Abstract
Background
A tumor necrosis factor-alpha is a multifunctional pro-inflammation cytokine, which has been considered as one of pathogenic factors for various diseases. The promoter -1031(T/C) polymorphism in the tumor necrosis factor-alpha gene was reported that it plays a part in reproduction-related diseases. Among these, polycystic ovary syndrome (PCOS) is known to be a common gynecological disease of women in reproductive age women. Here, we performed a comparative study of -1031(T/C) polymorphism of TNF-alpha gene with PCOS in a Korean population.
Methods
The -1031(T/C) polymorphism of TNF-alpha gene was analyzed by polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) in a total of 217 PCOS patients and 144 matched female controls of healthy women. And statistical analysis was performed using HapAnalyzer. X2 test and logistic regression were utilized analyze the association between two groups. A p-value under 0.05 was considered statistically significant.
Results
The genotype and allelic frequencies were in Hardy-Weinberg equilibrium (HWE). There was strong association between the -1031(T/C) polymorphism in the promoter region of TNF-alpha gene and PCOS (p-value = 0.0003, odd ratio (OR) = 2.53). In addition, the frequency of C allele was significantly higher in PCOS patients compared with controls. Sequence analyses also showed the -1031(T/C) polymorphism of TNF-alpha gene.
Conclusion
This is the first study on the -1031(T/C) polymorphism of TNF-alpha gene in PCOS. We concluded that the -1031(T/C) polymorphism of TNF-alpha gene is associated with PCOS in a Korean population. Therefore, it is possible that it may be considered as a clinical biomarker to diagnose for PCOS, and is helpful in understanding the etiology for the pathogenesis of PCOS.
Keywords: Polycystic ovary syndrome, TNF-alpha, tumor necrosis factor, PCR-RFLP
Background
Polycystic ovary syndrome (PCOS) is one the most common gynecological disorder and affects up to 5% women in reproductive ages [1-4]. Generally, PCOS patients show the symptoms of obesity, increased risk of type 2 diabetes mellitus, menstrual irregularity, and anovulation [5-9]. A number of groups focused on the studies for single nucleotide polymorphisms and expected that could be associated with PCOS. However, its etiology is still not fully identified [10,11].
Up to now, several association studies were reported that the some of polymorphisms of tumor necrosis factor-α (TNF-α) are related with gynecological diseases including pre-eclampsia, endometriosis [12]. It is a multifunctional proinflammation cytokine and has a significant source of genetic variability [13,14]. Many studies suggested that the TNF-α may be considered as an immunological and molecular indicator for gynecological-related diseases. A previous study showed that -857(C/T) and -863(C/A) polymorphisms in TNF-α gene showed no effect on endometriosis patients in a Japanese population [15]. Additional genetic studies of -238(G/A) and -308(G/A) promoter polymorphisms in a TNF-α gene have no association with endometriosis in a Caucasian population [16]. And -308(G/A) promoter polymorphism does not affect genetic susceptibility to polycystic ovaries [17]. In contrast, only -1031(T/C) polymorphism in a TNF-α gene plays a part of endometriosis in Asian populations [15,18]. Even though there are no extensive genetic studies performed on PCOS, previous studies suggest that the -1031(T/C) polymorphism in a TNF-α gene may affect PCOS. Here, we investigated the relationship between SNP in -1031(T/C) of TNF-α gene and PCOS to investigated -1031(T/C) polymorphism in a TNF-α gene is associated with PCOS.
Methods
Subjects
All samples were recruited from at Fertility Center of CHA General Hospital in Seoul, Korea. The study appraised 217 PCOS patients and 144 healthy Korean women as case and control groups based on the revised diagnostic criteria according to the 2003 ASRM/ESHRE Rotterdam consensus.
Written informed consent was obtained from all of the participating women. Blood samples were collected in tubes containing EDTA as an anti-clotting factor and stored at -20°C until use. Genomic DNA was isolated from the blood samples of PCOS patients and controls. PCOS patients and controls in this study were all Korean women and this study with human blood samples was authorized by an Institutional Review Board (IRB).
Biochemical determinations
The clinical and biochemical characteristics of the PCOS patients and controls are indicated in Table 1. Blood samples were collected from both PCOS patients and controls. Hormone and glucose levels, including plasma LH, FSH, PRL, E2, TSH, testosterone, and DHEA-S were measured as indicators of distinction.
