Abstract
Determining the source of regenerated luminal epithelial cells in the adult prostate during androgen deprivation and replacement will provide insights into the origin of prostate cancer cells and their fate during androgen deprivation therapy. Prostate stem cells in the epithelial layer have been suggested to give rise to luminal epithelium. However, the extent of stem cell participation to prostate regrowth is not clear. In this report, using prostate-specific antigen-CreERT2-based genetic lineage marking/tracing in mice, preexisting luminal epithelial cells were shown to be a source of regenerated luminal epithelial cells in the adult prostate. Prostatic luminal epithelial cells could survive androgen deprivation and were capable of proliferating upon androgen replacement. Prostate cancer cells, typically exhibiting a luminal epithelial phenotype, may retain this intrinsic capability to survive and regenerate in response to changes in androgen signaling, providing part of the mechanism for the ultimate failure of androgen deprivation therapy in prostate cancer.
Androgens regulate prostate homeostasis in the adult mouse. Androgen deprivation causes dramatic prostate regression via massive apoptosis of prostatic luminal epithelial cells, and with subsequent androgen replacement the prostate undergoes rapid regrowth and the luminal epithelial cells are repopulated. The prostate can undergo multiple cycles of regression and regrowth in response to repeated rounds of androgen deprivation and replacement (1). However, the origin of regenerated luminal epithelial cells remains unresolved.
Defining the lineage of regenerated luminal epithelial cells has significant implications in elucidating the origin of prostate cancer cells. Stem/progenitor cells in the prostate are thought to be responsible for luminal epithelial cell regeneration and may serve as the source of putative prostate cancer stem cells. Thus, identification and characterization of stem/progenitor cells for the prostate luminal epithelium has been a major focus in elucidating the origin of prostate cancer.
Androgen receptor (AR)-negative prostate stem cells in the basal epithelial cell layer are thought to be the source of AR-positive luminal epithelium in the regenerated prostate (2–6). These putative progenitor cells have been characterized by their expression of stem cell markers and ability to differentiate in vitro and to generate structures resembling prostatic ducts in tissue recombinants (5, 7–10). There is no definitive evidence, however, that basal epithelial stem cells produce the newly formed luminal epithelial cells in the androgen-regenerated prostate in vivo. Alternatively, it has been proposed that adult luminal cells develop independently of basal cells and are derived from androgen deprivation-resistant luminal stem cells capable of self-renewal (11, 12). Based on several cell kinetic studies of the rodent prostate, others have proposed that fully differentiated luminal cells can proliferate in vivo, and basal and luminal cells are independent lineages capable of self-renewal (13–15). The contribution of various mechanisms in prostate luminal epithelial regeneration remains unclear.
In the present paper, prostate-specific antigen (PSA)-CreERT2-based genetic lineage marking/tracing was used to track the fate of luminal epithelial cells during regression and regrowth of the adult mouse prostate. This study shows the survival and repopulation of preexisting prostatic luminal epithelial cells during androgen deprivation and replacement.
Results
Genetic lineage tracing of luminal epithelial cells in adult mouse prostate
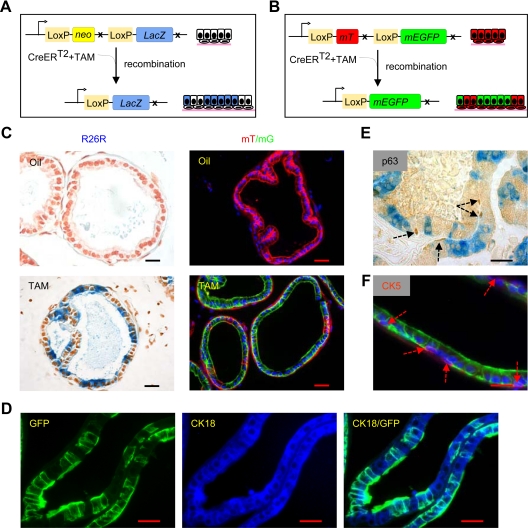
A recently developed genetic lineage tracing strategy (16) was used to follow the fate of mouse prostatic luminal epithelial cells during androgen withdrawal and androgen replacement (hereafter called “androgen manipulation”). To control for the specificity of prostatic luminal epithelial cell labeling and the time of labeling, a previously reported PSA-CreERT2 transgenic mouse strain expressing tamoxifen (TAM)-inducible CreER recombinase driven by a 6-kb human PSA promoter/enhancer was used (17, 18). PSA-CreERT2 mice were crossed with ROSA26R-lacZ or mT/mG mice to generate bigenic PSA-CreERT2/R26-LacZ (Fig. 1A) or PSA-CreERT2/R26-enhanced green fluorescent protein (GFP) (Fig. 1B) mice. The efficiency of PSA-CreERT2-based genetic labeling was greatest in the lateral prostate and was specific to the luminal epithelial cells (Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). As a control, no x-gal staining or GFP expression was observed in the PSA-CreERT2/R26R or PSA-CreERT2/R26R-GFP mouse injected with corn oil alone (Fig. 1C). Labeled cells were CK18 positive (Fig. 1D) and CK5- and p63 negative (Fig. 1, E and F), indicating they were luminal and not basal cells (6).
