Abstract
Hyperprolactinemia, usually caused by a pituitary lactotroph tumor, leads to galactorrhea and infertility. Increased prolactin (PRL) levels may be due to enhanced PRL expression or proliferation of PRL-secreting cells. We hypothesize that PRL expression and PRL-secreting cell proliferation are linked. Using microarray-based gene expression profiling, we identified CCAAT-enhancer-binding protein δ (CEBPD) transcription factor as a critical gene that regulates both PRL expression and lactotroph cell proliferation. CEBPD expression levels are decreased approximately 7-fold in experimental rat prolactinoma cells. Forced expression of this transcription factor in PRL-secreting cells (GH3 and MMQ) inhibited PRL expression and cellular proliferation, and CEBPD knockdown by small interfering RNA leads to increased PRL expression in both cell lines. To determine mechanisms underlying this observation, we determined binding of CEBPD to the PRL promoter and also showed marked suppression (96%) of PRL promoter activity. CEBPD and Pit1 interact and attenuate each other's binding to the PRL promoter. CEBPD also suppresses expression of proliferation-related genes, including c-Myc, survivin, as well as cyclins B1, B2, and D1. These results show that PRL expression and cell proliferation are controlled in part by CEBPD.
Enhanced cellular expression of prolactin (PRL) parallels the increased PRL-secreting cell mass observed in prolactinomas (1). During pregnancy, PRL mRNA levels also parallel the progressive PRL-secreting cell hyperplasia (2), and decreased PRL levels observed after lactation are concordant with lactotroph regression (3).
Regulation of pituitary PRL production involves regulation of PRL transcription as well as factors controlling lactotroph proliferation. PRL transcription is regulated by transcriptional factors including Pit1, estrogen receptor-α (ERa), c-Ets, and GA-binding protein (GABP) (4–6), and Pit1 mutations cause pituitary hormone deficiency in both mice and humans (6–8). However, Pit1 alone is insufficient to enable PRL gene transcription and also interacts with nuclear hormone receptors and a number of coregulators (4, 5). ERa, a nuclear hormone receptor regulating PRL, interacts with Pit1 through an AF-2 domain (8). Complexed Pit1 and ERa formation involves coactivators/corepressors, with steroid receptor coactivator 1 and glucocorticoid receptor interacting protein 1 stimulating, and receptor interacting protein 140 inhibiting, PRL promoter activity (8). Binding of Ets-1/Pit-1 to the composite element and to each other determines basal promoter activity (9). Ets-1 and Pit-1 factors are required for Ras responsiveness of the PRL promoter (9), whereas the Ets-1 and GABP complex preferentially regulates basal PRL transcription (10).
Lactotroph proliferation is induced by estrogen and epidermal growth factor (EGF) and suppressed by dopamine (4). Estrogen is a powerful mitogen for the anterior pituitary (11), and estrogen treatment induces prolactinomas in Fisher 344 rats (11, 12). EGF promotes lactotroph proliferation through the protein kinase C-ERK1/2-Pit-1 mediated pathway (13), and this effect can be blocked by EGF receptor inhibitors (14). Dopamine blocks lactotroph proliferation by inducing apoptosis (15). In addition to their roles in regulating lactotroph proliferation, estrogen and EGF enhance, and dopamine agonists suppress, PRL synthesis (11, 13, 14, 16).
Mechanisms underlying the linkage between PRL transcription and lactotroph proliferation are unclear. We hypothesized that PRL expression and lactrotroph proliferation are linked and employed gene expression profiling and molecular biology techniques to identify CCAAT-enhancer-binding protein δ (CEBPD) as a candidate transcription factor that coordinates both PRL expression and lactotroph cell proliferation.
Results
CEBPD is down-regulated in rat and human prolactinomas
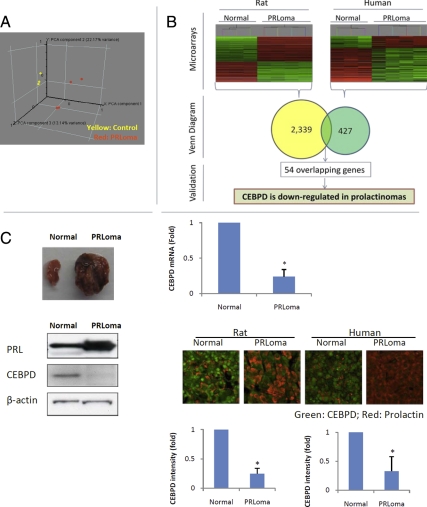
We analyzed gene expression patterns of published microarray data (17) of diethylstilbestrol-treated pituitary cells derived from the rat (ACI strain). We detected a distinct gene expression pattern compared with vehicle control-treated cells (Fig. 1A). Using one-way ANOVA and applying a cutoff (false discovery rate <0.005, fold change >2 or <−2), 2339 genes were identified as differentially expressed between control and diethylstilbestrol-treated ACI rat pituitary cells (see Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org, for a complete list). Analyzing previously published microarray data derived from human prolactinomas (18), and comparing gene profiles of three normal human pituitaries and four prolactinomas, 428 genes were identified differentially expressed (false discovery rate <0.05; fold change >2 or <−2). We sought conserved genes in rat and human prolactinoma (Fig. 1B), and 54 genes were identified as either up- or down-regulated in both rat and human prolactinomas, including a marked down-regulation of CEBPD (Fig. 1B).
Fig. 1.
CEBPD is down-regulated in prolactinomas. A, Principal Component Analysis (PCA) of microarray results derived from prolactinomas and normal rat pituitaries (Normal). B, Combined microarray data of human and rat prolactinomas identified CEBPD as a down-regulated gene in both species. C, CEBPD down-regulation in prolactinoma (PRLoma) was confirmed at the mRNA level by real-time PCR and protein levels were confirmed by Western blotting and immunofluorescence. The CEBPD (green) intensity in each PRL-positive (red) cell was quantified using ImageJ (mean ± sd; n = 3, *, P < 0.05).
To determine whether CEBPD is down-regulated in prolactinomas of other rat strains, Fischer rats were treated with estrogen. Compared with control vehicle-treated rats, in estrogen-treated Fischer rats, pituitary size increased 16-fold (119 vs. 7.2 mg; Fig. 1C, upper left). Serum PRL levels increased 78-fold (778 vs. 10 ng/ml, Fig. 1C), and pituitary PRL mRNA expression increased 10-fold. CEBPD mRNA and protein levels decreased 7-fold and 5-fold, respectively, as evaluated by real-time PCR and Western blot (Fig. 1C). Normal rat pituitary glands, including PRL-positive cells, exhibited strong nuclear staining for CEBPD. The intensity of CEBPD staining correlated inversely with PRL levels in rat prolactinomas and was weaker than in normal pituitary (Fig. 1C). Similar results were found in human pituitary tissue. Normal human pituitary tissue samples (n = 3) exhibited strong nuclear CEBPD staining, whereas six of 10 human prolactinomas were negative for CEBPD, and the other four exhibited weak CEBPD immunostaining (Fig. 1C).
