Abstract
Differentiation of human endometrial stromal cells (HESC) into decidual cells represents a highly coordinated process essential for embryo implantation. We show that decidualizing HESC down-regulate the histone methyltransferase enhancer of Zeste homolog 2 (EZH2), resulting in declining levels of trimethylation of histone 3 on lysine 27 (H3K27me3) at the proximal promoters of key decidual marker genes PRL and IGFBP1. Loss of H3K27me3 was associated with a reciprocal enrichment in acetylation of the same lysine residue, indicating active remodeling from repressive to transcriptionally permissive chromatin. Chromatin immunoprecipitation coupled with DNA microarray analysis demonstrated that decidualization triggers genome-wide changes in H3K27me3 distribution that only partly overlap those observed upon EZH2 knockdown in undifferentiated HESC. Gene ontology revealed that gain of the repressive H3K27me3 mark in response to decidualization and upon EZH2 knockdown in undifferentiated cells was enriched at the promoter regions of genes involved in transcriptional regulation and growth/cell proliferation, respectively. However, loss of the H3K27me3 mark (indicating increased chromatin accessibility) in decidualizing cells and upon EZH2 knockdown occurred at selective loci enriched for genes functionally implicated in responses to stimulus. In agreement, EZH2 knockdown in undifferentiated HESC was sufficient to augment the induction of decidual marker genes in response to cyclic AMP and progesterone signaling. Thus, loss of EZH2-dependent methyltransferase activity in the endometrium is integral to the process of chromatin remodeling that enables the transition from a proliferative to a decidual phenotype in response to differentiation cues.
The postovulatory rise in progesterone levels drives the differentiation of the endometrium in preparation of pregnancy, a highly coordinated and sequential process characterized by secretory transformation of the glandular epithelium, influx of specialized immune cells, decidualization of the stroma, and vascular remodeling (1–3). Although progesterone signaling is indispensible, the sequential nature of this differentiation process is thought to reflect the actions of locally produced cytokines and growth factors. For example, decidualization, which denotes the differentiation of endometrial stromal cells into epitheloid decidual cells, is a classical progesterone-dependent process, although this process is initiated in vivo only approximately 9 d after the ovulatory surge in circulating progesterone levels (4–6). Similarly, purified human endometrial stromal cells (HESC) in culture are also largely refractory to progesterone signaling, despite abundantly expressing the nuclear progesterone receptors (PR) A and B (4, 7, 8). However, progesterone does acquire control over the expression of decidual markers genes, such as PRL and IGFBP1, upon simultaneous activation of the cAMP pathway (4, 9–11). Endometrial cAMP levels also increase in vivo during the luteal phase, probably reflecting the induction of local factors that activate adenylate cyclase in stromal cells, such as relaxin, corticotropin-releasing hormone, and prostaglandin E2 (5).
Multiple mechanisms have been shown to underpin the convergence of the cAMP and progesterone signal pathways in HESC. For example, cAMP inhibits ligand-dependent sumoylation of PR (11, 12), a posttranslational modification that limits the transactivation capacity of this nuclear receptor (13). Increased cAMP levels also induce the expression of several transcription coregulators of PR (including forkhead box protein O1, signal transducer and activator of transcription 5, and CCAAT/enhancer binding protein β), and disrupt the binding of the receptor to specific corepressors, such as nuclear receptor corepressor and silencing mediator for retinoid and thyroid receptors (14–18). Although all these observations indicate that PR activity in HESC is tightly controlled, they do not explain well the highly temporal regulation of the decidual process during the cycle. One, as yet untested, possibility is that decidual transformation of the endometrial stroma in the cycle is dependent on coordinated chromatin modifications that create permissive and repressive transcriptional environments enabling large gene networks to respond to differentiation signals.
Numerous DNA and histone modifying enzymes govern the accessibility of the transcriptional machinery to chromatin, thus determining whether a gene is silenced, activated, or poised to respond to a stimulus (19–21). One of the most widely studied histone modifiers is enhancer of Zeste homolog 2 (EZH2), which along with the embryonic ectoderm development and the zinc finger protein suppressor of Zeste 12 homolog, makes up the Polycomb-repressive complex 2. Within this repressive complex, EZH2 serves as the active enzyme that catalyzes the trimethylation of histone 3 on lysine 27 (H3K27me3) leading to gene silencing (22–24). Importantly, aberrant expression of EZH2 occurs in a variety of hormone-dependent malignancies, including endometrial, breast, and prostate cancers (25–29). Furthermore, ovarian hormones regulate EZH2 expression and activity in a variety of cell types (30). These observations prompted us to examine the expression of EZH2 in human endometrium and to determine whether cycle-dependent changes in EZH2 methylation activity play a role in differentiation of HESC into specialized decidual cells.
Results
Cycle-dependent expression of EZH2 in human endometrium
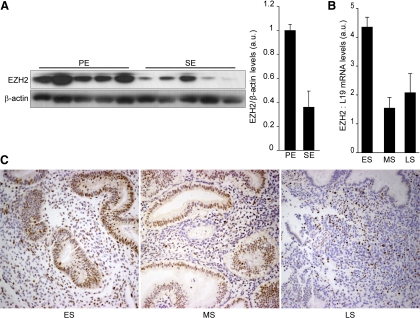
EZH2 expression during the menstrual cycle was examined in five proliferative and five secretory endometrial biopsies. Western blot analysis of whole-tissue lysates showed a marked decrease in the expression of this methyltransferase during the secretory phase of the cycle (Fig. 1 A). To further explore the dynamics of this down-regulation, we focused on the expression of EZH2 during this phase. Real-time quantitative PCR (RT-qPCR) analysis on timed endometrial biopsies revealed a 3-fold decrease in EZH2 transcript levels with the onset of the midsecretory phase. This reduction in EZH2 mRNA level was then maintained for the remainder of the cycle (Fig. 1B). Tissue sections obtained between d 14 and 27 were subjected to immunohistochemistry. As shown in Fig. 1C, EZH2 immunoreactivity was prominent in the epithelial glandular compartment during the early secretory phase. It was also abundantly expressed in stromal cells, although the staining was less homogenous. As the cycle progressed, a marked loss of EZH2 expression was apparent in epithelial cells, resulting in virtual lack of signal in this cellular compartment by the end of the cycle. A similar trend was apparent in the stroma, although individual cells strongly expressing EZH2 were still present during the late-secretory phase. Taken together, these data suggest that the progression of the menstrual cycle results in a gradual but marked loss of EZH2 expression in differentiating human endometrium.
Fig. 1.
