The primate pituitary contains a functional kisspeptin signaling system, which is dynamically regulated, is influenced by estrogens, and contributes to control of gonadotropic/reproductive and somatotropic/growth axes.
Abstract
Kisspeptins (Kps) have emerged as key players in the control of reproductive-axis function, in which they operate as primary regulators of hypothalamic GnRH release. In addition, recent data indicate that Kps can also directly act on the pituitary to stimulate LH and GH release in primary pituitary cell culture prepared from rats, cows, and sheep. We present herein evidence that Kps (specifically Kp-10) can also stimulate LH and GH release in primary pituitary cell cultures prepared from female baboons (Papio anubis), a species that more closely models human physiology. The stimulatory effect of Kp-10 on LH and GH release was dose and time dependent and enhanced the hormonal responses to their major regulators (GnRH for LH; GHRH/ghrelin for GH) without affecting the release of other pituitary hormones (TSH, FSH, ACTH, prolactin). Use of pharmacological intracellular signaling blockers indicated Kp-10 signals through phospholipase C, protein kinase C, MAPK, and intracellular Ca2+ mobilization, but not adenylyl cyclase, protein kinase A, extracellular Ca2+ influx (through L-type channels), or nitric oxide synthase, to stimulate both LH and GH release. Interestingly, blockade of mammalian target of rapamycin or phosphoinositol 3-kinase activity fully abolished the stimulatory effect of Kp-10 on LH but not GH release. Of note, estradiol enhanced the relative LH response to Kp-10, alone or in combination with GnRH. In sum, our data are the first to provide evidence that, in a primate model, there is a functional Kp-signaling system within the pituitary, which is dynamically regulated and may contribute to the direct control of gonadotropic and somatotropic axes.
Kisspeptins (Kps) are the peptide products of the Kiss1 gene, which signal through the G protein-coupled receptor Kiss1r (also known as GPR54) to regulate reproductive function. The importance of Kps/GPR54 is emphasized by the observation that some forms of hypogonadotropic hypogonadism are caused by inactivating mutations of GPR54 in humans and rodents (1, 2). Subsequent studies, conducted in diverse mammalian and nonmammalian species, have confirmed that Kps are essential gatekeepers of proper reproductive maturation and function, including brain sexual differentiation, puberty onset, and neuroendocrine control of gonadotropin secretion and its gating by metabolic and seasonal cues (3).
It is clear that Kps act at the level of the hypothalamus to stimulate GnRH release. Detailed neuroanatomical studies in rodents, sheep, primates, and fish led to the identification of discrete populations of hypothalamic neurons that express Kiss1/kisspeptin, and presence of functional Kiss1r have been documented in GnRH neurons. Specifically, in mammals, a prominent population of Kiss1 neurons has been demonstrated in the arcuate nucleus in both sexes (4, 5). In addition, in rodents another group of kisspeptin neurons are located in the anteroventral periventricular (AVPV) nucleus and adjacent areas, in which this neuronal population seems to be more abundant in females (4, 5). In fact, direct appositions between Kiss1 terminals in the AVPV nucleus and GnRH neurons have been described in rodents (6). Similarly, kisspeptin and GnRH axons have been found in close association within the median eminence of monkeys (7). These findings, coupled with an abundance of convincing functional data, have led to the notion that Kiss1 neurons within the hypothalamus are pivotal afferents in the circuitry governing GnRH secretion, in which they operate as major nodal points for the integration and transmission of key regulatory signals, from sex steroids to metabolic hormones (3). Evidence is also accumulating showing Kiss1 and Kiss1r are expressed in other brain (extrahypothalamic) and peripheral tissues, thus suggesting additional sites of action, if not different biological roles, for Kps.
In this regard, the possibility that Kps could exert direct modulatory effects on gonadotropin secretion at the pituitary level was put forward by initial studies of characterization of the pharmacological effects of Kps on LH and FSH release in the rat (8). Since then, several groups have documented the ability of Kps to elicit LH secretion by pituitary tissue in vitro in rodent, bovine, and ovine species (9–13). In addition, Kps have been detected in the portal blood of the pituitary in sheep (12), and recent immunohistochemical studies in the monkey suggest a potential direct effect on the pituitary (7). Furthermore, expression of Kiss1 and Kiss1r mRNAs has been documented in the pituitary of rats, sheep, and humans under the control of GnRH and/or sex steroids (14–17). Collectively these data suggest a plausible functional role of Kp signaling at the pituitary level. Admittedly, however, some other studies have been unable to detect any direct action of Kps in the pituitary of rats (18, 19). Moreover, the physiological relevance of such direct effects, at least regarding their involvement in the induction of the preovulatory LH surge, has also been questioned in sheep (12). Evidence also suggests that the pituitary actions of Kps might involve the modulation of other neuroendocrine axes, such as the somatotroph system (9, 10, 20, 21), but this action also remains controversial.
In the present work, we aimed to verify the direct pituitary actions of Kps on hormone release using the female baboon as a model. We hypothesized that kisspeptin signaling in pituitary cells may play a role in the fine tuning of the gonadotropic axis, complementary to its dominant, pivotal function at the hypothalamus. In addition, based on our previous data in the rat (9, 10, 20, 21), we considered the possibility that Kp may directly regulate pituitary somatotroph function. To test these hypotheses, both LH and GH secretory responses were monitored in primary cell cultures of female baboon pituitaries after Kp-10 treatment, alone or in the presence of well-known modulators of gonadotroph (GnRH) or somatotroph [GHRH, somatostatin (SST), or ghrelin] function. Furthermore, because there is solid evidence indicating that Kps can activate a wide variety of intracellular signals via GPR54 in primary cultures as well as heterologous cell models transfected with GPR54 (3, 22), the relative contribution of major intracellular signaling pathways to Kp-10-mediated LH and GH release were studied using pharmacological blockers.