Overexpression of CEBPD suppresses PRL expression and cell proliferation
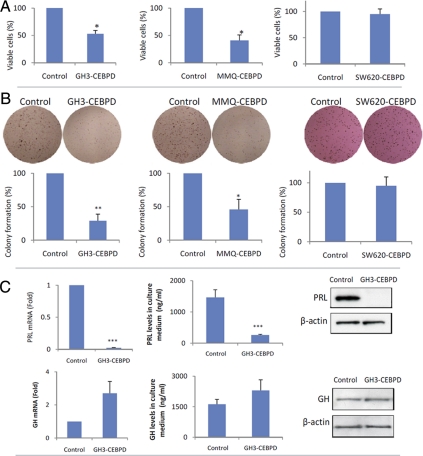
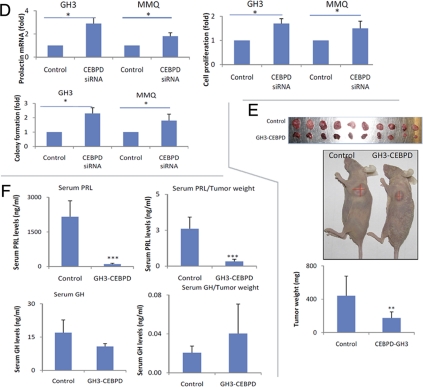
CEBPD was stably expressed in GH3 (GH3-CEBPD) and MMQ (MMQ-CEBPD) pituitary cells. GH3-CEBPD cells proliferated 47% more slowly than GH3 cells containing empty vector (GH3-EV). In a colony formation assay, GH3-CEBPD cells formed 71% fewer colonies compared with GH3-EV cells (P < 0.01) (Fig. 2B). Similarly, MMQ-CEBPD cells proliferated 39% more slowly and formed 54% fewer colonies than MMQ-EV cells (P < 0.05) (Fig. 2B). GH3-CEBPD cells had 43-fold decreased PRL mRNA expression (Fig. 2C) and nondetectable PRL protein by Western blotting compared with the control cells (Fig. 2C). PRL secretion into the culture medium also decreased 6-fold (Fig. 2C). In contrast, levels of GH mRNA, protein or culture media concentration were unchanged (Fig. 2C). CEBPD small interfering RNA (siRNA) suppressed CEBPD mRNA levels approximately 75% in both GH3 and MMQ cells, increased PRL expression 2.9- and 1.8-fold, cell proliferation 1.7- and 1.5-fold, and colony formation 2.3- and 1.8-fold, respectively (Fig. 2D), confirming a suppressive role of CEBPD on both PRL expression and cell proliferation. In contrast, overexpressing CEBPD in nonpituitary SW620 colon cancer cells did not influence either their proliferation or colony formation (Fig. 2, A and B).
Fig. 2.
Overexpression of CEBPD suppresses cell proliferation and PRL expression. A, GH3 and MMQ cells overexpressing CEBPD (GH3-CEBPD or MMQ-CEBPD) proliferated slower than corresponding cells expressing EV (control, GH3-EV, or MMQ-EV). Antiproliferative effects of CEBPD were not observed in SW620 cells (mean ± sd, n = 3; *, P < 0.05). B, GH3-CEBPD, MMQ-CEBPD, and SW620-CEBPD cells and controls expressing EV (GH3-EV, MMQ-EV, or SW620-EV) were subjected to soft agar colony formation assay. Colonies were counted manually under a microscope (mean ± sd, n = 3; *, P < 0.05; **, P < 0.01). C, Total RNA was extracted from GH3-CEBPD and GH3 cells expressing EV (control) and analyzed using real-time PCR for PRL and GH mRNA levels. PRL and GH from GH3-CEBPD and control cells were analyzed using Western blot, and medium PRL and GH levels were measured by RIA (mean ± sd, n = 3; ***, P < 0.001). D, Scrambled (control) or CEBPD siRNA was transfected into GH3 or MMQ cells, cell proliferation was measured by WST-1 assay, and total RNA was extracted for real-time PCR analysis (mean ± sd, n = 3; *, P < 0.05). E, Twenty one days after sc injection into nude mice, GH3-CEBPD cells developed smaller tumors than GH3-EV (control) cells (mean ± sd, n =10; **, P < 0.01). F, Serum was collected from tumor-harboring mice, and PRL levels were measured (mean ± sd, n = 10; **, P < 0.001). PRL levels per mg tumor weight were calculated. G, GH3-CEBPD tumors secreted less PRL than GH3-EV (control), but GH levels per mg tumor tissue were similar.
Stably expressing CEBPD transfectants were inoculated sc into nude mice and developed 2.5-fold smaller tumors compared with control GH3-EV inoculants (174 vs. 440 mg, P < 0.01; Fig. 2E). Serum PRL levels in the GH3-CEBPD group (103 ng/ml) were decreased approximately 21-fold compared with the control cohort (2157 ng/ml, P < 0.001; Fig. 2F). When normalized to tumor weight, PRL secretion levels were 0.33 ng/ml per 1 mg tumor tissue in the GH3-CEBPD group and 2.6 ng/ml in the control group (Fig. 2F), suggesting that decreased serum PRL levels in CEBPD-GH3-inoculated mice were due to both decreased tumor volume, as well as suppression of PRL expression. Serum GH levels decreased 1.6-fold in the GH3-CEBPD group (17 vs. 11 ng/ml, Fig. 2F). However, when serum GH levels were normalized to the corresponding tumor weights, GH levels per mg tumor tissue were not changed (Fig. 2F), suggesting that the moderately decreased serum GH levels could be attributed to the decreased tumor volume.
CEBPD and Pit1 modulate each other's function
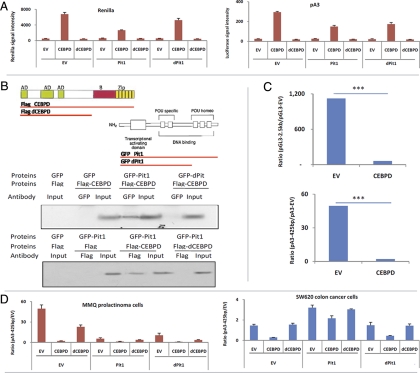
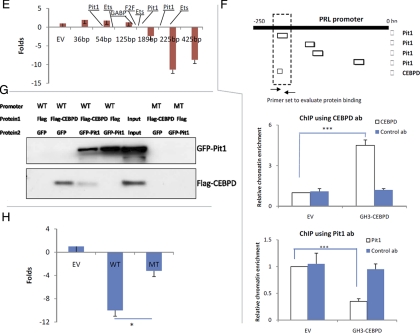
Effects of CEBPD, Pit1, and their respective mutants were tested on levels of TK-Renilla (Fig. 3A). When cotransfecting into MMQ pituitary cells, CEBPD alone induced Renilla activity about 5-fold (Fig. 3A, upper panel). Pit1 alone had no effect, but when cotransfected with CEBPD, it inhibited the stimulatory activity of CEBPD 3-fold on Renilla levels, suggesting a potential interaction with CEBPD. A Pit1 mutant devoid of its N-terminal region (dPit1) or CEBPD mutant without the bZip domain (amino acids 192–268, dCEBPD) both lost their inhibitory activities on CEBPD-induced Renilla. To test whether these observations are promoter specific, the empty luciferase reporter vector pA3-Luc was used to study promoter usage (Fig. 3 A, lower panel). Pit1 did not alter pA3-Luc activity; in contrast, CEBPD induced pA3-Luc about 10 fold. Cotransfection of Pit1 and CEBPD induced pA3-Luc approximately 1.5-fold, suggesting that Pit1 antagonized the CEBPD effect on pA3-Luc. dPit1 did not reduce the effect of CEBPD on expression of pA3-Luc, suggesting that the Pit1 N-terminal is critical in regulating CEBPD functions. dCEBPD also did not induce luciferase expression of pA3-Luc, suggesting that the bzip domain is important for CEBPD to regulate pA3-Luc activity. Effects of CEBPD on Tk-Renilla and EV mirrored each other, suggesting that these effects are Tk-promoter independent and likely resulted from CEBPD interactions with the plasmid backbone.
