Prolactin and glucose have differential and complementary effects on β-cell DNA synthesis and gene expression.
Abstract
The mechanisms by which lactogenic hormones promote β-cell expansion remain poorly understood. Because prolactin (PRL) up-regulates β-cell glucose transporter 2, glucokinase, and pyruvate dehydrogenase activities, we reasoned that glucose availability might mediate or modulate the effects of PRL on β-cell mass. Here, we used male rat islets to show that PRL and glucose have differential but complementary effects on the expression of cell cyclins, cell cycle inhibitors, and various other genes known to regulate β-cell replication, including insulin receptor substrate 2, IGF-II, menin, forkhead box protein M1, tryptophan hydroxylase 1, and the PRL receptor. Differential effects on gene expression are associated with synergistic effects of glucose and PRL on islet DNA synthesis. The effects of PRL on gene expression are mirrored by β-cell overexpression of signal transducer and activator of transcription 5b and are opposed by dexamethasone. An ad-small interfering RNA specific for cyclin D2 attenuates markedly the effects of PRL on islet DNA synthesis. Our studies suggest a new paradigm for the control of β-cell mass and insulin production by hormones and nutrients. PRL up-regulates β-cell glucose uptake and utilization, whereas glucose increases islet PRL receptor expression and potentiates the effects of PRL on cell cycle gene expression and DNA synthesis. These findings suggest novel targets for prevention of neonatal glucose intolerance and gestational diabetes and may provide new insight into the pathogenesis of β-cell hyperplasia in obese subjects with insulin resistance.
β-Cell mass and insulin production increase markedly during two critical windows of the human lifespan: the perinatal period and pregnancy. A surge of β-cell replication and the emergence of glucose-stimulated insulin secretion (GSIS) during the perinatal period (1, 2) are essential for neonatal glucose tolerance and establishment of β-cell reserve throughout the lifespan. Preterm infants, who lack the β-cell surge, are at risk for glucose intolerance in the newborn period and have double the risk of developing diabetes in adulthood (3). Likewise, increases in β-cell mass and GSIS during mid and late pregnancy, when the mother develops severe insulin resistance, are required to defend against glucose intolerance and gestational diabetes (4–6).
The increases in β-cell mass and GSIS in humans during the perinatal period and pregnancy coincide with a surge in the levels of placental lactogen (PL) and prolactin (PRL), which stimulate β-cell replication and GSIS in human and rodent islets and insulinoma cells (7–11). Overexpression of lactogenic hormones in β-cells induces β-cell replication and insulin production (12, 13). Conversely, deletion [knockout (KO)] of the PRL receptor (PRLR), which mediates actions of PL as well as PRL, reduces β-cell mass and GSIS in pregnant and nonpregnant mice (14, 15). The decrease in β-cell mass in PRLR KO mice results from reductions in β-cell replication rather than increases in β-cell apoptosis. The insulin secretory response to glucose is blunted in vivo and in vitro, and glucose tolerance is reduced. Insulin sensitivity, on the other hand, is normal (14, 15).
We have postulated (5, 16) that β-cell expansion and induction of insulin production by PL and PRL facilitate fetal growth and defend against the development of neonatal glucose intolerance and gestational diabetes. However, the molecular mechanisms by which lactogens increase β-cell mass and GSIS are poorly understood. PRL up-regulates β-cell glucose transporter 2, glucokinase, and pyruvate dehydrogenase activity and thereby promotes β-cell glucose uptake and utilization (16–19). Moreover, both PRL (16) and glucose (20) increase the phosphorylation (and thereby reduce the activity) of forkhead box protein (Fox)O1 (16, 20), which inhibits β-cell replication and increases β-cell apoptosis (21–24). We therefore reasoned that glucose availability might mediate or modulate the effects of PRL on β-cell mass. To test that hypothesis, we explored the interactions of PRL and glucose in the control of islet DNA synthesis and expression of various β-cell cyclins, cell cycle inhibitors, and other genes known to modulate β-cell replication, including pancreatic and duodenal homeobox 1 (PDX-1) (25), insulin receptor substrate 2 (IRS-2) (26, 27), IGF-II (28), FoxM1 (29), menin (30), and tryptophan hydroxylase 1 (Tph1) (31). FoxM1 and menin were reported to be up- and down-regulated, respectively, by PRL in mouse islets cultured in 10% serum (29, 30). Islet Tph1 is up-regulated markedly in pregnancy and is induced by PRL in islets of pregnant and nonpregnant female mice (31, 32). In this study, we found differential but overlapping and synergistic effects of glucose and PRL on cell cycle gene expression and islet DNA synthesis, suggesting a new paradigm by which hormones and nutrients act in concert to regulate β-cell mass.
