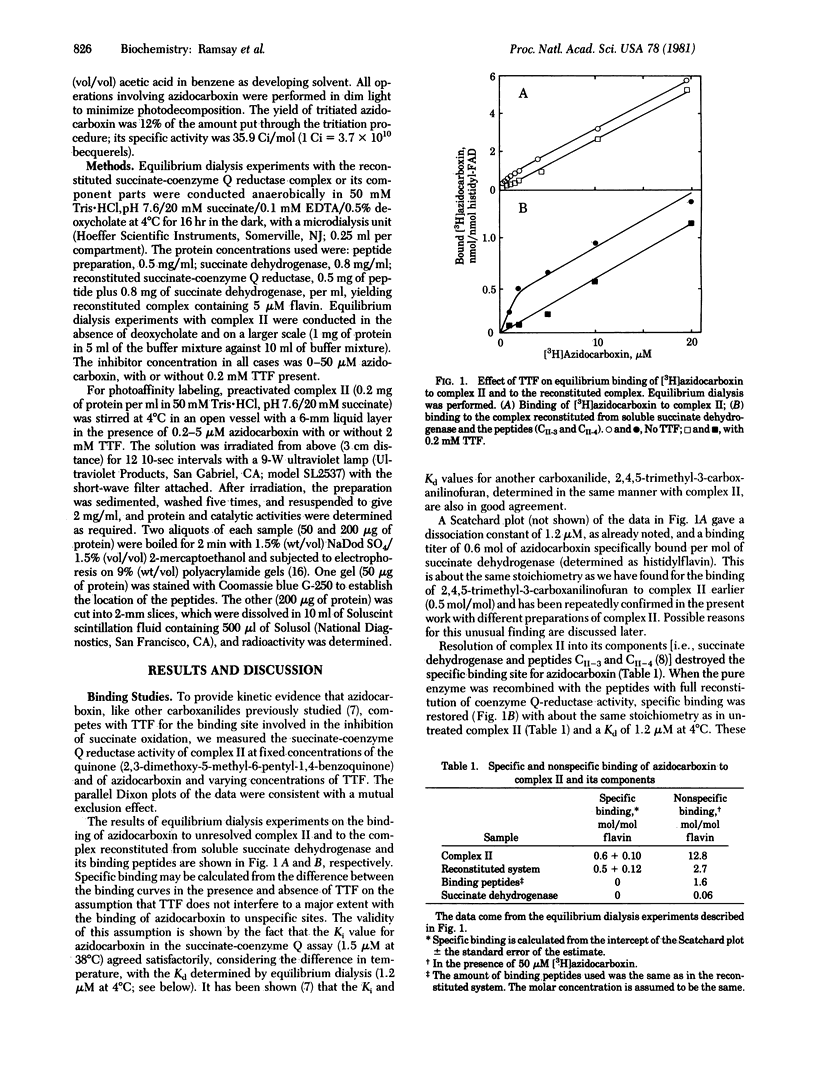
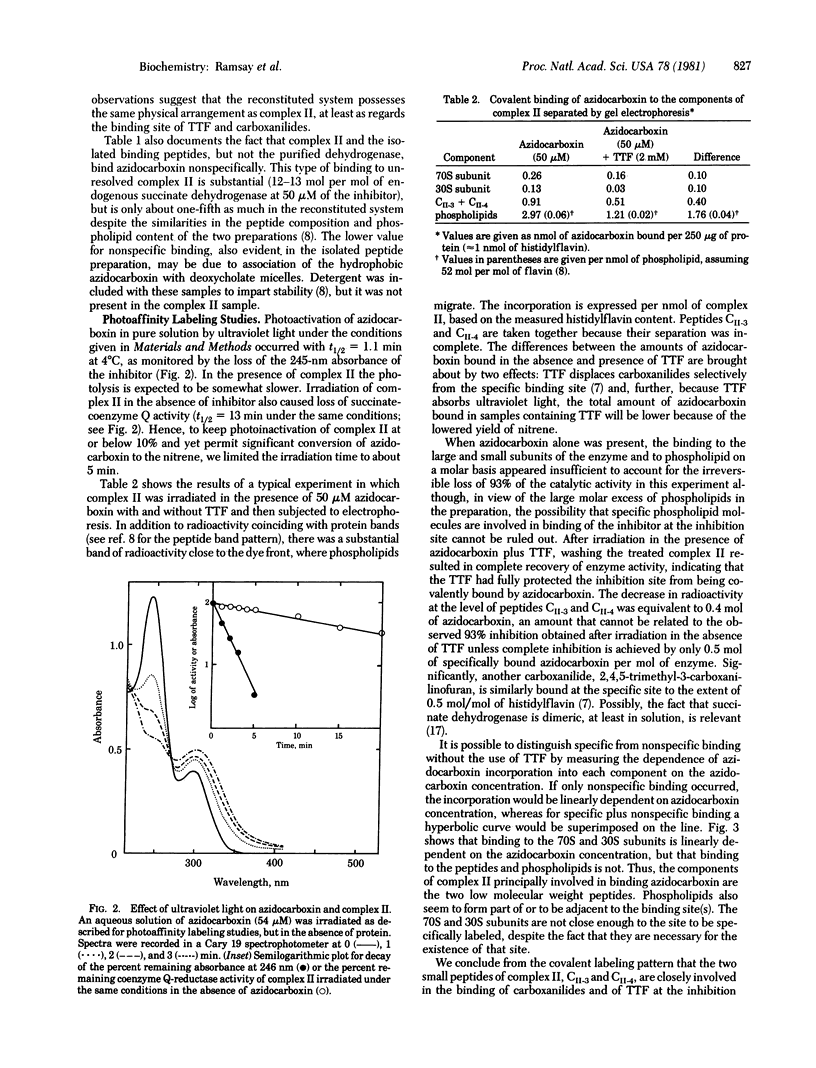
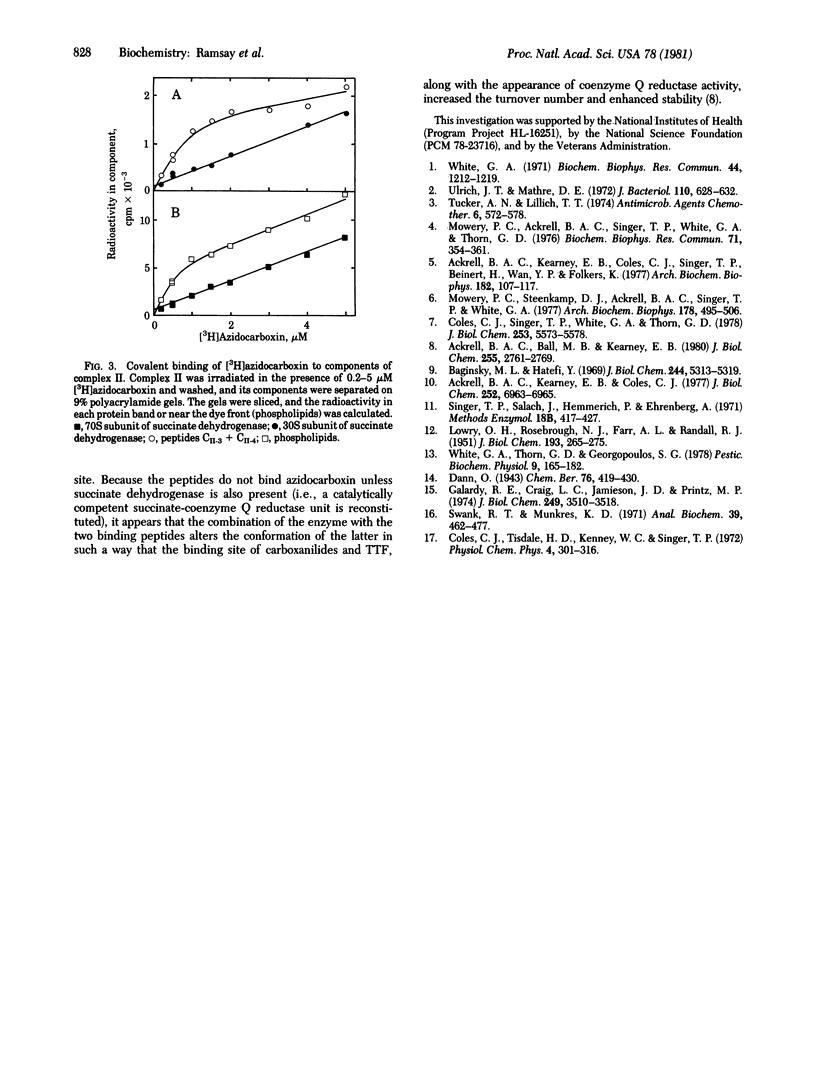
Abstract
Oxathiin carboxanilides are systemic fungicides that inhibit the oxidation of succinate by interrupting electron transport between succinate dehydrogenase [succinate:(acceptor) oxidoreductase, EC 1.3.99.1] and coenzyme Q. Kinetic and electron paramagnetic resonance studies have established that the specific binding site of carboxanilides and of thenoyltrifluoroacetone responsible for the inhibition is the same. Although the binding of carboxanilides to membrane preparations of the dehydrogenase is very tight (Ki = 0.01-0.1 microM), it is noncovalent. Identification of the membrane component(s) to which specific binding occurs has therefore required the introduction of a photoaffinity label onto the carboxanilide molecule. By using [G-3H]3'-azido-5,6-dihydro-2-methyl-1,4-oxathiin-3-carboxanilide, it was found, in accord with earlier data with other carboxanilides, that unresolved complex II specifically binds about 0.6 mol of the inhibitor per mol of succinate dehydrogenase in equilibrium dialysis experiments. The resolved components of the complex, succinate dehydrogenase and the two binding peptides CII-3 and CII-4, failed to bind the inhibitor; however, when these were recombined with reconstitution of coenzyme Q reductase activity, the initial binding titer was restored. Azidocarboxanilide-inhibited complex II was irradiated to generate covalent linkages with the binding site, and the components of the complex were separated on polyacrylamide gel. Most of the specifically bound inhibitor was found in the low molecular weight binding peptides and phospholipids.