that the activation of various kinases including Janus-like kinase (JAK)-1, JAK-2, c-Src, extracellular signal-regulated kinases 1 and 2, AKT, and epidermal growth factor receptor, implicated in STAT3 activation, were inhibited by the flavone. It is noteworthy that pervanadate suppressed the ability of PMF to inhibit the phosphorylation of STAT3, suggesting that protein tyrosine phosphatase was involved. PMF induced the expression of SHP-1 and was linked to the dephosphorylation of STAT3, because its deletion by small interfering RNA abolished the PMF-induced constitutive and inducible STAT3 inhibition. STAT3 inhibition led to the suppression of proteins involved in proliferation (cyclin D1 and c-myc), survival (survivin, Mcl-1, Bcl-xL, Bcl-2, and cIAP-2), and angiogenesis (vascular endothelial growth factor). Finally, PMF inhibited proliferation and induced apoptosis of MM cells. PMF also significantly potentiated the apoptotic effects of Velcade and thalidomide in MM cells. Overall, these results suggest that PMF is a novel blocker of STAT3 activation and thus may have potential in suppression of tumor cell proliferation and reversal of chemoresistance in MM cells.
Introduction
Signal transducers and activators of transcription (STAT) is a family of transcription factors that was first discovered in 1994 and ever since has been linked with a wide variety of physiological and pathological processes (Aggarwal et al., 2006). Of six different STATs that have been identified, one of them, STAT3, has been most closely associated with inflammation, immunity, and tumorigenesis (Yu et al., 2009) and has been labeled as an oncogene (Bromberg et al., 1999). Although activation of STAT3 by most cytokines is transient, persistent activation of STAT3 is observed in most tumors (Lee et al., 2010). Constitutive activation of the STAT3 contributes to the transformation, survival, proliferation, chemoresistance, and angiogenesis of various tumors (Ram et al., 2000).
The phosphorylation of STAT3 is mediated through the activation of nonreceptor protein tyrosine kinases, including Janus-like kinase (JAK)-1, -2, and -3, TYK2, and c-Src kinase. Agents that disrupt this pathway would be good candidates as STAT3 inhibitors. STAT3 is activated by several factors including IL-6, a cytokine essential for the survival of many cell lineages, including multiple myeloma (MM) (Catlett-Falcone et al., 1999). Upon activation, STAT3 undergoes phosphorylation at serine 727 and at tyrosine 705, dimerization, nuclear translocation, and DNA binding, which in turn leads to transcription of various genes, including apoptosis inhibitors (survivin, Mcl-1, Bcl-xL, Bcl-2, and cIAP-2), cell cycle regulators (cyclin D1 and c-myc), and inducers of angiogenesis [vascular endothelial growth factor (VEGF)]. Hence, activation of STAT3 plays an important role in chemoresistance, and inhibition of STAT3 may overcome this chemoresistance (Aggarwal et al., 2006).
Because STAT3 is dispensable in most normal tissue, targeted inhibition of STAT3 is an attractive therapy for patients with cancer. Thus, agents that can suppress STAT3 activation have potential as cancer therapeutics. The possibility of using natural agents to suppress tumor growth and overcome chemoresistance, through the suppression of STAT3, without the debilitating side effects of conventional chemotherapy, is an attractive strategy. One possible source for such an agent is Gardenia obtusifolia (Rubiaceae family). Extracts of this plant are used to inhibit implantation (Luechtefeld et al., 1981) and suppress ulcers (Takase et al., 1989) and as an antibacterial agent (Laurens et al., 1985). One of the compounds isolated from this plant, 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone (PMF), is cytotoxic to various cancer cell lines (Lichius et al., 1994; Shi et al., 1995; Zhang et al., 1999), exhibits anti-HIV activity (Tuchinda et al., 2004), is a potent inducer of apoptosis and abrogates the nuclear factor-κB cell signaling pathway (Phromnoi et al., 2011). PMF isolated from another medicinal plant, Polanisia dodecandra (native to North America), has been reported to exhibit anticancer activity by binding to tubulin and inhibiting its polymerization (Lichius et al., 1994; Shi et al., 1995; Zhang et al., 1999). Because of the reported potential of PMF against cancer cells and the fact that STAT3 plays a critical role in tumor cell development, we postulated that this flavone may modulate STAT3 cell signaling pathways. We provide evidence that PMF can suppress both constitutive and inducible STAT3 activation through the activation of a protein tyrosine phosphatase, leading to suppression of various gene products linked to tumor cell survival, proliferation, and angiogenesis.
Materials and Methods
Reagents.
Penicillin, streptomycin, RPMI 1640 and Dulbecco's modified Eagle's medium were obtained from Invitrogen (Carlsbad, CA). Fetal bovine serum was supplied by Atlanta Biologicals (Norcross, GA). Horseradish peroxidase-conjugated anti-mouse secondary antibodies were purchased from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). Goat anti-rabbit horseradish peroxidase conjugate was purchased from Bio-Rad (Hercules, CA). Antibodies against phospho-STAT3 (tyrosine 705), STAT3, phospho-ERK1/2, ERK1/2, JAK2, SHP-1, cyclin D1, c-myc, poly(ADP-ribose) polymerase (PARP), caspase-3, Mcl-1, Bcl-2, Bcl-xL, c-IAP2, AKT, JNK, and phospho-JNK (Thr183/Tyr185) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-survivin was purchased from R&D Systems (Minneapolis, MN). An anti-VEGF and anti-EGFR was purchased from NeoMarkers (Fremont, CA). Antibodies to phospho-Src (Tyr416), Src, phospho-JAK1 (Tyr1022/1023), JAK1, phospho-JAK2 (Tyr1007/1008), and phospho-EGFR (Tyr1068) were purchased from Cell Signaling Technology (Danvers, MA). The phospho-Akt (Ser473) antibody was obtained from Imgenex (San Diego, CA). Bacteria-derived recombinant human IL-6 was kindly provided by Novartis Pharmaceuticals (Basel, Switzerland). The small interfering RNA (siRNA) for SHP-1 and the scrambled control were obtained from Ambion (Austin, TX). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Cell Lines.
The cell lines used in our studies were established from human multiple myeloma (U266, RPMI8226, MM1S) and human head and neck cancer (SCC4); they were obtained from the American Type Culture Collection (Manassas, VA). U266, RPMI8226, and MM1S cells were cultured in RPMI 1640; SCC4 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 1% sodium pyruvate. Culture media were supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Extraction and Isolation of PMF.
The leaves of G. obtusifolia were collected from the Doi Suthep-Pui National Park (Chiang Mai, Thailand). Voucher herbarium specimen of the plant was identified by J. F. Maxwell, and deposited in the Chiang Mai University Herbarium (Chiang Mai, Thailand). The samples were washed, air-dried, and chopped into small pieces. They were oven-dried at temperature below 50°C and powdered. The dried powder was macerated with 95% ethanol. The ethanolic solutions were combined and evaporated at 50°C under reduced pressure to give a dark brown residue. A portion of the crude extract was separated by liquid-liquid partition procedure. Based on the bioassay-guide isolation, the crude chloroform extract was subjected to further isolation with column chromatography on SiO2. Gradient elution was performed with different compositions of a mobile phase as a gradient of increasing polarity. Separated fractions were evaluated by thin-layer chromatography. Repeated separations were performed using CHCl3/ethyl acetate with increasing polarity up to a ratio of 5:5 to yield a pure fraction of PMF. The purity and the structure of these yellow crystals was measured and identified by thin-layer chromatography, high-performance liquid chromatography, mass spectroscopy, and NMR analysis.