Materials and Methods
Materials
RPMI 1640, DMEM, l-glutamine, antibiotic/antimycotic solution, fetal bovine serum (FBS), and Trizol reagent were from Life Technologies (Rockville, MD). Rat PRL was from the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD). Dexamethasone (DEX) was from Sigma (St. Louis, MO). RNeasy Micro kit was from QIAGEN (Valencia, CA). High Capacity cDNA Archive kits and SYBR Green PCR Master mixes were from Applied Biosystems, Inc. (Foster City, CA). Vivapure AdenoPACK columns were from Sartorius Stedim Biotech (Bohemia, NY). Antihexon fluorescein isothiocyanate-conjugated antibody was from Virostat (Portland, ME).
Cell culture
Primary rat islets were isolated from approximately 250-g-male Wistar rats as described (16, 19, 33), as approved by Duke University Institutional Animal Care and Use Committee. The preincubation medium (used during the first 24 h after isolation) was RPMI 1640 containing 6.8 mm glucose, 10% FBS, 10 mm HEPES, and 1% antibiotic/antimycotic solution. After washing, the islets were incubated for 24 or 48 h with hormones or diluents in serum-free RPMI containing varying glucose concentrations (2.5, 5.5, or 17.8 mm) and 0.5% BSA.
Parental rat insulinoma (INS-1) cells (from Claes Wollheim, Geneva, Switzerland) and an INS-1 cell line with high glucose responsivity (832-13 cells; from Christopher Newgard, Duke University Medical Center) were used for additional experiments. The 832-13 cells were employed for studies of signal transducer and activator of transcription (Stat)5b overexpression, as described (16). INS-1 cells were grown in RPMI 1640 (11.1 mm glucose) with 10% FBS, 50 μm 2-mercaptoethanol, 1 mm sodium pyruvate, 10 mm HEPES, and 1% antibiotic/antimycotic solution. At 80% confluence, the cells were washed and incubated with hormones or diluents for 24 h in serum-free media [DMEM with 5.5 mm glucose, 0.1% human serum albumin (HSA) (16, 19), 10 μg/ml human transferrin, 50 μm ethanolamine, 0.1 nm tri-iodothyronine, 50 μm phosphoethanolamine, and 1% antibiotic/antimycotic solution].
DNA synthesis in isolated islets
Rat islets were cultured for 24 h in RPMI containing 10% FBS and 6.8 mm glucose. The islets were washed three times with PBS and incubated for 48 h in serum-free RPMI containing 2.5, 5.5, or 17.8 mm glucose in the presence or absence of rat PRL (20 nm). Medium was replaced each day. Methyl [3H]-thymidine was added (0.25 μCi/ml) during the last 4 h of culture. Islets were washed three times in cold PBS. DNA was precipitated with cold 10% trichloroacetic acid, washed, and dissolved in 0.3 n NaOH. [3H]-thymidine incorporated into DNA was normalized to total cellular protein.
Quantification of mRNA expression
Islets were washed with serum-free basal media and incubated for 24 or 48 h with varying concentrations of glucose in the presence or absence of rat PRL. Islet RNA was isolated using RNeasy Micro kit (QIAGEN), and INS-1 cell RNA was isolated as described (19) and reverse transcribed with High-Capacity cDNA Archive kit (Applied Biosystems, Inc.). Quantitative real-time PCR (qRT-PCR) employed the ABI 7300 Real-Time PCR System. Oligonucleotide primers were designed using Primer Express from Applied Biosystems, Inc. Amplicon lengths averaged 60 bp; all primer pairs spanned introns. Negative controls were processed without reverse transcriptase. All samples from a single experiment were run using a single PCR mixture. Expression levels were normalized against levels of actin and quantified using the comparative threshold cycle (CT) method. Table 1 shows the sequences of primers used for qRT-PCR and mean baseline CT values in rat islets.