Table 1.
Clinical and biochemical characteristic of PCOS patients (n = 217) and normal controls (n = 144)
| Characteristics | PCOS patients (n = 217) | Controls (n = 144) |
|---|---|---|
| Age (y) | 32.47 ± 3.04 (29-35) | 33.09 ± 3.36 (29-36) |
| BMI (kg/m2) | 34.99 ± 5.84 (24.38-56.23) | 18.77 ± 2.13 (15.04-25.08) |
| Waist/hip ratio (WHR) | 1.23 ± 0.09 (1.00-2.24) | 0.72 ± 0.06 (0.63-0.82) |
| FSH (mIU/ml) | 8.75 ± 2.75 (1.50-29.98) | 5.93 ± 1.64 (2.71-12.22) |
| LH (mIU/ml) | 12.61 ± 9.87 (1.50-37.67) | 2.93 ± 1.34 (1.01-6.43) |
| E2 (pg/ml) | 64.69 ± 52.11 (7.53-133.36) | 29.4 ± 13.7 (3.84-58.05) |
| Prolactin (ng/ml) | 20.22 ± 14.52 (3.46-108.80) | 11.08 ± 5.86 (3.71-42.02) |
| TSH (μIU/ml) | 3.39 ± 2.15 (0.69-15.74) | 1.7 ± 0.85 (0.02-3.67) |
| DHEA-S (μg/dl) | 298.48 ± 115.34 (68.26-568.41) |
142.96 ± 50.22 (60.86-233.11) |
| Testosterone (ng/ml) | 0.87 ± 0.31 (0.09-1.29) | 0.22 ± 0.13 (0.01-0.48) |
Numerical data were presented as means ± standard deviation (SD). Abbreviations: BMI, Body mass index: WHR, Waist-hip ratio: FSH, Follicle-stimulating hormone: LH, Luteinizing hormone: E2, Estradiol: TSH, Thyroid-stimulating hormone; DHEA-S, Dehydroepiandrosterone-sulfate
Genetic analysis
To investigate the association between the -1031(T/C) polymorphism of TNF-a gene and PCOS, we collected 217 PCOS patients sample and 144 control samples, and polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) analysis was performed. The -1031(T/C) polymorphism of TNF-α gene was amplified by PCR with a set of primers, 5'-TAT GTG ATG GAC TCA CCA GG-3' and 5'-CCT CTA CAT GGC CCT GTC TT-3'. The 30 cycles of PCR was perform at 96°C for 5 min, 94°C for 30 sec, 63°C for 40 sec, and 72°C for 1 min and final cycle of 72°C for 10 min. Amplified PCR products were purified using AccuPrep Bioneer's PCR purification kit (Bioneer, Daejeon, Korea), and digested with Bbs I (New England BioLabs, Beverly, MA, USA) for 14 hrs at 37°C.
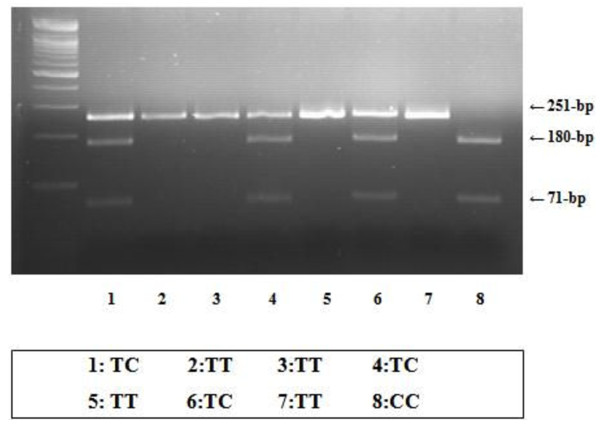
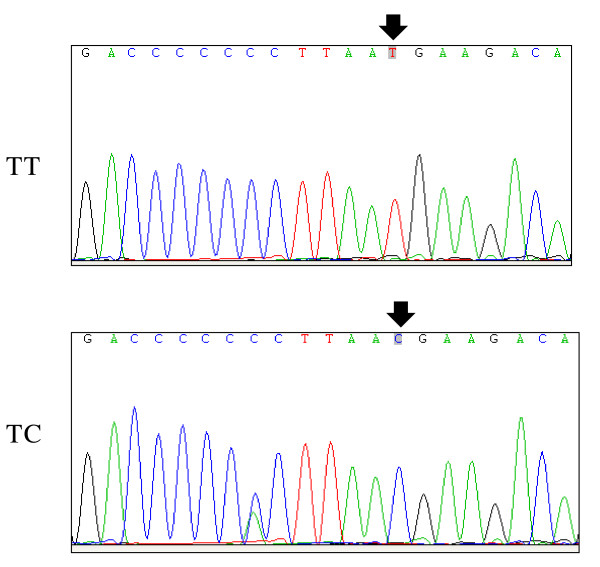
DNA fragments were electrophoresed on 2% agarose gels containing ethidium bromide in 0.5× Tris-Borate-EDTA buffer. Stained fragments were visualized under ultraviolet transilluminator. The fragments of 251- and 13-bp revealed homozygosity for the T allele and 180-, 71- and 13-bp fragments indicated homozygosity for the C allele (Figure 1). In addition, PCR products of each genotype were sequenced to confirm the genotyping results from the RFLP analysis.
