Prader-Willi syndrome causes endocrine disruption, including growth hormone deficiency and abnormal stress responses; similar endocrine dysfunction in mice missing the PWS gene Magel2 is also shown.
Abstract
Hypothalamic dysfunction may underlie endocrine abnormalities in Prader-Willi syndrome (PWS), a genetic disorder that features GH deficiency, obesity, and infertility. One of the genes typically inactivated in PWS, MAGEL2, is highly expressed in the hypothalamus. Mice deficient for Magel2 are obese with increased fat mass and decreased lean mass and have blunted circadian rhythm. Here, we demonstrate that Magel2-null mice have abnormalities of hypothalamic endocrine axes that recapitulate phenotypes in PWS. Magel2-null mice had elevated basal corticosterone levels, and although male Magel2-null mice had an intact corticosterone response to restraint and to insulin-induced hypoglycemia, female Magel2-null mice failed to respond to hypoglycemia with increased corticosterone. After insulin-induced hypoglycemia, Magel2-null mice of both sexes became more profoundly hypoglycemic, and female mice were slower to recover euglycemia, suggesting an impaired hypothalamic counterregulatory response. GH insufficiency can produce abnormal body composition, such as that seen in PWS and in Magel2-null mice. Male Magel2-null mice had Igf-I levels similar to control littermates. Female Magel2-null mice had low Igf-I levels and reduced GH release in response to stimulation with ghrelin. Female Magel2-null mice did respond to GHRH, suggesting that their GH deficiency has a hypothalamic rather than pituitary origin. Female Magel2-null mice also had higher serum adiponectin than expected, considering their increased fat mass, and thyroid (T4) levels were low. Together, these findings strongly suggest that loss of MAGEL2 contributes to endocrine dysfunction of hypothalamic origin in individuals with PWS.
Prader-Willi syndrome (PWS) is a complex genetic disorder, whose clinical features suggest abnormalities of the hypothalamic-pituitary axis. These include GH deficiency with short stature, increased fat mass and reduced lean mass, hypogonadotropic hypogonadism, childhood-onset hyperphagia and obesity, and sleep disorders. Structural abnormalities of the brain documented in PWS include frequent ventriculomegaly and cortical abnormalities. Pituitary abnormalities are common, but structural abnormalities of the hypothalamus are not typically found (1–3). Children with PWS typically have decreased spontaneous GH secretion and low peak GH response in stimulation tests (4, 5), accompanied by reduced serum IGF-I and low IGF-binding protein 3 (6–8). GH deficiency contributes to abnormal body composition consisting of increased body fat mass and reduced lean mass, and treatment with GH partially normalizes body composition and short stature (9–11). In contrast, obesity in the general population is often associated with a relative GH deficiency but normal or high levels of IGF-I, suggesting defective feedback mechanisms in GH pathways that can resolve on weight loss.
In normal adults, serum leptin levels increase with increasing adiposity, but levels of adiponectin, particularly in its multimeric high molecular weight form, are inversely correlated with fat mass (12, 13). Lower adiponectin levels are typically found in type 2 diabetics and correlate with insulin resistance in obese individuals (14). Adiponectin acts primarily through receptors present in liver and in muscle (13), but also acts in the paraventricular nucleus of the hypothalamus to stimulate food intake and decrease energy expenditure during fasting (15, 16). Obese individuals with PWS have high serum leptin levels, but despite their increased adiposity, plasma adiponectin levels are higher in PWS than in obese controls, although lower than or similar to levels in lean individuals (17–21). Consistent with higher adiponectin levels, obese adults with PWS are more insulin sensitive than matched obese controls (22–24).
Central adrenal insufficiency has been proposed to contribute to sudden deaths in children with PWS (25–28). One recent study used an overnight single-dose metyrapone test to inhibit cortisol production, stimulating ACTH production. About 60% of tested PWS individuals showed an insufficient ACTH response to decreased cortisol production (27). However, other studies found no evidence for adrenal insufficiency (29) or normal increases in plasma cortisol in response to synthetic ACTH (30). The frequency of central hypothyroidism in the PWS population is reported to be up to 24%, although various studies report thyroid hormone levels that vary from low to normal (8, 31–33).
Because PWS is a multigene disorder, studies of gene-targeted knockout mice have been used to elucidate the relative contribution of loss of individual genes to the complex clinical findings in PWS. MAGEL2 is one of several imprinted genes that are typically inactivated in people with PWS (34, 35). In the mouse, Magel2 is most highly expressed in the hypothalamus, with maximal levels in the suprachiasmatic nucleus, which controls circadian rhythm, and in the arcuate nucleus, which controls energy homeostasis (34). The encoded protein melanoma antigen, subfamily L (MAGEL)2/Magel2 is a member of the MAGE family of proteins, which act as adaptor proteins for complexes that participate in signal transduction (36, 37). We previously described decreased activity, progressive infertility, and blunted circadian rhythm in mice lacking Magel2 (38, 39). We also found that Magel2-null mice are obese, with increased body fat, increased serum leptin, and decreased lean mass but normal length (40). We have now tested the hypothesis that Magel2 is important for endocrine axes regulated by the hypothalamus, by measuring differences in endocrine responses between Magel2-null and wild-type control mice. We now report endocrine dysfunction in Magel2-null mice, including profound insulin-induced hypoglycemia and a marked delay in the counterregulatory hypoglycemia response. We also discovered abnormalities of hypothalamic pituitary endocrine axes in Magel2-null mice, including elevated basal corticosterone levels in both sexes and reduced ghrelin-stimulated GH release with reduced Igf-I levels in female mice. Our work suggests that Magel2 is essential for the normal feedback mechanisms that regulate endocrine axes controlled by the hypothalamus.
Materials and Methods
Mouse breeding, genotyping, and housing
All animal studies were conducted in accordance with the Canadian Council on Animal Care Guidelines and Policies with approval from the Animal Policy and Welfare Committees for the University of Alberta. The Magel2-null mice have been maintained on a C57Bl/6 background for at least 15 generations and were genotyped as described (40). Mice carrying a paternally inherited lacZ-knockin allele are functionally null for Magel2 and are referred to as Magel2-null; littermates that are wild type for Magel2 were used as controls. Mice were weaned between 3 and 4 wk of age and then housed two to three per cage with food and water ad libitum, maintained under 12-h light, 12-h dark cycle conditions, and tested between 3 and 10 months of age. For tail vein blood collection, the time between blood sample collections was at least 6 d, with up to 100 μl (average 50 μl) blood collected each time.
Stress-induction protocol
For corticosterone levels, morning basal samples were collected between 0900 and 1100 h. Tail blood was collected from partially restrained mice. Restraint stress-induced samples were collected from mice restrained in 50-ml conical tubes for 30 min. Insulin-induced samples were collected from partially restrained mice, 30 min after insulin injection. Samples from unstressed (partially restrained, basal), restrained, and insulin-treated mice were collected at least 1 wk apart. All stress-induced experiments were performed between 0800 and 1200 h, which is the low point of corticosterone levels in rodents (41). For the dexamethasone suppression test, mice were injected ip with 0.15 mg/kg dexamethasone in 100 μl saline. Tail blood was collected 4 h after injection. For the ACTH stimulation test, mice were injected intraperitoneally with 25 mg/kg metyrapone, then corticosterone levels were measured after 1 h and ACTH levels after 6 h.
Intraperitoneal insulin tolerance test (IPITT) and ip glucose tolerance test (IPGTT)
For the IPITT, mice were fasted for 2 h and then human insulin (0.75 U/kg, in 100 μl saline; Sigma-Aldrich, St. Louis, MO) was injected ip. For the IPGTT, mice were fasted for 6 h and then injected ip with 1 mg/g glucose in 0.9% saline. For both tests, blood glucose was measured by the glucose oxidase method (Contour Glucometer; Bayer, Toronto, Canada) after 0, 15, 30, 60, and 120 min, in a small drop of blood from the tail vein.