Immunocytochemistry for STAT3 Localization.
The effect of PMF on the nuclear translocation of STAT3 was examined by an immunocytochemical method using an epifluorescence microscope (Labophot-2; Nikon, Tokyo, Japan) as described previously (Pandey et al., 2009).
Electrophoretic Mobility Shift Assay for STAT3-DNA Binding.
STAT3-DNA binding was analyzed by electrophoretic mobility shift assay (EMSA) as described previously (Pandey et al., 2009).
Western Blot Analysis.
Whole-cell protein (30 μg) was resolved on 7.5% or 10% SDS-PAGE gel, transferred to a nitrocellulose membrane, blocked with 5% nonfat milk, and probed with specific antibodies. The blots were washed, exposed to horseradish peroxidase-conjugated secondary antibodies, and finally detected by enhanced chemiluminescent reagent (GE Healthcare).
Cytotoxicity Assay.
The cytotoxicity of PMF was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium uptake method as described earlier (Pandey et al., 2009).
Flow Cytometric Analysis.
To determine the effect of PMF on the cell cycle progress, U266 cells were treated with PMF in different doses for 48 h, washed, and stained with propidium iodide as described previously (Kunnumakkara et al., 2009). The cells were analyzed using a fluorescence-activated cell sorter (BD Biosciences, San Jose, CA).
Transfection with SHP-1 siRNA.
SCC4 cells were plated in six-well plates and allowed to adhere for 24 h (Kunnumakkara et al., 2009). On the day of transfection, 12 μl of HiPerFect transfection reagent (QIAGEN, Valencia, CA) were added to 5 nM SHP-1 siRNA in a final volume of 100 μl of culture medium. After 48 h of transfection, cells were treated with PMF for 8 h, and whole-cell extracts were prepared for SHP-1, STAT3, and phospho-STAT3 analysis by Western blotting.
Apoptosis Assay.
Apoptosis was measured using Live/Dead assay according to the manufacturer's instructions (Invitrogen) (Kunnumakkara et al., 2009). Cells were analyzed under a fluorescence microscope (Labophot-2).
Statistical Analysis.
The statistical analysis was done by one-way analysis of variance test using SPSS version 15.0 (SPSS Inc., Chicago, IL).
Results
Although PMF has been shown to exhibit anticancer activity, whether it can modulate STAT3 signaling pathway is unknown. Therefore, the objective of this study was to investigate the effect of this flavone on the modulation of STAT3 pathway. For most studies, we used human multiple myeloma U266 cells because they express constitutively active STAT3, and the role of STAT3 pathways in this cancer is well understood. However, for gene-silencing studies, we used squamous cell carcinoma, SCC-4. These cells express constitutively active STAT3 and have been successfully used for the delivery of siRNA. To produce inducible STAT3, we used IL-6, because this cytokine has been examined extensively for STAT3 activation.
Identification of PMF.
PMF was obtained in the form of yellow crystals. The isolated compound was identified through analysis of its Rf values, melting point, ultraviolet absorption, infrared absorption, nuclear magnetic resonance, and mass spectra compared with data published previously (Shi et al., 1995; Zhang et al., 1999). The electron impact-mass spectrum of PMF exhibited a molecular ion peak at m/z 404, supporting the molecular formula of C20H2OO9. The IR spectrum showed strong absorption bands of OH (3100–3700 cm−1, broad), C=O (1650–1705 cm−1, medium), C=C (1600–1500 cm−1, strong), and C-O (1200–1400 cm−1, strong). The UV spectrum consisted of two absorption maxima (λmax) at 348 nm (band I) and 260–278 nm (band II). Inspection of the signals in the 1H NMR and carbon signals in the 13C NMR spectrum allowed us to deduce the structure of PMF. Its spectral data were in agreement with those obtained from the reference compound reported in the literature (Lichius et al., 1994; Shi et al., 1995; Tuchinda et al., 2004) (Fig. 1A).
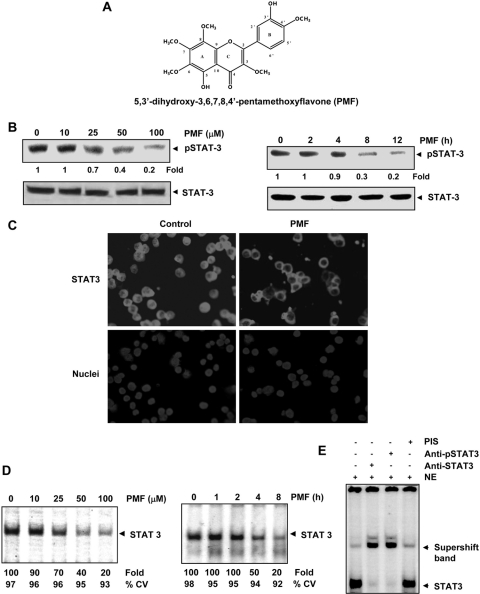
Fig. 1.
A, structure of PMF. B, PMF inhibits constitutive STAT3 activation. U266 cells (2× 106/ml) were treated with the indicated concentrations of PMF for 8 h (left) or with 100 μΜ PMF for the indicated times (right), after which whole-cell extracts were prepared and subjected to Western blotting as described under Materials and Methods. Blot was probed for phospho-STAT3. The same blots were stripped and reprobed with STAT3 antibody. Densitometric quantitation in fold change of each band is given below the blot. C, PMF suppresses STAT3 nuclear translocation. U266 cells (105/ml) were treated with 100 μΜ PMF for 8 h and immunocytochemistry was performed with STAT3 antibody. D, PMF inhibits constitutively active STAT3 in U266 cells. U266 cells (2 × 106/ml) were treated with the indicated concentrations of PMF for 8 h (left) or with 100 μΜ PMF for the indicated durations (right) and analyzed for nuclear STAT3 levels by EMSA. E, nuclear extract from U266 cells were incubated with STAT3, phospho-STAT3 antibodies, or preimmune serum. The nuclear extract was then assayed for STAT3-DNA binding using EMSA. The results shown are representative of three independent experiments.
PMF Inhibits Constitutive STAT3 Phosphorylation.
Whether PMF can suppress constitutive STAT3 activation in U266 cells, which are known to express constitutive STAT3 (Pandey et al., 2009), was investigated. As shown in Fig. 1B, PMF inhibited the constitutive activation of STAT3, as indicated by a decrease in phosphorylated STAT3, in a dose- (left) and time-dependent (right) manner. This flavone had no effect on the expression of STAT3 protein. Exposure of cells to 100 μM PMF for approximately 8 h was optimum to suppress constitutive STAT3 activation significantly. Under these conditions, cells were fully viable. Hence, we selected this condition for further experiments.
PMF Suppresses the Nuclear Translocation of STAT3.