Fig. 1.
Specificity of TAM-inducible PSA-CreERT2 system in mice. A and B, Schematic diagrams showing recombination by active CreERT2 fusion protein and lineage marking of cells in PSA-CreERT2/R26R LacZ and PSA-CreERT2/Rosa26R mT/mG mice. In the presence of TAM, CreERT2 fusion protein causes recombination resulting in removal of the neo or mT gene and permanent, heritable expression of lacZ or GFP, which results in labeling of cells with active PSA promoter. C, TAM-induced lineage marking of luminal epithelial in the lateral prostate. Lineage-marked cells are dark blue via x-gal staining in tissue sections of lateral prostate of 10-wk-old PSA-CreERT2/R26R lacZ or green in PSA-CreERT2/Rosa26R mT/mG mice treated with TAM. Controls are corn oil-injected mice. D, Immunofluorescence staining for CK18 in lateral prostate sections of PSA-CreERT2/Rosa26R mT/mG mice. E, Immunostaining for p63 in lateral prostate sections of PSA-CreERT2/R26R lacZ mice. F, Immunofluorescence staining for CK5 in lateral prostate sections of PSA-CreERT2/Rosa26R mT/mG mice. p63 and CK5 staining are indicated by dashed arrows. Scale bars, 25 μm. mEGFP, membrane-tagged enhanced GFP.
PSA-CreERT2-based genetic labeling of prostatic luminal epithelial cells is androgen dependent
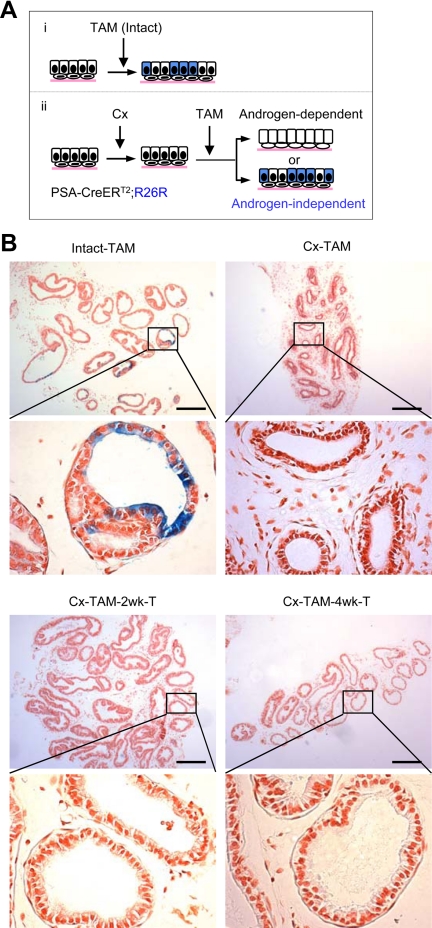
The PSA-CreERT2-based labeling should be androgen dependent because the PSA promoter/enhancer is androgen dependent and inactive in the androgen-deprived prostate (18). To confirm that labeling induced by TAM in the PSA-CreERT2 system was androgen dependent, 8-wk-old male PSA-CreERT2/R26R mice were castrated, randomized, and injected with TAM 3 wk after castration (Cx) at 3 mg/40 g body weight (Fig. 2). One group was euthanized 10 d after the last injection of TAM (Cx-TAM). The other two groups were implanted with testosterone 2 or 4 wk after the TAM injection and then euthanized 10 d after testosterone implantation (Cx-TAM −2 wk-T, and Cx-TAM −4 wk-T). The lateral prostates were dissected, processed, and analyzed. Compared with approximately 20% labeling by TAM in a noncastrated, intact group, less than 1% of luminal epithelial cells were labeled when TAM was injected 3 wk after the Cx, verifying that labeling was androgen dependent (Fig. 2B and Supplemental Table 1). The lack of labeling should not be related to TAM delivery to the castrated prostate because Cx-resistant Nkx3.1(CARN)-expressing cells in castrated prostate can be efficiently labeled by TAM administration (12). Testosterone replacement 2 or 4 wk after TAM administration had no significant effect on the frequency of labeled luminal cells in the prostate (still <1%), indicating that TAM should be metabolized and/or excreted from the animals within 2 wk after TAM injection (Fig. 2B and Supplemental Table 1). Similar results were observed when TAM was injected at a higher concentration (9 mg/40 g body weight), except that labeling of the luminal cells was slightly increased to approximately 3% (Supplemental Table 1). In this model, therefore, PSA-CreERT2-based labeling is androgen dependent and occurs specifically in AR-positive luminal epithelial cells upon transient TAM induction.