Figure 1.
The -1031(T/C) polymorphism of the TNF-α gene. Shown is 2% agarose gel on the electrophoresis stained with ethidium bromide following Bbs I digestion. The 251- and 13-bp indicate the homozygosity of the T allele. However, 13-bp band was not observed in 2% gel. 180-, 71-, and 13-bp indicated the homozygosity of the C allele. And the T/C genotype indicates three bands of 251-, 180-, and 71-bp.
Statistical analysis
Statistical analysis was performed using HapAnalyzer. X2 test and logistic regression were utilized to analyze the association between two groups. A p-value under 0.05 was considered statistically significant.
Results
According to the instruction of the ASRM/ESHRE Rotterdam consensus in 2003, PCOS patients showed following two of the three phenotypes: clinical or biochemical hyperandrogenism, oligo- or amenorrhea and polycystic ovarian morphology through ultrasonography. To validate the clinical and biochemical characteristics between PCOS patients and controls, all subjects were measured for body mass index (BMI), waist/hip ratio (WHR), glucose, and hormone levels including FSH, LH, E2, PRL, TSH, testosterone and DHEA-S are shown in Table 1. Consequently, the PCOS patient group showed distinct difference compared with the control group. The level of TSH, DHEA-S, and testosterone were slightly higher in the PCOS group. Furthermore, 33 patients (15.2%) in the PCOS group had hyperandrogenism and oligo- or amenorrhea, 18 patients (8.3%) in the PCOS group had hyperandrogenism and polycystic ovaries, 141 patients (65%) in the PCOS group had oligo- or amenorrhea and polycystic ovaries, 22 patients (10.1%) in the PCOS group had hyperandrogenism, oligo- or amenorrhea and polycystic ovaries which are summarized in Table 2.
Table 2.
Comparison of clinical characteristics between PCOS patients (n = 217) and controls (n = 144)
| Characteristics | PCOS patients (n = 217) | Controls (n = 144) |
|---|---|---|
| Hyperandrogenism and Oligo- or amenorrhea | n = 33 (15.2%) | n = 0 (0.0%) |
| Hyperandrogenism and Polycystic ovaries | n = 18 (8.3%) | n = 0 (0.0%) |
| Oligo- or amenorrhea and polycystic ovaries | n = 141 (65%) | n = 0 (0.0%) |
| Hyperandrogenism, Oligo- or amenorrhea and polycystic ovaries | n = 22 (10.1%) | n = 0 (0.0%) |
For the RFLP analysis for the -1031(T/C) polymorphism of TNF-α gene, we recruited 217 patients and 144 control samples. In the present study, the frequency of T/T, T/C, and C/C genotypes of -1031(T/C) polymorphism in TNF-α gene was confirmed by sequence analysis and it showed different proportion (p-value = 0.0003, odd ratio (OR) = 2.53) between PCOS and control groups (Figure 2, Table 3). According to the X2 test, both groups were in Hardy-Weinberg equilibrium (HWE). The frequency of T/T genotype between PCOS and control groups was 66.3% and 84.7%, respectively. The frequency of T/C genotype between PCOS and control groups was 32.7% and 15.3%, respectively. And, the frequency of C/C genotype between PCOS and control groups was 0.9% and 0%, respectively. Our results demonstrated that there was significant difference between PCOS and control groups, indicating that there is strong association between the -1031(T/C) polymorphism in the promoter region of TNF-α gene and PCOS.