Materials and Methods
Culture reagents
All reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Kp-10, GnRH, GHRH, ghrelin, and SST were purchased from Sigma and Phoenix Pharmaceuticals (Burlingame, CA). α-MEM, HEPES, horse serum, and penicillin-streptomycin were obtained from Invitrogen (Grand Island, NY). Inhibitors of intracellular signaling pathways were purchased from Cayman Chemical (U73122 and L-arginine methyl ester hydrochloride; Ann Arbor, MI) or Tocris Bioscience (rapamycin; Ellisville, MO).
Animals and tissue collection
Pituitaries were obtained from randomly cyclic female baboons (Papio anubis, 7–12 yr of age) within 15 min after sodium pentobarbital overdose. The selected baboons represent control animals from a breeding colony. All procedures were conducted under the Institutional Animal Care and Use Committee at the University of Illinois at Chicago (Chicago, IL). After the animals were killed, pituitaries were immediately excised and placed in sterile cold (4 C) basic media consisting of α-MEM, 0.15% BSA, 6 mm HEPES, 10 IU/ml penicillin, and 10 μg/ml streptomycin. Pituitaries were then washed twice in fresh media and divided into smaller fragments with surgical blades. Some fragments were rapidly frozen in liquid nitrogen and stored at −80 C until RNA isolation (see below for details), whereas the remaining fragments were dispersed into single cells for culture as described below. Dissection of the hypothalamic area was done consistently in every animal by excising a central region of the hypothalamus with a cube form (parallel lateral and coronal cuts separated 5 mm from each other, centered around midline), which provided a tissue fragment of approximately 0.15 cm3, likely containing several distinct neuronal nuclei.
Primary pituitary cell culture
Anterior pituitaries were dispersed into single cells by enzymatic and mechanical disruption, as previously described (23–25). Cells were plated onto 24-well tissue culture plates at 200,000 cells/well density in 0.5 ml of basic medium containing 10% horse serum. After a 48 h-incubation (37 C), medium was removed and cells were preincubated for 1 h in fresh, warm (37 C), serum-free medium to stabilize basal hormone secretion. After the preincubation period, medium was replaced with serum-free medium containing treatments. Experiments specifically tested the effects of: experiment A) Kp-10 alone (from 10−14 to 10−6 m) for 4 h (dose response experiment); experiment B) Kp-10 alone (10−8 m) for 30 min or 4, 12, 24, to 48 h (time course experiment); or experiment C) Kp-10 alone (10−8 m) or in combination with GnRH, GHRH, ghrelin (10−8 m), or SST (10−7 m) for 4 h. These doses were selected according to previous studies (23). In addition, in experiment D, to study the intracellular signaling pathways involved in the actions of Kp on pituitary function, medium containing the inhibitors of key intracellular signaling pathways was added following the 1-h preincubation period (medium alone was used in the vehicle treated controls). Ninety minutes later, the medium was replaced with medium alone (vehicle) or containing the selected inhibitor combined with Kp-10 (10−8 m) and incubated for an additional 4-h period. Finally, in experiment E, to study the effect of sex steroids on Kp-10-mediated hormone release, cells were preincubated with medium containing estradiol (E2; 10 nm) for 36 h, before the day of the experiment in the presence of serum (medium without E2 was used in the vehicle treated controls). After preincubation, medium was removed and cells were preincubated for 1 h in fresh, warm (37 C), serum-free medium (with or without E2) to stabilize basal hormone secretion. Then the medium was replaced with medium containing Kp-10 (10−8 m, with or without E2) and incubated for 4 additional hours. In all experiments, after the corresponding incubation period, medium was collected for hormone analysis (see below). Total RNA was extracted from selected cultures treated with Kp (10−8 m) for expression analysis of pituitary hormone transcripts. Controls consisted of cells cultured in serum-free basic medium. Each treatment was repeated at least three times on different pituitary cell preparations (3–4 wells/treatment per experiment).
Hormone analysis
Culture medium was recovered, centrifuged (2000 × g per 5 min) and stored at −80 C for subsequent analysis of LH, FSH, GH, ACTH, prolactin (PRL), and TSH concentrations using human commercial ELISAS [Diagnostic Systems Laboratories, Webster, TX (for GH, reference: DSL-10-19100) or DRG Instruments, Mountainside, NJ (reference no. EIA-1289, EIA-1787, EIA-1288, EIA-1291, EIA-3647, and EIA-1790 for LH, GH, FSH, PRL, ACTH, and TSH, respectively)] following the manufacturer's instruction. All information regarding specificity, detectability, and reproducibility for each of the assays can be accessed at the web sites of the indicated companies.