Fig. 3.
CEBPD interacts with Pit1 and suppresses PRL promoter activity. A, EV, CEBPD, Pit1, dCEBPD (CEBPD without bzip domain), and dPit1 (Pit1 without pou domain) constructs were cotransfected with TK-renilla plasmids into MMQ or SW620 cells. Cells were harvested 24 h after transfection, and Renilla activity was measured. CEBPD increased Renilla activity, and Pit1 antagonized effects of CEBPD in both cell lines. B, Pit1 was tagged with GFP (GFP-Pit1), and CEBPD was tagged with Flag (Flag-CEBPD). Plasmids expressing GFP, Flag, GFP-Pit1, Flag-CEBPD, GFP-dPit1, or Flag-dCEBPD were cotransfected into HEK293 cells. Coimmunoprecipitations were performed using GFP antibody and detected using Flag antibody (upper panel) or vice versa (lower panel). C, EV, CEBPD, and luciferase vectors containing different PRL promoter lengths were cotransfected into MMQ cells. After 24 h, samples were collected and luciferase activity was measured. In both conditions (transfection with EV or CEBPD), luciferase values obtained with PRL promoters were normalized by dividing values obtained with empty luciferase vectors. CEBPD suppressed 2.5-kb PRL promoter activity 18-fold and the 425-bp promoter 24-fold. D, EV, CEBPD, Pit1, dCEBPD (CEBPD without bzip domain), and dPit1 (Pit1 without pou domain) constructs were cotransfected with the 425-bp PRL promoter into MMQ cells, and promoter activity was measured by luciferase assay. E, CEBPD plasmids were cotransfected with pA3 luciferase vectors containing the indicated PRL promoter. The locations of the well-characterized functional elements are indicated. F, Analysis of −250- to 0-bp PRL promoter indicated that the CEBPD-binding motif overlaps with the Pit1 site. A primer set (arrow bar) targeting that region was designed for chromatin immunoprecipitation (ChIP) using either CEBPD antibody (ab) or Pit1 ab. G, PRL promoter fragments (WT) or mutant (MT) were labeled with biotin and incubated with nuclease extracts derived from HEK293 cells overexpressing Flag, Flag-CEBPD, GFP, or GFP-Pit1. Promoter fragments were pulled down using streptavidin beads and bound proteins were analyzed by Western blotting. H, Luciferase EV, vectors containing wild type −225-bp PRL promoter (WT), or vectors containing a PRL promoter mutant (MT, without CEBPD-binding site) were cotransfected with CEBPD plasmid. After 24 h, samples were collected and luciferase activity measured (mean ± sd, n = 3; *, P < 0.05; ***, P < 0.001).
Results of the luciferase assay suggested that CEBPD and Pit1 could be interacting. Pit1 and dPit1 were tagged with green fluorescent protein (GFP). CEBPD and dCEBPD were tagged with Flag. Pit1, CEBPD, and their mutants were therefore coexpressed in human embryonic kidney (HEK)293 cells and immunoprecipitated with GFP or Flag antibody, Western blotted and probed with either Flag or GFP antibody. CEBPD co-immunoprecipitated with Pit1, but not with dPit1; and Pit1 coimmunoprecipitated with CEBPD, but not with dCEBPD (Fig. 3B). In summary, the N terminus of Pit1 and bzip domain of CEBPD appear critical for their mutual interaction.
CEBPD suppresses PRL promoter activity
As CEBPD enhances Renilla activity, luciferase activity of the PRL promoter was normalized to its corresponding empty luciferase vector. CEBPD suppressed the 2.5-kb PRL promoter 18-fold and the 425-bp promoter by 24-fold in MMQ cells (Fig. 3C), suggesting that CEBPD-responsive elements are located within the 425-bp region. We evaluated whether CEBPD and Pit1 coordinates PRL promoter activity using this 425-bp promoter region (Fig. 3D). In MMQ cells (Fig. 3D), dCEBPD exhibited 12-fold less suppressive activity on the PRL promoter than CEBPD wild type (2- vs. 24-fold). Pit1 suppressed PRL promoter activity 9-fold and its mutant form suppressed PRL 5-fold. The combination of CEBPD and Pit1 resulted in 39-fold suppression of PRL promoter activity. Surprisingly, the combination of CEBPD and dPit1 suppressed PRL promoter activity 55-fold, whereas the combination of dCEBPD and dPit1 suppressed the promoter 14-fold. In nonpituitary cells (SW620), CEBPD suppressed PRL promoter activity 5-fold (Fig. 3D, lower panel). dCEBPD alone failed to suppress the PRL promoter. In contrast to results in pituitary MMQ cells, Pit1 stimulated PRL promoter activity 2-fold, and dPit1 lost its stimulatory effects on the PRL promoter. When combined with Pit1, CEBPD modestly suppressed the PRL promoter, and dCEBPD lost its suppressive effects on Pit1. Because dCEBPD and dPit1 lost suppressive and stimulatory PRL promoter effects, respectively, their combination did not alter PRL promoter activity, as expected.