Fig. 2.
Androgen dependency of PSA-CreERT2-mediated genetic marking. A, Schematic diagram showing possible outcomes of lineage marking of cells in testis-intact and castrated PSA-CreERT2/R26R LacZ mice. B, TAM induction of genetic marking in testis-intact and castrated PSA-CreERT2/R26R LacZ mice. TAM was injected 3 wk after Cx (Cx-TAM) along with testis-intact control (Intact-TAM) mice. To verify the transient nature of TAM induction, Cx-TAM-2 wk-T and Cx-TAM-4 wk-T groups were generated by implanting testosterone pellets into Cx-TAM mice 2 or 4 wk after the TAM induction. Mice were killed 10 d after TAM injection in Cx-TAM and Intact-TAM groups or 10 d after the testosterone replacement in Cx-TAM-2 wk-T and Cx-TAM-4 wk-T groups. Scale bars, 200 μm. T, Testosterone.
Regenerated luminal epithelial cells are derived from preexisting luminal epithelial cells in the adult prostate
To determine whether newly formed luminal epithelial cells in the regenerated prostate are derived from preexisting luminal epithelial cells or from other cell types, the fate and frequency of the genetically labeled luminal epithelial cells during cycles of androgen manipulation were examined: specifically, the percent of luminal epithelial cells expressing β-galactosidase and/or GFP as compared with the total number of luminal epithelial cells (Fig. 3). In this model, heritable labeling of fully differentiated prostate luminal epithelial cells through expression of lacZ or GFP is achieved by ip injection of TAM (Fig. 3, A and B). These labeled cells are tracked through cycles of androgen manipulation by x-gal staining or expression of GFP in the prostate. Cell populations derived from preexisting luminal epithelial cells at the time of TAM injection can be identified (Fig. 3A). Newly formed luminal epithelial cells in the regenerated prostate should be labeled if and only if they are derived from preexisting labeled differentiated luminal cells. New luminal epithelial cells derived from any AR-negative cells, including stem cells and basal cells, would not be labeled. These different mechanisms of luminal epithelial cell regeneration would generate distinguishable populations of luminal cells within the prostate after cycles of androgen manipulation based on the presence and frequency of labeled cells. If newly formed luminal epithelial cells are derived from preexisting AR-positive luminal cells, the frequency of labeled luminal cells within the prostate should remain the same. Because the AR-negative stem or basal epithelial cells are incapable of activating the PSA promoter/enhancer, newly formed luminal epithelial cells derived entirely from those cells would not contain any labeled luminal cells. Finally, if a combination of stem/basal cells and preexisting luminal epithelial cells replenish luminal epithelial cells within the regenerating prostate, the frequency of labeled luminal cells should be decreased.
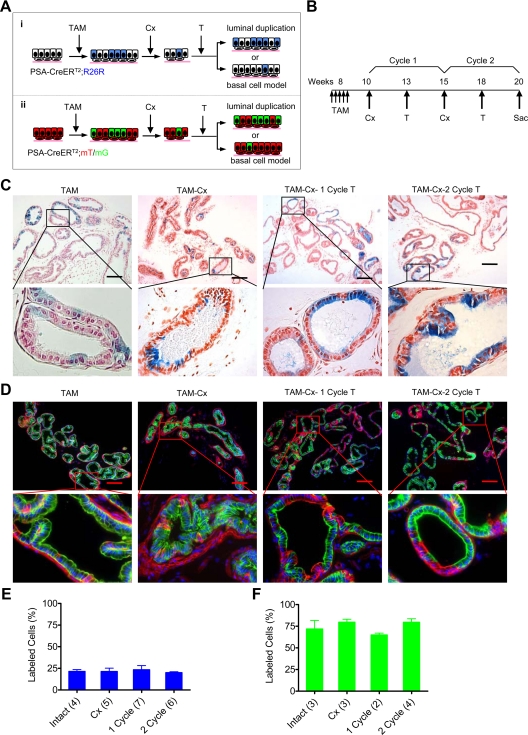
Fig. 3.