Table 1.
Analysis of gene expression in rat islets by quantitative real time PCR
| rGene | Accession no. | Primer sequence (5′ to 3′) | Mean CT islets |
|---|---|---|---|
| PRLR-L | NM_001034111 | CCT GCA TCT TTC CAC CAG TTC | 26 |
| GCA CTC AGC AGT TCT TCA GAC TTG | |||
| FoxO1 | XM_001056726 | TGT GCC CTA CTT CAA GGA TAA GG | 26 |
| GTG GCG AAT TGA ATT CTT CCA | |||
| p18Ink4c | NM_131902 | ACT CTG AAA TTC TGC CTC AAG TCA | 26 |
| CCA AGG CTC GGC CAT TCT | |||
| p21CIP1 | NM_080782 | GAC ATC TCA GGG CCG AAA AC | 22 |
| AAT CGG CGC TTG GAG TGA TA | |||
| p27KIP1 | NM_031762 | GGA GCA GTG TCC AGG GAT GA | 22 |
| CCT TTT GTT TTG CGA AGA AGA ATC | |||
| p57KIP1 | AJ488291 | TGC TGC GGC CAA TGC | 28 |
| CGT TCG ACG CCT TGT TCT C | |||
| Cyclin A2 | NM_053702 | CCG CAG CAG AGG CTC AAG | 31 |
| CAT CGT TTA TAG GAA GGT CCT TAA GAG | |||
| Cyclin B1 | NM_171991 | TGC TGC AGG AGA CCA TGT ACA | 30 |
| GCA CAC AAC TGT CCT GCA TGA | |||
| Cyclin B2 | NM_001009470 | CAG GCA GCT TGA GGC TTT G | 31 |
| CGT CCG TTT ATG TCT CTT CCA TCT | |||
| Cyclin D1 | NM_171992 | CGC AAA CAT GCA CAG ACC TT | 26 |
| GAG GGT GGG TTG GAA ATG AA | |||
| Cyclin D2 | NM_022267 | GCT ACC TGG ATG CTA GAG GTC TGT | 24 |
| GGT AAT TCA TGG CCA GAG GAA A | |||
| Cyclin D3 | NM_012766 | GCC CTC TGT GCC ACA GAT TAC | 26 |
| GCC CGT GGC GAT CAT G | |||
| Cyclin E1 | NM_001100821 | CCA TGC CAA GGG AGA GGA A | 30 |
| GGA GCC ACC TTC TTC TTT CAT GT | |||
| CDK1 | NM_019296 | CGC GTC CCA CGT CAA GA | 26 |
| AGA CCA GCA TTT TCG AGA GCA A | |||
| CDK2 | NM_199501 | AAG ACG GAC GGA GCT TGT TAT C | 26 |
| TTG GCT GAA ATC CGC TTG TT | |||
| CDK4 | NM_053593 | GTTG GCT GTA TCT TCG CAG AGA | 25 |
| CAG CCT CAG AGT TCC CAC AGA | |||
| IRS-2 | NM_001076309 | CGC AAG CAT CGA CTT CTT GTC | 26 |
| GCC CGC AGC ACT TTA CTC TT | |||
| IGF-II | NM_031511 | TGT CTAC CTC TCA GGC CGT ACT T | 23 |
| CCA GGT GTC GAA TTT GAA GAA CTT G | |||
| PDX-1 | NM_022852 | GCC AGT GGG CAG GAG GTG C | 24 |
| CCT TCT CCA GCT CCA GCA GCT | |||
| Menin | NM_019208 | TTC AGC TTC ATC ACA GGC ACT AA | 24 |
| TGG CAG GCC CCT ACC A | |||
| FoxM1 | NM_031633 | GGA ACT GGA AGA GAA GGA GAA TTG | 27 |
| TGA GGG CGC CTC AAC CT | |||
| Tph1 | NM_001100634 | TCA GAA ACT GGC AAC GTG CTA | 31 |
| CTG CCC ATC TTG CTT GCA | |||
| Actin | NM_031144 | CGT GAA AAG ATG ACC CAG ATC A | 18 |
| GCC TGG ATG GCA ACG TAC ATG |
The table shows the oligonucleotide primer pairs, all of which encode rat genes. The mean CT values shown in the table were obtained in islets incubated for 24 h in serum-free RPMI 1640 (5.5 mm glucose) supplemented with 0.5% BSA. For primer sequence: top, forward; bottom, reverse.