RNA isolation, reverse transcription, and real-time PCR
Total RNA from primary pituitary cell cultures and whole tissue was extracted using the Absolutely RNA RT-PCR miniprep kit (Stratagene, La Jolla, CA) with deoxyribonuclease treatment, as previously described (23–25). The amount of RNA recovered was determined by the Ribogreen RNA quantification kit (Molecular Probes, Eugene, OR). Total RNA was reverse transcribed in a 20-μl volume using random hexamer primers and the cDNA first-strand synthesis kit (MRI Fermentas, Hanover, MD). The cDNA obtained was treated with ribonuclease H (1 U; MRI Fermentas), and duplicate aliquots (1 μl) were amplified by real-time RT-PCR (rtRT-PCR) using the Stratagene Brilliant SYBR green QPCR master mix. Details regarding the development, validation, and application of rtRT-PCR to measure expression levels of different baboon transcripts, including cyclophilin A (used as a housekeeping gene), have been recently reported by our laboratory (23–25). New baboon sequences obtained in the present study (LH, FSH, TSH, and Kiss1r) were submitted to GenBank. Primer sets for baboon LH, FSH, GH, ACTH, proopiomelanocortin (POMC), PRL, TSH, and cyclophilin A used in this study, as well as the GenBank accession numbers, are provided in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Statistical analysis
To normalize mRNA values within each treatment and minimize intragroup variations, the values obtained were compared with vehicle-treated controls (set at 100%), and the results are reported as the mean ± sem in all experiments. Each treatment group was tested in a minimum of three separate pituitary cultures, each prepared from a different animal, and within each pituitary cell preparation (experiment), treatments were replicated in at least three to four wells. The entire study was conducted on pituitaries from 11 baboons collected over a 3-yr period. Data were assessed for heterogeneity of variance, and if found, values were log transformed. Differences between treatment groups were assessed by ANOVA (one way or two way ANOVA) with repeated measures, followed by Fisher's test for multiple comparisons. P < 0.05 was considered significant. All statistical analyses were performed using GB-STAT software package (Dynamic Microsystems, Inc., Silver Spring, MD).
Results
Baboon primary pituitary cell cultures as putative model for Kp actions on human pituitary
Pituitaries from female baboons expressed Kiss1r at high levels compared with those found in the central region of the hypothalamus [the putative site of action of Kps (984 ± 197 vs. 530 ± 248 copies per 0.05 μg total RNA, respectively)] (8). Comparison of partial baboon mRNA sequences of all pituitary hormone genes [generated in this and previous studies (23–25)] and of Kiss1r with the corresponding human sequences revealed a close homology (LH, 98%; GH, 98%; FSH, 99%; PRL, 97%; POMC, 97%; TSH, 100%; and Kiss1r 98%), with a higher degree of divergence vs. Kiss1r sequences from nonprimate species (88–90% homology when compared with transcripts of pig, rat, mouse, and sheep).
To confirm that primary pituitary cells of baboons maintain a differentiated phenotype after dispersion and culture, absolute mRNA levels (copy numbers per 0.05 μg total RNA) of LH, GH, FSH, PRL, POMC, TSH, Kiss1r, and cyclophilin A (used as a housekeeping gene) were compared between whole-tissue extracts and extracts prepared from pituitary cultures 4 h after incubation in serum-free medium, and results are shown in Table 1. Transcript levels did not vary significantly between in vivo and in vitro samples, indicating that the cell preparation and culture conditions did not impact the expression of pituitary hormone genes or kiss1r. Together these results support that the culture system used allows for the maintenance of correct functions of the different pituitary cell types (gonadotrophs, somatotrophs, lactotrophs, corticotrophs, and thyrotrophs) and serves as a positive control that accurate quantitative rtRT-PCR measures reflect physiologically relevant differences in gene expression.
Table 1.
Absolute cDNA copy number per 0.05 μg total RNA of gene transcripts in the whole pituitary vs. primary pituitary cell cultures (control groups) of female baboons, as determined by quantitative rtRT-PCR
| Whole pituitary | Primary pituitary cell cultures | |
|---|---|---|
| LH | 18.683 ± 3.429 | 20.345 ± 4.122 |
| GH | 339.800 ± 88.456 | 298.362 ± 42.639 |
| FSH | 58.770 ± 33.607 | 42.792 ± 12.381 |
| PRL | 2.183.973 ± 699.518 | 1.893.283 ± 159.750 |
| POMC | 32.168 ± 11.223 | 35.271 ± 8.391 |
| TSH | 54.985 ± 32.007 | 41.958 ± 7.830 |
| Kiss1r | 984 ± 197 | 1.163 ± 241 |
| Cyclophilin A | 146.731 ± 20.648 | 166.320 ± 12.381 |
Values represent means ± sem [n = 12 separate whole pituitary extracts and n = 5 separate primary pituitary cell cultures (three to five wells per experiment)].
Direct effect of Kps on pituitary hormone release
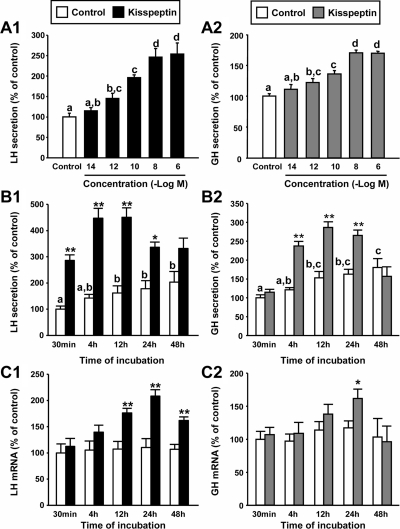
Incubation of cultured baboon pituitary cells with increasing doses of Kp-10 (Kp) for 4 h revealed clear stimulatory effects on LH and GH release in a concentration-dependent manner (at doses equal to or above 10−12 m; Fig. 1, A1 and B2, respectively). The lowest dose of Kp that caused a maximal increase of both LH and GH secretion was 10−8 m (247 ± 21 and 170 ± 4% compared with controls set at 100%, respectively). Accordingly, a stimulatory concentration of 10−8 m Kp was chosen to further analyze the action of the peptide on baboon LH and GH release.
Fig. 1.
Direct actions of Kp-10 (10 nm) on baboon LH and GH synthesis and secretion. A, Effect of 4 h treatment with Kp on LH (A1) and GH (A2) release. B, Time-dependent effect of Kp on LH (B1) and GH (B2) release. C, Time-dependent effect of Kp on LH (C1) and GH (C2) mRNA levels. Data are expressed as percent of control (set at 100%) at 4 h (A) or 30 min (B and C). Values represent the mean ± sem (n = 4 individual experiments, three to four wells/experiment). Values that do not share a common letter (a, b, c, d) are statistically different. Asterisks indicate values that significantly differ from their respective control values (same incubation time period). *, P < 0.05; **, P < 0.01.