Because tyrosine phosphorylation causes dimerization of STAT3, which then leads to nuclear translocation, we examined whether PMF inhibits nuclear translocation of STAT3 in U266 cells. The results of immunocytochemistry showed that PMF inhibited nuclear translocation of STAT3 (Fig. 1C).
PMF Inhibits Binding of STAT3 to the DNA.
STAT3 after phosphorylation is translocated to the nucleus, in which it binds to the DNA and regulates STAT3-dependent gene expression (Yu et al., 1995). Whether PMF inhibits DNA binding activity of STAT3 was examined by EMSA. Nuclear extracts prepared from U266 cells showed STAT3-DNA binding activity, and PMF inhibited the binding in a dose- (Fig. 1D, left) and time-dependent (Fig. 1D, right) manner.
To confirm that the protein-DNA complex as visualized by EMSA contains STAT3, we preincubated nuclear extracts from U266 cells with antibodies against STAT3 and phospho-STAT3. Incubation with the antibodies shifted the band to a higher molecular mass, whereas preimmune serum had no effect on STAT3-DNA binding (Fig. 1E). These results suggest that the protein-DNA complex as visualized by EMSA, indeed contained STAT3.
PMF-Induced Inhibition of STAT3 Phosphorylation Is Reversible.
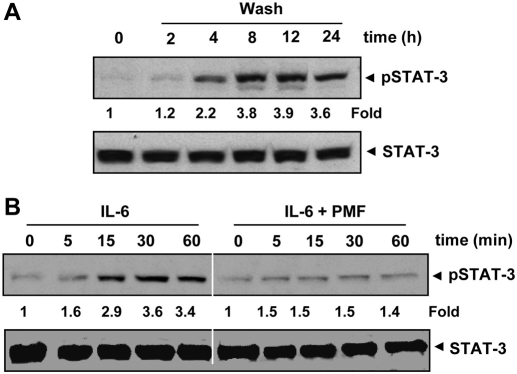
Whether PMF-induced inhibition of STAT3 phosphorylation was reversible was also examined. Our results showed that removal of the compound reversed the PMF-induced inhibition of STAT3 phosphorylation (Fig. 2A). The STAT3 protein levels remained constant under these conditions.
Fig. 2.
A, PMF-induced inhibition of STAT3 phosphorylation is reversible. U266 cells (2 × 106/ml) were treated with 100 μΜ PMF for 8 h and washed with phosphate-buffered saline twice to remove PMF before resuspension in fresh medium. Cells were harvested at the indicated times after which Western blotting was performed for phospho-STAT3. Densitometric quantitation in fold change of each band is given below the blot. B, PMF down-regulates IL-6-induced phospho-STAT3. MM1S cells (2 × 106/ml) were treated with IL-6 (10 ng/ml) or pretreated with 100 μΜ PMF for 8 h followed by IL-6 for the indicated times, whole-cell extracts were prepared, and phosphorylated STAT3 was detected by Western blot. The same blots were stripped and reprobed with STAT3 antibody to verify equal protein loading. Densitometric quantitation in fold change of each band is given below the blot.
PMF Inhibits IL-6-Induced STAT3 Phosphorylation.
IL-6, a growth factor for MM cells, is overexpressed in various cancers and is a potent inducer of STAT3 (Kawano et al., 1988). Whether PMF could inhibit IL-6-induced STAT3 phosphorylation was investigated. We found that IL-6 induced phosphorylation of STAT3 as early as 15 min, and pretreatment of cells with PMF for 8 h suppressed IL-6–induced STAT3 phosphorylation (Fig. 2B).
PMF Suppresses Constitutive Activation of JAK1 and JAK2.
Because STAT3 is activated by soluble tyrosine kinases of the Janus family, also called JAK (Ihle, 1996), we examined the effects of PMF on JAK phosphorylation. The results showed that PMF inhibited constitutive phosphorylation of JAK1 and JAK2 in a dose- (Fig. 3A, left) and time-dependent (Fig. 3A, right) manner. The levels of total JAK1 and JAK2, however, remained unchanged.
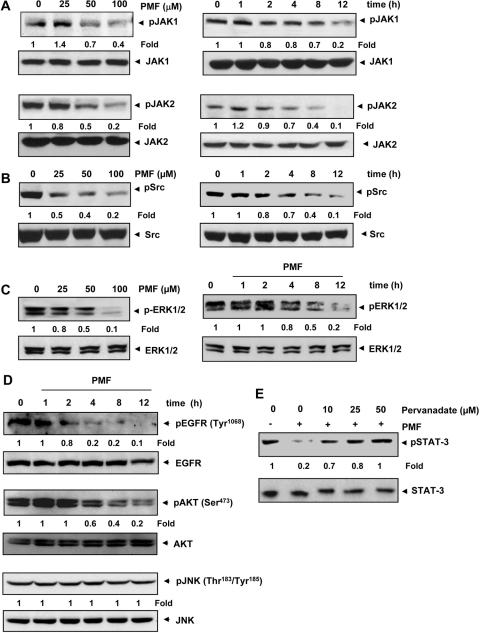
Fig. 3.
A, PMF suppresses the activation of JAK1 and JAK2 in a dose- and time-dependent manner. U266 cells (2 × 106/ml) were treated with PMF at the indicated doses (left) or with 100 μΜ PMF for the indicated time intervals (right). Whole-cell protein extracts were used for detection of phospho-JAK1 and -JAK2 by Western blotting using specific antibodies. The same blots were analyzed for JAK1 and JAK2 protein expression. Densitometric quantitation in fold change of each band is given below the blot. B, PMF suppresses phospho-Src levels in a dose- and time-dependent manner. U266 cells (2 × 106/ml) were treated with the indicated doses of PMF (left) or with 100 μΜ PMF for the indicated times (right), after which whole-cell extracts were prepared and subjected to Western blotting for phospho-Src antibody. The same blots were stripped and reprobed with Src antibody. Densitometric quantitation in fold change of each band is given below the blot. C, PMF suppresses phospho-ERK1/2 levels in a time-dependent manner. U266 cells (2 × 106/ml) were treated with the indicated doses of PMF (left) or with 100 μΜ PMF for the indicated times (right), after which Western blotting was performed for phospho-ERK1/2 antibody. The same blots were stripped and reprobed with ERK1/2 antibody. Densitometric quantitation in fold change of each band is given below the blot. D, PMF suppresses phosphorylation of EGFR and AKT. U266 cells were treated with 100 μM PMF for the indicated time, and Western blotting was carried out using indicated antibodies. Densitometric quantitation in fold change of each band is given below the blot. E, pervanadate reverses the phospho-STAT3 inhibitory effect of PMF. U266 cells (2 × 106/ml) were treated with the indicated concentration of pervanadate and 100 μΜ PMF for 8 h, after which whole-cell extracts were prepared and subjected to Western blotting for phospho-STAT3 and STAT3. Densitometric quantitation in fold change of each band is given below the blot. The results shown are representative of three independent experiments.
PMF Suppresses Constitutive Activation of c-Src.
Because activation of Src has also been linked with STAT3 activation (Schreiner et al., 2002), we examined the effect of PMF on constitutive activation of c-Src kinase in U266 cells. We found that U266 cells exhibited constitutive activation of cSrc kinase and that PMF suppressed the activation in a dose- (Fig. 3B, left) and time-dependent (Fig. 3B, right) manner.