GH assays
Mice were injected ip with saline (control), 60 μg/kg GHRH (Sigma-Aldrich), or 120 μg/kg rat ghrelin (Tocris Bioscience, Ellisville, MO) in a final volume of 100 μl saline. Injections occurred between 1300 and 1700 h, and tail blood was collected between 5 and 15 min after injection.
Measurements of hormones in blood samples
Collected blood was allowed to clot at room temperature for 30 min and then centrifuged at 2000 × g for 10 min. Serum was aliquoted and stored at −20 C until it was assayed for hormone content. Serum samples were analyzed using the following ELISAs: high sensitivity corticosterone (IDS, Inc., Fountain Hills, AZ), ACTH (Calbiotech, Spring Valley, CA), Igf-I (Antigenix America, Huntington Station, NY), GH (Millipore, Billerica, MA), T4 (Calbiotech), and high molecular weight/total adiponectin (Alpco Diagnostics, Salem, NH) according to manufacturers' instructions. Intra- and interassay coefficients of variance were provided by the manufacturers (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Reagents supplied in each ELISA kit were used to generate standard curves, and concentrations of hormones in mouse serum samples were interpolated from these curves.
Statistical analysis
Results are expressed as the mean ± sem unless otherwise stated. Mice of each sex were analyzed separately. Basal values for endocrine factors (corticosterone, ACTH, glucose, adiponectin, Igf-I, and T4) were compared between genotypes using a two-sided Student's t test. GH levels (basal, ghrelin stimulated, and GHRH stimulated) were not normally distributed so were compared between genotypes using a nonparametric Mann-Whitney test. Corticosterone levels measured after either stress treatment (restraint or insulin) were compared with basal levels of corticosterone using a two-sided Student's t test, for each genotype and each sex. Corticosterone levels measured after dexamethasone injection were not normally distributed so were compared with levels after saline injection using a nonparametric Mann-Whitney test, for each genotype and each sex. Corticosterone levels measured after metyrapone injection were compared with levels after saline injection with a two-sided Student's t test, for each genotype and each sex. Differences between genotypes in the IPITT were calculated using repeated measures ANOVA with Bonferroni post hoc tests within GraphPad Prism 4 software. The glucose recovery rate in the IPITT was calculated as the difference between glucose values at 60 and 120 min after insulin injection, divided by 60, and described in millimols per minute. Area under the curve (AUC) in the IPGTT was calculated using the trapezoidal rule (42) and compared between genotypes using a two-sided unpaired Student's t test. In all cases, P < 0.05 was considered statistically significant.
Results
Basal and stress-induced corticosterone levels in Magel2-null and control mice
We first noted that corticosterone levels measured in tail blood samples from partially restrained mice were significantly higher in Magel2-null mice compared with sex-matched control littermates (Fig. 1, A and B, and Table 1). Moreover, values in control mice were higher than expected from other studies, where typically corticosterone levels are measured in blood obtained after rapid decapitation (43). We tested whether the partial restraint required for tail blood sampling was sufficient to induce a more pronounced stress response in Magel2-null than in control mice, thus giving the appearance of high basal corticosterone levels. We collected trunk blood samples after rapid decapitation with minimal restraint. In the control mice, the corticosterone levels in trunk blood were lower than those in tail blood samples (Supplemental Fig. 1). In contrast, corticosterone levels from either trunk or tail blood samples were comparable in the Magel2-null mice. Corticosterone levels measured in tail blood after control saline injection were similar to the partially restrained, uninjected levels found in each genotype, i.e. about 2-fold higher in Magel2-null mice compared with sex-matched control mice. The corticosterone levels that we measured in tail blood samples from partially restrained mice were therefore used as “basal” values for all subsequent comparisons, so as to measure only the effect of the treatment rather than the combined effect of tail blood collection and the treatment.
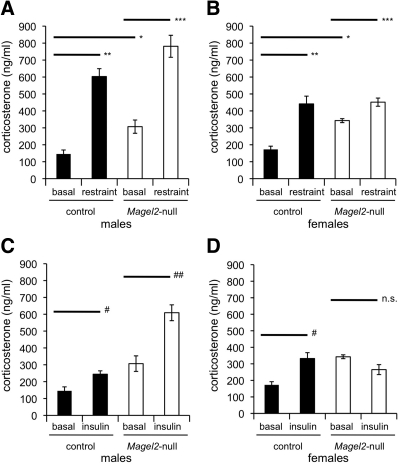
Fig. 1.
Stress responses in Magel2-null and control mice. Values represent the mean ± sem; n = 5–8 group. Black bars represent wild-type control, white bars represent Magel2-null. A and B, basal levels of serum corticosterone were elevated in both female and male Magel2-null mice compared with control (Student's t test; *, P < 0.01). Serum corticosterone levels were determined 30 min after restraint stress and compared within sex and genotype to basal levels by Student's t test. As expected, restraint significantly increased corticosterone levels over basal levels in the control mice (4.1 ± 1.1-fold and 2.6 ± 0.6-fold over basal in control male and female mice, respectively, both significantly increased over basal; **, P < 0.001). Restraint also increased corticosterone levels over basal in the Magel2-null mice [2.5 ± 0.3-fold (Magel2-null male) and 1.3 ± 0.1-fold (Magel2-null female) over basal, both ***, P < 0.002 comparing restrained levels with basal levels]. C and D, Serum corticosterone levels were determined 30 min after insulin injection. As expected, corticosterone levels in control mice were elevated, with an average increase of 1.7 ± 0.5-fold (male) and 1.9 ± 0.3-fold (female) over basal levels after 30 min (both sexes #, P < 0.03). An appropriate increase was seen in the male Magel2-null mice (2.0 ± 0.2-fold increase; ##, P < 0.01). However, Magel2-null female mice had no significant (n.s.) change in corticosterone levels after insulin administration.
Table 1.
Endocrine changes in Magel2-null mice
| Parameter | Female | Male |
|---|---|---|
| Basal corticosterone | Elevated | Elevated |
| Corticosterone response to restraint | Equivalent | Equivalent |
| Corticosterone response to insulin/hypoglycemia | No response | Equivalent |
| Corticosterone response to dexamethasone | No response | Equivalent |
| Corticosterone response to metyrapone | Equivalent | n.d. |
| ACTH | Elevated | n.d. |
| ACTH response to metyrapone | Equivalent | n.d. |
| Initial glucose response to insulin | Equivalent | Equivalent |
| Minimum glucose after insulin | Reduced | Reduced |
| Glucose recovery rate | Reduced | Reduced |
| IPGTT-AUC | Normal | Elevated |
| Adiponectin (total and HMW) | Elevated | Equivalenta |
| Igf-I | Reduced | Equivalentb |
| GH | Equivalent | n.d. |
| GH response to ghrelin | Reduced | n.d. |
| GH response to GHRH | Equivalent | n.d. |
| T4 | Reduced | n.d. |
Data are summarized from a variety of experiments described in the text. HMW, High molecular weight; n.d., not done.
Higher than expected considering high adiposity.
Lower than expected considering high adiposity.
Stressful situations activate the hypothalamic-pituitary-adrenal (HPA) axis, causing a rapid increase in circulating glucocorticoid levels. We compared basal corticosterone levels with those measured after 30 min of physical restraint, within each genotype and each sex. As expected, control mice demonstrated a significantly higher corticosterone after restraint stress, as did Magel2-null mice of both sexes (Fig. 1, A and B).
As a more specific test of the reactive stress response and in parallel with an IPITT described below, we measured corticosterone levels 30 min after injection of a dose of insulin sufficient to cause hypoglycemia (0.75 U/kg) (42). At this dose, mice of both genotypes displayed behaviors characteristic of hypoglycemia, including reduced movement and tremors, and as described below, experienced a significant reduction in their blood glucose. We compared insulin-stimulated corticosterone levels with basal levels, within each genotype and each sex (Fig. 1, C and D). We first confirmed that insulin treatment increased corticosterone levels in control male and female mice, diagnostic of an intact HPA response (44). An appropriate increase of about 2-fold was observed in the male Magel2-null mice (Fig. 1C). However, Magel2-null female mice had no change in corticosterone levels after insulin administration (Fig. 1D).