Cycle-dependent expression of EZH2 in human endometrium. A, Protein lysates from proliferative (PE) and secretory (SE) endometrium were subjected to Western blot analysis and immunoprobed for EZH2. β-Actin served as a loading control. Panel on the right shows densitometric analysis of Western blots using ImageJ. B, EZH2 transcript levels in timed endometrial biopsies obtained during the early secretory (ES) (n = 5), midsecretory (MS) (n = 5), and late secretory (LS) (n = 5) phases of the cycle were determined by RT-qPCR. The data, normalized to L19 mRNA, are expressed in arbitrary units (a.u.); mean ± sem. C, Endometrial tissue sections obtained at different phases of the cycle were immunostained for EZH2 (brown). The data shown in this figure are representative of three or more independent experiments.
Loss of EZH2 expression in decidualizing HESC
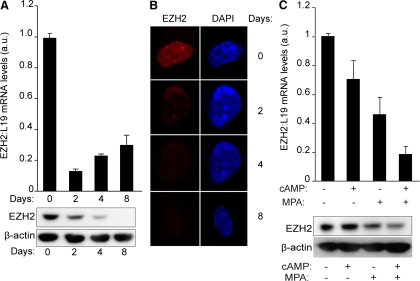
Elevated intracellular cAMP levels initiate differentiation of HESC, whereas progesterone is essential for the maintenance and enhancement of the decidual phenotype (5). Because EZH2 expression is down-regulated in the stromal compartment of differentiating endometrium, we examined whether this would be the case upon decidualization of primary HESC in vitro. Primary cultures were stimulated with 8-Bromo-cAMP (8-Br-cAMP) and the progestin medroxyprogesteroneacetate (MPA) for various time points (Fig. 2A). Total mRNA and protein were extracted from parallel cultures to examine the expression of EZH2. Unexpectedly, transcript levels were already 80% lower in cells decidualized for 2 d, and this level of repression was maintained throughout the entire time course (Fig. 2A, top panel). The decline in EZH2 mRNA expression was paralleled at protein level, although the reduction was more gradual. Upon 8 d of differentiation, expression of this methyltransferase was below the level of detection on Western blot analysis (Fig. 2A, bottom panel). The loss of EZH2 upon decidualization of HESC was further confirmed by confocal microscopy (Fig. 2B).
Fig. 2.
EZH2 is down-regulated upon decidualization. A, Confluent HESC cultures were either untreated (d 0) or decidualized with 8-Br-cAMP and MPA for the indicated time points. EZH2 expression at mRNA and protein levels were determined in parallel cultures by RT-qPCR (upper panel) and Western blot analysis (lower panel). The RT-qPCR data show the fold change (±sem of triplicate measurements) in EZH2 transcript levels upon treatment relatively to expression in untreated cells. B, EZH2 expression in endometrial stromal cells cultured in the presence or absence of 8-Br-cAMP and MPA for 2, 4, and 8 d. The intensity of EZH2 (red channel) and DAPI nuclear staining (blue channel) were captured by confocal microscopy. C, Confluent HESC cultures either untreated or decidualized with 8-Br-cAMP (cAMP), MPA, or a combination for 2 d. EZH2 expression at mRNA and protein levels were determined in parallel cultures by RT-qPCR (upper panel) and Western blot analysis (lower panel). The data show the fold change (±sem of triplicate measurements) in EZH2 transcript levels upon treatment relatively to expression in untreated cells. a.u., Arbitrary units.
To provide insights into the mechanism of EZH2 repression, primary cultures were treated with 8-Br-cAMP or MPA, alone or a combination, for 48 h. EZH2 transcripts decreased modestly upon treatment with 8-Br-cAMP, whereas MPA reduced the expression level by approximately 50%. However, a combination 8-Br-cAMP and MPA yielded an additive effect, resulting in approximately 80% reduction in EZH2 mRNA levels (Fig. 2C, top panel). Again, the regulation of EZH2 transcripts was recapitulated at protein level (Fig. 2C, bottom panel). Thus, both the cAMP and progesterone signal transduction pathways play a role in EZH2 down-regulation in differentiating HESC, with progesterone being the dominant signal.
Interplay between acetylation and trimethylation of H3K27
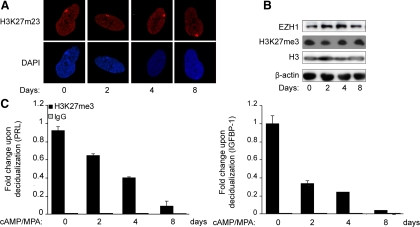
EZH2 catalyzes trimethylation of H3K27, a histone tail modification associated with repressive chromatin and gene silencing (31). To examine whether loss of EZH2 impacts on global cellular H3K27me3 levels, confocal microscopy as well as Western blot analysis were carried out on undifferentiated HESC and cultures treated with 8-Br-cAMP and MPA for various time points. Unexpectedly, differentiation of HESC for as long as 8 d was not associated with a discernible change in overall methylation of H3K27 (Fig. 3, A and B). This suggested that other enzymes with histone H3 methylase activity are likely to be present in decidualizing cells. As shown in Fig. 3B, EZH1, a functional homolog of EZH2 (32), is indeed expressed in HESC, and the abundance of this methyltransferase does not decrease upon treatment with 8-Br-cAMP and MPA.
Fig. 3.
H3K27 methylation in undifferentiated and decidualizing HESC. A, Primary HESC treated with 8-Br-cAMP and MPA for the indicated time points were fixed in formaldehyde, stained, and subjected to confocal microscopy. The upper panel represents H3K27me3 staining (red channel), whereas the lower panel shows the corresponding nuclear DAPI staining (blue channel). B, Total protein lysates of HESC treated as above were subjected to Western blot analysis and immunoprobed for EZH1, H3K27me, and total H3. β-Actin was used as a loading control. C, Chromatin extracted from HESC first decidualized for 2, 4, or 8 d was immunoprecipitated with antibodies against H3K27me3 and IgG. The chromatin, normalized to the input, was analyzed by qPCR (ChIP-qPCR) with primers specific for the promoter regions of PRL (left panel) and IGFBP1 (right panel). Data are expressed as the fold change (±sem of triplicate measurements) relatively to the abundance of chromatin-bound H3K27me3 in untreated cells. The data shown in this figure are representative of three independent experiments.
Next, we examined whether loss of EZH2 impacts on the methylation status of H3K27 at the transcriptional start site (TSS) of highly induced decidua-specific genes. PRL and IGFBP1 are the most widely studied decidual marker genes, and their transcriptional regulation has been extensively studied (14, 33–36). Notably, prolactin (PRL) in the endometrium is transcribed from an alternative promoter upstream of a noncoding exon located approximately 6 kb upstream of the pituitary-specific TSS (33). Chromatin immunoprecipitation (ChIP) analysis was carried out using an H3K27me3-specific antibody followed by amplification of a 98-bp fragment encompassing the −332- to −270-bp regulatory region of the decidual-specific PRL TSS, which contains several response elements required for cAMP- and progesterone-dependent regulation (12, 15, 36, 37). Time-course analysis revealed that HESC differentiation is associated with a gradual but dramatic loss of this mark at this locus (Fig. 3C). Loss of H3K27me3 was even more rapid and pronounced at the proximal IGFBP1 promoter (−263 to −33 bp relatively to the TSS). Thus, the marked decline of H3K27me3 at specific loci in the absence of a global change in levels suggests that decidualization is associated with a dynamic, albeit specific, redistribution of the mark.