Effects of Stat5b overexpression
An adenovirus expressing green fluorescent protein (GFP) and a murine constitutively active STAT5b adenovirus (from Nils Billestrup, Novo Nordisk, Denmark), described previously (34), were added to INS-1-derived 832-13 cells at 60–70% confluence. Adenovirus-containing medium was removed 24 h after infection by washing; cells were cultured for an additional 24 h in serum-free medium. The cells were then washed and incubated for 24 h in fresh basal media in the presence or absence of rat PRL (20 nm). mRNA levels were assessed by qRT-PCR.
Small interfering RNA (siRNA)-mediated suppression of cyclin D2 in rat primary islets and INS-1 cells
Oligonucleotides containing the rat cyclin D2 target sequence GGA TCC CTT GTA TAT GCG AA were annealed and ligated into FF805, an EH006-derived RNA interference plasmid containing the human H1 RNA promoter (35). The resulting plasmid was cotransfected into HEK293 cells along with JM17, a replication-deficient variant of the adenoviral genome (36), giving rise to recombinant adenovirus. An adenovirus expressing a “scrambled” siRNA (from Tom Becker, Duke University Medical Center) with no known homology (GAG ACC CTA TCC GTG ATT A) was used as a control. Adenoviruses were purified using the Vivapure AdenoPACK columns (Sartoris-Stedim). Titers were determined by infecting HEK293 cells and staining with antihexon fluorescein isothiocyanate-conjugated antibody (Virostat).
Isolated rat islets were cultured in complete RPMI medium (10% FBS, 6.8 mm glucose) and treated for 18 h with either an adenovirus expressing a scrambled siRNA or a-cyclin D2 siRNA at a concentration of 107 infectious particles per 100 islets. Islets were washed and incubated for 48 h in serum-free basal medium containing PRL or diluent; [3H]-thymidine was added for the final 4 h. Cyclin D2 mRNA levels and [3H]-thymidine incorporation were measured as described above.
Statistical analysis
All assays were performed in triplicate or quadruplicate unless otherwise noted. Data are expressed as mean ± sem of all values obtained in two to five independent experiments. In each experiment, the groups contained four to six samples per treatment group. Differences among sample means were tested by ANOVA, followed by the Bonferroni tests of multiple comparisons. P < 0.05 was considered statistically significant.
Results
PRL induction of islet DNA synthesis. Regulation of cell cycle inhibitor mRNAs and induction of cell cyclins
Previous studies showed that PRL and PL increase DNA synthesis and replication of pancreatic islets in culture (7–11, 37). In a representative experiment, PRL stimulated a 78% increase in [3H]-thymidine incorporation (controls, 27.4 ± 4.4 cpm/μG protein; PRL, 48.8 ± 2.8 cpm/μG protein; P < 0.01) in rat islets during a 48-h incubation in serum-free medium. To explore molecular mechanisms by which PRL stimulates islet replication, we conducted a series of studies of the hormone's effects on expression of cell cyclins and cell cycle inhibitors in rat islets in vitro. Islets were incubated in serum-free medium in the presence or absence of rat PRL for 24 or 48 h; mRNA levels were quantified by qRT-PCR and normalized to actin mRNA.
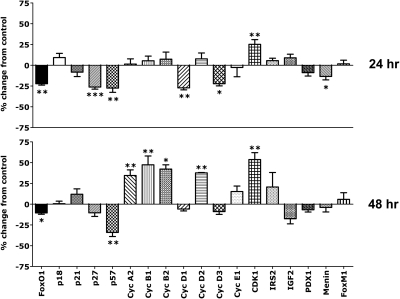
The results are summarized in Fig. 1. Most interesting is that the initial effects (24 h) of PRL in islets cultured in 5.5 mm glucose included down-regulation of the cell cycle inhibitors FoxO1, p27, p57, and, to a lesser and variable degree, menin. There were no significant effects on p18 or p21, and no induction of cell cyclins was noted at this initial time point. However, PRL stimulated a small increase (25.2%, P < 0.05) in expression of cyclin-dependent kinase 1 (CDK1). Suppression of cell cycle inhibitors was followed (at the 48-h time point) by increases in cyclins A2, B1, B2, and D2 mRNAs and further increases in CDK1; p57 mRNA levels remained suppressed. Thus, PRL induction of islet DNA synthesis involves down-regulation of cycle inhibitors before, or concurrent with, increases in cell cyclins. There were no effects of PRL alone on islet cyclin E1, CDKs 2 or 4, PDX-1, IRS-2, IGF-II, or FoxM1 mRNAs. Levels of Tph1 mRNA were quite low in male rat islets (baseline CT, 31). We found no consistent effects of PRL on Tph1 expression.