HPA axis responses to low or high corticosterone
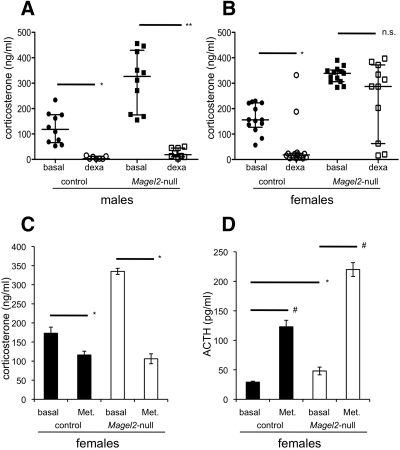
We next tested whether Magel2-null mice have intrinsic abnormalities in their glucocorticoid responses. We measured in vivo responses to acute changes in circulating glucocorticoids, administering dexamethasone to test negative feedback regulation of the HPA axis or metyrapone to test feedback regulation in response to a reduction in corticosterone. Both sexes of control mice, and the Magel2-null male mice, responded to a dexamethasone injection with significant lower corticosterone measured after 4 h (Fig. 2, A and B, and Table 1). However, mean corticosterone levels were not significantly reduced in Magel2-null female mice after dexamethasone injection, suggesting that they either do not properly sense or do not properly respond to high circulating corticosteroids. To further test the responsiveness of the HPA axis in the female mice, we administered the 11-β-hydroxylase inhibitor metyrapone, which inhibits glucocorticoid production, thereby stimulating ACTH production (45). Metyrapone effectively suppressed corticosterone levels measured 1 h after injection, both in the control and Magel2-null female mice (Fig. 2C). Basal ACTH levels were elevated in the mutant mice compared with control, consistent with their high basal corticosterone levels (Fig. 2D). As expected, the metyrapone-induced reduction in corticosterone correlated with a significant rise in ACTH levels in control female mice, measured after 6 h. We observed a similar significant rise in ACTH in the Magel2-null female mice (Fig. 2D). Finally, no abnormalities were observed in the size and appearance of the adrenal or pituitary glands dissected from Magel2-null mutant mice (data not shown). Overall, these results suggest that the lack of a stress response to insulin-induced hypoglycemia in female Magel2-null mice may result from dysfunction in glucose sensing in the hypothalamus, because the pituitary and adrenal responses to other stressors and to a reduction in circulating glucocorticoid levels appear to be intact in these mice.
Fig. 2.
Investigation of the HPA axis. A and B, Symbols represent individual mice [control (circles) and Magel2-null (squares)] with the median and interquartile range shown; n = 7–14 per group. Posttreatment serum corticosterone levels were compared with basal levels within sex and genotype by nonparametric Mann-Whitney test. As expected, corticosterone levels were lower after dexamethasone (dexa) in both sexes of control mice, measured 4 h after injection [*, P < 0.001; U = 0 (male) and U = 21 (females)]. Corticosterone levels were also lower after dexamethasone in male Magel2-null mice (**, P < 0.001; U = 0). However, corticosterone levels were not lower after dexamethasone treatment in female Magel2-null mice [not significant (n.s.)]. C, Corticosterone levels were significantly reduced by metyrapone (Met.) injection measured after 1 h, in female mice of both genotypes (*, P < 0.05). D, Baseline ACTH levels were higher in female Magel2-null mice compared with control (Student's t test; *, P < 0.02). ACTH levels increased significantly in female mice 6 h after metyrapone injection, compared with baseline levels within each genotype (#, P < 0.001).
Blood glucose response to insulin
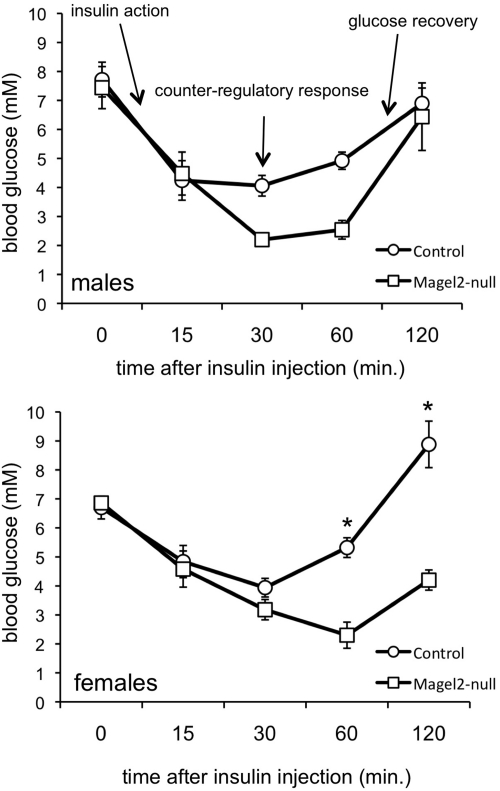
In conjunction with testing for a corticosterone response to insulin-induced hypoglycemia described above, we measured the blood glucose response at intervals up to 120 min after insulin injection (42). We then compared the responses between genotypes within each sex (Fig. 3 and Table 1). There was a significant effect of genotype on the overall glucose response to insulin in the female mice (P = 0.002 by repeated measures ANOVA). We further analyzed the IPITT curves in both sexes to derive specific data regarding basal glucose levels, insulin action as reflected in the initial fall in blood glucose, the glucose threshold for the initiation of a counterregulatory response, and the glucose recovery rate. Blood glucose levels after the 2-h fast did not differ between genotypes in either sex. There were no significant differences between genotypes in the initial insulin action on blood glucose in the first 15 min. A glucose threshold of 4.4 mmol/liter is generally sufficient to induce a robust counterregulatory hormonal response in mice (42). Control mice became hypoglycemic, with a mean minimum blood glucose of 3.8 ± 0.3 mmol/liter (female mice) and 3.6 ± 0.5 mmol/liter (male mice). However, both sexes of Magel2-null mice became more profoundly hypoglycemic than control, dropping to a mean minimum value of 2.1 ± 0.3 mmol/liter in males (P = 0.02 compared with control) and 2.2 ± 0.3 mmol/liter in females (P = 0.01 compared with control). We then calculated the glucose recovery rate as a measure of the counterregulatory response to hypoglycemia, by measuring the slope of the glucose curve in the second hour. In control female mice, blood glucose levels returned to preinjection glucose levels at a calculated glucose recovery rate of 65 ± 6 μmol/l · min (females) during the second hour. Female Magel2-null mice remained hypoglycemic up to 120 min after injection, with a glucose recovery rate reduced to 32 ± 1 μmol/l · min (P = 0.01 compared with control). However, both sexes of Magel2-null mice do recover from hypoglycemia without intervention despite glucose as low as 1.1 mmol/liter in some mutant mice, suggesting a delayed rather than deficient counterregulatory response. To summarize, mutant mice became more hypoglycemic than sex-matched control mice after insulin injection, suggesting a blunted counterregulatory response. As well, female Magel2-null mice experienced delayed recovery from insulin-induced hypoglycemia compared with control littermates.
Fig. 3.
IPITT in control (circles) and Magel2-null (squares) mice. Values represent the mean ± sem; n = 5–8 per group. There was no difference in the overall response in the male mice (two-way ANOVA with repeated measures). However, there was a significant effect of genotype in female mice [F(1,32) = 20; P = 0.002, two-way ANOVA with repeated measures], and post hoc testing revealed differences between genotypes at 60 and 120 min (*, P < 0.002). Additional interpretation of the IPITT is provided in the text.