Additional experiments analyzed effects of CEBPD on the PRL promoter using PRL-secreting MMQ cells. CEBPD suppressed the truncated 225-bp and the 189-bp PRL promoter 7.8-fold and 2.2-fold, respectively, but the suppressive effects of CEBPD were lost if only the 125-bp, 54-bp, and 36-bp fragments of the promoter were tested (Fig. 3E). These results indicated that the −125- to −225-bp region of the PRL promoter contained the sequence motif allowing CEBPD to mediate PRL suppression. One CEBPD- and four Pit1-binding motifs were identified in the 225 bp of the PRL promoter (Fig. 3F). One of the Pit1- and CEBPD-binding motifs overlapped, suggesting the presence of a potential competitive region. Chromatin immunoprecipitation at this region (−125 to −225 bp) showed a 4.5-fold increased CEBPD binding (P < 0.001, Fig. 3F), and a 3-fold decreased Pit1 binding (P < 0.01, Fig. 3 F), suggesting that CEBPD reduced Pit1 binding to DNA. A modified binding assay using a biotin-labeled PRL promoter fragment (−217 bp to −186 bp, TCTCATTTCCTTTTGCTGTAATTAATCAA AATCCTT) was incubated with nuclear extracts derived from cells overexpressing either Pit1 or CEBPD. The DNA-protein complex was precipitated using streptavidin beads and analyzed by Western blotting. CEBPD overexpression reduced Pit1 binding to the oligo and vice versa (Fig. 3G). Deletion of the CEBPD-Pit1 binding region abolished binding of both CEBPD and Pit1 to the promoter fragment (Fig. 3G) and reduced suppressive effects of CEBPD on PRL promoter (Fig. 3H). Oligonucleotides containing the CEBPD motif alone pulled down CEBPD but not Pit1, and oligonucleotides containing the Pit1 motif alone pulled down Pit1, but not CEBPD, suggesting that CEBPD and Pit1 do not compete on single CEBPD- or Pit1-binding sites (Supplemental Figs. 1 and 2).
CEBPD down-regulates c-Myc, survivin, and cyclin B1, B2, and D1
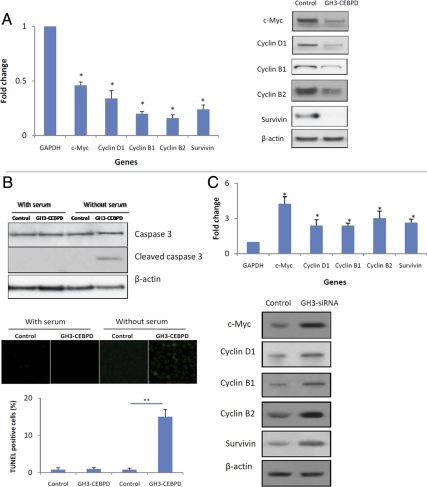
CEBPD interacts with the E2F1/Rb complex and suppresses E2F1 targeted genes in nonpituitary tumor cells (19). We found that E2F1 targeted genes were suppressed in GH3 pituitary tumor cells stably expressing CEBPD compared with GH3 vector control cells (GH3-EV). c-Myc mRNA and protein were suppressed 2-fold and 4-fold, respectively (Fig. 4A), and cyclins D1, B1, and B2 were suppressed about 3- to 6-fold at both the RNA and protein levels (Fig. 4A). Survivin mRNA and protein were suppressed about 4- to 5-fold (Fig. 4A). No cell cycle differences were observed (Supplemental Fig. 3), suggesting that inhibition of cell proliferation by CEBPD was not cell cycle specific. Because survivin is antiapoptotic, GH3-CEBPD cells were examined for apoptosis. Serum-starved GH3-CEBPD cells were more apoptotic than GH3-EV, as evidenced by enhanced levels of cleaved caspase 3 and increased the number of terminal deoxynucleotide transferase-mediated dUTP nick end labeling-positive cells (Fig. 4B). Also, CEBPD knockdown in GH3 cells had increased c-Myc (4-fold), survivin (3-fold), cyclin B1 (2-fold), cyclin B2 (3-fold), and cyclin D1 (2-fold) compared with control cells (Fig. 4C).
Fig. 4.
CEBPD downstream signals. GH3 cells were transfected with CEBPD (GH3-CEBPD). Cells transfected with EV were used as control. Total RNA and protein were extracted 24 h after transfection and analyzed using real-time PCR or Western blot for c-Myc, cyclin D1, cyclin B1, cyclin B2, and survivin. B, GH3 cells transfected with either EV (control) or stably expressing CEBPD (GH3-CEBPD) were grown in media containing 10% serum (with serum) or 5% BSA (without serum). Proteins were analyzed by Western blotting for caspase 3 and cleaved caspase 3. Terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL) assay was performed and apoptotic cells were counted. C, GH3 cells were transfected with CEBPD siRNA (GH3-siRNA). Cells transfected with scrambled siRNA were used as control. Total RNA and protein were extracted 24 h after transfection and analyzed by real-time PCR or Western blot for c-Myc, cyclin D1, cyclin B1, cyclin B2, and survivin (mean ± sd, n = 3; *, P < 0.05; **, P < 0.01).
CEBPD is downstream of estrogen/ERa
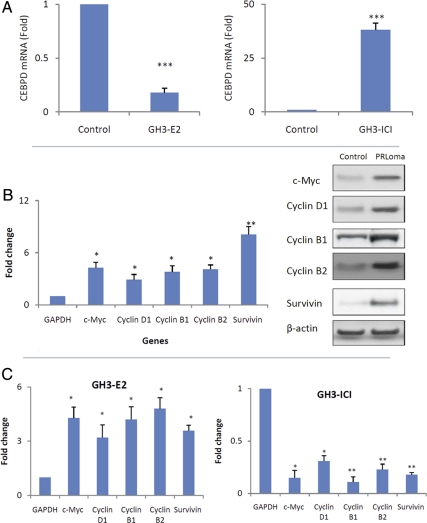
Because estrogen regulates the lactotroph cell population (4, 20), and our expression data showed that CEBPD was down-regulated in estrogen-induced rat prolactinomas (Fig. 1), we tested whether CEBPD acts downstream of estrogen. GH3 cells were cultured with either estrogen or ERa inhibitor (ICI 182780). Estrogen reduced CEBPD mRNA levels 5-fold (Fig. 5A). In parallel, genes normally suppressed by CEBPD were increased including c-Myc (4-fold), survivin (4-fold), cyclin B1 (4-fold), cyclin B2 (5-fold), and cyclin D1 (3-fold) (Fig. 5C). We found similar results in estrogen-induced rat prolactinomas, in which expression of c-Myc (4-fold), survivin (8-fold), cyclin B1 (4-fold), cyclin B2 (4-fold), and cyclin D1 (3-fold) increased (Fig. 5B). In contrast, CEBPD levels were increased 39-fold in GH3 cells after exposure to ICI 182780 (Fig. 5A). This striking induction was associated with decreased expression of genes normally suppressed by CEBPD including decreased c-Myc (7-fold), survivin (5-fold), cyclin B1 (9-fold), cyclin B2 (4-fold), and cyclin D1 (3-fold) (Fig. 5C). These results suggest that estrogen regulates CEBPD and its downstream pathways.
Fig. 5.