PMF Suppresses Constitutive Activation of ERK1/2.
Apart from tyrosine phosphorylation, STAT3 is also known to undergo phosphorylation at serine residues through the ERK pathway (Chung et al., 1997). We therefore investigated whether PMF modulates constitutive activation of ERK1/2 kinase in U266 cells. As shown in Fig. 3C, PMF inhibits the constitutive phosphorylation of ERK1/2 kinase in U266 cells in a dose- (Fig. 3C, left) and time-dependent manner (Fig. 3C, right).
PMF Suppresses Constitutive Activation of EGFR.
Because activation of receptor tyrosine kinases such as EGFR that are upstream to ERK has been reported to be important in the pathogenesis of multiple myeloma, we examined whether PMF can affect the activation of these receptors. We found that MM cells exhibited constitutive phosphorylation of EGFR, whereas PMF suppressed this phosphorylation in a time-dependent manner (Fig. 3D).
PMF Suppresses Activation of Akt.
We next examined whether PMF has potential to affect activation of Akt, another kinase upstream to ERK. The results indicated that PMF suppressed activation of Akt in a time-dependent manner. Total Akt was, however, unchanged under similar conditions (Fig. 3D).
PMF Does Not Affect JNK Activation.
We also examined whether PMF can modulate activation of JNK. We found that phosphorylation at Thr183/Tyr185 and total JNK protein levels were not affected by PMF treatment. These results indicate JNK is not involved in the suppression of ERK and STAT3 activation by PMF (Fig. 3D).
Tyrosine Phosphatase Inhibitor Abrogates PMF-Induced Inhibition of STAT3 Phosphorylation.
Protein tyrosine phosphatases (PTPs) have been implicated in STAT3 activation (Han et al., 2006). Therefore, we examined whether PMF-induced inhibition of STAT3 tyrosine phosphorylation could be due to activation of a PTP. Treatment of U266 cells with the broad-acting tyrosine phosphatase inhibitor sodium pervanadate reversed the PMF-induced inhibition of STAT3 phosphorylation (Fig. 3E). This suggests that PTPs are involved in PMF-induced inhibition of STAT3 phosphorylation.
PMF Induces the Expression of SHP-1.
SHP-1 is a nontransmembrane PTP that has been linked with regulation of STAT3 activation. Because PMF induced suppression in the STAT3 activation was reversed by a PTP inhibitor, we examined whether PMF has potential to induce SHP-1. As shown in Fig. 4A, PMF indeed induced the expression of SHP-1 both in a dose- (Fig. 4A, left) and time- (Fig. 4A, right) dependent manner.
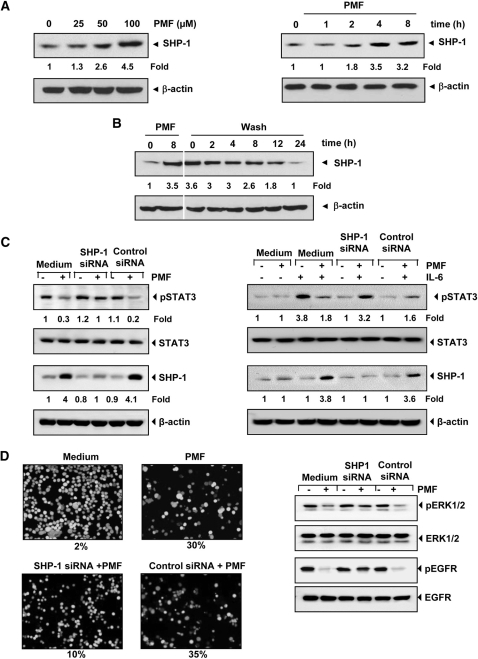
Fig. 4.
A, PMF induces the expression of SHP-1 in U266 cells in a dose- and time-dependent manner. U266 cells (2 × 106/ml) were treated with PMF for 8 h with different concentrations of PMF (left) or with 100 μΜ PMF for the indicated times (right), after which whole-cell extracts were prepared and Western blotting was performed for SHP-1 antibody. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. Densitometric quantitation in fold change of each band is given below the blot. B, PMF-induced SHP-1 activation is transient. U266 cells (2 × 106/ml) were treated with 100 μΜ PMF for 8 h and washed with phosphate-buffered saline twice to remove PMF before resuspension in fresh medium. Cells were harvested at indicated times and subjected to Western blotting against SHP-1. Blot was stripped and reprobed for β-actin antibody. Densitometric quantitation in fold change of each band is given below the blot. C, effect of SHP-1 knockdown on PMF-induced expression of constitutive STAT3 (left) and on inducible STAT3 (right). SCC4 cells (2 × 105/ml) were transfected with either scrambled or SHP-1-specific siRNA (5 nM). After 48 h, cells were treated with 100 μΜ PMF for 8 h and whole-cell extracts were subjected to Western blot analysis for SHP-1. The same blots were stripped and reprobed with β-actin antibody. The same whole-cell extracts were subjected to phospho-STAT3 and STAT3 (left). A293 cells (2 × 105/ml) were transfected with either scrambled or SHP-1-specific siRNA (5 nM). After 48 h, cells were treated with 100 μΜ PMF for 8 h and then IL-6 (10 ng/ml) for 30 min. Whole-cell extracts were subjected to Western blot analysis for phospho-STAT3 and STAT3. The same whole-cell extracts were subjected to SHP-1. The same blots were stripped and reprobed with β-actin antibody (right). Densitometric quantitation in fold change of each band is given below the blot. D, knockdown of SHP-1 inhibited the PMF-induced apoptosis and activation of ERK1/2 and EGFR. SCC4 cells (2 × 105/ml) were transfected with either scrambled or SHP-1-specific siRNA (5 nM). After 48 h, cells were treated with 100 μΜ PMF for 24 h at 37°C. Cells were stained with a Live/Dead assay reagent and then analyzed under a fluorescence microscope (left). SCC4 cells were transfected with either scrambled or SHP-1 specific siRNA (5 nM). After 48 h, cells were treated with 100 μΜ PMF for 12 h at 37°C. Whole cell extract were prepared and analyzed by Western blotting using indicated antibodies (right). The results shown are representative of three independent experiments.
Induction of SHP-1 by PMF Is Transient.
Because inhibition of STAT3 phosphorylation by PMF was reversible (Fig. 2B), we determined whether it was due to transient induction of SHP-1. Our results showed that PMF induced SHP-1 protein maximally at 8 h, and removal of the compound down-regulated its expression (Fig. 4B). Thus, the dephosphorylation of STAT3 correlates well with the appearance of SHP-1.
Silencing of SHP-1 Reverses the Inhibition of Constitutive STAT3 by PMF.
We showed above that the dephosphorylation of STAT3 by PMF correlates with the appearance of SHP-1. Whether the silencing of SHP-1 expression by siRNA would abrogate the inhibitory effect of PMF on STAT3 activation was investigated. Western blotting showed that PMF-induced SHP-1 expression was abolished in the cells treated with SHP-1 siRNA; treatment with scrambled siRNA had no effect (Fig. 4C, left). We also found that PMF failed to suppress STAT3 activation in cells treated with SHP-1 siRNA (Fig. 4C, left). These results further corroborate our earlier evidence for the critical role of SHP-1 in suppression of STAT3 phosphorylation by PMF.