GTT and adiponectin levels
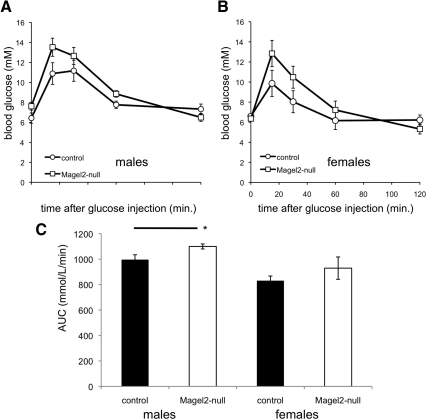
The normal decline in glucose in response to insulin suggests that Magel2-null mice maintain insulin sensitivity despite their increased adiposity. To further explore glucose homeostasis, we administered an IPGTT to fasted Magel2-null and control mice (42). There was no significant difference between genotypes in starting blood glucose, after a 6-h fast (Fig. 4, A and B, and Table 1). An area under the glucose tolerance curve (AUC) calculation demonstrated a modestly impaired IPGTT in the male mice and normal IPGTT in the female mice (Fig. 4C).
Fig. 4.
IPGTT. A and B, GTT in control (circles) and Magel2-null (squares) mice. Values represent the mean ± sem; n = 5–8 per group. C, Area under the glucose tolerance curve (AUC) calculations indicate that male Magel2-null mice have slightly reduced glucose tolerance (higher AUC) compared with control, but no difference was seen in the female mice. *, P < 0.05.
Total adiponectin levels are typically negatively correlated with fat mass in rodents and humans, reduced in individuals with impaired glucose tolerance, but high in obese rats with defective leptin receptors (46). We therefore measured adiponectin levels to determine whether increased fat mass (both sexes of Magel2-null mice) and impaired glucose tolerance (in male Magel2-null mice) is associated with low adiponectin. Both total and high-molecular weight adiponectin were higher in female Magel2-null mice than female control (total adiponectin in Magel2-null, 56 ± 2 μg/ml; control 45 ± 2 μg/ml; P = 0.003), but neither measurement differed between genotypes in male mice. The ratio of high molecular weight to total adiponectin was not different from control in either sex of Magel2-null mice. In summary, despite their increased fat mass, male Magel2-null mice have adiponectin levels comparable with lean control and display mildly impaired glucose tolerance, and female Magel2-null mice have higher adiponectin, together with normal glucose tolerance.
GH release in response to ghrelin and GHRH
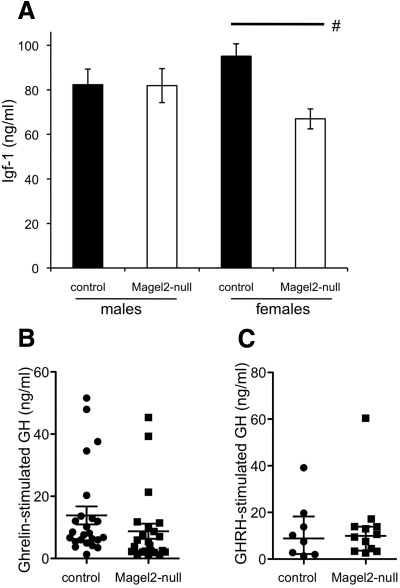
The GH-Igf-I axis is a key endocrine system controlled by the hypothalamus and is dysfunctional in PWS. GH release is pulsatile, and steady-state levels can be difficult to measure in rodents, so circulating levels of Igf-I can be used as a surrogate indicator of long-term GH secretion. Although Igf-I levels were similar in male Magel2-null and control littermates, female Magel2-null mice had a 1.4-fold lower Igf-I compared with control (Fig. 5A and Table 1). This result prompted us to investigate stimulated GH release in the female mice. We found equivalent basal GH levels in both genotypes, although as expected from the highly pulsatile nature of GH release in rodents, there was a large variation in these levels [median and interquartile range for control females, 0.55 ng/ml (0.16–0.82); for Magel2-null females, 0.23 ng/ml (0.10–0.68)]. Intraperitoneal injection of ghrelin normally elicits a robust increase in circulating GH levels in mice, by targeting GHRH neurons in the arcuate nucleus of the hypothalamus (47, 48). Indeed, ghrelin injection increased GH levels in female mice of both genotypes compared with basal levels of less than 1 ng/ml (Fig. 5B). However, the median values of ghrelin-stimulated GH were 1.9-fold lower in Magel2-null compared with control mice. Next, we investigated the GH response to injected GHRH, which acts directly on cognate receptors in the anterior pituitary to stimulate GH release. GH response to stimulation with GHRH was comparable between genotypes (Fig. 5C). These suggest that pituitary stimulation of GH release (by GHRH) is normal, whereas hypothalamic stimulation of the GH pathway (by ghrelin) is impaired in Magel2-null female mice, eventually leading to reduced circulating Igf-I levels.
Fig. 5.
Analysis of the GH pathway. A, Serum Igf-I levels were determined as a long-term indicator of GH axis function. Values represent the mean ± sem; n = 5–8 per group. Black bars represent control, and white bars represent Magel2-null. Magel2-null male mice had Igf-I levels similar to those of control littermates, whereas Magel2-null female mice had reduced Igf-I (Student's t test comparing control with Magel2-null, #, P = 0.002). B, Stimulated GH levels were measured after ghrelin injection. Symbols represent individual female mice [control (circles) and Magel2-null (squares)]. The error bars indicate the median and interquartile range. Median levels of ghrelin-stimulated GH were reduced in the Magel2-null mice compared with control (nonparametric Mann-Whitney test, U = 170, P = 0.02; n = 23–24 per group). C, Stimulated GH levels were measured after GHRH injection. Symbols represent individual female mice [control (circles) and Magel2-null (squares)]. The error bars indicate the median and interquartile range. There was no difference between genotypes in GHRH-stimulated GH levels (n = 8–12 per group).
Hypothalamus-pituitary-thyroid measurements
Low levels of thyroid hormones stimulate the production of hypothalamus-derived TRH and pituitary-derived thyroid-stimulating hormone, stimulating the production of thyroid hormones. Mean T4 levels were reduced 1.3-fold in female Magel2-null mice compared with control (Magel2-null, 2.9 ± 0.2 μg/dl compared with control, 3.9 ± 0.2 μg/dl; P < 0.002).
Discussion
Obesity is frequently associated with endocrine dysfunction, but differences in endocrine measures between typical obese and PWS obese individuals suggest that factors beyond excessive food consumption vs. energy expenditure contribute to obesity in PWS. In particular, decreased IGF-I, increased adiposity, and decreased lean mass all reflect GH deficiency that becomes partially normalized in PWS children undergoing GH replacement therapy (9–11). PWS individuals tend to have greater insulin sensitivity (22–24) and higher adiponectin levels (17–21) compared with body mass index-matched controls. Other endocrine abnormalities are less well defined in PWS, but include incomplete puberty, variable central adrenal insufficiency, and possibly central hypothyroidism. Many of these findings may be hypothalamic in origin, but a physiological or genetic basis for this dysfunction has been elusive. We now show that loss of the PWS candidate gene Magel2 in mice causes abnormalities in endocrine axes controlled by the hypothalamus, which overlap with those observed in rodents with mutations in leptin signaling and GH pathways.
Magel2 is required for normal function of the HPA axis
The HPA axis monitors and responds to physiological and psychological stress and can be altered in obesity. Basal plasma corticosterone levels were elevated in both male and female Magel2-null mice (Fig. 1 and Table 1), consistent with behavioral changes, we previously reported that include increased baseline freezing times and reluctance to explore novel objects (49). High basal corticosterone levels are also found in rodents with deficiencies in leptin signaling pathways [e.g. db/db mice and fa/fa rats (50)] but are not typical of other obese mice (51–53). Acute responses to changes in circulating glucocorticoids were examined using a dexamethasone suppression test and a metyrapone stimulation test (Fig. 2). Administration of dexamethasone lowered circulating corticosterone in male but not female Magel2-null mice. Inhibition of corticosterone synthesis with metyrapone lowered corticosterone and provoked an appropriate increase in ACTH in both control and Magel2-null female mice. Together, these results suggest that both sexes of Magel2-null mice have high circulating glucocorticoid levels, and in addition, female mice do not respond to an artificial increase in circulating glucocorticoids, suggesting impaired glucocorticoid sensing or negative feedback regulation. Importantly, both the pituitary and hypothalamus can respond to changes in glucocorticoid levels, so neither test (dexamethasone or metyrapone) is specifically diagnostic for hypothalamic dysfunction.