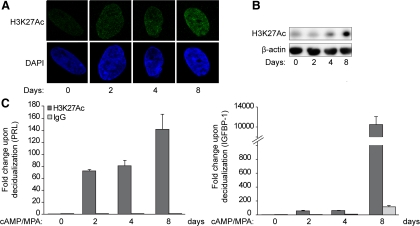
Acetylation of H3K27 (H3K27ac) antagonizes Polycomb-repressive complex 2-dependent gene silencing. Furthermore, acetylation and trimethylation of H3K27 are mutually exclusive, because both modifications compete for binding to the same lysine residue (38, 39). To determine whether complementarity between these marks exists in HESC, we first analyzed global levels of H3K27ac in undifferentiated and decidualizing cells. Confocal microscopy revealed an increase in H3K27ac upon decidualization in HESC apparent after 2 d but even more pronounced after 4 and 8 d of differentiation (Fig. 4A). This was further confirmed by Western blot analysis, demonstrating a gradual increase in the abundance of this mark in decidualizing cultures (Fig. 4B). To verify whether loss of H3K27me3 is accompanied by a gain in acetylation at specific loci, ChIP with an H3K27ac-specific antibody followed by qPCR amplification of the proximal decidual PRL and IGFBP1 promoters was carried out. As shown in Fig. 4C, differentiation of HESC is indeed associated with rapid and marked increase in H3K27ac signal upstream of the TSS of both genes. This was especially true for the IGFBP1 promoter, where the abundance of this modification increased by several multitudes over time.
Fig. 4.
Dynamic changes in H3K27 acetylation upon decidualization of HESC. A, Endometrial stromal cells were cultured in the presence or absence of 8-Br-cAMP and MPA for the indicated time points. The intensity of H3K27ac (green channel) and the corresponding DAPI nuclear staining (blue channel) were captured by confocal microscopy. B, Whole-cell protein lysates of HESC treated as above were subjected to Western blot analysis using a H3K27ac-specific antibody. β-Actin served as a loading control. C, ChIP was carried out on HESC decidualized for the indicated time points using antibodies against H3K27ac and IgG. qPCR was carried out on the ChIPed DNA with primers specific for the proximal promoter regions of PRL (left panel) and IGFBP1 (right panel) and normalized to the input. Data are expressed as the fold change (±sem of triplicate measurements) relatively to the abundance of chromatin-bound H3K27ac in untreated cells. The data shown in this figure are representative of three independent experiments.
EZH2 down-regulation is permissive for decidualization
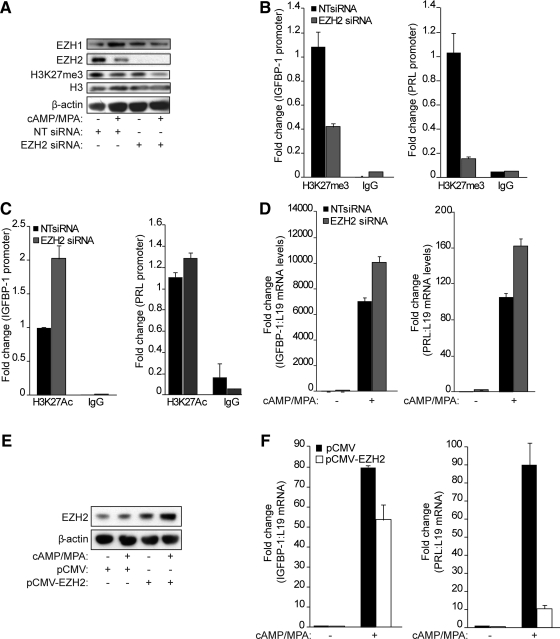
Next, we explored the functional consequences of EZH2 down-regulation on the expression of decidual marker genes. First, we used small interfering RNA (siRNA) to silence EZH2 expression in primary cultures, which were then left untreated or decidualized with 8-Br-cAMP and MPA for 2 d. The knockdown was highly effective and reduced EZH2 expression below the level of detection on Western blot analysis (Fig. 5A). Interestingly, EZH2 silencing was sufficient to decrease H3K27 trimethylation on the decidual PRL and IGFBP1 promoters by 60 and 90%, respectively (Fig. 5B). Notably, EZH2 knockdown enhanced acetylation of the same residue on H3 at the IGFBP1 but only marginally on PRL promoter (Fig. 5C). Furthermore, knockdown of this methyltransferase in undifferentiated cells enhanced the subsequent induction of both PRL and IGF binding protein (IGFBP)1 transcripts in response to 8-Br-cAMP and MPA treatment (Fig. 5D). There was also a consistent but very discrete increase in basal expression levels of these marker genes upon EZH2 knockdown. To confirm that EZH2 inhibits decidualization, primary HESC cultures were transfected with an expression vector encoding EZH2 and then differentiated with 8-Br-cAMP and MPA for 2 d. Overexpression was confirmed by Western blot analysis (Fig. 5E). RT-qPCR analysis of parallel cultures demonstrated that exogenous EZH2 expression reduces the induction of IGFBP1 mRNA and, even more pronounced, of PRL transcripts in differentiating HESC (Fig. 5F). Together, the data demonstrate that loss of EZH2 alone is insufficient to induce a decidual phenotype yet determines the cellular responsiveness to differentiation stimuli.
Fig. 5.
EZH2 down-regulation is permissive for the induction of decidual marker genes. A, Total cell lysates from primary HESC cultures first transfected with either NTor EZH2 siRNA and then treated with cAMP and MPA for 2 d were subjected to Western blot analysis and probed with EZH1-, EZH2-, H3K27me3-, and H3-specific antibodies. β-Actin served as a loading control. B, ChIP with H3K27me3 and IgG antibodies was performed on cultures treated as above. The precipitated DNA fragments were analyzed by qPCR and normalized to the input. The primers used were specific for the proximal promoter regions of IGFBP1 (left panel) and PRL (right panel). Data are expressed as the fold change (±sem of triplicate measurements) relatively to the abundance of chromatin-bound H3K27me3 in untreated cells. C, ChIP was carried out with H3K27ac-specific antibody under the experimental conditions described above. D, PRL and IGFBP-1 transcript levels were measured by RT-qPCR in HESC first transfected with either NT or EZH2 siRNA and then treated with cAMP and MPA for 2 d. The data show fold-change (±sem of triplicate measurements) in expression relative to untreated cells transfected with NT siRNA. E, Protein lysates extracted from cells first transfected with an empty control vector (pCMV) or a plasmid encoding EZH2 (pCMV-EZH2) vector and then treated with decidualization stimuli for 2 d were subjected to Western blot analysis and immunoprobed for EZH2. β-Actin was used as a loading control. F, Total RNA from parallel cultures was examined for the expression of IGFBP-1 (left panel) and PRL (right panel) transcripts by RT-qPCR. The data (mean ± sem) are presented as fold induction, and the results are representative of three repeat experiments.