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Ball M. B., Kearney E. B. Peptides from complex II active in reconstitution of succinate-ubiquinone reductase. J Biol Chem. 1980 Apr 10;255(7):2761–2769. [PubMed] [Google Scholar]
- Ackrell B. A., Kearney E. B., Coles C. J. Isolation of reconstitutively active succinate dehydrogenase in highly purified state. J Biol Chem. 1977 Oct 25;252(20):6963–6965. [PubMed] [Google Scholar]
- Ackrell B. A., Kearney E. B., Coles C. J., Singer T. P., Beinert H., Wan Y. P., Folkers K. Kinetics of the reoxidation of succinate dehydrogenase. Arch Biochem Biophys. 1977 Jul;182(1):107–117. doi: 10.1016/0003-9861(77)90288-0. [DOI] [PubMed] [Google Scholar]
- Baginsky M. L., Hatefi Y. Reconstitution of succinate-coenzyme Q reductase (complex II) and succinate oxidase activities by a highly purified, reactivated succinate dehydrogenase. J Biol Chem. 1969 Oct 10;244(19):5313–5319. [PubMed] [Google Scholar]
- Coles C. J., Singer T. P., White G. A., Thorn G. D. Studies on the binding of carboxin analogs to succinate dehydrogenase. J Biol Chem. 1978 Aug 25;253(16):5573–5578. [PubMed] [Google Scholar]
- Coles C. J., Tisdale H. D., Kenney W. C., Singer T. P. Studies on succinate dehydrogenase: XXI. Quaternary structure of succinate dehydrogenase. Physiol Chem Phys. 1972;4(4):301–316. [PubMed] [Google Scholar]