Figure 2.
Sequence analysis for the -1031(T/C) polymorphism of TNF-α gene using forward primer.
Table 3.
Allele frequencies of -1031(T/C) polymorphism of TNF-α gene in PCOS group (n = 217) and control group (n = 144)
| PCOS patient group | Control group | |
|---|---|---|
| Genotypes, n (%) | ||
| TT | 144 (66.5%) | 122 (84.7%) |
| TC | 71 (32.7%) | 22 (15.3%) |
| CC | 2 (0.9%) | 0 (0.0%) |
| Alleles, n (%) | ||
| T | 359 (82.7%) | 266 (92.4%) |
| C | 75 (17.3%) | 22 (7.6%) |
| Total | OR (95% CI) = 2.53 (1.53-4.17), p-value = 0.0003 | |
Discussion
TNF-α is a multifunctional proinflammation cytokine and plays an important role in wide-range of various diseases [19,20]. It exists not only in oocytes [21-24] and granulosa cells [25-28], but also in follicular fluid of human ovary [29-31]. It is considered to be related to ovarian apoptosis, increased ovarian steroid secretion, and anovulation [32,33]. Furthermore, it was reported that serum level of TNF- α was shown to increase in patients with PCOS. The TNF-α gene is known to cause a decrease in insulin receptor tyrosine phosphorylation and an increase in serine phosphorylation of insulin receptor substrate, leading to inhibition of downstream insulin signaling and glucose uptake [34].
TNF-α expression is regulated at both the transcriptional and post-transcriptional levels. Moreover, TNF-α gene transcription is regulated by the promoter region which consists of an 1100 base pair of DNA [35,36].
Up to now, several studies of the -1031(T/C) polymorphism of TNF-α were reported including Behcet's disease [37], large joint arthropathy [38], and Crohn's disease [39]. Crohn's disease and Behcet's disease patients showed the increased frequency of -1031C allele compared with controls. In contrast, patients with ulcerative collitis and hyperandrogenism showed lower frequency of -1031C allele than that of controls [39,40]. In the present study, we investigated the influence of -1031(T/C) polymorphism of TNF-α on PCOS by biochemical, clinical, and molecular genetic approaches. Our data showed that the -1031(T/C) polymorphism in the promoter region of TNF-α gene is associated with PCOS in a Korean population. Our study indicated that the C allele may provide protection from PCOS, and this promoter polymorphism has been associated with the number of other diseases including inflammatory bowel diseases [41], rheumatoid arthritis [42], and breast cancer [43]. Further studies are required to determine the functional significance of the -1031C allele in PCOS. This is the first study to demonstrate an association between the -1031(T/C) polymorphism in the TNF-α gene and PCOS. Genetic predisposition can be investigated with association studies, and our results suggest that it could be one of the etiological factors for PCOS and that the -1031(T/C) polymorphism may play an important role for the progression of PCOS.
Up to now, there is no transcriptional factor binding to the -1031(T/C) polymorphism of TNF-α gene. It is very important to investigate the molecular mechanism of the polymorphism for PCOS. Further studies are required for the functional significance of the polymorphisms in TNF-α and may be suggested to be a credible molecular and immunological marker. And further association studies of PCOS with related genes required for finding the pathogenesis of PCOS and SNPs, which have association with PCOS important for prediction and suggestion as a biomarker for diagnosis of the disease.
In conclusion, this is the first report on the association of -1031(T/C) polymorphism of TNF- α gene with PCOS. These data might be very informative for the advancement of future genetic treatment for PCOS patients.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JY designed the study and drafted the manuscript. JY and JC performed molecular genetic studies. KL and JS designed the study and offered the blood samples. KB designed the study, contributed the data analysis, and wrote the manuscript. All authors read and authorized the final manuscript.
Contributor Information
Ji-Hyun Yun, Email: jhyun6575@gmail.com.
Jin-Woo Choi, Email: jchoi@sps.edu.