Genome-wide redistribution of H3K27me3 upon decidualization and in response to EZH2 knockdown
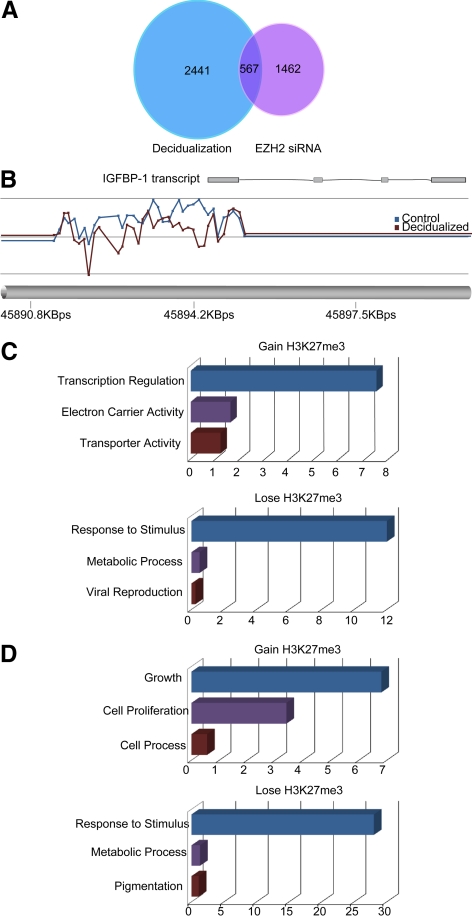
Our results indicated that loss of EZH2 expression in decidualizing cells results in loss of H3K27me3 signal at specific loci, yet overall levels of this repressive histone modification seem to be maintained. To determine whether decidualization is associated with genome-wide redistribution of H3K27 methylation, we carried out ChIP-chip arrays on undifferentiated HESC and cells treated with 8-Br-cAMP and MPA for 8 d, a time point at which EZH2 expression is virtually undetectable (Fig. 2A). Chromatin from three independent, paired undifferentiated, and decidualizing cultures was immunoprecipitated with the H3K27me3 antibody, labeled, and hybridized to a Roche NimbleGen Human ChIP-chip 3x720K RefSeq promoter array (Roche, Wellyn Garden City, UK). We identified a significant change (P < 0.05) in H3K27me3 signal at 3008 genomic regions, which included the IGFBP1 promoter (Fig. 6, A and B, and Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Of these, there was a reduction in methylation in 75% and enrichment in 25% upon differentiation of HESC into decidual cells. Gene ontology (GO) analysis revealed that gain of this repressive modification was strongly enriched at promoters of genes involved in transcriptional regulation (enrichment score, 7.5; P < 0.001), whereas loss of the mark was prominent at genes implicated in response to stimulus (enrichment score, 12.5; P < 0.001) (Fig. 6C). The decidua-specific PRL promoter, however, was not represented on the array.
Fig. 6.
Genome-wide redistribution of H3K27me3 in decidualizing HESC and upon EZH2 knockdown in undifferentiated HESC. A, Venn diagram indicating the number of genomic loci significantly (P < 0.05) altered in H3K27 methylation as determined by ChIP-chip analysis of HESC either decidualized with 8-Br-cAMP and MPA for 8 d (left) or upon EZH2 knockdown (right). B, Example of altered H3K27me3 peaks in the genomic region encompassing the IGFBP1 promoter. The blue line represents the peaks in the undifferentiated cells, whereas the red line indicates the corresponding relative level of H3K27me3 in paired cultures decidualized with 8-Br-cAMP and MPA for 8 d. C, GO analysis of genes in the proximity of genomic loci characterized by significant loss (bottom panel) or gain (top panel) in H3K27me3 upon decidualization of HESC. D, GO enrichment analysis of promoter regions altered in H3K27 methylation in response to differentiation as well as siRNA-mediated EZH2 knockdown in undifferentiated cultures.
To determine whether this genome-wide redistribution of H3K27me3 upon decidualization of HESC is caused by the loss of EZH2, we repeated the ChIP-chip arrays, but this time on chromatin immunoprecipitated from three independent undifferentiated primary cultures first transfected with nontargeting (NT) or EZH2 siRNA. Silencing EZH2 expression was sufficient to significantly alter the abundance of H3K27me3 at 2029 distinct genomic regions, and a comparable number of loci displayed a reduction or enrichment in histone modification, 53 and 47%, respectively (Supplemental Table 1). Furthermore, cross-referencing of the two datasets revealed altered H3K27me3 at 567 genomic regions in both decidualizing HESC and upon EZH2 knockdown in undifferentiated cultures (Fig. 6A). However, the direction of change was discordant in 229 regions (40%). In other words, EZH2 knockdown in undifferentiated cells only recapitulated the change in H3K27 methylation at 338 of 3008 (11%) genomic regions altered upon decidualization (Supplemental Table 2). These 338 highly EZH2-responsive regions were also subjected to GO analysis, where loss and gain of the H3K27me3 repressive mark was found to occur prominently in the proximity of genes involved in response to stimuli (enrichment score, 28; P < 0.001) and cellular growth (enrichment score, 6.8; P < 0.05), respectively (Fig. 6D).
Discussion
Like other ligand-activated nuclear receptors, progesterone triggers a conformational change in PR, which leads to dissociation of chaperone proteins, receptor dimerization, and binding to specific DNA recognition sequences in the promoters of target genes (40). However, interaction of the activated PR with DNA is in itself insufficient to alter gene expression, because nuclear receptors do not possess chromatin-modifying activities necessary to enable or prevent recruitment of the basal transcriptional machinery (41–44). Remodeling of local chromatin depends on recruitment of coregulators, broadly divided into coactivators and corepressors, to the DNA-bound receptor (41, 42). Based on their mechanisms of action, nuclear receptor coactivators can be categorized into three major function complexes. 1) the switch/sucrose nonfermentable complex, which remodels the local chromatin structure through adenosine triphosphate-dependent histone acetylation. 2) The Sarcoma complex, which contains acetyltransferases [e.g. CREB binding protein (CBP), p300, and the p300/CBP-associated factor] and methyltransferases (e.g. coactivator-associated arginine methyltransferase 1 and protein arginine N-methyltransferase 1). And 3) mediator complex, involved in the activation of RNA polymerase II and initiation of transcription. Although the mechanism of agonist-bound PR-dependent gene repression is still unclear, it is widely assumed to involve interaction with NCoR, SMRT, or possibly nuclear receptor interacting protein 1, corepressors capable of recruiting DNA- and histone-methyltransferases complexes (45, 46).