- Galardy R. E., Craig L. C., Jamieson J. D., Printz M. P. Photoaffinity labeling of peptide hormone binding sites. J Biol Chem. 1974 Jun 10;249(11):3510–3518. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mowery P. C., Ackrell B. A., Singer T. P. Carboxins: powerful selective inhibitors of succinate oxidation in animal tissues. Biochem Biophys Res Commun. 1976 Jul 12;71(1):354–361. doi: 10.1016/0006-291x(76)90290-4. [DOI] [PubMed] [Google Scholar]
- Mowery P. C., Steenkamp D. J., Ackrell A. C., Singer T. P., White G. A. Inhibition of mammalian succinate dehydrogenase by carboxins. Arch Biochem Biophys. 1977 Jan 30;178(2):495–506. doi: 10.1016/0003-9861(77)90220-x. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tucker A. N., Lillich T. T. Effect of the systemic fungicide carboxin on electron transport function in membranes of Micrococcus denitrificans. Antimicrob Agents Chemother. 1974 Nov;6(5):572–578. doi: 10.1128/aac.6.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich J. T., Mathre D. E. Mode of action of oxathiin systemic fungicides. V. Effect on electron transport system of Ustilago maydis and Saccharomyces cerevisiae. J Bacteriol. 1972 May;110(2):628–632. doi: 10.1128/jb.110.2.628-632.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. A. A potent effect of 1,4-oxathiin systemic fungicides on succinate oxidation by a particulate preparation from Ustilago maydis. Biochem Biophys Res Commun. 1971 Sep;44(5):1212–1219. doi: 10.1016/s0006-291x(71)80215-2. [DOI] [PubMed] [Google Scholar]