Estrogen (E2) regulates CEBPD. A, GH3 cells were cultured in charcoal-stripped media, treated with E2 (10 nm) or estrogen receptor inhibitor ICI 182780 (ICI, 10 nm) for 48 h, and total RNA or protein extracted and analyzed by real-time PCR and Western blotting, respectively, for CEBPD. B, c-Myc, cyclins D1, B1, and B2, and survivin levels were measured in E2-induced rat prolactinoma (PRLoma). C, c-Myc, cyclins D1, B1, and B2, and survivin levels were measured in E2-treated GH3 cells (GH3–E2) and ICI-treated GH3 cells (GH3-ICI) (mean ± sd, n = 3; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
Discussion
Our results indicate that CEBPD regulates PRL expression and proliferation of PRL-secreting cells. CEBPD overexpression suppresses PRL expression and inhibits PRL-secreting cell proliferation in vitro and in vivo. Injection of CEBPD-overexpressing cells into nude mice caused decreased levels of serum PRL resulting from suppression of both pituitary cell proliferation and PRL expression. Our results also indicate that CEBPD is a downstream target of estrogen. CEBPD levels were decreased in estrogen-induced rat prolactinoma. In GH3 cells, estrogen suppression of CEBPD was reversed by an estrogen receptor inhibitor (ICI 182780). Downstream target genes of CEBPD were also modulated by either estrogen or ICI 182780, suggesting that CEBPD may mediate estrogen regulation of PRL secretion. Knockdown of CEBPD using siRNA up-regulated PRL expression levels and increased GH3 and MMQ proliferation. Because increased PRL levels were more pronounced than increased cell proliferation, a direct involvement on PRL regulation is likely. However, effects of CEBPD siRNA on PRL expression were not as dramatic as these caused by CEBPD overexpression, which could be due to the low endogenous levels of CEBPD or already high PRL levels in these cells.
Results of luciferase assays suggested that Pit1 and CEBPD modulated each other's activity. CEBPD bzip domain and Pit1 carboxy-terminal region (amino acid residues 255–291) were required for their mutual interaction. CEBPD suppressed PRL promoter activity by directly binding the PRL promoter and antagonizing Pit1 binding. The CEBPD transcription factor can behave both as an inhibitor or enhancer of expression of targeted genes (19, 21). In contrast to its suppressive effects on PRL promoter, our results show that CEBPD enhanced basal transcription activity of several reporter gene constructs, and this effect was antagonized by Pit1. Deletion of Pit1 carboxy-terminal region (255–291) blunted its activity against CEBPD, further confirming the requirement of the Pit1 C terminus in regulating CEBPD functions. The results were consistently observed using three different plasmid constructs and three different cell lines (with or without endogenous Pit1), suggesting broad Pit1 effects on CEBPD. Although the bzip domain is critical for CEBPD function, deletion of this domain did not abolish all CEBPD activity in MMQ cells, suggesting involvement of additional mechanisms. Endogenous Pit1 in MMQ cells did not affect exogenous Pit1 effects on CEBPD, which could be due to elevated total Pit1 when expressing an exogenous Pit1, and CEBPD and CEBPA are known to transactivate promoterless luciferase vectors (22). Our unpublished data shows that CEBPB also transactivates these promoterless luciferase vectors, suggesting a common effect of CEBP family proteins.
The six CEBP family members share approximately 90% sequence identity in the C-terminal amino acid residues, which contains the bZIP domain (21). CEBP proteins regulate cell differentiation in adipocytes, myelomonocytic cells, hepatocytes, mammary epithelial cells, ovarian luteal cells, keratinocytes, neuronal cells, and intestinal epithelial cells (21). However, only CEBPD was found to be down-regulated in prolactinomas in the microarray screening, suggesting a specific role of CEBPD in PRL-secreting cells. CEBPD suppressed proliferation of PRL-secreting cells but not colon cancer cells, suggesting a cell-specific function. This observation is consistent with reports that CEBPD exhibits broad cell-specific functions (23, 24). Because expression of Pit1 is restricted to certain cell types (25–28), Pit1 may be important for cell-specific functions of CEBPD. Because Pit1 is required for cell-specific pituitary differentiation, overexpression or knockdown of CEBPD may have broad consequences including differentiation of more proximal pituitary precursor cells.
The Rb-E2F1 pathway plays important roles in pituitary tumor development (29). Deregulated E2F activity induces hyperplasia and senescence-like features in the murine pituitary gland (30), and Rb heterozygous mice develop spontaneous intermediate lobe pituitary tumors (31, 32). CEBPD binds and regulates the Rb-E2F1 complex (19), and we confirmed that CEBPD down-regulates expression of two know E2F targeted genes, c-Myc and cyclin D1. In addition to c-Myc and cyclin D1, cyclins B1 and B2 levels are also suppressed by CEBPD. Although cyclin D1 is involved in G1/S phase transition (33) and cyclin B1 and B2 play important roles in control of G2/M phase (34, 35), specific cell cycle changes were not noted in cells overexpressing CEBPD, suggesting that CEBPD may slow cell proliferation by decreasing cyclin D1, B1, and B2 without changing the distribution of cells in each phase of the cell cycle.
Transcriptional regulation of PRL involves multiple transcription factors including c-Ets and GABP (10). Pit1 and c-Ets interact and mediate ras stimulation of the PRL promoter (36). Both c-Ets and GABP bind the basal transcription element of the PRL promoter and regulate PRL expression. How CEBPD interacts with these transcription factors is unclear. High mobility group AT-hook (HMGA)-1 and -2 transgenic mice develop pituitary tumors that secrete both GH and PRL (37, 38), suggesting their roles in regulating somatolactotroph. HMGA and CEBPD seem to antagonize the function of each other. Indeed, CEBPD interacts with E2F1-Rb complex and suppresses E2F1 downstream targets (19), whereas HMGA interact with pRB and induce E2F1 activity (39). CEBPD suppresses both cyclins B1 and B2, but HMGA up-regulates cyclin B2 (40). As CEBPD exhibited no effect on GH expression, HMGA and CEBPD may independently regulate pituitary function. ERa interacts with Pit1 and up-regulates PRL transcription (8). Our results show that CEBPD interacts with Pit1 to suppress PRL transcription. Because CEBPD is suppressed by estrogen, activation of ERa may thus promote an ERa-Pit1 complex by removing CEBPD. However, further investigation is required to determine whether there is a balance between ERa-Pit1 and CEBPD-Pit1 complexes. Overexpressing Pit1 or dPit1 suppressed the −425 PRL promoter in MMQ but not in SW620 cells. Further investigation is required to elucidate whether an overabundant Pit1 may actually disrupt transcription machinery that favors PRL transactivation.
In summary, CEBPD coordinately down-regulates pituitary PRL expression and proliferation of PRL-secreting cells. Our findings suggest a novel pathway that may facilitate developing new strategies in treating hyperprolactinemia patients.
Materials and Methods
Cell lines and reagents
GH3 rat lacto-somatotroph, MMQ lactotroph, and SW620 colon cancer cells were purchased from American Type Culture Collection (Manassas, VA) and maintained in conditions as recommended. GH3 cells stably expressing either EV (GH3-EV) or CEBPD (GH3-CEBPD) were generated by transfection with lipofectamine and selection with G418. Antibodies used in this study include: CEBPD, PTTG1, GFP, survivin, cyclin D1, B1, and B2 (Abcam, Cambridge, MA), PRL and GH (A.F. Parlow, National Hormone & Peptide Program, Torrance, CA), Pit1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and Flag (Cell Signaling Technology, Danvers, MA). Estrogen and ERa inhibitor (ICI 182780) were from Sigma Aldrich. (St Louis, MO). The siRNA targeting CEBPD was from Ambion (Austin, TX). Luciferase vectors containing PRL promoters were from Arthur Gutierrez-Hartmann. PRL promoter mutant without CEBPD binding motif were generated using QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) with primers CTCTCATTTCCTTTTCCTTCCTTTCTGG and CCAGAAAGGAAGGAAAAGGAAATGAGAG.