Silencing of SHP-1 Blocks the Effects of PMF on Inducible STAT3.
Next, we determined whether knockdown of SHP-1 expression by siRNA also abolished the effect of PMF on inhibition of IL-6-induced STAT3 activation. Our results of Western blot showed that IL-6 induced the STAT3 activation in SCC4 cells and PMF inhibited the STAT3 activation through up-regulation of SHP-1 expression. However, silencing the SHP-1 expression abrogated the effect of PMF on IL-6-induced STAT3 activation. Scrambled siRNA had no effect (Fig. 4C, right). These results indicate the critical role of SHP-1 in PMF-induced suppression of inducible STAT3.
SHP-1 siRNA Reduces PMF-Induced Apoptosis.
We showed above that SHP-1 plays a critical role in suppression of STAT3 phosphorylation by PMF. Next, we examined whether PMF has potential to induce apoptosis by live/dead assay. If so, whether silencing of SHP-1 will affect PMF-induced apoptosis was also investigated. We found that PMF induced apoptosis by 30% and that knockdown of SHP-1 almost completely reversed the apoptotic effects of PMF (Fig. 4D, left).
Silencing of SHP-1 Abrogates the PMF-Induced Down-Regulation of ERK1/2 and EGFR Activation.
Next, we examined whether silencing SHP1 would abrogate the inhibitory effect of PMF on phosphorylation of ERK1/2 and EGFR. Although PMF inhibited the phosphorylation of both ERK1/2 and EGFR, silencing SHP-1 abrogated this inhibition (Fig. 4D, right). Scrambled siRNA, however had no effect. These results suggest that SHP-1 may have a role in PMF-induced suppression of ERK1/2 and EGFR activation.
PMF Suppresses the Expression of Proliferative Gene Products.
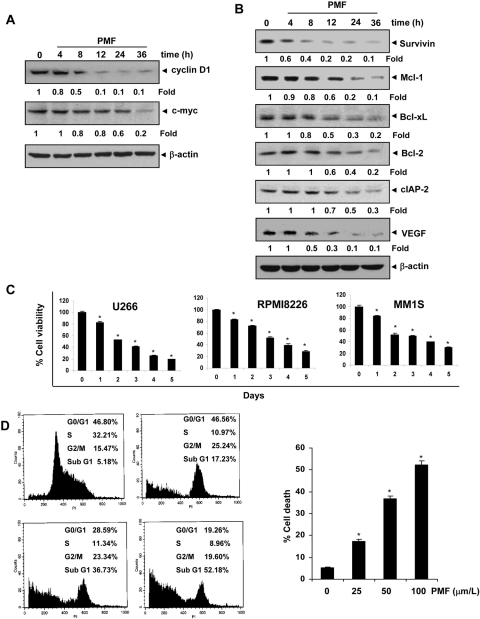
STAT3 activation has been linked with the proliferation of tumor cells. This effect of STAT3 is mediated through its ability to induce the expression of cyclin D1 and c-myc, which is required for cell proliferation (Bromberg et al., 1999; Aggarwal et al., 2006). We therefore examined the effect of PMF on constitutive expression of cyclin D1 and c-myc in U266 cells. Our results showed that PMF treatment suppressed the expression of cyclin D1 and c-myc in a time-dependent manner (Fig. 5A).
Fig. 5.
A. PMF suppresses STAT3-regulated proliferative gene products. U266 cells (2 × 106/ml) were treated with 100 μΜ PMF for the indicated time intervals, after which whole-cell extracts were prepared and subjected to Western blotting for cyclin D1 and c-myc. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. Densitometric quantitation in fold change of each band is given below the blot. B, PMF suppresses STAT3-regulated survival and angiogenic gene products. U266 cells (2 × 106/ml) were treated with 100 μΜ PMF for the indicated time intervals, after which whole-cell extracts were prepared, and subjected to Western blotting against survivin, Mcl-1, Bcl-2, Bcl-xL, cIAP-2, and VEGF antibody. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. Densitometric quantitation in fold change of each band is given below the blot. C, PMF suppresses cell proliferation of multiple myeloma cells. U266, RPMI 8226, and MM1S cells were treated with 50 μΜ PMF and then subjected to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay on days 0 to 5 to analyze the proliferation of cells. Results represent the mean ± S.D. of three different experiments performed in triplicate. *, P < 0.001 was considered statistically significant. D, PMF causes significant accumulation of multiple myeloma cells in the sub-G1 phase. U266 cells (2 × 106/ml) were treated with PMF for 48 h with different concentrations of PMF, after which the cells were washed, fixed, stained with propidium iodide, and analyzed for DNA content by flow cytometry. *, significant against control (p < 0.05).
PMF Down-Regulates the Expression of Antiapoptotic Gene Products.
It has been shown that cell survival gene products including Bcl-xL, Bcl-2, survivin, Mcl-1, and cIAP-2 are regulated by STAT3 (Aggarwal et al., 2006). Hence, whether down-regulation of STAT3 activation by PMF leads to down-regulation of these gene products was examined. The results showed that PMF inhibited the expression of Bcl-xL, Bcl-2, survivin, Mcl-1, and cIAP-2 in a time-dependent manner (Fig. 5B).
PMF Down-Regulates the Expression of VEGF.
VEGF, a major mediator of angiogenesis, is regulated by STAT3 activation. Therefore, we examined the effect of PMF on constitutive VEGF expression in U266 cells. Our results show that PMF inhibited the expression of VEGF in U266 cells in a time-dependent manner (Fig. 5B).
PMF Inhibits the Proliferation of MM Cells.
Because PMF suppressed the expression of STAT3-regulated cyclin D1 and c-myc expression related to cell proliferation (Fig. 5A), whether PMF inhibits the proliferation of MM cells was investigated. The results indicated that PMF at 50 μM suppressed the proliferation of U266, RPMI8226, and MM1S cells in a time-dependent manner (Fig. 5C).
PMF Causes the Accumulation of the Cells in the Sub-G1 Phase of the Cell Cycle.
Next, we determined the effect of PMF on cell cycle phase distribution. We found that PMF caused significant accumulation of cells in the sub-G1 phase after treatment for 48 h (Fig. 5D).
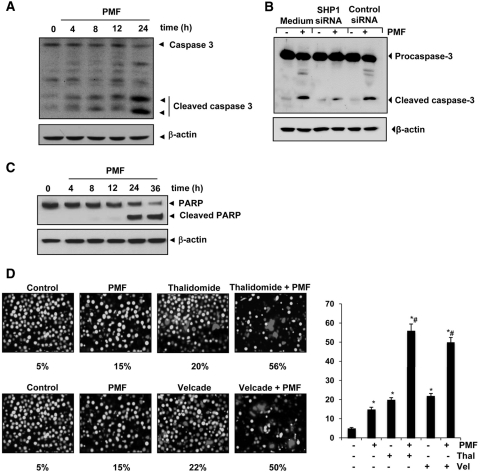
PMF Activates Caspase-3 and Causes PARP Cleavage.