In this model, nuclear receptors like PR are viewed as pioneer factors responsible for initiating the process of chromatin remodeling near the TSS of target genes. However, this paradigm is being profoundly challenged by novel techniques that allow genome-wide mapping of binding of nuclear receptors to DNA (19, 47, 48). Contrary to expectations, a majority of nuclear binding events does not occur proximal to TSS of target genes but at large distances from promoters (48–50). Moreover, rather than the activated receptor inducing a permissive chromatin environment that enables transcription, most activated receptors will bind at preexisting sites that are constitutively accessible. For example, the binding pattern of the activated glucocorticoid receptor to DNA, and subsequent gene regulation, was recently shown to be highly cell specific and comprehensively predetermined by basal differences between cell types in chromatin structure (47). When extrapolated to the cycling endometrium, these observations strongly suggest that acquisition of responsiveness to differentiation signals must be preceded by, or at least occur in concert with, genome-wide remodeling of the chromatin.
This study provides evidence that loss of EZH2 activity in HESC contributes to the chromatin changes necessary for expression of a decidual phenotype in response to differentiation cues. Although EZH2 transcript levels fell rapidly in response to cAMP and progestin treatment, protein levels declined more gradually. The kinetics of this response correlated well with the decline in H3K27me3 at the PRL and IGFBP1 promoters and, inversely, with the level of transcriptional activation of these marker genes (4, 12). Furthermore, a parallel gain in the competing activation mark H3K27ac was observed at both promoters upon decidualization of HESC, although the amplitude of this response was more pronounced at the TSS of IGFBP1. Interestingly, a recent study reported that different modifications are predictive of the expression levels of genes that are either rich or poor in the cytosine guanine dinucleotide (CpG) content of their promoters. In particular, H3K27ac is strongly associated with activation of high but not low CpG content promoters (51). In keeping with this model, H3K27ac upon HESC differentiation was not only more pronounced at the IGFBP1 promoter, which has 68 CpG, but EZH2 knockdown was sufficient to enrich the mark. In contrast, the decidua-specific PRL promoter is poor in CpG content, and enrichment in H3K27ac was strictly dependent on cAMP and MPA stimulation. In a previous study, we demonstrated that CBP/p300, coactivators that catalyze H3K27ac, are indispensible for PRL expression in differentiating HESC (52), suggesting that recruitment of these histone modifiers to decidual promoters requires binding of specific transcriptional complexes induced by cAMP and MPA signaling. In other words, although loss of EZH2-dependent methyltransferase activity contributes to creating a transcriptionally permissive chromatin environment, these changes alone are insufficient to trigger the expression of decidual marker genes in undifferentiated HESC or even upon stimulation with only MPA (data not shown).
Down-regulation of EZH2 expression in decidualizing HESC was not paralleled by a decline in global cellular H3K27me3 levels as determined by Western blot analysis or confocal imaging. However, our ChIP-chip array study suggested a net reduction in chromatin-bound methylated H3K27 after 8 d of differentiation because three-time more genomic regions were reduced than enriched in this modification. Nevertheless, residual H3 methylase activity remained apparent in differentiating HESC even with EZH2 expression below the level of detection. This residual activity is likely accounted for by the continuous expression of EZH1. Like EZH2, EZH1 integrates in PRC2, although there is evidence that these complexes differ in their repressive roles. EZH1 has relative weak intrinsic histone methyltransferase activity and is thought to elicit its role by compacting chromatin through interaction with nucleosomes (53). Interestingly, EZH2 expression is widely associated with cellular proliferation, whereas EZH1 is reportedly more abundant in nonproliferative cells (53). This general pattern of expression fits well with our observation that EZH2 but not EZH1 is lost upon differentiation of HESC.
In addition to DNA methylation, over 60 different residues within histone tails have been identified as targets for various posttranslational modifications, including methylation, acetylation, ubiquitination, phosphorylation, and sumoyaltion (21, 54). Mining of existing gene expression revealed that decidualization of HESC is associated with altered expression of members of several classes of chromatin modifying enzymes, including DNA methyltransferases (e.g. DNMT3a and DNMT3b), DNA binding proteins (e.g. UFRH1), and histone modifying enzymes (e.g. SUV420H1). Similarly, the expression of DNA methyltransferase 1 and methyl-CpG-binding domain protein 2, a methyl-CpG binding protein, in human endometrium has been shown to be cycle dependent (55). Because of the interdependency of various chromatin modifications (51, 56), it was anticipated that EZH2 knockdown in undifferentiated HESC would at best only partially recapitulate the changes in H3K27me3 observed upon decidualization. This was indeed the case. Only 11% of the genomic regions altered in H3K27me in response to prolonged cAMP and MPA stimulation were modified in a similar manner upon EZH2 knockdown in untreated cells. These loci could be viewed as highly EZH2 dependent for remodeling. Interestingly, GO analysis revealed that those regions that gain the transcriptionally repressive H3K27me3 mark upon decidualization of HESC are enriched for genes associated with growth. Even more strikingly, the same analysis of regions that lost the mark, thus acquiring a transcriptionally permissive chromatin environment, revealed a preponderance of genes functionally associated with responsiveness to stimuli. Thus the result of the ChIP-chip arrays further supports the notion that down-regulation of EZH2 is a key event that renders endometrial cells responsive to differentiation and other environmental cues.
Taken together, our data show that cAMP and progesterone signaling reshapes the chromatin landscape of HESC, which in turn enables regulation of large gene networks that underpin the expression of a decidual phenotype. Extrapolated to the in vivo situation, our findings suggest that cycle-dependent changes in chromatin structure are a major determinant in the cellular responsiveness to differentiation signals and the subsequent acquisition of a decidual phenotype. In light of the crucial role of EZH2 in this process, it seems likely that perturbations in this or other chromatin remodeling enzymes underpin reproductive failure.
Materials and Methods
Primary cell culture
This study was approved by the Hammersmith and Queen Charlotte's & Chelsea Research Ethics Committee (1997/5065). Endometrial samples were obtained from premenopausal consented women without uterine pathology. Written informed consent was obtained from all participating subjects before tissue collection. HESC were isolated, and established cultures were maintained as described previously (15). For in vitro decidualization, cultures were treated with 0.5 mm 8-Br-cAMP (Sigma, St. Louis, MO) and 1 μm MPA (Sigma). Where the tissue was used directly for experiments, it was classified as early secretory when taken between the 14th and 18th d of the menstrual cycle, as midsecretory between the 18th and the 24th d, and as late secretory if they were obtained on d 24 or later of the cycle.