Microarray data analysis
The microarray data for ACI rat prolactinoma (41) were downloaded from GEO (access number GSE4028). The microarray data for human prolactinoma (18) were kindly provided by Dr. Nelson M. Oyesiku. These microarray data were imported into Genespring 7.3 (Agilent Technologies, Palo Alto, CA). All genes were normalized to their median, and data quality was assessed using Principle Component Analysis and sample clustering. Differentially expressed genes were identified by parametric testing (not assuming equal variance). Results were subjected to Multiple Testing Correction using the Benjamini and Hochberg method .
Estrogen-induced prolactinomas
Animal protocols were approved by Institutional Animal Care and Use Committee (IACUC) at Cedars-Sinai Medical Center. Briefly, 4- to 5-wk ovariectomized female Fischer-344 rats (Harlan Sprague Dawley, Inc., Indianapolis, IN) were implanted sc with a 17β-estradiol-filled SILASTIC brand capsule (Medical Grade Tubing Special; length, 3 cm; outer diameter, 0.125 in.; inner diameter, 0.062 in.; Dow Corning Corp., Midland, MI) under isoflurane inhalational anesthesia. Blood (500 μl) was collected by retro-orbital bleeding every 2 wk for hormone assessment. Rats were euthanized 2 months after estrogen implantation, and cardiac blood and pituitary glands were collected and analyzed. Fragments of each pituitary were fixed in formalin, embedded in paraffin, and either preserved in RNA Later solution (Ambion) or frozen in liquid nitrogen.
Hormone assays
RIA for rat GH and PRL were performed in duplicate, using reagents provided by Dr. Parlow in the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA). Iodination of rat GH and PRL (5 μg) with iodine-125 (500 μCi; PerkinElmer Life & Analytical Sciences, Boston, MA) mixed with 0.1 mg of Iodo-Gen (Pierce Chemical Co., Rockford, IL) was performed using 10 ml columns prepared by G-75 Sephadex (Sigma Chemical Co.). Mouse GH and PRL concentrations in serum were measured using ELISA kits (Millipore Corp. (Bedford, MA) and R&D Systems (Indianapolis, IN), respectively).
Cell proliferation
Cell proliferation was evaluated using WST-1 cell proliferation reagent (CLONTECH Laboratories, Inc., Palo Alto, CA). Briefly, 3000 cells (GH3-EV or GH3-CEBPD) in 100 μl culture medium were seeded into 96-well plates, cultured for 48 h, and 10 μl premixed WST-1 cell proliferation reagent were added. Cells were incubated for a further 4 h, shaken and absorbance measured at 450 nm using a Victor 3 multiwell plate reader (PerkinElmer). Cell viability was calculated for each well as A450 treated cells/A450 control cells × 100% (A450: OD value at 450 nm).
Colony formation
Six-well plates were coated with 1.5 ml 0.6% low melting soft agar; 5000 cells were mixed with 2 ml of 0.4% low melting soft agar and plated in precoated six-well plates for 10 d. Colonies were stained and counted manually.
Subcutaneous xenografted tumors
Female athymic mice (5- to 6 wk of age) (The Jackson Laboratory, Bar Harbor, ME) were separated into two groups, one for GH3-EV and the other for GH3-CEBPD cells. Cells were mixed with the same amount of Matrigel to reach a concentration of 1 × 107 cells/ml. Cell suspension (100 μl) was injected sc into the left flank to generate tumors. Mice were monitored daily and weighed twice weekly. Tumor sizes were measured twice weekly by caliper measurements using the formula: tumor volume = (length × width2)/2, as previously reported (42). At the end of the study, 3 wk after cell injection, animals were euthanized under CO2 inhalation followed by cervical dislocation to ensure death (consistent with the Panel on Euthanasia of the American Veterinary Medical Association). Blood and tumor tissue were collected and analyzed.
Chromatin immunoprecipitation
Chromatin immunoprecipitaton was performed with ChIP-IT kits (Active Motif, Carlsbad, CA). Cells were fixed and chromatin sonicated to 0.3–2 kb with the peak at 0.8 kb. Rabbit IgG provided in the kit was used as a negative control antibody. Chromatin samples were immunoprecipitated using antibodies for CEBPD or Pit1, RNA was digested by addition of ribonuclease A, and cross-links were removed by incubating at 65 C for 4 h. Proteins were digested with proteinase K, DNA purified, and subjected to real-time PCR detection.
Luciferase reporter assay
Luciferase assays were carried out using Dual Luciferase Reporter System (Promega Corp.) according to the manufacturer's protocol. Briefly, cells were seeded in 24-well plates and incubated at 37 C overnight. Each well was cotransfected with 100 ng luciferase plasmids (plasmids with PRL promoter or EV), 100 ng expression plasmids (CEBPD, Pit1, mutants, or combinations) and 5 ng pRL-TK plasmids (Promega) encoding Renilla (internal control to assess transfection efficiency). After 24 h, whole-cell lysates were collected and measured using an Orion Microplate Luminometer (Berthold Detection System). Transfections were performed in triplicate and repeated three times. Relative luciferase activity was calculated using the formula: relative luciferase levels = Luc-promoter/Luc-pGL3 basic.
DNA affinity assay
PRL promoter fragment (−217 bp to −186 bp, TCTCATTTCCTTTTGCTGTAATTAATCAAAATCCTT) and a mutant without CEBPD binding site (TCTCATTTCCTTTTATCACGGCCGATCCGGTCCTT) were synthesized with 3′-ends labeled with biotin and annealed to form double strands. The oligotides without biotin were used as negative control. CEBPD, Pit1, or their mutants were overexpressed in HEK293, and nuclear protein was extracted using a nuclear extract kit (Active Motif). Protein concentration was measured by Coomassie Plus Assay Kit (Pierce). Oligonucleotide (1μg) was incubated with 1 mg nuclear proteins for 20 min at room temperature in binding buffer containing 12% glycerol, 12 mm HEPES (pH 7.9), 4 mm Tris (pH 7.9), 150 mm KCl, 1 mm EDTA, 1 mm dithiothreitol, and 10 μg polydeoxyinosinic deoxycytidylic acid competitor. Streptavidin-agarose (30 μl) (Sigma) was added and incubated at 4 C for 4 h. The protein-DNA-streptavidin-agarose complex was washed three times with binding buffer and loaded onto NuPAGE Novex Bis-Tris Gel (Invitrogen, Carlsbad, CA) and Western blotting was performed.
Western blot
Protein extracts were resolved by Nupage 4–12% Bis-Tris Gel (Invitrogen), samples were electroblotted onto polyvinylidene difluoride membrane (Invitrogen), and membranes were blocked and incubated with primary antibody. Donkey antirabbit or antimouse (GE Healthcare, Piscataway, NJ) antibodies were conjugated to horseradish peroxide to reveal immunocomplexes by enhanced chemiluminescence (Pierce). Detected bands were quantified using Image J v1.43 as instructed in the software manual.