Kyung-Ju Lee, Email: drlkj4094@cha.ac.kr.
Joong-Sik Shin, Email: shinjs@cha.ac.kr.
Kwang-Hyun Baek, Email: baek@cha.ac.kr.
Acknowledgements
We would like to thank members of the Fertility Center and Stem Cell Institute at CHA University and CHA General Hospital. This study was supported by a grant from Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (01-PJ10-PG6-01GN13-0002).
References
- Futterweit W. Polycystic ovary syndrome: clinical perspectives and management. Obestet Gynecol Surv. 1999;54:403–413. doi: 10.1097/00006254-199906000-00024. [DOI] [PubMed] [Google Scholar]
- Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84:1897–1899. doi: 10.1210/jc.84.6.1897. [DOI] [PubMed] [Google Scholar]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Lergo RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clan Endocrinal Metal. 1999;84:165–169. doi: 10.1210/jc.84.1.165. [DOI] [PubMed] [Google Scholar]
- Chang WY, Knochenhauer ES, Bartolucci AA, Azziz R. Phenotypic spectrum of polycystic ovary syndrome: clinical and biochemical characterization of the three major clinical subgroups. Fertil Steril. 2005;83:1717–1723. doi: 10.1016/j.fertnstert.2005.01.096. [DOI] [PubMed] [Google Scholar]
- Stankiewicz M, Norman R. Diagnosis and management of polycystic ovary syndrome: a practical guide. Drug. 2006;66:903–912. doi: 10.2165/00003495-200666070-00002. [DOI] [PubMed] [Google Scholar]
- Sheehan MT. Polycystic ovary syndrome: diagnosis and management. Clin Med Res. 2004;2:13–27. doi: 10.3121/cmr.2.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan JA, Chen TT, Chadwell NL, Torry DS, Caudle MR. IL-1 beta, TNF-alpha and IL-2 in peritoneal fluid and macrophage conditioned media of women with endometriosis. Am J Rerprod Immunol. 1995;34:381–385. doi: 10.1111/j.1600-0897.1995.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Braun DP, Ding J, Dmowski. Peritoneal fluid-mediates enhancement of eutopic and ectopic endometrial cell proliferation in dependent on tumor necrosis factor-alpha in women with endometriosis. Fertil Steril. 2002;78:727–732. doi: 10.1016/S0015-0282(02)03318-6. [DOI] [PubMed] [Google Scholar]
- Beutler B. TNF immunity and inflammatory disease: lessions of the past decade. J Invest Med. 1995;43:227–235. [PubMed] [Google Scholar]
- Rasmussen SK, Urhammer SA, Jensen JN, Hansen T, Borch-Johnsen K, Pedersen O. The -238 and -308 G/A polymorphisms of tumor necrosis factor alpha gene promoter are not associated with features of the insulin resistance syndrome or altered birth weight in Danish Caucasians. J Clin Endocrinol Metab. 2000;85:1731–1734. doi: 10.1210/jc.85.4.1731. [DOI] [PubMed] [Google Scholar]
- Bischof FZ, Simpson JL. Heritability and molecular genetic studies of endometriosis. Hum Reprod Update. 2000;6:37–44. doi: 10.1093/humupd/6.1.37. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Thusu K, Abdel-Rahman E, Prabhala A, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor in normal-weight women with polycystic ovary syndrome. Metabolism. 1999;48:437–441. doi: 10.1016/S0026-0495(99)90100-2. [DOI] [PubMed] [Google Scholar]
- Chen G, Wilson R, Wang SH, Zheng HZ, Walker JJ, McKillop JH. Tumour necrosis factor-alpha (TNF-alpha) gene polymorphism and expression in pre-eclampsia. Clin Exp Immunol. 1996;104:154–159. doi: 10.1046/j.1365-2249.1996.d01-647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar T, Yoshida S, Kennedy S, Negoro K, Zhuo W, Hamana S, Motoyama S, Nakago S, Barlow D, Maruo T. The tumor necrosis factor-a promoter-1031C polymorphism is associated with decreased risk of endometriosis in a Japanese population. Hum Reprod. 2004;19:2509–2514. doi: 10.1093/humrep/deh478. [DOI] [PubMed] [Google Scholar]