Transient transfection of primary cultures
Primary HESC were transfected by calcium phosphate coprecipitation using the ProFection Mammalian Transfection kit (Promega, Madison, WI) according to manufacturer's instructions. For EZH2, gene-silencing HESC were transiently transfected with the following siRNA: siCONTROL NT siRNA pool (Dharmacon, Lafayette, CO) and EZH2 siGENOME SMARTpool siRNA (Dharmacon). For EZH2 overexpression, the EZH2 in pCMV-SPORT6 (Open Biosystems, Huntsville, AL) vector was used.
Western blot analysis
Whole-cell protein extracts were obtained by direct lysis in Laemmli buffer. Proteins were resolved by SDS-PAGE, transferred to a polyvinylidene fluorite membrane, and probed with antibodies raised against EZH2 (NCL-L-EZH2; Leica Biosystems, Newcastle Upon Tyne, UK), EZH1 (ab13665; Abcam, Cambridge, UK), H3K27me3 (07-449; Millipore, Billerica, MA), H3K27ac (ab4729; Abcam), H3 (ab1791; Abcam), and β-actin (ab-8226; Abcam). The proteins were visualized by incubation with horseradish peroxidase-conjugated secondary antibodies (Roche) and the chemiluminescence detected using the ECL+ kit (GE Healthcare, Indianapolis, IN).
Real-time quantitative PCR
RNA was extracted using STAT-60 (AMS Biotech, Abingdon, UK) and following manufacturer's instructions. cDNA was synthesized using the SuperScript First-Strand Synthesis for RT-PCR (Invitrogen, Carlsbad, CA) with oligo-dT primers (Invitrogen) after having treated the extracted RNA with amplification grade deoxyribonuclease I (Invitrogen). Quantitative PCR was carried out on Bio-Rad Opticon Monitor 3 Real-Time PCR System (Bio-Rad, Hercules, CA). PCR reactions were set up using SYBR Green JumpStart Taq (Sigma), 0.20 μm each primer, 0.5 μl of template in a 15-μl reaction. The following program was run on the thermocycler: 50 C for 2 min, 95 C for 10 min followed by 40 cycles of 95 C for 15 sec, 60 C for 1 min, and 72 C for 30 sec. Input variance was normalized against the expression of the L19 gene, which encodes for a nonregulated universally expressed ribosomal protein. Primers used to amplify EZH2, IGFBP-1, and PRL were as follows: EZH2 forward (F) (5′-TTC ATG CAA CAC CCA ACA CT-3′) and EZH2 reverse (R) (5′-CTC CCT CCA AAT GCT GGT AA-3′), IGFBP1* F (5′-CGA AGG CTC TCC ATG TCA CCA-3′) and IGFBP1* R (5′TGT CTC CTG TGC CTT GGC TAA AC-3′), and dPRL* F (5′-AAG CTG TAG AGA TTG AGG AGC AAA C-3′) and dPRL* R (5′-TCA GGA TGA ACC TGG CTG ACT A-3′).
Confocal microscopy
HESC were cultured on four-well chamber slides and decidualized for 2, 4, and 8 d. The cells were fixed with 4% paraformaldehyde (Thermo Fisher Scientific, Waltham, MA). Subsequently, they were permeabilized with 0.5% Triton X and blocked with 7.5% normal goat serum and 3% BSA in PBS. The slides were hybridized with the primary antibody and then with the secondary one conjugated with Alexa Fluor 594. The chambers were mounted in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI). Alexa Fluor 594 and DAPI were visualized under a Leica SP5 confocal microscope with a ×63 oil-immersion objective.
Immunohistochemistry
Paraffin-embedded, formalin-fixed endometrial tissue sections were placed on 1% wt/vol polylysine coated slides. Immunostaining was performed using Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA), according to manufacturer's instructions. The sections were stained with EZH2 antibody (NCL-L-EZH2; Leica Biosystems).
Chromatin immunoprecipitation
HESC were cultured in 10-cm culture dishes, fixed with 1% formaldehyde, and incubated for 10 min at 37 C. After having stopped the fixation with 125 mm glycine, the nuclei were isolated by incubating at 4 C for 10 min in 1 ml of Swelling buffer [25 mm HEPES (pH 7.9), 1.5 mm MgCl2, 10 mm KCl, and 0.1% Nonidet]. The cells were then scraped and homogenized with a dounce homogenizer. The samples were centrifuged for 3 min at 16,000 × g at 4 C and the nuclei collected in a pellet, resuspended in 500 μl of sodium dodecyl sulfate (SDS) lysis buffer [1% SDS, 1% Triton X-100, 0.5% deoxycholate, 10 mm EDTA, and 500 mm Tris-HCl (pH 8.1)] and sonicated for 30 min (with 30-sec cycles) at 4 C on high power in a Diagenode Bioruptor sonicator. The resulting suspension was centrifuged for 15 min at 16,000 × g at 4 C and the supernatant diluted 10 times in IP buffer [0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-HCl (pH 8.1), and 167 mm NaCl] and then precleared at 4 C for 3 h with Protein A Dynabeads (Invitrogen). The chromatin was then complexed overnight at 4 C with the antibody bound to Protein A Dynabeads and washed with the following buffers: low salt buffer [0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl (pH 8.1), and 150 mm NaCl], high salt buffer [0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl (pH 8.1), and 500 mm NaCl], LiCl buffer [250 mm LiCl, 1% Nonidet, 1% deoxycholate, 1 mm EDTA, and 10 mm Tris-HCl (pH 8.1)], and Tris-EDTA buffer [10 mm Tris-HCl (pH 8), and 1 mm EDTA] before eluting the chromatin with 250 μl of Elution buffer (1% SDS and 100 mm NaHCO3) and incubating at room temperature for 15 min; 200 mm NaCl was added to reverse cross-link the proteins and the DNA. After an overnight incubation at 65 C, 10 mm EDTA, 40 mm Tris-HCl (pH 8), and 40 μg/ml Protease K (Sigma) were added and the sample incubated for a further hour at 55 C before proceeding with the DNA purification using QIAquick PCR Purification kit (QIAGEN, Venlo, Netherlands). All the buffers were supplemented with protease inhibitors, 1 mm phenylmethanesulfonylfluoride and 10 mm sodium butyrate. The following antibodies were used in the ChIP experiments: H3K27me3 (07-449; Millipore) and H3K27ac (ab4729; Abcam) and as negative control the rabbit polyclonal antimouse IgG (M7023; Sigma) was used. The purified DNA was amplified by RT-qPCR (as described above), and the following primers were used: EZH2 F (5′-TTC ATG CAA CAC CCA ACA CT-3′) and EZH2 R (5′-CTC CCT CCA AAT GCT GGT AA-3′), IGFBP1* F (5′-CGA AGG CTC TCC ATG TCA CCA-3′) and IGFBP1* R (5′TGT CTC CTG TGC CTT GGC TAA AC-3′), and dPRL* F (5′-AAG CTG TAG AGA TTG AGG AGC AAA C-3′) and dPRL* R (5′-TCA GGA TGA ACC TGG CTG ACT A-3′). The primer sequences used to amplify the promoter regions of PRL and IGFBP1 were as follows: dPRL-332/270 A F (5′-TGC TTT AAC ATT TTT GCC TAG TAA-3′) and dPRL-332/270 A R (5′-AAA TGG AGT GTC TAA AAA CGT TGA-3′), and IGFBP1 Prom (263) F (5′-CGT CAT CCC CCT CCC AGC TGA G-3′) and IGFBP1 Prom (33) R (5′-GCA CAG GCC GCG CCA CTT GCA CC-3′) (57).