Abnormalities in glucocorticoid-mediated stress responses are a significant cause for concern during illness in people with PWS and may have hypothalamic, pituitary, and adrenal components. We examined the central response to two types of stress in Magel2-null mice. Restraint stress activates the HPA axis through input from peripheral sensory information, the nucleus of the solitary tract in the brain stem, and forebrain responses, integrated in the paraventricular nucleus of the hypothalamus (54, 55). These neurons produce hormones required for ACTH release from the anterior pituitary under both basal and stressed conditions. In contrast, hypoglycemia directly activates glucose-sensing neurons in the ventromedial hypothalamus (VMH), which initiate a counterregulatory response, including HPA activation, to normalize blood glucose (56–58). Genetic and physical lesions in the hypothalamus can impair the hypoglycemia response, even in animals that have normal glucose homeostasis under fed conditions (59, 60). Female Magel2-null mice exhibit a delayed, inadequate corticosterone response to the physiological stress provoked by insulin-induced hypoglycemia (Figs. 1 and 3). In this experiment, Magel2-null mice became more profoundly hypoglycemic than their control littermates, and some mutant mice declined to 1.1 mmol/liter blood glucose in the IPITT, nearing the limit for the induction of hypoglycemic shock. Notably, we only used a 2-h fast before the IPITT rather than a more typical 6- to 12-h fast, which would be expected to cause mortality from insulin-induced hypoglycemia in the mutant mice. Thus, the defective hypoglycemia response in the Magel2-null mice may actually be more severe than demonstrated here. Further, mutant mice had delayed onset of recovery, reduced glucose recovery rate, and in females, failure to release corticosterone and extended time to reattain euglycemia (Figs. 1 and 3). As glucose-sensing neurons in the VMH are primarily responsible for the counterregulatory response to hypoglycemia, further studies could help to determine whether neurons are insensitive to hypoglycemia in the Magel2-null mice and whether the glucose-induced excitatory or inhibitory responses are both deficient (56). The VMH is also considered the satiety center of the hypothalamus (61). It is possible that the deficient counterregulatory hypoglycemia response that we observe is only one manifestation of a VMH deficiency, motivating future testing of satiety responses in these mice. In summary, our observation that counterregulatory hypoglycemia responses are impaired in Magel2-null mice strongly suggests faulty integration of physiological signals in the hypothalamus in these mice but apparently normal regulation of the HPA axis at the level of the pituitary gland and the feedback loops that regulate ACTH production. In addition, these stress responses take place in a background of chronically elevated basal corticosterone in the mutant mice.
Body composition and glucose homeostasis in Magel2-null mice
Magel2-null mice have abnormal body composition, with fat mass 2.8 sds above the mean for control mice, and lean mass 3.2 sds below control mean (40). They consume less food than their wild-type littermates, but their food consumption is excessive considering their decreased activity levels (40). In the IPITT, both sexes of mutant mice responded normally to the initial glucose-lowering effect of insulin (Fig. 3). In the IPGTT, male mutant mice showed mildly impaired glucose tolerance compared with lean control (Fig. 4). Part of this difference may reflect the fact that the mutant mice have reduced muscle mass, the primary site for glucose uptake, so are less able to respond to doses of glucose calibrated for body mass rather than muscle mass (42). Although their leptin levels are increased in proportion to their fat mass (40), adiponectin levels, which are typically negatively correlated with fat mass and insulin resistance (12, 13), are instead elevated (female) or similar (male) to levels in lean control mice (Table 1). In summary, Magel2-null mice have better insulin sensitivity and more adiponectin than expected, considering that they have greatly increased adiposity.
Loss of MAGEL2 may be a genetic basis for defective counterregulation of hypoglycemia in PWS
Hypersensitivity to exogenous insulin has been noted in PWS individuals, including reductions of 50% in serum glucose in almost all insulin-treated PWS cases but in only a minority of obese controls (22), and severe insulin-induced hypoglycemia in earlier case reports (62). Blunting of this counterregulatory hypoglycemia response is a major concern for diabetics who have suffered repeated hypoglycemic episodes. Our demonstration that loss of Magel2 in mice impairs the counterregulatory response to hypoglycemia may motivate increased awareness of the importance of avoiding hypoglycemic episodes in individuals with PWS who are not frankly diabetic, but in whom the hypoglycemia counterregulatory response may be congenitally impaired by loss of the MAGEL2 gene. Monitoring for hypoglycemia in individuals with PWS is additionally imperative during fasting, e.g. during acute illness or before medical procedures, because coexisting impaired responses to other physiological stresses could lead to increased morbidity in this vulnerable population.
Magel2 and the GH axis
Diet-induced obese rodents typically have high Igf-I levels in proportion to their increased fat mass (63), whereas obese rodents with impaired GH secretion secondary to mutations in the GH pathway, or with defects in leptin signaling, have low Igf-I levels (64–66). We found low (female) or equivalent (male) Igf-I levels in Magel2-null mice compared with lean controls (Fig. 5 and Table 1), which could suggest GH deficiency or defective leptin signaling. We next measured GH release in female mice, in response to stimulation with ghrelin. Ghrelin acts on neuronal receptors to produce GHRH and thereby stimulate release of GH from the anterior pituitary (48). We documented a reduced GH response in the ghrelin stimulation assay in Magel2-null female mice but found a normal response in the GHRH stimulation test, suggesting abnormal hypothalamic but normal pituitary function in GH synthesis and release. Overall, Magel2-null mice exhibit an increased fat to lean mass ratio, low Igf-I levels considering their increased fat mass, and in females, a reduced response in a GH stimulation assay, all suggestive of a GH deficiency of hypothalamic origin. Igf-I levels were also recently measured in mice carrying a gene-targeted deletion of a different PWS candidate gene, MBII-85/SNORD116 (67). In contrast to Magel2-null mice, these mutant mice suffer from severe postnatal growth retardation and low fat mass. Similar to calorie restricted lean mice (68, 69), MBII-85/SNORD116 mice exhibit reduced serum Igf-I. GH release was not reported in this strain.
Endocrine phenotypes of Magel2-null mice resemble those found in rodents with congenital leptin resistance
The interrelationships among endocrine axes complicate the understanding of the underlying functional defect from loss of Magel2. For example, lower Igf-I levels are found in rodents that are hypothyroid and in females that do not go through proestrus (70), and both chronic HPA axis activation and circadian disruption are associated with obesity (71, 72). We did note a marked overlap between the endocrine profile of Magel2-null mice and that of rodents with congenital leptin resistance, in contrast to rodents with diet-induced obesity. Like Magel2-null mice, rodents with leptin receptor mutations [Zucker (fa/fa) rats or db/db mice] have increased corticosterone and ACTH (50), high plasma adiponectin (46), reduced GH secretion and low Igf-I (65, 66), and low T4 (73). We previously reported that Magel2-null mice display delayed puberty in females despite adequate fat stores, reduced fertility in both sexes (39), and reduced locomotor activity with an attenuated diurnal rhythm in 12-h light, 12-h dark cycle (38), all of which are phenotypes also described in db/db mice (74–76). Like the Magel2-null mice, there are sex differences in the endocrine responses in congenitally leptin resistant rodents (73, 77). Leptin receptor mutations are rare in humans but cause obesity and similar endocrine findings to those in rodents, including early onset obesity with hyperphagia, reduced percentage lean mass, delayed puberty due to hypogonadotrophic hypogonadism, low IGF-I and low stimulated GH secretion in some cases, and hypothalamic hypothyroidism but normal ACTH and cortisol levels and normal glucose homeostasis (78, 79).