Survival and regeneration of luminal epithelial cells during cycles of prostate regeneration. A, Predictions from self-duplication model and basal cell model for luminal cell repopulation in regenerating prostate. Regenerated luminal epithelial cells derived from genetically labeled preexisting luminal cells, with either lacZ (blue) or GFP (green), can be determined after regression and regeneration of prostate. Regeneration by self-duplication from preexisting (labeled) cells predicts that the fraction of labeled cells would remain constant in new epithelium. In the basal stem cell model of regeneration, luminal epithelial cells are derived from stem cells that do not express the PSA promoter-driven CreER gene, and the percentage of labeled cells would decrease after each cycle of regression and regeneration. B, Time line employed for TAM labeling and cycles of serial regression and regeneration. C and D, Fate of genetically labeled luminal epithelial cells during cycles of lateral prostate regression and regeneration. Shown are images of x-gal staining and GFP in PSA-CreERT2/R26R LacZ and PSA-CreERT2/Rosa26R mT/mG mouse model, respectively. E and F, Quantitation of the results in C and D, respectively. Number of animals is indicated in parentheses. The percentage of labeled cells was not significantly different between groups in E (P = 0.92) or in F (P = 0.55). Error bars indicate sd. Scale bars, 200 μm. T, Testosterone; Sac, euthanized.
In intact, noncastrated mice, approximately 22% of the luminal epithelial cells were labeled with x-gal (Fig. 3, C and E, and Supplemental Table 1). The percent of labeled luminal epithelial cells after one cycle or two cycles of androgen manipulation remained virtually the same (Fig. 3, C and E). This finding was confirmed in PSA-CreERT2/R26R-GFP mice (Fig. 3, D and F). Although the efficiency of GFP genetic labeling by TAM was much higher than lacZ labeling, i.e. about 70% of the luminal epithelial cells, the percent of GFP labeling remained constant between intact and androgen-manipulated mice (Fig. 3, D and F, and Supplemental Table 2). Based on these observations, it appears that the majority of newly formed luminal epithelial cells in regenerating mouse prostate are derived from preexisting luminal epithelial cells. There should be significant cellular turnover during regression and regrowth of the prostate (Supplemental Fig. 2): approximately 90% of the luminal epithelial cells are estimated to undergo apoptosis after androgen deprivation (13) and, as a result, the majority of luminal epithelial cells are newly formed after each cycle of regeneration. The proliferation required for this repopulation to occur was demonstrated by bromodeoxyuridine (BrdU) positivity in the genetically labeled GFP-positive cells (Fig. 4). Taken together, newly regenerated luminal epithelial cells are predominantly derived from preexisting luminal cells.
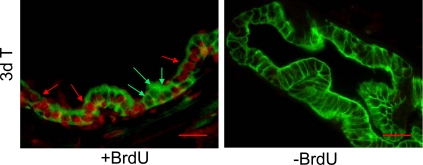
Fig. 4.
Proliferation of GFP-labeled luminal epithelial cells after androgen replacement. BrdU was injected 3 d and 4 d after testosterone replacement, and the prostate was isolated 4 h after the second injection (two-cycle group as described in Fig. 3). Red arrows indicate BrdU-labeled, GFP-positive cells (top panel). Green arrows indicate BrdU-negative, GFP-positive cells. Animals without BrdU injection were processed in parallel as negative control. Scale bars, 25 μm. T, Testosterone.
These data also demonstrated the survival of a subset of luminal epithelial cells in the regressed prostate 3 wk after Cx (Fig. 3, C–F). The percent of genetically labeled luminal epithelial cells in the regressed prostate remained constant compared with the intact prostate, indicating similar rates of survival in labeled and unlabeled luminal epithelial cells. This suggests some fully differentiated luminal epithelial cells have intrinsic mechanisms to survive androgen deprivation. In addition, this finding indicates that the PSA promoter is equally active in luminal cells that will undergo apoptosis and those that will survive Cx.
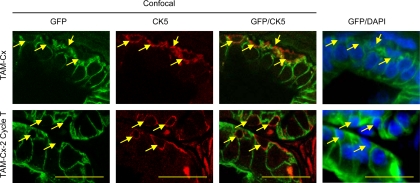
In this study, no GFP-labeled basal epithelial cells could be identified in the prostate after Cx and/or regeneration (Fig. 5). Basal cells were stained by CK5 antibody in sections from castrated and two-cycle regenerated prostates. Confocal microscopy examination of at least 50 CK5-positive basal epithelial cells in each mouse did not find any GFP-positive basal cells. Some of the CK5-positive cells can be surrounded by GFP-positive cells at one focal plane but not at another focal plane. Thus, surviving labeled luminal epithelial cells do not seem to undergo transdifferentiation to become basal epithelial cells during Cx or regeneration.
Fig. 5.
Confocal microscopic analysis of CK5-stained lateral prostate sections of PSA-CreERT2/Rosa26R mT/mG mice after Cx (TAM-Cx) and two cycles of regeneration (TAM-Cx-2 Cycle T). The lateral prostate sections were from castrated or regenerated prostates as described in Fig 3. Shown are representative images of confocal microscopy of GFP and CK5 staining and regular fluorescent microscopy of GFP and DAPI staining. Arrows indicate CK5-positive basal cells. In confocal images, CK5-positive cells can be surrounded by GFP-positive cells in one focal plane but not in another focal plane. Scale bars, 25 μm. T, Testosterone.