ChIP-chip array
Chromatin was immunoprecipitated with an H3K27me3-specific antibody. ChIP DNA was amplified using the WGA2 kit (Sigma), following manufacturer's instructions. The ChIP-chip assays were performed in triplicate, each representing a primary culture from an individual patient. A total of 12 primary cultures was assayed. Labeling of ChIP DNA and input, hybridization to NimbleGen Human ChIP-chip 3x720K RefSeq promoter arrays (Roche), and scanning were performed by NimbleGen in their service laboratory. Peak detection and statistical analysis was carried out using Partek Genomic Suite (Partek, St. Louis, MO).
Acknowledgments
We thank the patients and clinicians that participated in this study.
This work was supported by the Genesis Trust (G.G.) and a National Institute for Health Research Biomedical Research Centre funding scheme (J.J.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 8-Br-cAMP
- 8-Bromo-cAMP
- CBP
- CREB binding protein
- ChIP
- chromatin immunoprecipitation
- CpG
- cytosine guanine dinucleotide
- DAPI
- 4′,6-diamidino-2-phenylindole
- EZH2
- enhancer of Zeste homolog 2
- F
- forward
- GO
- gene ontology
- HESC
- human endometrial stromal cell
- H3K27ac
- acetylation of H3K27
- H3K27me3
- trimethylation of histone 3 on lysine 27
- IGFBP
- IGF binding protein
- MPA
- medroxyprogesteroneacetate
- NT
- nontargeting
- PR
- progesterone receptor
- PRL
- prolactin
- R
- reverse
- RT-qPCR
- real-time quantitative PCR
- SDS
- sodium dodecyl sulfate
- siRNA
- small interfering RNA
- TSS
- transcriptional start site.
References
- 1. Gellersen B, Brosens IA, Brosens JJ. 2007. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25:445–453 [DOI] [PubMed] [Google Scholar]
- 2. Wynn RM. 1974. Ultrastructural development of the human decidua. Am J Obstet Gynecol 118:652–670 [DOI] [PubMed] [Google Scholar]
- 3. Brosens JJ, Gellersen B. 2006. Death or survival—progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol 36:389–398 [DOI] [PubMed] [Google Scholar]
- 4. Brosens JJ, Hayashi N, White JO. 1999. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 140:4809–4820 [DOI] [PubMed] [Google Scholar]
- 5. Gellersen B, Brosens J. 2003. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 178:357–372 [DOI] [PubMed] [Google Scholar]
- 6. de Ziegler D, Fanchin R, de Moustier B, Bulletti C. 1998. The hormonal control of endometrial receptivity: estrogen (E2) and progesterone. J Reprod Immunol 39:149–166 [DOI] [PubMed] [Google Scholar]
- 7. Mote PA, Balleine RL, McGowan EM, Clarke CL. 1999. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab 84:2963–2971 [DOI] [PubMed] [Google Scholar]
- 8. Li X, O'Malley BW. 2003. Unfolding the action of progesterone receptors. J Biol Chem 278:39261–39264 [DOI] [PubMed] [Google Scholar]
- 9. Brosens I. 1999. Management of ovarian endometriomas and pregnancy? Fert Steril 71:1166–1167 [PubMed] [Google Scholar]
- 10. Brar AK, Frank GR, Kessler CA, Cedars MI, Handwerger S. 1997. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine 6:301–307 [DOI] [PubMed] [Google Scholar]
- 11. Jones MC, Fusi L, Higham JH, Abdel-Hafiz H, Horwitz KB, Lam EW, Brosens JJ. 2006. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci USA 103:16272–16277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cloke B, Huhtinen K, Fusi L, Kajihara T, Yliheikkilä M, Ho KK, Teklenburg G, Lavery S, Jones MC, Trew G, Kim JJ, Lam EW, Cartwright JE, Poutanen M, Brosens JJ. 2008. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology 149:4462–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB. 2002. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem 277:33950–33956 [DOI] [PubMed] [Google Scholar]
- 14. Christian M, Zhang X, Schneider-Merck T, Unterman TG, Gellersen B, White JO, Brosens JJ. 2002. Cyclic AMP-induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein β in differentiating human endometrial stromal cells. J Biol Chem 277:20825–20832 [DOI] [PubMed] [Google Scholar]
- 15. Christian M, Pohnke Y, Kempf R, Gellersen B, Brosens JJ. 2002. Functional association of PR and CCAAT/enhancer-binding protein β isoforms: promoter-dependent cooperation between PR-B and liver-enriched inhibitory protein, or liver-enriched activatory protein and PR-A in human endometrial stromal cells. Mol Endocrinol 16:141–154 [DOI] [PubMed] [Google Scholar]
- 16. Pohnke Y, Kempf R, Gellersen B. 1999. CCAAT/enhancer-binding proteins are mediators in the protein kinase A-dependent activation of the decidual prolactin promoter. J Biol Chem 274:24808–24818 [DOI] [PubMed] [Google Scholar]
- 17. Mak IY, Brosens JJ, Christian M, Hills FA, Chamley L, Regan L, White JO. 2002. Regulated expression of signal transducer and activator of transcription, Stat5, and its enhancement of PRL expression in human endometrial stromal cells in vitro. J Clin Endocrinol Metab 87:2581–2588 [DOI] [PubMed] [Google Scholar]
- 18. Wagner BL, Norris JD, Knotts TA, Weigel NL, McDonnell DP. 1998. The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor. Mol Cell Biol 18:1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wiench M, Miranda TB, Hager GL. 2011. Control of nuclear receptor function by local chromatin structure. FEBS J 278:2211–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharov AA, Nishiyama A, Piao Y, Correa-Cerro LS, Amano T, Thomas M, Mehta S, Ko MS. 2011. Responsiveness of genes to manipulation of transcription factors in ES cells is associated with histone modifications and tissue specificity. BMC Genomics 12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kouzarides T. 2007. Chromatin modifications and their function. Cell 128:693–705 [DOI] [PubMed] [Google Scholar]
- 22. Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. 2002. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298:1039–1043 [DOI] [PubMed] [Google Scholar]
- 23. Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. 2002. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111:185–196 [DOI] [PubMed] [Google Scholar]