Immunofluorescence
Tissue sections were deparaffinized in xylene, hydrated in graded ethanol, and heated in Target Retrieval Solution (DakoCytomation) to retrieve antigen at 95 C for 40 min. Slides were permeabilized with 1% Triton-X-100 in PBS for 30 min and incubated in blocking buffer (10% goat serum, 1% BSA, 0.1% Triton-X-100 in PBS) for 1 h. After washing with PBS, slides were hybridized at 4 C overnight with antibodies against indicated proteins. Primary antibody was omitted for negative controls. Alexa Fluor antimouse antibody with either green fluorescence or antirabbit antibody with red fluorescence (Molecular Probes, Inc., Eugene, OR) were used as secondary antibodies and incubated at room temperature avoiding light for 1 h. Slides were mounted with ProLong Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole (Invitrogen), and nuclei were dyed by 4′,6-diamidino-2-phenylindole with blue fluorescence. Images were taken under the same setting using a confocal microscope. The CEBPD intensity (green) in each PRL-positive (red) cell was quantified using ImageJ according to the manual.
Data analysis.
Except for the microarray study, all results were expressed as mean ± sd. Two-group comparison was assessed by nonpaired, two tailed Student's t test. The growth difference between multiple groups was assessed by ANOVA.
Acknowledgments
We thank Drs. Nelson M. Oyesiku at Emory University (Atlanta, GA) for sharing the microarray data for human prolactinomas, Peter Rotwein at Oregon Health & Science University (Portland, OR) for the CEBPD plasmids, and Day Richard at Indiana University (Bloomington, IN) for the Pit1 plasmids.
This work was supported by National Institutes of Health Grant K99CA138914 (to Y.T.), CA 75979 (to S.M.), and The Doris Factor Molecular Endocrinology Laboratory (to S.M.); CA026038–32 (to H.P.K.), an A*STAR Investigator Grant (to H.P.K.) and DK37667 (to A.G.).
Author contributions: Y.T. designed and carried out the experiments and wrote the manuscript; J.Z. performed microarray analysis and data integration; J.M. provided technical support; H.F. collaborated in establishing the estrogen-induced rat prolactinoma model; S.-G. R. performed RIA for all hormones; A.G.-H. kindly provided the PRL promoter constructs and helped in interpreting the data related to transcriptional regulation of PRL; H.P.K. was involved in data interpretation and discussion of results; S.M. was pivotal in experiment design, data interpretation, discussion of results and writing the manuscript.
Disclosure Summary: The authors declare no commercial conflicts.
Footnotes
- CEBPD
- CCAAT-enhancer-binding protein δ
- EGF
- epidermal growth factor
- ERa
- estrogen receptor-α
- EV
- empty vector
- GABP
- GA-binding protein
- GFP
- green fluorescent protein
- HEK
- human embryonic kidney
- HMGA
- high mobility group AT-hook protein
- PRL
- prolactin
- siRNA
- small interfering RNA.
References
- 1. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA. 2011. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:273–288 [DOI] [PubMed] [Google Scholar]
- 2. Stefaneanu L, Kovacs K, Lloyd RV, Scheithauer BW, Young WF, Jr, Sano T, Jin L. 1992. Pituitary lactotrophs and somatotrophs in pregnancy: a correlative in situ hybridization and immunocytochemical study. Virchows Arch B Cell Pathol Incl Mol Pathol 62:291–296 [DOI] [PubMed] [Google Scholar]
- 3. Haggi ES, Torres AI, Maldonado CA, Aoki A. 1986. Regression of redundant lactotrophs in rat pituitary gland after cessation of lactation. J Endocrinol 111:367–373 [DOI] [PubMed] [Google Scholar]
- 4. Ben-Jonathan N, LaPensee CR, LaPensee EW. 2008. What can we learn from rodents about prolactin in humans? Endocr Rev 29:1–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scully KM, Jacobson EM, Jepsen K, Lunyak V, Viadiu H, Carrière C, Rose DW, Hooshmand F, Aggarwal AK, Rosenfeld MG. 2000. Allosteric effects of Pit-1 DNA sites on long-term repression in cell type specification. Science 290:1127–1131 [DOI] [PubMed] [Google Scholar]
- 6. Drouin J. 2006. Molecular mechanisms of pituitary differentiation and regulation: implications for hormone deficiencies and hormone resistance syndromes. Front Horm Res 35:74–87 [DOI] [PubMed] [Google Scholar]
- 7. Pfäffle R, Klammt J. 2011. Pituitary transcription factors in the aetiology of combined pituitary hormone deficiency. Best Pract Res Clin Endocrinol Metab 25:43–60 [DOI] [PubMed] [Google Scholar]
- 8. Schaufele F. 1999. Regulation of estrogen receptor activation of the prolactin enhancer/promoter by antagonistic activation function-2-interacting proteins. Mol Endocrinol 13:935–945 [DOI] [PubMed] [Google Scholar]
- 9. Duval DL, Jean A, Gutierrez-Hartmann A. 2003. Ras signaling and transcriptional synergy at a flexible Ets-1/Pit-1 composite DNA element is defined by the assembly of selective activation domains. J Biol Chem 278:39684–39696 [DOI] [PubMed] [Google Scholar]
- 10. Schweppe RE, Gutierrez-Hartmann A. 2001. Pituitary Ets-1 and GABP bind to the growth factor regulatory sites of the rat prolactin promoter. Nucleic Acids Res 29:1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarkar DK. 2006. Genesis of prolactinomas: studies using estrogen-treated animals. Front Horm Res 35:32–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S. 1999. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med 5:1317–1321 [DOI] [PubMed] [Google Scholar]
- 13. De Paul AL, Gutierrez S, Sabatino ME, Mukdsi JH, Palmeri CM, Soaje M, Petiti JP, Torres AI. 2011. Epidermal growth factor induces a sexually dimorphic proliferative response of lactotroph cells through PKC/ERK1/2/Pit-1 in vitro. Exp Physiol 96:226–239 [DOI] [PubMed] [Google Scholar]
- 14. Vlotides G, Siegel E, Donangelo I, Gutman S, Ren SG, Melmed S. 2008. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res 68:6377–6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Radl DB, Ferraris J, Boti V, Seilicovich A, Sarkar DK, Pisera D. 2011. Dopamine-induced apoptosis of lactotropes is mediated by the short isoform of D2 receptor. PLoS One 6:e18097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gruszka A, Ren SG, Dong J, Culler MD, Melmed S. 2007. Regulation of growth hormone and prolactin gene expression and secretion by chimeric somatostatin-dopamine molecules. Endocrinology 148:6107–6114 [DOI] [PubMed] [Google Scholar]