Discussion
Using the PSA-CreERT2-based genetic lineage tracing system, this study generated evidence for the survival and proliferation of preexisting luminal epithelial cells during cycles of regression and regrowth of adult prostate in the mouse model. This finding demonstrates the importance of preexisting luminal cells as an origin of luminal cell regeneration in adult prostate, which will have significant implications in prostate biology and prostate cancer research.
Determining the source of regenerated prostate luminal epithelial cells will help in identifying the origin of prostate cancer cells. Because regenerated luminal cells can be derived from preexisting luminal cells, they are also likely to be an important origin of prostate cancer cells. Prostate cancer cells, which typically exhibit a luminal epithelial phenotype, should retain the intrinsic capability of luminal epithelial cells to survive androgen deprivation and to regenerate upon androgen replacement. This provides a reasonable explanation for the capacity of prostate cancer cells to survive under androgen-depleted conditions and to proliferate once androgen signaling is abnormally activated. Taking the above considerations together, understanding the molecular and cellular mechanisms responsible for luminal epithelial cell survival and proliferation in response to androgen manipulation will provide insights into the mechanism of prostate cancer progression, particularly to the lethal Cx resistance phenotype.
The surviving, labeled luminal epithelial cells comprised approximately 70% of the luminal epithelial cells in the castrated and regenerated prostate, suggesting the proliferative capacity of luminal cells. This was supported by BrdU labeling of a majority of GFP-labeled prostate luminal epithelial cells in a castrated prostate 3 d after testosterone replacement (Fig. 4). This BrdU labeling experiment was not intended to track cell fate because BrdU labeling is not specific to luminal cells and can be diluted during cell divisions (14). The BrdU labeling result was consistent with previous data that the maximum of luminal epithelial cell proliferation occurred around 3 d after androgen replacement (19, 20). Taken together, the majority of surviving luminal epithelial cells in the regressed prostate is capable of proliferating and participating in the repopulation of luminal epithelial cells during prostate regeneration. This finding argues an important role for surviving preexisting luminal epithelial cells in prostate regeneration.
A popular model of prostate luminal epithelial regeneration involves stem cells, transit amplifying cells, intermediate cells, and secretory luminal cells (4). Transit amplifying/intermediate cells are derived from AR-negative undifferentiated cells whereas the surviving luminal epithelial cells are derived from preexisting luminal cells. Thus, the surviving luminal epithelial cells should be distinctly different from transit-amplifying/intermediate cells. Based on the PSA-CreERT2 genetic lineage tracing, AR-negative stem cells did not seem to contribute appreciably to luminal epithelial cell regeneration, at least during the first two cycles of regression and regeneration. Transit amplifying and intermediate cells are also unlikely to contribute significantly during the first two cycles of regression and regrowth. Transit amplifying cells, which are derived from AR-negative stem cells and reside in the basal compartment, can give rise to intermediate cells in the luminal compartment (4). Both transit amplifying cells and intermediate cells are CK5 positive but do not express AR or express it at a very low level (4). These cells are thought to respond to andromedin rather than androgens directly. Thus, transit-amplifying and intermediate cells should not be labeled or labeled at very low efficiency by the PSA-CreERT2 system, which is consistent with the observation that GFP-labeled epithelial cells were CK-5 negative (Fig. 5). Despite heterogeneous GFP labeling in the prostate, the majority of the labeled GFP-positive cells were obviously CK5 negative in both regressed and fully-grown prostate. Although confocal microscopy did not detect any CK-5-positive GFP-labeled cells (Fig. 5), their existence cannot be ruled out. Regardless, this argues that the majority of surviving, labeled epithelial cells in the luminal compartment of castrated prostate are not CK5-positive intermediate or transit-amplifying cells, suggesting that prostate regeneration is driven largely by preexisting luminal epithelial cells.
The finding in this study is not consistent with previous reports suggesting that AR-negative putative stem/progenitor/basal epithelial cells give rise to a majority of luminal epithelial cells and to structures resembling prostate glandular architecture based on tissue recombination experiments in the presence of urogenital sinus mesenchyme cells (7). Urogenital sinus mesenchyme cells have potent induction capabilities toward prostatic differentiation; they can even induce the differentiation of bladder epithelial cells into cells expressing prostate epithelial proteins (21). According to the results presented here, however, basal cells are unlikely to be an important source of newly regenerated luminal epithelial cells in the prostate in vivo, at least during the first two cycles of regression and regeneration. Furthermore, labeled luminal epithelial cells in the adult prostate do not seem to undergo transdifferentiation to become basal epithelial cells during cycles of regression and regeneration. Thus, regeneration of PSA-CreERT2-labeled luminal epithelial cells appears independent of basal cell lineage in the prostate.