- Wieser F, Fabjani G, Tempfer C, Schneeberger C, Zeillinger R, Huber JC, Wenzl R. Tumor necrosis factor-a promoter polymorphisms and endometriosis. J Soc Gynecol Investig. 2002;9:313–318. doi: 10.1016/S1071-5576(02)00174-0. [DOI] [PubMed] [Google Scholar]
- Milner CR, Craig JE, Hussey ND, Norman RJ. No association between the -308 polymorphism in the tumour necrosis factor alpha (TNF alpha) promoter region and polycystic ovaries. Mol Hum Reprod. 1999;5:5–9. doi: 10.1093/molehr/5.1.5. [DOI] [PubMed] [Google Scholar]
- Lee KH, Choi YM, Kim SH, Hong MA, Oh ST, Lim YT, Moon SY. Association of tumor necrosis factor-a gene polymorphisms with advanced stage endometriosis. Hum Reprod. 2008;23:977–981. doi: 10.1093/humrep/den016. [DOI] [PubMed] [Google Scholar]
- Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O'Brine-Greco B, Foley SF, Ware CF. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-A. [DOI] [PubMed] [Google Scholar]
- Trowsdale J. Genomic structure and function in the MHC. Trends Genet. 1993;9:117–122. doi: 10.1016/0168-9525(93)90205-V. [DOI] [PubMed] [Google Scholar]
- Kondo H, Maruo T, Mochizuki M. Immunohistochemical evidence for the presence of tumor necrosis factor-alpha in the infant and adult human ovary. Endocr J. 1995;42:771–780. doi: 10.1507/endocrj.42.771. [DOI] [PubMed] [Google Scholar]
- Roby KF, Terranova PF. Localisation of immunoreactive tumor necrosis factor (I-TNF) in the human ovary. Biol Reprod. 1989;40(Suppl 1):171. [Google Scholar]
- Zolti M, Bider D, Seidman DS, Mashiach S, Ben-Rafael Z. Cytokine levels in follicular fluid of polycystic ovaries in patients treated with dexamethasone. Fertil Steril. 1992;57:501–504. doi: 10.1016/s0015-0282(16)54891-2. [DOI] [PubMed] [Google Scholar]
- Fernández-Real JM, Gutierrez C, Ricart W, Casamitjana R, Fernández-Castañer M, Vendrell J, Richart C, Soler J. The TNF-alpha gene Nco I polymorphism influences the relationship among insulin resistance, percent body fat, and increased serum leptin levels. Diabetes. 1997;46:1468–1472. doi: 10.2337/diabetes.46.9.1468. [DOI] [PubMed] [Google Scholar]
- Herrmann SM, Ricard S, Nicaud V, Mallet C, Arveiler D, Evans A, Ruidavets JB, Luc G, Bara L, Parra HJ, Poirier O, Cambien F. Polymorphisms of the tumour necrosis factor-alpha gene, coronary heart disease and obesity. Eur J Clin Invest. 1998;28:59–66. doi: 10.1046/j.1365-2362.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- Hoffstedt J, Eriksson P, Hellström L, Rössner S, Rydén M, Arner P. Excessive fat accumulation is associated with the TNF alpha-308 G/A promoter polymorphism in women but not in men. Diabetologia. 2000;43:117–120. doi: 10.1007/s001250050015. [DOI] [PubMed] [Google Scholar]
- Kaipia A, Chun SY, Eisenhauer K, Hsueh AJ. Tumor necrosis factor-alpha and its second messenger, ceramide, stimulate apoptosis in cultured ovarian follicles. Endocrinology. 1996;137:4864–4870. doi: 10.1210/en.137.11.4864. [DOI] [PubMed] [Google Scholar]
- Roby KF, Terranova PF. Effects of tumor necrosis factor-alpha in vitro on steroidogenesis of healthy and atretic follicles of the rat: theca as a target. Endocrinology. 1990;126:2711–2118. doi: 10.1210/endo-126-5-2711. [DOI] [PubMed] [Google Scholar]