Contribution of loss of MAGEL2 to PWS
A major physiological role for leptin is to regulate the neuroendocrine axis during starvation, and endocrine dysfunction in congenitally leptin resistant rodents is comparable with the endocrine starvation response (80, 81). The hypothesis that endocrine phenotypes in Magel2-null mice resemble a state of starvation is also consistent with the idea that PWS (a MAGEL2-deficient state) represents a genetic model of starvation caused by hypothalamic dysfunction (82). We propose that MAGEL2 deficiency in PWS causes hypothalamic phenotypes through a mechanism that overlaps with congenital leptin resistance. We further propose that loss of other PWS genes, including SNORD116 and NDN, may also contribute to hypothalamic dysfunction and to the failure to thrive (67, 83), high ghrelin levels (67), neonatal respiratory deficiency (84, 85), pain insensitivity (86), pituitary abnormalities, and developmental delay, which are all commonly found in PWS but not in individuals with leptin receptor mutations. Of note, a young woman was recently described who carries a chromosomal rearrangement that caused deletion of MAGEL2 and two nearby genes but left intact clusters of small nucleolar RNAs that are proposed to be important in PWS (87). This woman (patient 1) was investigated for PWS because of feeding problems in infancy, developmental delay, obesity with body mass index over the 97th percentile at 7 and 12 yr of age, excessive eating without documented hyperphagia, and precocious puberty but did not meet clinical criteria for PWS. Although only a single report, this phenotype strongly suggests that loss of MAGEL2 contributes to PWS through effects on energy balance, leading to increased adiposity and excessive weight gain in childhood.
Magel2 deficiency impairs processes controlled by several regions of the hypothalamus
Magel2 is most highly expressed in sites in the hypothalamus that include the suprachiasmatic, arcuate and ventromedial nuclei. The observed phenotypes are consistent with our hypothesis that Magel2 is important for homeostatic functions controlled by the hypothalamus, in addition to those attributed to reduced leptin responses outlined above. Blunted circadian rhythm (38) suggests a contribution from defective output from suprachiasmatic nucleus neurons. The weak, delayed response to hypoglycemia points to defective integration in the ventromedial nucleus. Although adiponectin has a major mode of action in muscle and liver, it also acts in the paraventricular and arcuate nuclei of the hypothalamus (15, 88, 89). Many peripheral signals, including leptin, insulin, adiponectin, reproductive hormones, low glucose, and ghrelin converge on the hypothalamus to maintain endocrine homeostasis (52, 88, 89). We propose that hypothalamic dysfunction causes reduced sensitivity to peripheral hormones, thereby creating the complex phenotype that ultimately disturbs multiple interdependent endocrine axes in Magel2-null mice.
Acknowledgments
We thank Dr. Andrea Haqq (University of Alberta) and Dr. Jennifer Miller (University of Florida, Gainesville, FL) for critical comments on the manuscript and members of the Wevrick laboratory for helpful discussions. We gratefully acknowledge donations to the Wevrick laboratory for Prader-Willi Syndrome research, made by the family of Samuel Jenness in his honor.
This work was supported by The Canadian Diabetes Association. Metabolic analyses at the University of Cincinnati Mouse Metabolic Phenotyping Center were supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health Grants DK5963 and DK59630. A.A.T. was supported by a Scriver Family M.D./Ph.D. Studentship Award from the Canadian Institutes for Health Research Institute of Genetics in partnership with the Canadian Gene Cure Foundation and the Canadian Genetic Disease Network, and by a studentship from the Alberta Heritage Foundation for Medical Research.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve
- HPA
- hypothalamic-pituitary-adrenal
- IPGTT
- ip glucose tolerance test
- IPITT
- ip insulin tolerance test
- MAGEL
- melanoma antigen, subfamily L
- PWS
- Prader-Willi syndrome
- VMH
- ventromedial hypothalamus.
References
- 1. Miller JL, Couch JA, Schmalfuss I, He G, Liu Y, Driscoll DJ. 2007. Intracranial abnormalities detected by three-dimensional magnetic resonance imaging in Prader-Willi syndrome. Am J Med Genet A 143:476–483 [DOI] [PubMed] [Google Scholar]
- 2. Miller L, Angulo M, Price D, Taneja S. 1996. MR of the pituitary in patients with Prader-Willi syndrome: size determination and imaging findings. Pediatr Radiol 26:43–47 [DOI] [PubMed] [Google Scholar]
- 3. Iughetti L, Bosio L, Corrias A, Gargantini L, Ragusa L, Livieri C, Predieri B, Bruzzi P, Caselli G, Grugni G. 2008. Pituitary height and neuroradiological alterations in patients with Prader-Labhart-Willi syndrome. Eur J Pediatr 167:701–702 [DOI] [PubMed] [Google Scholar]
- 4. Theodoridis CG, Brown GA, Chance GW, Rudd BT. 1971. Plasma growth hormone levels in children with the Prader-Willi syndrome. Aust Paediatr J 7:24–27 [DOI] [PubMed] [Google Scholar]
- 5. Burman P, Ritzén EM, Lindgren AC. 2001. Endocrine dysfunction in Prader-Willi syndrome: a review with special reference to GH. Endocr Rev 22:787–799 [DOI] [PubMed] [Google Scholar]
- 6. Eiholzer U, Stutz K, Weinmann C, Torresani T, Molinari L, Prader A. 1998. Low insulin, IGF-I and IGFBP-3 levels in children with Prader-Labhart-Willi syndrome. Eur J Pediatr 157:890–893 [DOI] [PubMed] [Google Scholar]
- 7. Sode-Carlsen R, Farholt S, Rabben KF, Bollerslev J, Schreiner T, Jurik AG, Christiansen JS, Höybye C. 2010. Body composition, endocrine and metabolic profiles in adults with Prader-Willi syndrome. Growth Horm IGF Res 20:179–184 [DOI] [PubMed] [Google Scholar]
- 8. Miller JL, Goldstone AP, Couch JA, Shuster J, He G, Driscoll DJ, Liu Y, Schmalfuss IM. 2008. Pituitary abnormalities in Prader-Willi syndrome and early onset morbid obesity. Am J Med Genet A 146A:570–577 [DOI] [PubMed] [Google Scholar]
- 9. Carrel AL, Myers SE, Whitman BY, Eickhoff J, Allen DB. 2010. Long-term growth hormone therapy changes the natural history of body composition and motor function in children with prader-willi syndrome. J Clin Endocrinol Metab 95:1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eiholzer U, l'Allemand D, Schlumpf M, Rousson V, Gasser T, Fusch C. 2004. Growth hormone and body composition in children younger than 2 years with Prader-Willi syndrome. J Pediatr 144:753–758 [DOI] [PubMed] [Google Scholar]
- 11. Mogul HR, Lee PD, Whitman BY, Zipf WB, Frey M, Myers S, Cahan M, Pinyerd B, Southren AL. 2008. Growth hormone treatment of adults with Prader-Willi syndrome and growth hormone deficiency improves lean body mass, fractional body fat, and serum triiodothyronine without glucose impairment: results from the United States multicenter trial. J Clin Endocrinol Metab 93:1238–1245 [DOI] [PubMed] [Google Scholar]
- 12. Hu E, Liang P, Spiegelman BM. 1996. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271:10697–10703 [DOI] [PubMed] [Google Scholar]
- 13. Yamauchi T, Kadowaki T. 2008. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes 32(Suppl 7):S13–S18 [DOI] [PubMed] [Google Scholar]
- 14. Saltiel AR. 2001. You are what you secrete. Nat Med 7:887–888 [DOI] [PubMed] [Google Scholar]
- 15. Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. 2004. Adiponectin acts in the brain to decrease body weight. Nat Med 10:524–529 [DOI] [PubMed] [Google Scholar]
- 16. Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. 2007. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6:55–68 [DOI] [PubMed] [Google Scholar]