Treatment with Kp for different incubation times (from 30 min to 48 h) revealed a stimulatory effect on LH release between 30 min and 24 h (Fig. 1, B1), whereas the stimulatory effect of Kp on GH release was observed between 4 and 24 h of incubation (Fig. 1, B2). In both cases, hormone release was no longer significantly increased after 48 h of treatment. Interestingly, Kp was able to increase LH mRNA levels between 12 and 48 h after the exposure, whereas it was able to significantly stimulate GH expression only after 24 h of incubation (Fig. 1, C1 and C2).
The direct actions of Kp on primate pituitary function were restricted to stimulation of LH and GH release because this same experimental regime failed to significantly alter spontaneous FSH, PRL, ACTH, and TSH release or their basal gene expression levels at any of the tested doses and time points (Supplemental Fig. 1). The absolute impact of Kp on LH or GH secretion at a maximal dose (10 nm; 4 h incubation) ranged between 2.2- and 4.9-fold for LH secretion and between 1.6- and 3.2-fold for GH release when compared with vehicle-treated controls, depending on the individual pituitary preparation (Supplemental Table 2). The magnitude of response did not correlate with the age of the tissue donor but may be associated with the stage of the estrus cycle or the metabolic environment, in which this information was not available to us.
Interaction of Kp with major regulators of LH (GnRH) and GH (GHRH, ghrelin, and SST) release
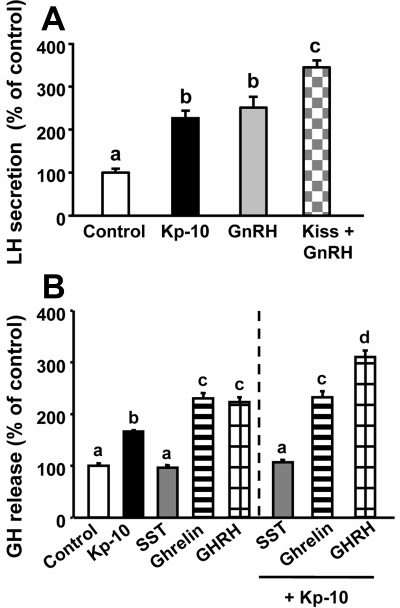
Comparison of the effects of equimolar doses of Kp and GnRH revealed that both peptides induced similar increases of LH release, whereas Kp and GnRH coadministration elicited an additive increase in LH release, compared with the effects of each peptide alone (Fig. 2A). In contrast, Kp was less potent than GHRH and ghrelin in inducing GH release in vitro and only caused an additive stimulation on GH release in combination with GHRH but not with ghrelin (Fig. 2B). As expected, SST fully inhibited the stimulatory action of Kp on GH secretion (Fig. 2B).
Fig. 2.
Interaction of Kp-10 (10 nm) with regulators of gonadotrope and somatotrope function in primary pituitary cell cultures from baboons. A, Effect of 4 h treatment of Kp and/or GnRH (10 nm) on LH secretion. B, Effect of 4 h treatment of Kp and/or GHRH, ghrelin (10 nm), or SST (100 nm) on GH secretion. Values are expressed as percentage of controls, set at 100% within each experiment, and represent the mean ± sem of four independent experiments (three to four wells/experiment). Values that do not share a common letter (a, b, c, d) differ significantly (P < 0.05).
Intracellular signaling pathways involved in Kp-induced LH and GH release
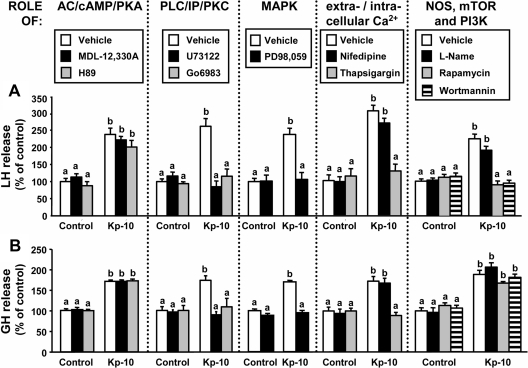
Treatment of pituitary cell cultures with specific inhibitors of phospholipase C (PLC), protein kinase C, MAPK, and intracellular Ca2+ mobilization but not with blockers of adenylyl cyclase (AC), protein kinase A, extracellular Ca2+ influx (through L-type channels), or nitric oxide synthase (NOS) completely suppressed the stimulatory effects caused by Kp on baboon LH and GH release (Fig. 3). Interestingly, blockade of mammalian target of rapamycin (mTOR) or phosphoinositol 3-kinase (PI3K) activity completely abolished the stimulatory effect of Kp on LH secretion (Fig. 3A) but not that of GH release (Fig. 3B). Administration of these inhibitors alone did not modify basal LH or GH secretion (Fig. 3).
Fig. 3.
Intracellular signaling pathways of Kp-10-stimulated baboon LH and GH release. Effect of the inhibition of AC (MDL-12,330A; 10 μm), protein kinase A (H89; 15 μm), PLC (U73122; 50 μm), protein kinase C (Go6983; 20 μm), MAPK (PD-98,059; 10 μm), extracellular Ca2+ L-type channels (nifedipine; 1 μm), intracellular Ca2+ channels (thapsigargin; 10 μm), NOS (L-arginine methyl ester hydrochloride; 10 μm), mTOR (rapamycin; 10 nm), and PI3K (wortmannin; 1 μm) on kisspeptin-stimulated LH (A) and GH (B) release. On the day of the experiment, inhibitors were added to the incubation media 90 min before Kp treatment (4 h; 10 nm). Values are expressed as percentage of vehicle-treated controls without inhibitor (set at 100%) within each experiment, and represent the mean ± sem of three to five independent experiments (three to four wells/treatment per experiment). Values that do not share a common letter (a or b) significantly differ (P < 0.05).