Whether suppression of STAT3-regulated antiapoptotic gene products and induction of apoptosis in U266 cells by PMF is correlated with caspase activation was also examined. We found that PMF activated caspase-3 in a time-dependent manner (Fig. 6A). It is noteworthy that PMF-induced caspase-3 activation was completely suppressed after silencing SHP-1 (Fig. 6B). PMF also led to the cleavage of native PARP into 87-kDa fragments, which is a hallmark of apoptosis (Fig. 6C). These results suggest that PMF-induced apoptosis is mediated through caspase-3 pathway and that SHP-1 may have a role in this pathway.
Fig. 6.
A, PMF induces caspase-3 activation and PARP cleavage. U266 cells were treated with 100 μΜ PMF for the indicated times, and whole-cell extracts were prepared, separated on SDS-PAGE, and analyzed by Western blotting using caspase-3 antibody. B, knockdown of SHP-1 inhibit PMF-induced cleavage of caspase-3. SCC4 cells were transfected with either scrambled or SHP-1-specific siRNA (5 nM). After 48 h, cells were treated with 100 μΜ PMF for 24 h at 37°C. Whole-cell extracts were prepared and analyzed by Western blotting using procaspase-3 antibody. C, PMF induces caspase-3 activation and PARP cleavage. U266 cells were treated with 100 μΜ PMF for the indicated times, and whole-cell extracts were prepared, separated on SDS-PAGE, and analyzed by Western blotting using PARP antibody. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. D, PMF potentiates the apoptotic effect of thalidomide and Velcade. U266 cells (2 × 106/ml) were treated with 100 μΜ PMF and 10 ng/ml thalidomide or 20 nM Velcade alone or in combination for 24 h at 37°C, and the apoptosis was analyzed by the Live/Dead assay. *, significant against untreated control; #, significant against respective drug treated cells at p < 0.05. The results shown are representative of three independent experiments.
PMF Potentiates the Apoptotic Effect of Velcade and Thalidomide in MM Cells.
Because STAT3 activation has been linked with chemoresistance, we investigated whether PMF can reverse chemoresistance. Velcade, an inhibitor of proteasome, and thalidomide, an inhibitor of tumor necrosis factor expression, have been approved for the treatment of MM in patients (Cavo, 2006). However, these drugs produce several side effects, and the disease inevitably relapses in most cases because the patients eventually develop resistance to these drugs. Therefore, we examined whether PMF can potentiate the effect of these drugs. As shown in Fig. 6D, PMF significantly enhanced the apoptotic effects of thalidomide from 20 to 56% and of Velcade from 22 to 50%.
Discussion
Although STAT3 is a critical mediator of the oncogenic phenotype of many cancers, it is disposable for the function of most normal cells. A more effective response of the immune system against the tumor is also mediated through the suppression of STAT3 (Kortylewski et al., 2005). Thus, finding effective inhibitors of STAT3 may provide useful targeted agents for cancer therapy. In the present study, we report the identification of a novel inhibitor of STAT3. We found that PMF suppressed both constitutive and IL-6-inducible STAT3 activation through the activation of SHP-1 in parallel with the inhibition of JAK1, JAK2, c-Src, and ERK1/2 activation. PMF also induced apoptosis and down-regulated the expression of STAT3-regulated gene products, including cyclin D1, c-myc, survivin, Mcl-1, Bcl-2, Bcl-xL, cIAP-2, and VEGF.
Numerous hydroxylated polymethoxyflavones have been isolated primarily from the Citrus genus such as nobiletin, tangeretin, artemetin, and sinensetin (Takanaga et al., 2000; Choi et al., 2002; Xiao et al., 2009; Zheng et al., 2009), from the spice thyme such as 5,4′-dihydroxy-6,7,3′-trimethoxyflavone, 5,4′-dihydroxy-6,7,8,3′-tetramethoxyflavone, 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone, and luteolin and from Gardenia spp. and other plants (Lichius et al., 1994; Shi et al., 1995; Tuchinda et al., 2004). Most of them showed cytotoxicity to various tumor cells (Lichius et al., 1994; Shi et al., 1995; Tuchinda et al., 2004; Sergeev et al., 2006; Xiao et al., 2009). How they produce the cytotoxic effects is not well established. We found that this flavone has the potential to inhibit STAT3 activation in MM cells, as evident by STAT3 phosphorylation at Tyr705, by nuclear translocation, and by DNA binding. The inhibition was transitory, however. We found that PMF also suppressed STAT3 activation induced by IL-6, one of the many tumor cell growth factors that activate STAT3 (Chatterjee et al., 2002). Thus, our studies provide an insight into one possible mechanism that the down-regulation of STAT3 may play a role in the suppression of proliferation and induction of apoptosis by PMF in MM cells.
How PMF inhibits activation of STAT3 was investigated in detail. The activation of Janus-activated kinases has been closely linked with STAT3 activation (Ihle, 1996), and we found that PMF inhibited the activation of constitutively active JAK1 and JAK2 in MM cells. This is in agreement with a report that 5-chloro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]-N4-(5-methyl-1H-pyrazol-3-yl)pyrimidine-2,4-diamine (AZD1480), a JAK2 inhibitor, can suppress STAT3 signaling and oncogenesis (Hedvat et al., 2009). Besides JAK, c-Src has also been implicated in STAT3 activation. PMF also inhibited c-Src activation. In addition to phosphorylation at Tyr705, STAT-3 undergo phosphorylation at Ser727 by mitogen-activated protein kinase family proteins (Chung et al., 1997). The inhibition of ERK activation by PMF suggests its role in inhibiting STAT-3 phosphorylation at Ser727. We also investigated how PMF inhibits ERK activation. Several kinases including EGFR, AKT, and JNK have been reported to function upstream to ERK. We found that this flavone inhibited activation of EGFR and AKT. Thus it is likely that PMF suppresses ERK activation through inhibition of EGFR and AKT activation.
We also found that the PMF-induced inhibition of STAT3 activation involves a PTP. Numerous PTPs have been implicated in STAT3 signaling, including SHP-1, SHP-2, T-cell PTP, PTEN, CD45, and PTP (Aggarwal et al., 2009). We found that PMF induced SHP-1 that was correlated with a down-regulation in constitutive STAT3 phosphorylation in multiple myeloma cells. That the transfection with SHP-1 siRNA reversed the STAT3 inhibitory effect of PMF and abolished apoptosis further confirms the role of this phosphatase in PMF-induced down-regulation of STAT3 activation. In agreement with our observations, loss of SHP-1 has been shown to enhance JAK3/STAT3 signaling in anaplastic lymphoma kinase-positive anaplastic large-cell lymphoma (Han et al., 2006). It is noteworthy that the multikinase inhibitor sorafenib was also found to inhibit STAT3 through activation of a PTP (Yang et al., 2010a). In fact, the role of PTP was also implicated in the action of sunitinib, another multikinase inhibitor approved for the treatment of solid tumors, as an inhibitor of STAT3 activation (Yang et al., 2010b). Two distinct promoters direct the expression of human (Banville et al., 1995) and murine (Martin et al., 1999) SHP-1, and two types of transcripts are initiated from the promoters. Although how PMF induces SHP-1 was not investigated in the present study, it is likely that PMF either directly or through mediation of other regulatory proteins target SHP-1 promoters.