A limitation of the lineage tracing of luminal epithelial cells is its inability to detect the existence of rare stem cells such as the CARN. These potential stem cells are very rare, comprising only approximately 0.7% of the epithelial cells in the castrated mouse prostate (12). The PSA-CreERT2-labeled luminal cells seemed to behave differently from CARN, although both types of the labeled cells are capable of regenerating. Genetically labeled CARN can differentiate into both basal and luminal epithelial cells in prostate regeneration, and the labeled cells were increased from 0.37% to 3.3% after the initial cycle of regeneration and then maintained in the subsequent cycles of regression and regeneration (12). In contrast, PSA-CreERT2-labeled cells maintained their abundance at approximately 70% and luminal phenotype in the prostate during regression or regeneration (Fig. 3), but did not seem to differentiate into basal cells. Further studies will be necessary to determine the relationship between CARN and PSA-CreERT2-labeled cells.
Another limitation to this study is that only two cycles of regression-regeneration were performed. Prostatic luminal epithelial cells may have short-term proliferation capacity, which can be exhausted after multiple cycles of regression-regeneration of the prostate. However, there is no evidence suggesting that the mechanism of prostate regeneration in early cycles of Cx-androgen replacement is different from that in the late cycles. Further studies will be needed to address this issue.
The finding of luminal epithelial regeneration from preexisting luminal cells raises several important questions. For example, are prostatic luminal cells surviving Cx predetermined or stochastic? Is it possible that labeled luminal cells will exhaust their regeneration potential after multiple cycles of regression and regeneration? Also, is regeneration of adult prostate during androgen manipulation different from early prostate development in urogenital sinus? To address these important questions, additional studies will be needed.
In summary, newly-formed luminal epithelial cells are predominantly derived from preexisting luminal epithelial cells during the first two cycles of regression and regrowth in the adult mouse prostate. This study demonstrates the survival and proliferation of luminal epithelial cells in response to Cx and androgen replacement, respectively. Understanding the mechanisms of androgen action in prostatic luminal epithelial cell regeneration may shed light on the progression of prostate cancer cells to Cx resistance.
Materials and Methods
Animal work
All animal studies were performed according to an approved IACUC protocol. Generation of PSA-CreERT2 mice has been previously described (17), and Rosa26R-lacZ (22) and mT/mG (23) mice were obtained from The Jackson Laboratory (Bar harbor, ME). PSA-CreERT2 mice were cross-bred with ROSA26R-lacZ mice (The Jackson Laboratory) to generate double transgenic PSA-CreERT2/R26R-lacZ mice. Genotyping was confirmed by PCR using tail genomic DNA. Primers specific for Cre recombinase mice were upstream primer TTGCCTGCATTACCGGTCGATG and downstream primer TCCAGCCACCAGCTTGCATG. Double-fluorescent mT/mG mice, which express red fluorescence before, and green fluorescence after, TAM-induced Cre-mediated recombination were also generated to confirm results. Membrane-targeted tdTomato (mT)/membrane-targeted enhanced GFP (mG) mice were crossed with PSA-CreERT2 mice to generate PSA-CreERT2/Rosa26R mT/mG mice. Genotyping was confirmed by PCR of tail genomic DNA with primers; wild-type forward, CTCTGCTGCCTCCTGGCTTCT; wild-type reverse, CGAGGCGGATCACAAGCAATA; and mutant reverse, TCAATGGGCGGGGGTCGTT.
TAM induction of Cre activity in mice containing the PSA-CreERT2 allele was performed as previously described (24). Briefly, TAM (Sigma Chemical Co., St. Louis, MO) suspended in corn oil 3 mg/40 g of body weight or vehicle alone was injected ip daily for 5 consecutive days in adult mice. Mice were randomized into groups and either euthanized 10 d after the last TAM injection or subjected to Cx with sc testosterone pellet implantation 3 wk after Cx. For cycles of prostate regrowth and regression, testosterone pellets were removed from the mice 2 wk after implantation at the end of the regrowth phase, then removed, after which the prostate was allowed to regress for 3 wk before reimplantation of another testosterone pellet. Testosterone pellets were made as previously described (25). Approximately 7.5 mg testosterone (Sigma) were tightly packed into a silicone tube with an inner diameter of 1.58 mm and outer diameter of 3.18 mm (Helix Medical, Carpinteria, CA).
To verify androgen dependency and the transient nature of TAM-induced labeling, TAM was either injected or administered by oral gavage 3 wk after Cx in control groups. At 2 wk or 4 wk after the last dose of TAM, mice were either euthanized, or implanted with testosterone pellet. After regeneration of the prostate, mice were either euthanized or subjected to pellet removal for serial regression and/or regeneration of prostate.