- 17. Spady TJ, Pennington KL, McComb RD, Shull JD. 1999. Genetic bases of estrogen-induced pituitary growth in an intercross between the ACI and Copenhagen rat strains: dominant mendelian inheritance of the ACI phenotype. Endocrinology 140:2828–2835 [DOI] [PubMed] [Google Scholar]
- 18. Evans CO, Moreno CS, Zhan X, McCabe MT, Vertino PM, Desiderio DM, Oyesiku NM. 2008. Molecular pathogenesis of human prolactinomas identified by gene expression profiling, RT-qPCR, and proteomic analyses. Pituitary 11:231–245 [DOI] [PubMed] [Google Scholar]
- 19. Gery S, Tanosaki S, Hofmann WK, Koppel A, Koeffler HP. 2005. C/EBPdelta expression in a BCR-ABL-positive cell line induces growth arrest and myeloid differentiation. Oncogene 24:1589–1597 [DOI] [PubMed] [Google Scholar]
- 20. Melmed S. 2011. Pathogenesis of pituitary tumors. Nat Rev Endocrinol 7:257–266 [DOI] [PubMed] [Google Scholar]
- 21. Ramji DP, Foka P. 2002. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365:561–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li YC, Hayes S, Young AP. 1994. Transactivation of the ‘promoterless’ luciferase-encoding vectors pXP1 and pXP2 by C/EBP α. Gene 138:257–258 [DOI] [PubMed] [Google Scholar]
- 23. Ikezoe T, Gery S, Yin D, O'Kelly J, Binderup L, Lemp N, Taguchi H, Koeffler HP. 2005. CCAAT/enhancer-binding protein delta: a molecular target of 1,25-dihydroxyvitamin D3 in androgen-responsive prostate cancer LNCaP cells. Cancer Res 65:4762–4768 [DOI] [PubMed] [Google Scholar]
- 24. Sivko GS, DeWille JW. 2004. CCAAT/Enhancer binding protein δ (c/EBPδ) regulation and expression in human mammary epithelial cells. I. “Loss of function” alterations in the c/EBPδ growth inhibitory pathway in breast cancer cell lines. J Cell Biochem 93:830–843 [DOI] [PubMed] [Google Scholar]
- 25. Delhase M, Vergani P, Malur A, Hooghe-Peters EL, Hooghe RJ. 1993. The transcription factor Pit-1/GHF-1 is expressed in hemopoietic and lymphoid tissues. Eur J Immunol 23:951–955 [DOI] [PubMed] [Google Scholar]
- 26. Bamberger AM, Bamberger CM, Pu LP, Puy LA, Loh YP, Asa SL. 1995. Expression of pit-1 messenger ribonucleic acid and protein in the human placenta. J Clin Endocrinol Metab 80:2021–2026 [DOI] [PubMed] [Google Scholar]
- 27. Gil-Puig C, Blanco M, García-Caballero T, Segura C, Pérez-Fernández R. 2002. Pit-1/GHF-1 and GH expression in the MCF-7 human breast adenocarcinoma cell line. J Endocrinol 173:161–167 [DOI] [PubMed] [Google Scholar]
- 28. Gil-Puig C, Seoane S, Blanco M, Macia M, Garcia-Caballero T, Segura C, Perez-Fernandez R. 2005. Pit-1 is expressed in normal and tumorous human breast and regulates GH secretion and cell proliferation. Eur J Endocrinol 153:335–344 [DOI] [PubMed] [Google Scholar]
- 29. Guidi CJ, Mudhasani R, Hoover K, Koff A, Leav I, Imbalzano AN, Jones SN. 2006. Functional interaction of the retinoblastoma and Ini1/Snf5 tumor suppressors in cell growth and pituitary tumorigenesis. Cancer Res 66:8076–8082 [DOI] [PubMed] [Google Scholar]
- 30. Lazzerini Denchi E, Attwooll C, Pasini D, Helin K. 2005. Deregulated E2F activity induces hyperplasia and senescence-like features in the mouse pituitary gland. Mol Cell Biol 25:2660–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hereñú CB, Morel GR, Bellini MJ, Reggiani PC, Sosa YE, Brown OA, Goya RG. 2006. Gene therapy in the neuroendocrine system. Front Horm Res 35:135–142 [DOI] [PubMed] [Google Scholar]
- 32. Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. 1992. Effects of an Rb mutation in the mouse. Nature 359:295–300 [DOI] [PubMed] [Google Scholar]
- 33. Kim JK, Diehl JA. 2009. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol 220:292–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miyazaki T, Arai S. 2007. Two distinct controls of mitotic cdk1/cyclin B1 activity requisite for cell growth prior to cell division. Cell Cycle 6:1419–1425 [PubMed] [Google Scholar]
- 35. Gong D, Ferrell JE., Jr 2010. The roles of cyclin A2, B1, and B2 in early and late mitotic events. Mol Biol Cell 21:3149–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bradford AP, Conrad KE, Wasylyk C, Wasylyk B, Gutierrez-Hartmann A. 1995. Functional interaction of c-Ets-1 and GHF-1/Pit-1 mediates Ras activation of pituitary-specific gene expression: mapping of the essential c-Ets-1 domain. Mol Cell Biol 15:2849–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fedele M, Pentimalli F, Baldassarre G, Battista S, Klein-Szanto AJ, Kenyon L, Visone R, De Martino I, Ciarmiello A, Arra C, Viglietto G, Croce CM, Fusco A. 2005. Transgenic mice overexpressing the wild-type form of the HMGA1 gene develop mixed growth hormone/prolactin cell pituitary adenomas and natural killer cell lymphomas. Oncogene 24:3427–3435 [DOI] [PubMed] [Google Scholar]
- 38. Fedele M, Battista S, Kenyon L, Baldassarre G, Fidanza V, Klein-Szanto AJ, Parlow AF, Visone R, Pierantoni GM, Outwater E, Santoro M, Croce CM, Fusco A. 2002. Overexpression of the HMGA2 gene in transgenic mice leads to the onset of pituitary adenomas. Oncogene 21:3190–3198 [DOI] [PubMed] [Google Scholar]
- 39. Fedele M, Pierantoni GM, Visone R, Fusco A. 2006. E2F1 activation is responsible for pituitary adenomas induced by HMGA2 gene overexpression. Cell Div 1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Martino I, Visone R, Wierinckx A, Palmieri D, Ferraro A, Cappabianca P, Chiappetta G, Forzati F, Lombardi G, Colao A, Trouillas J, Fedele M, Fusco A. 2009. HMGA proteins up-regulate CCNB2 gene in mouse and human pituitary adenomas. Cancer Res 69:1844–1850 [DOI] [PubMed] [Google Scholar]
- 41. Strecker TE, Spady TJ, Schaffer BS, Gould KA, Kaufman AE, Shen F, McLaughlin MT, Pennington KL, Meza JL, Shull JD. 2005. Genetic bases of estrogen-induced pituitary tumorigenesis: identification of genetic loci determining estrogen-induced pituitary growth in reciprocal crosses between the ACI and Copenhagen rat strains. Genetics 169:2189–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Imaizumi M, Kondo T, Taguchi T, Hattori T, Abe O, Kitano M, Wakui A. 1993. A standardized method of using nude mice for the in vivo screening of antitumor drugs for human tumors. Surg Today 23:412–419 [DOI] [PubMed] [Google Scholar]