We also report for the first time that PMF suppresses the expression of several STAT3-regulated genes, including proliferative (cyclin D1 and c-myc) and antiapoptotic gene products (survivin, Mcl-1, Bcl-xL, Bcl-2, and cIAP-2) and an angiogenic gene product (VEGF). It is possible that the cytotoxic effects of PMF in various cancer cells (Lichius et al., 1994; Shi et al., 1995; Tuchinda et al., 2004) are due to suppression of these gene products.
Various tumors including MM express constitutively active STAT3. We showed that PMF suppressed the proliferation of the MM cells and induced sub-G1 cell cycle arrest. In addition, this flavone was found to activate caspase-3 and PARP cleavage and to induce apoptosis in MM cells, which is consistent with previous reports that tangeretin (5,6,7,8,4′-pentamethoxyflavone) induces G1 cell-cycle arrest and apoptosis in HL-60 cells (Pan et al., 2002). Monodemethylated PMFs increased the number of cells in the sub-G0/G1 phases of the cell cycle and down-regulated oncogenic proteins, and induced apoptosis by activation of caspase-3 and cleavage of PARP (Xiao et al., 2009). Likewise, hydroxylated PMFs were dramatically more active in inducing Ca2+-mediated apoptosis than nonhydroxylated PMF (Sergeev et al., 2006). However, whether flavones, which are hydroxylated and methoxylated at different positions, exhibit their activities through the suppression of STAT3, is unclear at present.
In addition, PMF seems to inhibit tubulin polymerization and impair microtubule stability (Shi et al., 1995; Zhang et al., 1999). It has been demonstrated that the expression of STAT3 is required for the stabilization of microtubules and cell migration. STAT3 can interact with stathmin and regulate microtubule dynamics by antagonizing its polymerization activity (Verma et al., 2009), and specific inhibition of STAT3 activity inhibited stathmin interaction with STAT3 and tubulin, thus disrupting its regulation of microtubule dynamics (Glasmacher et al., 2006). These studies suggested that STAT3 may have the potential as a therapeutic target for various pathological conditions involving T-cell responses including chronic inflammatory disease.
A proteasome inhibitor, ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxamido)propanamido)butyl)boronic acid (PS341, also called bortezomib or Velcade), and a tumor necrosis factor inhibitor (thalidomide) were approved for the treatment of MM (Cavo, 2006). However, prolonged exposure leads to the development of resistance and toxicity, and progression-free and overall survival times are short. We found that PMF can potentiate the apoptotic effect of bortezomib and thalidomide in multiple myeloma cells and thus provide a sound basis for pursuing the use of PMF further, either alone or in combination with other agents, to enhance treatment efficacy, reduce toxicity, and overcome the chemoresistance of relapsed or refractory MM.
Overall, our results show that PMF inhibits both inducible and constitutive STAT3 activation through the induction of tyrosine kinase phosphatase, which makes it a potentially effective suppressor of tumor cell survival, proliferation, and angiogenesis. In present study, 100 μM PMF was used against the cancer cell growth and proliferation. However, in vivo study showed that PMF (at 75 mg/kg) has potential to reduce tumor burden by 39% in colon in mice (Cai et al., 2009). These results indicate that the above-used dose could be clinically achievable. However, to extrapolate the present results, further in vivo studies may be warranted to provide important leads for using PMF as a treatment of cancer and other proinflammatory diseases.
Acknowledgments
We thank Walter Pagel for carefully proofreading the manuscript and providing valuable comments.
This work was supported by the National Institutes of Health National Cancer Institute [Grants CA016672, CA-124787-01A2]; the Center for Targeted Therapy of MD Anderson Cancer Center; and the Royal Golden Jubilee PhD Program of Thailand.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.073676.
- STAT
- signal transducers and activators of transcription
- EMSA
- electrophoretic mobility shift assay
- JAK
- Janus-like kinase
- MM
- multiple myeloma
- PARP
- poly(ADP-ribose) polymerase
- PMF
- 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone
- siRNA
- small interfering RNA
- VEGF
- vascular endothelial growth factor
- IL
- interleukin
- ERK1/2
- extracellular signal-regulated kinase 1/2
- EGFR
- epidermal growth factor receptor
- PAGE
- polyacrylamide gel electrophoresis
- PTP
- protein tyrosine phosphatase
- AZD1480
- 5-chloro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]-N4-(5-methyl-1H-pyrazol-3-yl)pyrimidine-2,4-diamine
- PS341
- ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxamido)propanamido)butyl)boronic acid.
Authorship Contributions
Participated in research design: Phromnoi, Limtrakul, and Aggarwal.
Conducted experiments: Phromnoi, Prasad, Gupta, Kannappan, and Reuter.
Performed data analysis: Phromnoi, Prasad, Gupta, and Limtrakul.
Wrote or contributed to the writing of the manuscript: Phromnoi, Prasad, Gupta, Limtrakul, and Aggarwal.
References
- Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B. (2009) Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship?. Ann NY Acad Sci 1171:59–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. (2006) Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann NY Acad Sci 1091:151–169 [DOI] [PubMed] [Google Scholar]
- Banville D, Stocco R, Shen SH. (1995) Human protein tyrosine phosphatase 1C (PTPN6) gene structure: alternate promoter usage and exon skipping generate multiple transcripts. Genomics 27:165–173 [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr (1999) Stat3 as an oncogene. Cell 98:295–303 [DOI] [PubMed] [Google Scholar]
- Cai H, Sale S, Schmid R, Britton RG, Brown K, Steward WP, Gescher AJ. (2009) Flavones as colorectal cancer chemopreventive agents–phenol-o-methylation enhances efficacy. Cancer Prev Res (Phila) 2:743–750 [DOI] [PubMed] [Google Scholar]
- Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna JL, Nuñez G, Dalton WS, Jove R. (1999) Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10:105–115 [DOI] [PubMed] [Google Scholar]
- Cavo M. (2006) Proteasome inhibitor bortezomib for the treatment of multiple myeloma. Leukemia 20:1341–1352 [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Hönemann D, Lentzsch S, Bommert K, Sers C, Herrmann P, Mathas S, Dörken B, Bargou RC. (2002) In the presence of bone marrow stromal cells human multiple myeloma cells become independent of the IL-6/gp130/STAT3 pathway. Blood 100:3311–3318 [DOI] [PubMed] [Google Scholar]
- Choi CH, Sun KH, An CS, Yoo JC, Hahm KS, Lee IH, Sohng JK, Kim YC. (2002) Reversal of P-glycoprotein-mediated multidrug resistance by 5,6,7,3′,4′-pentamethoxyflavone (Sinensetin). Biochem Biophys Res Commun 295:832–840 [DOI] [PubMed] [Google Scholar]