To compare Kp- and GnRH-activated signaling pathways, a similar pharmacological approach was used after GnRH stimulation of pituitary cell cultures, which is also analogous to that used in our previous reports documenting GHRH- and ghrelin-induced intracellular signals in pituitaries of baboons (23). However, owing to the limited availability of cell preparations, we were able to study only some selected signaling routes. In contrast to that observed for Kp, our data suggest that the extracellular Ca2+ influx (through L-type channels) and NOS, but not AC, are essential for the actions of GnRH on LH release from baboon gonadotrophs (Supplemental Fig. 2).
Effect of E2 on Kp-mediated LH and GH release
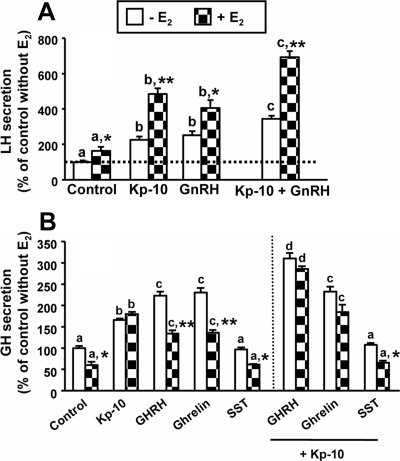
Sex steroids are key modulators of gonadotroph and somatotroph function. Thus, the influence of E2 on pituitary responsiveness to Kp in terms of LH and GH secretion was tested based on the previous experiments. Preincubation (36 h) with 10 nm E2 increased spontaneous LH release by 63% (Fig. 4A) and seemingly enhanced further the relative LH-releasing action of Kp administered alone or in combination with GnRH (Fig. 4A). In contrast, preincubation with E2 decreased basal GH release from cultured somatotrophs by 40% (Fig. 4B). Interestingly, although absolute stimulatory effects of GHRH and ghrelin on GH release in the presence of E2 were concomitantly decreased, GH responses to Kp, either alone or in combination with GHRH (but not with ghrelin), were preserved in the presence of E2, despite the marked decrease in basal GH release, thus resulting in an augmented capacity of somatotrophs to maintain its relative GH output response to Kp (Fig. 4B).
Fig. 4.
Interaction of Kp-10 (10 nm) with regulators of gonadotrope and somatotrope function in the absence or presence of estradiol (−E2 or +E2, respectively; 10 nm) in primary pituitary cell cultures from baboons. A, Effect of 4 h treatment of Kp and/or GnRH (10 nm) on LH secretion. B, Effect of 4 h treatment of Kp and/or GHRH, ghrelin (10 nm), or SST (100 nm) on GH secretion. Data are expressed as percentage of controls without E2 (set at 100%), and represent the mean ± sem of three to four independent experiments (three to four wells per experiment). Values that do not share a common letter (a, b, c, d) are statistically different. Asterisks indicate values that significantly differ from their respective control values (same treatment in the absence of estradiol). *, P < 0.05; **, P < 0.01.
In addition, the influence of E2 on the pattern of response to Kp of the remaining pituitary hormones was also evaluated (Supplemental Fig. 3). Similar to that observed for LH secretion, preincubation with E2 increased baseline FSH levels and uncovered an FSH-releasing effect of Kp. On the other hand, E2 did not alter basal PRL release; however, it sensitized lactotropes to positively respond to Kp. Finally, E2 failed to alter ACTH or TSH release, either in basal conditions or after Kp stimulation.
Discussion
The unexpected emergence in 2003 of Kps as novel, critical regulatory peptides for the reproductive axis imparted a renewed interest to the investigation of the neuroendocrine mechanisms underlying the control of gonadotropin secretion. Such an interest was boosted, among other ground-breaking findings, by the demonstration of the essential roles and extraordinarily potent gonadotropin-releasing effects of Kps in a wide variety of species, including rodents, sheep, monkeys, and humans. Compelling evidence accumulated during the last years offers no hesitation as to the need of hypothalamic Kps and Kiss1r to attain, and maintain, proper gonadotropin secretion and reproductive capacity. Yet the paramount importance of their hypothalamic actions might have obscured more subtle, albeit discernable, actions of Kps at other levels of the gonadotropic axis. Thus, despite early reports showing high levels of expression of Kiss1r in the pituitary, the number of functional studies directed at understanding the role and mechanism of action of Kps on pituitary hormone secretion are limited. Therefore, our present study provides comprehensive experimental evidence to support a plausible role of Kps in the direct pituitary control of gonadotroph (and somatotroph) function in the baboon, a primate model of likely interest for human physiology and translational medicine (23, 25–28).
It is well known that a number of central and peripheral factors can act directly on the pituitary to regulate the release of hypophyseal hormones either independently of, or cooperatively with, their putative hypothalamic drivers, e.g. ghrelin also stimulates GH from primate somatotrophs in a GHRH-independent manner (23). In line with this notion, mRNA expression and/or presence of proteins for both Kiss1r and Kiss1 have been described in the pituitary of humans, rodents, bovine, and ovine (10, 15–17, 29), and Kp has been shown to induce LH release in pituitary cultures of several species (9, 10, 21). Despite this latter evidence, the actual biological significance of this system in the pituitary still remains controversial because contradictory results have also been reported (7, 18, 19).