- Spaczynski RZ, Arici A, Duleba AJ. Tumor necrosis factor-alpha stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1999;61:993–998. doi: 10.1095/biolreprod61.4.993. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–1278. doi: 10.2337/diabetes.43.11.1271. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Yoo KJ, Kim SJ, Lee SH, Cha KY, Baek KH. Single nucleotide polymorphism in exon 17 of the insulin receptor gene is not associated with polycystic ovary syndrome in a Korean population. Fertil Steril. 2006;86:380–384. doi: 10.1016/j.fertnstert.2005.12.073. [DOI] [PubMed] [Google Scholar]
- Franks S, Gharani N, Waterworth D, Batty S, White D, Williamson R, McCarthy M. The genetic basis of polycystic ovary syndrome. Hum Reprod. 1997;12:2641–2648. doi: 10.1093/humrep/12.12.2641. [DOI] [PubMed] [Google Scholar]
- Xita N, Georgiou I, Tsatsoulis A. The genetic basis of polycystic ovary syndrome. Eur J Endocrinol. 2002;147:717–725. doi: 10.1530/eje.0.1470717. [DOI] [PubMed] [Google Scholar]
- Hart R, Hickey M, Franks S. Definition, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Prac Res Obstet Gynaecol. 2004;18:671–683. doi: 10.1016/j.bpobgyn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Laven JS, Imani B, Eijkemans MJ, Fauser BC. New approach to polycystic ovary syndrome and other forms of anovulatory infertility. Obstet Gynecol Surv. 2002;57:755–767. doi: 10.1097/00006254-200211000-00022. [DOI] [PubMed] [Google Scholar]
- Choi SW, Gu BH, Ramakrishna S, Park JM, Baek KH. Association between a single nucleotide polymorphism in MTHFR gene and polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2009;145:85–88. doi: 10.1016/j.ejogrb.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Ahmad T, Wallace GR, James T, Neville M, Bunce M, Mulcahy-Hawes K, Armuzzi A, Crawshaw J, Fortune F, Walton R, Stanford MR, Welsh KI, Marshall SE, Jewell DP. Mapping the HLA association in Behçet's disease: a role for tumor necrosis factor polymorphisms? Arthritis Rheum. 2003;48:807–813. doi: 10.1002/art.10815. [DOI] [PubMed] [Google Scholar]
- Orchard TR, Chua CN, Ahmad T, Cheng H, Welsh KI, Jewell DP. Uveitis and erythema nodosum in inflammatory bowel disease: clinical features and the role of HLA genes. Gastroenterology. 2002;123:714–718. doi: 10.1053/gast.2002.35396. [DOI] [PubMed] [Google Scholar]
- Negoro K, Kinouchi Y, Hiwatashi N, Takahashi S, Takagi S, Satoh J, Shimosegawa T, Toyota T. Crohn's disease is associated with novel polymorphisms in the 5'-flanking region of the tumor necrosis factor gene. Gastroenterology. 1999;117:1062–1068. doi: 10.1016/S0016-5085(99)70390-2. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Calvo RM, Sancho J, San Millán JL. TNF-alpha and hyperandrogenism: a clinical, biochemical, and molecular genetic study. J Clin Endocrinol Metab. 2001;86:3761–3767. doi: 10.1210/jc.86.8.3761. [DOI] [PubMed] [Google Scholar]
- Van Heel DA, Udalova IA, De Silva AP, McGovern DP, Kinouchi Y, Hull J, Lench NJ, Cardon LR, Carey AH, Jewell DP, Kwiatkowski D. Inflammatory bowel disease is associated with a TNF polymorphism that affects an interaction between the OCT1 and NF(-kappa)B transcription factors. Hum Mol Genet. 2002;11:1281–1289. doi: 10.1093/hmg/11.11.1281. [DOI] [PubMed] [Google Scholar]
- Vallvé JC, Paredes S, Girona J, Uliaque K, Ribalta J, Hurt-Camejo E, Masana L. Tumor necrosis factor-alpha -1031 T/C polymorphism is associated with smaller and more proatherogenic low density lipoprotein particles in patients with rheumatoid arthritis. J Rheumatol. 2008;35:1697–1703. [PubMed] [Google Scholar]
- Sirotkovic-Skerlev M, Cacev T, Krizanac S, Kulić A, Pavelic K, Kapitanovic S. TNF alpha promoter polymorphisms analysis in benign and malignant breast lesions. Exp Mol Pathol. 2007;83:54–58. doi: 10.1016/j.yexmp.2006.11.004. [DOI] [PubMed] [Google Scholar]