- Chung J, Uchida E, Grammer TC, Blenis J. (1997) STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol 17:6508–6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasmacher A, Hahn C, Hoffmann F, Naumann R, Goldschmidt H, von Lilienfeld-Toal M, Orlopp K, Schmidt-Wolf I, Gorschlüter M. (2006) A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol 132:584–593 [DOI] [PubMed] [Google Scholar]
- Han Y, Amin HM, Franko B, Frantz C, Shi X, Lai R. (2006) Loss of SHP1 enhances JAK3/STAT3 signaling and decreases proteosome degradation of JAK3 and NPM-ALK in ALK+ anaplastic large-cell lymphoma. Blood 108:2796–2803 [DOI] [PubMed] [Google Scholar]
- Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, Armstrong B, Bebernitz G, Weng S, Wang L, Ye M, McEachern K, Chen H, Morosini D, Bell K, Alimzhanov M, Ioannidis S, McCoon P, Cao ZA, Yu H, Jove R, Zinda M. (2009) The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 16:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle JN. (1996) STATs: signal transducers and activators of transcription. Cell 84:331–334 [DOI] [PubMed] [Google Scholar]
- Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, Asaoku H, Tang B, Tanabe O, Tanaka H. (1988) Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature 332:83–85 [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mulé J, Kerr WG, Jove R, Pardoll D, Yu H. (2005) Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med 11:1314–1321 [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Nair AS, Sung B, Pandey MK, Aggarwal BB. (2009) Boswellic acid blocks signal transducers and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1. Mol Cancer Res 7:118–128 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Laurens A, Mboup S, Tignokpa M, Sylla O, Masquelier J. (1985) [Antimicrobial activity of some medicinal species from the Dakar markets]. Die Pharmazie 40:482–484 [PubMed] [Google Scholar]
- Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, Jove R, Yu H. (2010) STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med 16:1421–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichius JJ, Thoison O, Montagnac A, Païs M, Guéritte-Voegelein F, Sévenet T, Cosson JP, Hadi AH. (1994) Antimitotic and cytotoxic flavonols from Zieridium pseudobtusifolium and Acronychia porteri. J Nat Prod 57:1012–1016 [DOI] [PubMed] [Google Scholar]
- Luechtefeld NW, Cambre RC, Wang WL. (1981) Isolation of Campylobacter fetus subsp jejuni from zoo animals. J Am Vet Med Assoc 179:1119–1122 [PubMed] [Google Scholar]
- Martin A, Tsui HW, Shulman MJ, Isenman D, Tsui FW. (1999) Murine SHP-1 splice variants with altered Src homology 2 (SH2) domains. Implications for the SH2-mediated intramolecular regulation of SHP-1. J Biol Chem 274:21725–21734 [DOI] [PubMed] [Google Scholar]
- Pan MH, Chen WJ, Lin-Shiau SY, Ho CT, Lin JK. (2002) Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis 23:1677–1684 [DOI] [PubMed] [Google Scholar]
- Pandey MK, Sung B, Ahn KS, Aggarwal BB. (2009) Butein suppresses constitutive and inducible signal transducer and activator of transcription (STAT) 3 activation and STAT3-regulated gene products through the induction of a protein tyrosine phosphatase SHP-1. Mol Pharmacol 75:525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phromnoi K, Reuter S, Sung B, Prasad S, Kannappan R, Yadav VR, Chanmahasathien W, Limtrakul P, Aggarwal BB. (2011) A novel pentamethoxyflavone down-regulates tumor cell survival and proliferative and angiogenic gene products through inhibition of activation of IκB kinase and sensitizes tumor cells to apoptosis by cytokines and chemotherapeutic agents. Mol Pharmacol 79:279–289 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ram PT, Horvath CM, Iyengar R. (2000) Stat3-mediated transformation of NIH-3T3 cells by the constitutively active Q205L Galphao protein. Science 287:142–144 [DOI] [PubMed] [Google Scholar]
- Schreiner SJ, Schiavone AP, Smithgall TE. (2002) Activation of STAT3 by the Src family kinase Hck requires a functional SH3 domain. J Biol Chem 277:45680–45687 [DOI] [PubMed] [Google Scholar]
- Sergeev IN, Li S, Colby J, Ho CT, Dushenkov S. (2006) Polymethoxylated flavones induce Ca2+-mediated apoptosis in breast cancer cells. Life Sci 80:245–253 [DOI] [PubMed] [Google Scholar]
- Shi Q, Chen K, Li L, Chang JJ, Autry C, Kozuka M, Konoshima T, Estes JR, Lin CM, Hamel E. (1995) Antitumor agents, 154. Cytotoxic and antimitotic flavonols from Polanisia dodecandra. J Nat Prod 58:475–482 [DOI] [PubMed] [Google Scholar]
- Takanaga H, Ohnishi A, Yamada S, Matsuo H, Morimoto S, Shoyama Y, Ohtani H, Sawada Y. (2000) Polymethoxylated flavones in orange juice are inhibitors of P-glycoprotein but not cytochrome P450 3A4. J Pharmacol Exp Ther 293:230–236 [PubMed] [Google Scholar]
- Takase H, Imanishi K, Miura O, Yumioka E, Watanabe H. (1989) Features of the anti-ulcer effects of Oren-gedoku-to (a traditional Chinese medicine) and its component herb drugs. Japan J Pharmacol 49:301–308 [DOI] [PubMed] [Google Scholar]
- Tuchinda P, Saiai A, Pohmakotr M, Yoosook C, Kasisit J, Napaswat C, Santisuk T, Reutrakul V. (2004) Anti-HIV-1 cycloartanes from leaves and twigs of Gardenia thailandica. Planta Medica 70:366–370 [DOI] [PubMed] [Google Scholar]
- Verma NK, Dourlat J, Davies AM, Long A, Liu WQ, Garbay C, Kelleher D, Volkov Y. (2009) STAT3-stathmin interactions control microtubule dynamics in migrating T-cells. J Biol Chem 284:12349–12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Yang CS, Li S, Jin H, Ho CT, Patel T. (2009) Monodemethylated polymethoxyflavones from sweet orange (Citrus sinensis) peel inhibit growth of human lung cancer cells by apoptosis. Mol Nutr Food Res 53:398–406 [DOI] [PubMed] [Google Scholar]
- Yang F, Brown C, Buettner R, Hedvat M, Starr R, Scuto A, Schroeder A, Jensen M, Jove R. (2010a) Sorafenib induces growth arrest and apoptosis of human glioblastoma cells through the dephosphorylation of signal transducers and activators of transcription 3. Mol Cancer Ther 9:953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Jove V, Xin H, Hedvat M, Van Meter TE, Yu H. (2010b) Sunitinib induces apoptosis and growth arrest of medulloblastoma tumor cells by inhibiting STAT3 and AKT signaling pathways. Mol Cancer Res 8:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, Jove R. (1995) Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269:81–83 [DOI] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9:798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Bastow KF, Tachibana Y, Kuo SC, Hamel E, Mauger A, Narayanan VL, Lee KH. (1999) Antitumor agents. 196. Substituted 2-thienyl-1,8-naphthyridin-4-ones: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J Med Chem 42:4081–4087 [DOI] [PubMed] [Google Scholar]
- Zheng G, Yang D, Wang D, Zhou F, Yang X, Jiang L. (2009) Simultaneous determination of five bioactive flavonoids in pericarpium Citri reticulatae from china by high-performance liquid chromatography with dual wavelength detection. J Agric Food Chem 57:6552–6557 [DOI] [PubMed] [Google Scholar]