Whole mount x-gal and GFP staining and histology
The lateral prostate was dissected and stained with x-gal as described previously (18, 26). Briefly, prostate lobes were dissected in cold PBS and fixed in lacZ fix buffer (0.2% glutaraldehyde; 50 mm EGTA, pH 7.3; 100 mm MgCl2 in PBS) on ice for 30 min with gentle shaking. Tissues were then washed three times for 20 min each in lacZ wash buffer (2 mm MgCl2, 0.01% sodium deoxycholate, 0.02% Nonidet P-40 in PBS). Staining was performed with 1 mg/ml x-gal, 5 mm potassium ferrocyanide, and 5 mm potassium ferricyanide in lacZ wash buffer at room temperature for 2 h, with gentle shaking and protection from light. After staining, samples were washed three times for 10 min in PBS to stop the reaction and postfixed in 4% paraformaldehyde in PBS before paraffin embedding. Sections (5 μm) were counterstained with neutral red. Slides were imaged using a Leica DM LB microscope (Leica Microsystems, Inc., Buffalo Grove, IL) equipped with QImaging micropublisher 3.3 digital camera (QImaging, Surrey, British Columbia, Canada).
For double fluorescent mice, tissue and cryosection preparation were as described previously (23). Briefly, prostates were fixed in 4% paraformaldehyde for 24 h, followed by 30% sucrose for 24 h. Lobes were then dissected for subsequent cryoembedding in OCT compound (Sakura Finetek USA, Inc., Torrance, CA). Sections (10 μm) were washed with PBS and mounted with Vectastain mounting media with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories).
Quantification of x-gal and GFP-labeled cells in lateral prostate
For x-gal labeling, five sections per animal per each group were scored manually with at least four animals per group. All visible prostatic ducts in lateral lobes were imaged at 40× magnification, and all clearly visibly distinguishable cells were counted. The percentage of x-gal-labeled cells was determined by dividing the number of labeled cells by the total number of cells scored in each region. The results were expressed as the mean and the sd at each time point, and comparison between groups were calculated using the one-way ANOVA and Bonferonni's Multiple Comparison Test as appropriate. GFP-labeled cells were counted similarly, with five sections per animal per each group with at least two animals per group.
Immunostaining
Immunohistochemical staining was performed on serial 10-μm cryosections prepared from frozen formaldehyde-fixed blocks, incubated in 0.5% Triton X 100 for 10 min, followed by antigen retrieval through boiling in antigen-unmasking solution (Vector Laboratories, Inc., Burlingame, CA). Slides were blocked with blocking reagents provided in the M.O.M. (Mouse on Mouse) immunodetection kit (Vector Laboratories) for mouse primary antibodies or in 10% normal serum for other antibodies, and then incubated with primary antibodies overnight at room temperature. Primary antibodies and dilutions used were p63 (1:50, clone A4A, sc-8431, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), BrdU (1:200, antibody 1893; Abcam, Cambridge, MA), CK5 (1:250, PRB-160P; Covance Laboratories, Inc., Madison, WI), and CK18 (1:100, antibody 668, Abcam). Slides were then incubated with secondary antibodies (diluted 1:200 in 0.3% horse or donkey serum, PBS) labeled with Alexa Fluor 350, Alexa Fluor 594, or Alexa Fluor 647 (Invitrogen, Carlsbad, CA). Sections were mounted with Vectastain mounting media either with or without DAPI.
BrdU labeling
Mice received an ip injection of 0.25 ml BrdU labeling reagent (Zymed Laboratories, Inc., South San Francisco, CA; Invitrogen) 28 h and 4 h before euthanasia.
Fluorescent and confocal microscopy
Immunofluorescent staining was imaged using a Zeiss Axioplan2 microscope (Carl Zeiss MicroImaging, LLC, Thornwood, NY) equipped with fluorescein isothiocyanate, tetamethylrhodamine isothiocyanate and DAPI filters, and images were merged using Axiovision Rel. 4.5 imaging software. For confocal microscopy, immunofluorescence staining was imaged using the Leica TCS-SL confocal microscope (Leica Microsystems Inc, Bannockburn, IL) with a 63×/1.4 NA oil immersion lens. Final composites were constructed with IMARIS (Bitplane) software Version 7.0.
Acknowledgments
We thank Dr. Dean Bacich for critical reading and insightful discussion.
This work was supported in part by National Institutes of Health Grants 5 R37 DK51193, R01 CA 108675, 1 P50 CA90386, and P30 CA047904. J.L. was a recipient of the Mellam Family Foundation Fellowship and L.E.P. is a trainee of National Institutes of Health T32 DK007774 training program.
Disclosure Summary: The authors declare that they have no competing interests.
Footnotes
- AR
- Andogen receptor
- BrdU
- bmodeoxyuridine
- CARN
- castration-resistant Nkx3.1
- Cx
- castration
- DAPI
- 4′,6-diamidino-2-phenylindole
- GFP
- green fluorescent protein
- PSA
- prostate-specific antigen
- TAM
- tamoxifen.