- 17. Pagano C, Marin O, Calcagno A, Schiappelli P, Pilon C, Milan G, Bertelli M, Fanin E, Andrighetto G, Federspil G, Vettor R. 2005. Increased serum resistin in adults with prader-willi syndrome is related to obesity and not to insulin resistance. J Clin Endocrinol Metab 90:4335–4340 [DOI] [PubMed] [Google Scholar]
- 18. Hoybye C, Bruun JM, Richelsen B, Flyvbjerg A, Frystyk J. 2004. Serum adiponectin levels in adults with Prader-Willi syndrome are independent of anthropometrical parameters and do not change with GH treatment. Eur J Endocrinol 151:457–461 [DOI] [PubMed] [Google Scholar]
- 19. Kennedy L, Bittel DC, Kibiryeva N, Kalra SP, Torto R, Butler MG. 2006. Circulating adiponectin levels, body composition and obesity-related variables in Prader-Willi syndrome: comparison with obese subjects. Int J Obes 30:382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haqq AM, Muehlbauer M, Svetkey LP, Newgard CB, Purnell JQ, Grambow SC, Freemark MS. 2007. Altered distribution of adiponectin isoforms in children with Prader-Willi syndrome (PWS): association with insulin sensitivity and circulating satiety peptide hormones. Clin Endocrinol 67:944–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Festen DA, van Toorenenbergen A, Duivenvoorden HJ, Hokken-Koelega AC. 2007. Adiponectin levels in prepubertal children with Prader-Willi syndrome before and during growth hormone therapy. J Clin Endocrinol Metab 92:1549–1554 [DOI] [PubMed] [Google Scholar]
- 22. Bray GA, Dahms WT, Swerdloff RS, Fiser RH, Atkinson RL, Carrel RE. 1983. The Prader-Willi syndrome: a study of 40 patients and a review of the literature. Medicine 62:59–80 [PubMed] [Google Scholar]
- 23. Talebizadeh Z, Butler MG. 2005. Insulin resistance and obesity-related factors in Prader-Willi syndrome: comparison with obese subjects. Clin Genet 67:230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schuster DP, Osei K, Zipf WB. 1996. Characterization of alterations in glucose and insulin metabolism in Prader-Willi subjects. Metabolism 45:1514–1520 [DOI] [PubMed] [Google Scholar]
- 25. Schrander-Stumpel CT, Curfs LM, Sastrowijoto P, Cassidy SB, Schrander JJ, Fryns JP. 2004. Prader-Willi syndrome: causes of death in an international series of 27 cases. Am J Med Genet A 124:333–338 [DOI] [PubMed] [Google Scholar]
- 26. Stevenson DA, Anaya TM, Clayton-Smith J, Hall BD, Van Allen MI, Zori RT, Zackai EH, Frank G, Clericuzio CL. 2004. Unexpected death and critical illness in Prader-Willi syndrome: report of ten individuals. Am J Med Genet A 124A:158–164 [DOI] [PubMed] [Google Scholar]
- 27. de Lind van Wijngaarden RF, Otten BJ, Festen DA, Joosten KF, de Jong FH, Sweep FC, Hokken-Koelega AC. 2008. High prevalence of central adrenal insufficiency in patients with Prader-Willi syndrome. J Clin Endocrinol Metab 93:1649–1654 [DOI] [PubMed] [Google Scholar]
- 28. Rudd BT, Chance GW, Theodoridis CG. 1969. Adrenal response to ACTH in patients with Prader-Willi syndrome, simple obesity, and constitutional dwarfism. Arch Dis Child 44:244–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Connell NA, Paterson WF, Wallace AM, Donaldson MD. 2010. Adrenal function and mortality in children and adolescents with Prader-Willi syndrome attending a single centre from 1991–2009. Clin Endocrinol 73:686–688 [DOI] [PubMed] [Google Scholar]
- 30. Nyunt O, Cotterill AM, Archbold SM, Wu JY, Leong GM, Verge CF, Crock PA, Ambler GR, Hofman P, Harris M. 2010. Normal cortisol response on low-dose synacthen (1 μg) test in children with Prader Willi syndrome. J Clin Endocrinol Metab 95:E464–E467 [DOI] [PubMed] [Google Scholar]
- 31. Butler MG, Theodoro M, Skouse JD. 2007. Thyroid function studies in Prader-Willi syndrome. Am J Med Genet A 143:488–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Festen DA, Visser TJ, Otten BJ, Wit JM, Duivenvoorden HJ, Hokken-Koelega AC. 2007. Thyroid hormone levels in children with Prader-Willi syndrome before and during growth hormone treatment. Clin Endocrinol 67:449–456 [DOI] [PubMed] [Google Scholar]
- 33. Diene G, Mimoun E, Feigerlova E, Caula S, Molinas C, Grandjean H, Tauber M. 2010. Endocrine disorders in children with Prader-Willi syndrome—data from 142 children of the French database. Horm Res Paediatr 74:121–128 [DOI] [PubMed] [Google Scholar]
- 34. Lee S, Kozlov S, Hernandez L, Chamberlain SJ, Brannan CI, Stewart CL, Wevrick R. 2000. Expression and imprinting of MAGEL2 suggest a role in Prader-willi syndrome and the homologous murine imprinting phenotype. Hum Mol Genet 9:1813–1819 [DOI] [PubMed] [Google Scholar]
- 35. Boccaccio I, Glatt-Deeley H, Watrin F, Roëckel N, Lalande M, Muscatelli F. 1999. The human MAGEL2 gene and its mouse homologue are paternally expressed and mapped to the Prader-Willi region. Hum Mol Genet 8:2497–2505 [DOI] [PubMed] [Google Scholar]
- 36. Doyle JM, Gao J, Wang J, Yang M, Potts PR. 2010. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol Cell 39:963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barker PA, Salehi A. 2002. The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res 67:705–712 [DOI] [PubMed] [Google Scholar]
- 38. Kozlov SV, Bogenpohl JW, Howell MP, Wevrick R, Panda S, Hogenesch JB, Muglia LJ, Van Gelder RN, Herzog ED, Stewart CL. 2007. The imprinted gene Magel2 regulates normal circadian output. Nat Genet 39:1266–1272 [DOI] [PubMed] [Google Scholar]
- 39. Mercer RE, Wevrick R. 2009. Loss of magel2, a candidate gene for features of Prader-Willi syndrome, impairs reproductive function in mice. PLoS ONE 4:e4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bischof JM, Stewart CL, Wevrick R. 2007. Inactivation of the mouse Magel2 gene results in growth abnormalities similar to Prader-Willi syndrome. Hum Mol Genet 16:2713–2719 [DOI] [PubMed] [Google Scholar]
- 41. Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH. 2009. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology 150:2153–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. 2009. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab 297:E849–E855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. 2006. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 103:18267–18272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Erturk E, Jaffe CA, Barkan AL. 1998. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab 83:2350–2354 [DOI] [PubMed] [Google Scholar]
- 45. Giordano R, Picu A, Bonelli L, Balbo M, Berardelli R, Marinazzo E, Corneli G, Ghigo E, Arvat E. 2008. Hypothalamus-pituitary-adrenal axis evaluation in patients with hypothalamo-pituitary disorders: comparison of different provocative tests. Clin Endocrinol 68:935–941 [DOI] [PubMed] [Google Scholar]
- 46. Oana F, Takeda H, Hayakawa K, Matsuzawa A, Akahane S, Isaji M, Akahane M. 2005. Physiological difference between obese (fa/fa) Zucker rats and lean Zucker rats concerning adiponectin. Metabolism 54:995–1001 [DOI] [PubMed] [Google Scholar]
- 47. Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. 2000. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141:4325–4328 [DOI] [PubMed] [Google Scholar]
- 48. Osterstock G, Escobar P, Mitutsova V, Gouty-Colomer LA, Fontanaud P, Molino F, Fehrentz JA, Carmignac D, Martinez J, Guerineau NC, Robinson IC, Mollard P, Méry PF. 2010. Ghrelin stimulation of growth hormone-releasing hormone neurons is direct in the arcuate nucleus. PLoS ONE 5:e9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mercer RE, Kwolek EM, Bischof JM, van Eede M, Henkelman RM, Wevrick R. 2009. Regionally reduced brain volume, altered serotonin neurochemistry, and abnormal behavior in mice null for the circadian rhythm output gene Magel2. Am J Med Genet B Neuropsychiatr Genet 150B:1085–1099 [DOI] [PubMed] [Google Scholar]
- 50. Duclos M, Timofeeva E, Michel C, Richard D. 2005. Corticosterone-dependent metabolic and neuroendocrine abnormalities in obese Zucker rats in relation to feeding. Am J Physiol Endocrinol Metab 288:E254–E266 [DOI] [PubMed] [Google Scholar]