- 24. Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. 2002. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Gene Dev 16:2893–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karanikolas BD, Figueiredo ML, Wu L. 2010. Comprehensive evaluation of the role of EZH2 in the growth, invasion, and aggression of a panel of prostate cancer cell lines. Prostate 70:675–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonzalez ME, Li X, Toy K, DuPrie M, Ventura AC, Banerjee M, Ljungman M, Merajver SD, Kleer CG. 2009. Downregulation of EZH2 decreases growth of estrogen receptor-negative invasive breast carcinoma and requires BRCA1. Oncogene 28:843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wei Y, Xia W, Zhang Z, Liu J, Wang H, Adsay NV, Albarracin C, Yu D, Abbruzzese JL, Mills GB, Bast RC, Jr, Hortobagyi GN, Hung MC. 2008. Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian, and pancreatic cancers. Mol Carcinogen 47:701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berezovska OP, Glinskii AB, Yang Z, Li XM, Hoffman RM, Glinsky GV. 2006. Essential role for activation of the Polycomb group (PcG) protein chromatin silencing pathway in metastatic prostate cancer. Cell Cycle 5:1886–1901 [DOI] [PubMed] [Google Scholar]
- 29. Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, Sabel MS, Livant D, Weiss SJ, Rubin MA, Chinnaiyan AM. 2003. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA 100:11606–11611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. 2010. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol 24:993–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F. 2006. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 439:871–874 [DOI] [PubMed] [Google Scholar]
- 32. Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, Tarakhovsky A, Fuchs E. 2009. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell 136:1122–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gellersen B, Kempf R, Telgmann R, DiMattia GE. 1994. Nonpituitary human prolactin gene transcription is independent of Pit-1 and differentially controlled in lymphocytes and in endometrial stroma. Mol Endocrinol 8:356–373 [DOI] [PubMed] [Google Scholar]
- 34. Lynch VJ, Brayer K, Gellersen B, Wagner GP. 2009. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PloS ONE 4:e6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao J, Mazella J, Tseng L. 2002. Hox proteins activate the IGFBP-1 promoter and suppress the function of hPR in human endometrial cells. DNA Cell Biol 21:819–825 [DOI] [PubMed] [Google Scholar]
- 36. Al-Sabbagh M, Fusi L, Higham J, Lee Y, Lei K, Hanyaloglu AC, Lam EW, Christian M, Brosens JJ. 2011. NADPH oxidase-derived reactive oxygen species mediate decidualization of human endometrial stromal cells in response to cyclic AMP signaling. Endocrinology 152:730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Telgmann R, Maronde E, Taskén K, Gellersen B. 1997. Activated protein kinase A is required for differentiation-dependent transcription of the decidual prolactin gene in human endometrial stromal cells. Endocrinology 138:929–937 [DOI] [PubMed] [Google Scholar]
- 38. Tie F, Banerjee R, Stratton CA, Prasad-Sinha J, Stepanik V, Zlobin A, Diaz MO, Scacheri PC, Harte PJ. 2009. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development 136:3131–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pasini D, Malatesta M, Jung HR, Walfridsson J, Willer A, Olsson L, Skotte J, Wutz A, Porse B, Jensen ON, Helin K. 2010. Characterization of an antagonistic switch between histone H3 lysine 27 methylation and acetylation in the transcriptional regulation of Polycomb group target genes. Nucleic Acid Res 38:4958–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maruyama T, Yoshimura Y. 2008. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J 55:795–810 [DOI] [PubMed] [Google Scholar]
- 41. Han SJ, Lonard DM, O'Malley BW. 2009. Multi-modulation of nuclear receptor coactivators through posttranslational modifications. Trends Endocrin Met 20:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lonard DM, O'Malley BW. 2006. The expanding cosmos of nuclear receptor coactivators. Cell 125:411–414 [DOI] [PubMed] [Google Scholar]
- 43. Thakur MK, Paramanik V. 2009. Role of steroid hormone coregulators in health and disease. Horm Res 71:194–200 [DOI] [PubMed] [Google Scholar]
- 44. Oñate SA, Tsai SY, Tsai MJ, O'Malley BW. 1995. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357 [DOI] [PubMed] [Google Scholar]
- 45. Li J, Wang J, Wang J, Nawaz Z, Liu JM, Qin J, Wong J. 2000. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J 19:4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kiskinis E, Hallberg M, Christian M, Olofsson M, Dilworth SM, White R, Parker MG. 2007. RIP140 directs histone and DNA methylation to silence Ucp1 expression in white adipocytes. EMBO J 26:4831–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. John S, Sabo PJ, Thurman RE, Sung MH, Biddie SC, Johnson TA, Hager GL, Stamatoyannopoulos JA. 2011. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet 43:264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wiench M, Hager GL. 2010. Expanding horizons for nuclear receptors. EMBO Rep 11:569–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hakim O, Sung MH, Hager GL. 2010. 3D shortcuts to gene regulation. Curr Opin Cell Biol 22:305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M. 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- 51. Karlić R, Chung HR, Lasserre J, Vlahovicek K, Vingron M. 2010. Histone modification levels are predictive for gene expression. Proc Natl Acad Sci USA 107:2926–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Christian M, Marangos P, Mak I, McVey J, Barker F, White J, Brosens JJ. 2001. Interferon-γ modulates prolactin and tissue factor expression in differentiating human endometrial stromal cells. Endocrinology 142:3142–3151 [DOI] [PubMed] [Google Scholar]
- 53. Margueron R, Li G, Sarma K, Blais A, Zavadil J, Woodcock CL, Dynlacht BD, Reinberg D. 2008. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell 32:503–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li B, Gogol M, Carey M, Pattenden SG, Seidel C, Workman JL. 2007. Infrequently transcribed long genes depend on the Set2/Rpd3S pathway for accurate transcription. Gene Dev 21:1422–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Kaam KJ, Delvoux B, Romano A, D'Hooghe T, Dunselman GA, Groothuis PG. 2011. Deoxyribonucleic acid methyltransferases and methyl-CpG-binding domain proteins in human endometrium and endometriosis. Fert Steril 95:1421–1427 [DOI] [PubMed] [Google Scholar]
- 56. Lee JS, Smith E, Shilatifard A. 2010. The language of histone crosstalk. Cell 142:682–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sakai N, Maruyama T, Sakurai R, Masuda H, Yamamoto Y, Shimizu A, Kishi I, Asada H, Yamagoe S, Yoshimura Y. 2003. Involvement of histone acetylation in ovarian steroid-induced decidualization of human endometrial stromal cells. J Biol Chem 278:16675–16682 [DOI] [PubMed] [Google Scholar]