References
- 1. Isaacs JT. 1985. Control of cell proliferation and cell death in the normal and neoplastic prostate: a stem cell model. In: Rodgers CH, Coffey DS, Cunha G, Grayhack JT, Henman JR, Horton R. eds. Benign prostatic hyperplasia. Washington DC: Department of Health and Human Services, National Institutes of Health; 85–94 [Google Scholar]
- 2. Bonkhoff H, Remberger K. 1996. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate 28:98–106 [DOI] [PubMed] [Google Scholar]
- 3. Litvinov IV, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL, Isaacs JT. 2006. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res 66:8598–8607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uzgare AR, Xu Y, Isaacs JT. 2004. In vitro culturing and characteristics of transit amplifying epithelial cells from human prostate tissue. J Cell Biochem 91:196–205 [DOI] [PubMed] [Google Scholar]
- 5. Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. 2007. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA 104:181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Hayward S, Cao M, Thayer K, Cunha G. 2001. Cell differentiation lineage in the prostate. Differentiation 68:270–279 [DOI] [PubMed] [Google Scholar]
- 7. Leong KG, Wang BE, Johnson L, Gao WQ. 2008. Generation of a prostate from a single adult stem cell. Nature 456:804–808 [DOI] [PubMed] [Google Scholar]
- 8. Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. 2004. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci 117:3539–3545 [DOI] [PubMed] [Google Scholar]
- 9. Burger PE, Xiong X, Coetzee S, Salm SN, Moscatelli D, Goto K, Wilson EL. 2005. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci USA 102:7180–7185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xin L, Lawson DA, Witte ON. 2005. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci USA 102:6942–6947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurita T, Medina RT, Mills AA, Cunha GR. 2004. Role of p63 and basal cells in the prostate. Development 131:4955–4964 [DOI] [PubMed] [Google Scholar]
- 12. Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. 2009. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evans GS, Chandler JA. 1987. Cell proliferation studies in the rat prostate: II. The effects of castration and androgen-induced regeneration upon basal and secretory cell proliferation. Prostate 11:339–351 [DOI] [PubMed] [Google Scholar]
- 14. Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, Shapiro E, Lepor H, Sun TT, Wilson EL. 2002. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol 157:1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verhagen AP, Ramaekers FC, Aalders TW, Schaafsma HE, Debruyne FM, Schalken JA. 1992. Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res 52:6182–6187 [PubMed] [Google Scholar]
- 16. Dor Y, Brown J, Martinez OI, Melton DA. 2004. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46 [DOI] [PubMed] [Google Scholar]
- 17. Ratnacaram CK, Teletin M, Jiang M, Meng X, Chambon P, Metzger D. 2008. Temporally controlled ablation of PTEN in adult mouse prostate epithelium generates a model of invasive prostatic adenocarcinoma. Proc Natl Acad Sci USA 105:2521–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cleutjens KB, van der Korput HA, Ehren-van Eekelen CC, Sikes RA, Fasciana C, Chung LW, Trapman J. 1997. A 6-kb promoter fragment mimics in transgenic mice the prostate-specific and androgen-regulated expression of the endogenous prostate-specific antigen gene in humans. Mol Endocrinol 11:1256–1265 [DOI] [PubMed] [Google Scholar]
- 19. Bruchovsky N, Lesser B, Van Doorn E, Craven S. 1975. Hormonal effects on cell proliferation in rat prostate. Vitam Horm 33:61–102 [DOI] [PubMed] [Google Scholar]
- 20. Sugimura Y, Cunha GR, Donjacour AA, Bigsby RM, Brody JR. 1986. Whole-mount autoradiography study of DNA synthetic activity during postnatal development and androgen-induced regeneration in the mouse prostate. Biol Reprod 34:985–995 [DOI] [PubMed] [Google Scholar]
- 21. Cunha GR, Lung B, Reese B. 1980. Glandular epithelial induction by embryonic mesenchyme in adult bladder epithelium of BALB/c mice. Invest Urol 17:302–304 [PubMed] [Google Scholar]
- 22. Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71 [DOI] [PubMed] [Google Scholar]
- 23. Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. 2007. A global double-fluorescent Cre reporter mouse. Genesis 45:593–605 [DOI] [PubMed] [Google Scholar]
- 24. Hayashi S, McMahon AP. 2002. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244:305–318 [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Gupta S, Hua V, Ramos-Garcia R, Shevrin D, Jovanovic BD, Nelson JB, Wang Z. 2010. Prolongation of off-cycle interval by finasteride is not associated with survival improvement in intermittent androgen deprivation therapy in LNCaP tumor model. Prostate 70:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. 1999. Z/AP, a double reporter for cre-mediated recombination. Dev Biol 208:281–292 [DOI] [PubMed] [Google Scholar]