- 51. Saito M, Bray GA. 1983. Diurnal rhythm for corticosterone in obese (ob/ob) diabetes (db/db) and gold-thioglucose-induced obesity in mice. Endocrinology 113:2181–2185 [DOI] [PubMed] [Google Scholar]
- 52. Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. 2001. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108:1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chiba T, Komatsu T, Nakayama M, Adachi T, Tamashiro Y, Hayashi H, Yamaza H, Higami Y, Shimokawa I. 2009. Similar metabolic responses to calorie restriction in lean and obese Zucker rats. Mol Cell Endocrinol 309:17–25 [DOI] [PubMed] [Google Scholar]
- 54. Ulrich-Lai YM, Herman JP. 2009. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. 2003. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–180 [DOI] [PubMed] [Google Scholar]
- 56. Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. 1994. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest 93:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. 2007. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 5:383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Routh VH. 2003. Glucosensing neurons in the ventromedial hypothalamic nucleus (VMN) and hypoglycemia-associated autonomic failure (HAAF). Diabetes Metab Res Rev 19:348–356 [DOI] [PubMed] [Google Scholar]
- 59. Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. 2004. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2:e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hochgeschwender U, Costa JL, Reed P, Bui S, Brennan MB. 2003. Altered glucose homeostasis in proopiomelanocortin-null mouse mutants lacking central and peripheral melanocortin. Endocrinology 144:5194–5202 [DOI] [PubMed] [Google Scholar]
- 61. King BM. 2006. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav 87:221–244 [DOI] [PubMed] [Google Scholar]
- 62. Sareen C, Ruvalcaba RH, Kelley VC. 1975. Some aspects of carbohydrate metabolism in Prader-Willi syndrome. J Ment Defic Res 19:113–119 [DOI] [PubMed] [Google Scholar]
- 63. Roberts DL, Dive C, Renehan AG. 2010. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med 61:301–316 [DOI] [PubMed] [Google Scholar]
- 64. Ahmad I, Finkelstein JA, Downs TR, Frohman LA. 1993. Obesity-associated decrease in growth hormone-releasing hormone gene expression: a mechanism for reduced growth hormone mRNA levels in genetically obese Zucker rats. Neuroendocrinology 58:332–337 [DOI] [PubMed] [Google Scholar]
- 65. Segev Y, Eshet R, Yakir O, Haim N, Phillip M, Landau D. 2007. Systemic and renal growth hormone-IGF1 axis involvement in a mouse model of type 2 diabetes. Diabetologia 50:1327–1334 [DOI] [PubMed] [Google Scholar]
- 66. Tannenbaum GS, Lapointe M, Gurd W, Finkelstein JA. 1990. Mechanisms of impaired growth hormone secretion in genetically obese Zucker rats: roles of growth hormone-releasing factor and somatostatin. Endocrinology 127:3087–3095 [DOI] [PubMed] [Google Scholar]
- 67. Ding F, Li HH, Zhang S, Solomon NM, Camper SA, Cohen P, Francke U. 2008. SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice. PLoS ONE 3:e1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Imrie H, Abbas A, Viswambharan H, Rajwani A, Cubbon RM, Gage M, Kahn M, Ezzat VA, Duncan ER, Grant PJ, Ajjan R, Wheatcroft SB, Kearney MT. 2009. Vascular insulin-like growth factor-I resistance and diet-induced obesity. Endocrinology 150:4575–4582 [DOI] [PubMed] [Google Scholar]
- 69. Fenton JI, Nuñez NP, Yakar S, Perkins SN, Hord NG, Hursting SD. 2009. Diet-induced adiposity alters the serum profile of inflammation in C57BL/6N mice as measured by antibody array. Diabetes Obes Metab 11:343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hapon MB, Gamarra-Luques C, Jahn GA. 2010. Short term hypothyroidism affects ovarian function in the cycling rat. Reprod Biol Endocrinol 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Raber J. 1998. Detrimental effects of chronic hypothalamic-pituitary-adrenal axis activation. From obesity to memory deficits. Mol Neurobiol 18:1–22 [DOI] [PubMed] [Google Scholar]
- 72. Maury E, Ramsey KM, Bass J. 2010. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res 106:447–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chomard P, Beltramo JL, Ben Cheikh R, Autissier N. 1994. Changes in thyroid hormone and thyrotrophin in the serum and thyroid glands of developing genetically obese male and female Zucker rats. J Endocrinol 142:317–324 [DOI] [PubMed] [Google Scholar]
- 74. Saiduddin S, Bray GA, York DA, Swerdloff RS. 1973. Reproductive function in the genetically obese “fatty” rat. Endocrinology 93:1251–1256 [DOI] [PubMed] [Google Scholar]
- 75. Johnson LM, Sidman RL. 1979. A reproductive endocrine profile in the diabetes (db) mutant mouse. Biol Reprod 20:552–559 [DOI] [PubMed] [Google Scholar]
- 76. Laposky AD, Bradley MA, Williams DL, Bass J, Turek FW. 2008. Sleep-wake regulation is altered in leptin-resistant (db/db) genetically obese and diabetic mice. Am J Physiol Regul Integr Comp Physiol 295:R2059–R2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cocchi D, Parenti M, Cattaneo L, De Gennaro Colonna V, Zocchetti A, Müller EE. 1993. Growth hormone secretion is differently affected in genetically obese male and female rats. Neuroendocrinology 57:928–934 [DOI] [PubMed] [Google Scholar]
- 78. Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O'Rahilly S. 2007. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougnères P, Lebouc Y, Froguel P, Guy-Grand B. 1998. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392:398–401 [DOI] [PubMed] [Google Scholar]
- 80. Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. 2010. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 21:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. 1996. Role of leptin in the neuroendocrine response to fasting. Nature 382:250–252 [DOI] [PubMed] [Google Scholar]
- 82. Holland A, Whittington J, Hinton E. 2003. The paradox of Prader-Willi syndrome: a genetic model of starvation. Lancet 362:989–991 [DOI] [PubMed] [Google Scholar]
- 83. Schaller F, Watrin F, Sturny R, Massacrier A, Szepetowski P, Muscatelli F. 2010. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum Mol Genet 19:4895–4905 [DOI] [PubMed] [Google Scholar]
- 84. Ren J, Lee S, Pagliardini S, Gérard M, Stewart CL, Greer JJ, Wevrick R. 2003. Absence of Ndn, encoding the Prader-Willi syndrome-deleted gene necdin, results in congenital deficiency of central respiratory drive in neonatal mice. J Neurosci 23:1569–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pagliardini S, Ren J, Wevrick R, Greer JJ. 2005. Developmental abnormalities of neuronal structure and function in prenatal mice lacking the prader-willi syndrome gene necdin. Am J Pathol 167:175–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kuwako K, Hosokawa A, Nishimura I, Uetsuki T, Yamada M, Nada S, Okada M, Yoshikawa K. 2005. Disruption of the paternal necdin gene diminishes TrkA signaling for sensory neuron survival. J Neurosci 25:7090–7099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kanber D, Giltay J, Wieczorek D, Zogel C, Hochstenbach R, Caliebe A, Kuechler A, Horsthemke B, Buiting K. 2009. A paternal deletion of MKRN3, MAGEL2 and NDN does not result in Prader-Willi syndrome. Eur J Hum Genet 17:582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hoyda TD, Ferguson AV. 2010. Adiponectin modulates excitability of rat paraventricular nucleus neurons by differential modulation of potassium currents. Endocrinology 151:3154–3162 [DOI] [PubMed] [Google Scholar]
- 89. Dridi S, Taouis M. 2009. Adiponectin and energy homeostasis: consensus and controversy. J Nutr Biochem 20:831–839 [DOI] [PubMed] [Google Scholar]