In the present work, we demonstrate that Kp-10 is able to directly stimulate specific populations of baboon pituitary cells in vitro. First, and most notably, Kp-10 significantly stimulated LH synthesis and release in a dose-dependent manner in cultured gonadotrophs. Moreover, this stimulation was additive to the GnRH action, suggesting the possibility that the pathways mediating LH release in response to GnRH and Kp at the gonadotroph level may be partially independent. Consistent with this, Kiss1r expression has been described in gonadotophs (12, 17) and immunocytochemical studies in rat pituitary have shown colocalization of LHβ and kiss1r, which would enable and support a direct action of Kp on gonadotrophs (17) because it is also substantiated by the ability of Kp-10 to evoke direct calcium responses from individual rat gonadotrophs in culture (10). Furthermore, our present data also demonstrate that the stimulation of LH elicited by Kp-10 upon Kiss1r activation at the pituitary is mediated by mTOR, PI3K, MAPK, PLC, and intracellular Ca2+ mobilization, a complex set of second-messenger pathways, which remarkably parallels that found previously to mediate the actions of kiss1r on GnRH neurons (11, 30, 31).
Interestingly, it should be noted that under the experimental conditions used in this study, Kp-10 failed to alter FSH release. Although LH and FSH are known to be costored in a subpopulation of bihormonal gonadotrophs and are often coregulated and released in response to diverse stimuli (32), it has also been shown in a number of studies that FSH release can follow a markedly different pattern from that of LH. For example, the frequency of GnRH can differentially affect LH and FSH release (33). Therefore, it is possible that the pattern of delivery of Kp to gonadotropes in vitro may also differentially regulate LH and FSH release. It should also be noted that preincubation of E2 unmasked a stimulatory effect of Kp on FSH release; therefore, it is possible that the steroidal milieu may differently influence LH and FSH response to Kps in vitro under the culture conditions used herein.
In addition to its effects on LH secretion, Kp-10 also induced GH synthesis and release, at doses equivalent to those effective for LH secretion. This stimulation, however, was of lower magnitude than that evoked by the primary GH secretagogues, GHRH and ghrelin. Noteworthy, Kp-dependent GH release is additive to GHRH stimulation but not with ghrelin action. Specifically, the coadministration of Kp-10 and GHRH leads to an additive effect, analogous to that previously observed after coadministration of ghrelin and GHRH (23). Interestingly, the set of second-messenger pathways required by Kp-10 to stimulate GH release seems to be more limited than that required for LH release, involving PLC and MAPK activation and intracellular Ca2+ mobilization. This divergence in the kiss1r-mediated signaling suggests that Kp-10 directly activates a distinct population of pituitary cells to induce GH release, presumably somatotrophs, which have been proposed to express kiss1r as well (12, 17, 23).
An important question arising from our data is to what extent the present observations represent a physiologically relevant phenomenon in a species, such as the baboon, of particular interest as a model to human biology (23, 25–28). Our present observations extend and refine previous findings describing the ability of Kp to directly elicit LH secretion at the pituitary in other species; this might be a contributing, fine-tuning mechanism for the well-characterized ability of Kps to potently stimulate gonadotropin release in primates. On the other hand, our results are suggestive of putative regulatory actions of Kps on other pituitary axes, such as the somatotrophic system. Admittedly, although the capacity of Kp to potently activate gonadotropin secretion (mainly through central mechanisms) is indisputable, its action on GH release remains controversial. Thus, although a number of in vitro studies have documented a significant stimulation of GH (10, 21), several in vivo studies, performed recently in cattle, pigs, and other primates (Macaca mulatta), did not observe this effect (7, 34, 35). In contrast, work by Kadokawa et al. (20) documented a stimulatory action of Kp (iv) on GH release in Holstein heifers, supporting the somatotrope action of Kp described by the in vitro assays. Of note, mice lacking a functional Kiss1r gene (Kiss1r knockout) show a significant reduction in body weight in adulthood (36), yet whether this effect is due to impairments in GH release during development and/or to the absence of the anabolic effect of sex steroids or other factors remains to be elucidated.
A possible explanation for the differential results between in vitro and in vivo studies might be related to the lower sensitivity of pituitary cells to Kp (picomolar range), compared with GnRH neurons in which Kp has been proven to be effective in the femtomolar range (11). Therefore, it is not unreasonable to think that peripherally injected Kp-10, which is considerably short lived, might not achieve concentrations high enough to stimulate somatotrope function. Of note, the main source of Kps acting on the pituitary could be local because Kiss1 and Kp have been described in gonadotrophs, somatotrophs, and lactotrophs (12). However, it should be noted that Kps are detectible in hypophysial portal blood in ewes (12), and kisspeptin-54 release has been reported to occur in vivo in the stalk-median eminence of female rhesus monkeys at the time of the pubertal increase in GnRH release (37). Taken together these data suggest that centrally derived Kps may reach their putative pituitary cell targets at sufficient quantities to exert their direct actions, an issue that certainly deserves investigation.
In addition, our results demonstrate that sex steroids modulated the impact of kiss1 on the pituitary. Specifically, E2 enhanced the stimulatory action of Kp-10 upon gonadotropin and prolactin release (the latter exclusively dependent on the presence of E2). Regulation of Kiss1 expression by E2 in the pituitary has been previously documented in rodent and bovine (9, 17) and coincides with the regulation of Kiss1 expression at the AVPV nucleus of female rodents (4). Importantly, gonadotroph-specific estrogen receptor-α knockout mice are infertile due to impairments in LH and FSH release (38), pointing to a crucial role for estradiol signaling at the gonadotrophs in the control of reproductive function, which might putatively involve its capacity to enhance Kp-induced gonadotropin secretion directly at the pituitary level. In line with this, E2 has been reported to enable kisspeptin-mediated increases in γ-aminobutyric acid and glutamate transmission to stimulate GnRH neurons (39) and to enhance GnRH/gonadotropin responsiveness to Kp-10 in rodents (40, 41). Moreover, results generated by other laboratories support a facilitative role of E2 acting on the pituitary to increase LH release in response to GnRH (42, 43), which is comparably observed in the present study. The molecular mechanism underlying this enhanced GnRH action seems to involve an increased E2-induced cAMP level (43).
In the current study, data are not available as to whether cAMP levels and/or, for instance, increased Kiss1r expression could mediate the E2-induced Kp-10 enhanced stimulatory action. Although previous studies did not show any variation in Kiss1r expression in relation to sex steroid milieu at the hypothalamic level in rats (44), recent work from our group suggest that E2 may enhance the ability of kp-10 to augment Kiss1r expression from cultured pituitary cells (our unpublished data). Nevertheless, further studies will be required to clarify this issue. In any case, our current data further extend those original observations, suggesting that such facilitative action of E2 on the Kp-mediated control of gonadotropin secretion might include a pituitary site of action as well. In fact, E2 also influences somatotroph responsiveness to Kp, although in a manner distinct from that observed for gonadotropes. Specifically the presence of E2 maintained Kp-induced GH output but markedly reduced basal GH release and blunted the GH response to the primary secretagogues, GHRH and ghrelin. The mechanisms behind these actions, as well as its physiological relevance, are yet to be elucidated, but the action on different pituitary cell populations and the apparent recruitment of distinct subsets of intracellular signaling systems might help to explain such divergence in E2 effects.
In summary, we report for the first time a detailed description of the stimulatory effect of Kps at the pituitary level in the female baboon. Our results add further, convincing evidence to reinforce previous observations obtained in nonprimate species and strongly suggest that, besides its major central actions, direct effects of Kps at the pituitary may represent an additional level of regulation in the control of hypophyseal hormone (prominently, LH) release. In addition, our study offers new insights on the molecular mechanisms whereby Kps elicit LH (and GH) secretion directly at the pituitary level in a species of special interest for human physiology and translational medicine.
Acknowledgments
We thank the veterinarian staff of the University of Illinois at Chicago, Biological Resource Center, for their invaluable help, and especially to Dr. Lisa Halliday.
This work was supported by Grants RYC-2007-00186, JC2008-00220, BFU2008-01136/BFI (to R.M.L.), FI06/00804 (to J.C.-C.), and FPU-AP20052473 (to M.D.G.); 7th European Union Marie Curie Fellowship Program (to V.M.N.); BFU 2008-00984/BFI (to M.T.-S.); National Institute of Diabetes and Digestive and Kidney Diseases Grant 30677; Veterans Affairs Merit Award (to R.D.K.) and Grants BFU2007-60180/BFI, BFU2010-19300, BIO-139, CTS-1705, and CTS-5051; and grants from IPSEN Pharmaceuticals, Milford, MA (to J.P.C.). Centro de Investigación Biomédica en Red is an initiative of the Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, Spain.
Disclosure Summary: The authors declare that they have no conflict of interest.
Footnotes
- AC
- Adenylyl cyclase
- AVPV
- anteroventral periventricular
- E2
- estradiol
- GPR54
- G protein-coupled receptor
- Kp
- kisspeptin
- mTOR
- mammalian target of rapamycin
- NOS
- nitric oxide synthase
- PI3K
- phosphoinositol 3-kinase
- PLC
- phospholipase C
- POMC
- proopiomelanocortin
- PRL
- prolactin
- rtRT-PCR
- real-time RT-PCR
- SST
- somatostatin.
References
- 1. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 3. Oakley AE, Clifton DK, Steiner RA. 2009. Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 5. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 6. Clarkson J, Herbison AE. 2006. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramaswamy S, Gibbs RB, Plant TM. 2009. Studies of the localisation of kisspeptin within the pituitary of the rhesus monkey (Macaca mulatta) and the effect of kisspeptin on the release of non-gonadotropic pituitary hormones. J Neuroendocrinol 21:795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2008. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol 29:48–69 [DOI] [PubMed] [Google Scholar]
- 9. Ezzat AA, Saito H, Sawada T, Yaegashi T, Goto Y, Nakajima Y, Jin J, Yamashita T, Sawai K, Hashizume T. 2010. The role of sexual steroid hormones in the direct stimulation by Kisspeptin-10 of the secretion of luteinizing hormone, follicle-stimulating hormone and prolactin from bovine anterior pituitary cells. Anim Reprod Sci 121:267–272 [DOI] [PubMed] [Google Scholar]
- 10. Gutiérrez-Pascual E, Martínez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagón MM, Castaño JP. 2007. Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol 19:521–530 [DOI] [PubMed] [Google Scholar]
- 11. Navarro VM, Castellano JM, Fernández-Fernández R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2005. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 146:156–163 [DOI] [PubMed] [Google Scholar]
- 12. Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. 2008. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology 149:1951–1959 [DOI] [PubMed] [Google Scholar]
- 13. Suzuki S, Kadokawa H, Hashizume T. 2008. Direct kisspeptin-10 stimulation on luteinizing hormone secretion from bovine and porcine anterior pituitary cells. Anim Reprod Sci 103:360–365 [DOI] [PubMed] [Google Scholar]
- 14. Bellingham M, Fowler PA, Amezaga MR, Rhind SM, Cotinot C, Mandon-Pepin B, Sharpe RM, Evans NP. 2009. Exposure to a complex cocktail of environmental endocrine-disrupting compounds disturbs the kisspeptin/GPR54 system in ovine hypothalamus and pituitary gland. Environ Health Perspect 117:1556–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. 2001. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 276:28969–28975 [DOI] [PubMed] [Google Scholar]
- 16. Quennell JH, Rizwan MZ, Relf HL, Anderson GM. 2010. Developmental and steroidogenic effects on the gene expression of RFamide related peptides and their receptor in the rat brain and pituitary gland. J Neuroendocrinol 22:309–316 [DOI] [PubMed] [Google Scholar]
- 17. Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. 2008. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol 20:381–393 [DOI] [PubMed] [Google Scholar]
- 18. Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. 2004. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun 320:383–388 [DOI] [PubMed] [Google Scholar]
- 19. Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. 2004. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 16:850–858 [DOI] [PubMed] [Google Scholar]
- 20. Kadokawa H, Matsui M, Hayashi K, Matsunaga N, Kawashima C, Shimizu T, Kida K, Miyamoto A. 2008. Peripheral administration of kisspeptin-10 increases plasma concentrations of GH as well as LH in prepubertal Holstein heifers. J Endocrinol 196:331–334 [DOI] [PubMed] [Google Scholar]
- 21. Kadokawa H, Suzuki S, Hashizume T. 2008. Kisspeptin-10 stimulates the secretion of growth hormone and prolactin directly from cultured bovine anterior pituitary cells. Anim Reprod Sci 105:404–408 [DOI] [PubMed] [Google Scholar]
- 22. Castaño JP, Martínez-Fuentes AJ, Gutiérrez-Pascual E, Vaudry H, Tena-Sempere M, Malagón MM. 2009. Intracellular signaling pathways activated by kisspeptins through GPR54: do multiple signals underlie function diversity? Peptides 30:10–15 [DOI] [PubMed] [Google Scholar]
- 23. Kineman RD, Luque RM. 2007. Evidence that ghrelin is as potent as growth hormone (GH)-releasing hormone (GHRH) in releasing GH from primary pituitary cell cultures of a nonhuman primate (Papio anubis), acting through intracellular signaling pathways distinct from GHRH. Endocrinology 148:4440–4449 [DOI] [PubMed] [Google Scholar]
- 24. Luque RM, Gahete MD, Hochgeschwender U, Kineman RD. 2006. Evidence that endogenous SST inhibits ACTH and ghrelin expression by independent pathways. Am J Physiol Endocrinol Metab 291:E395–E403 [DOI] [PubMed] [Google Scholar]
- 25. Luque RM, Gahete MD, Valentine RJ, Kineman RD. 2006. Examination of the direct effects of metabolic factors on somatotrope function in a non-human primate model, Papio anubis. J Mol Endocrinol 37:25–38 [DOI] [PubMed] [Google Scholar]
- 26. Braundmeier AG, Fazleabas AT. 2009. The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod 15:577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Comuzzie AG, Cole SA, Martin L, Carey KD, Mahaney MC, Blangero J, VandeBerg JL. 2003. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes Res 11:75–80 [DOI] [PubMed] [Google Scholar]
- 28. McClure HM. 1984. Nonhuman primate models for human disease. Adv Vet Sci Comp Med 28:267–304 [DOI] [PubMed] [Google Scholar]
- 29. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. 2001. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276:34631–34636 [DOI] [PubMed] [Google Scholar]
- 30. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roa J, Garcia-Galiano D, Varela L, Sánchez-Garrido MA, Pineda R, Castellano JM, Ruiz-Pino F, Romero M, Aguilar E, López M, Gaytan F, Diéguez C, Pinilla L, Tena-Sempere M. 2009. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology 150:5016–5026 [DOI] [PubMed] [Google Scholar]
- 32. Evans JJ. 1999. Modulation of gonadotropin levels by peptides acting at the anterior pituitary gland. Endocr Rev 20:46–67 [DOI] [PubMed] [Google Scholar]
- 33. Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. 1981. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385 [DOI] [PubMed] [Google Scholar]
- 34. Ezzat Ahmed A, Saito H, Sawada T, Yaegashi T, Yamashita T, Hirata T, Sawai K, Hashizume T. 2009. Characteristics of the stimulatory effect of kisspeptin-10 on the secretion of luteinizing hormone, follicle-stimulating hormone and growth hormone in prepubertal male and female cattle. J Reprod Dev 55:650–654 [DOI] [PubMed] [Google Scholar]
- 35. Lents CA, Heidorn NL, Barb CR, Ford JJ. 2008. Central and peripheral administration of kisspeptin activates gonadotropin but not somatotropin secretion in prepubertal gilts. Reproduction 135:879–887 [DOI] [PubMed] [Google Scholar]
- 36. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. 2007. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 37. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P, Ko C. 2008. Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females. Endocrinology 149:20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pielecka-Fortuna J, Chu Z, Moenter SM. 2008. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roa J, Vigo E, Castellano JM, Gaytan F, García-Galiano D, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M. 2008. Follicle-stimulating hormone responses to kisspeptin in the female rat at the preovulatory period: modulation by estrogen and progesterone receptors. Endocrinology 149:5783–5790 [DOI] [PubMed] [Google Scholar]
- 41. Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M. 2008. Opposite roles of estrogen receptor (ER)-α and ERβ in the modulation of luteinizing hormone responses to kisspeptin in the female rat: implications for the generation of the preovulatory surge. Endocrinology 149:1627–1637 [DOI] [PubMed] [Google Scholar]
- 42. Shibasaki HI, Silva de Sá MF. 1986. Effect of estradiol on the pituitary response to intravenous stimulation with luteinizing hormone-releasing hormone in menopausal women. Fertil Steril 46:385–391 [DOI] [PubMed] [Google Scholar]
- 43. Tang LK, Martellock AC, Horiuchi JK. 1982. Estradiol stimulation of LH response to LHRH and LHRH binding in pituitary cultures. Am J Physiol 242:E392–E397 [DOI] [PubMed] [Google Scholar]
- 44. Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2004. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]