Fig. 1.
Effect of PRL on gene expression in isolated rat islets. Islets were incubated for 24 or 48 h in serum-free RPMI (5.5 mm glucose, 0.5% BSA) in the presence or absence of rat PRL (20 nm). In each experiment, the groups contained four to six samples per treatment group. mRNA levels were measured by qRT-PCR. Control (diluent treated) values were adjusted so that the means equaled 1.0. Values for PRL-treated islets are expressed as percentage change from controls at 24 and 48 h. Cyc, Cyclin. Data represent mean ± sem of all values obtained in two to five independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
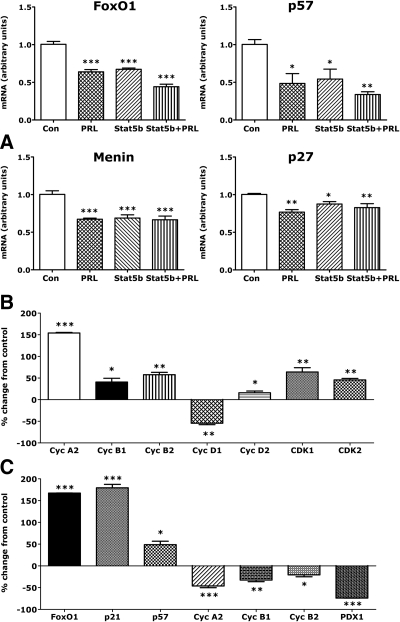
To ensure that PRL-dependent effects on islet cyclins and cyclin inhibitors reflected changes in islet β-cell gene expression, we compared the effects of PRL on gene expression in rat insulinoma cells with its effects in rat islets. As shown in Fig. 2A, PRL reduced expression of FoxO1, p27, and p57, and menin and stimulated increases (Fig. 2B) in cyclins A2 (+154%), B1 (+40%), B2 (+58%), D2 (+23%), and CDK1 (+64%) mRNAs (all P < 0.001) in INS-1 cells. As in rat islets, PRL reduced expression of cyclin D1 (−47%, P < 0.01) during a 24-h incubation. On the other hand, PRL increased CDK2 mRNA levels (+45%, P < 0.01) in INS-1 cells but not in islets and had no effect on INS-1 cell Tph1 mRNA.
Fig. 2.
Effect of PRL, STAT5b, and DEX on β-cell gene expression. A, INS-1 832-13 cells in the control (Con) and PRL groups were transfected with an adenovirus containing GFP. Cells in the Stat5b groups were transfected with an adenovirus containing a constitutively active form of Stat5b. The cells were then incubated for 24 h in serum-free basal media (5.5 mm glucose, 0.1% HSA) in the presence or absence of PRL (20 nm). Values represent mean + se (n = 4) of levels of mRNA relative to controls. Control values were adjusted so that the mean equaled 1.0. Values in other groups were calculated as a function of control values. *, P < 0.05; **, P < 0.01; ***, P < 0.001 relative to controls. Similar results were obtained in two experiments. B, INS-1 cells were incubated for 24 h in serum-free basal media (5.5 mm glucose, 0.1% HSA) in the presence or absence of PRL (20 nm). mRNA levels were measured by qRT-PCR. Control (diluent treated) values were adjusted so that the mean equaled 1.0. Other values represent the mean + se (n = 4) percentage change relative to controls. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Similar results were obtained in two experiments. C, Rat islets were incubated for 24 h in serum-free RPMI containing 5.5 mm glucose in the presence or absence of DEX (100 nm). mRNA levels were measured by qRT-PCR. Cyc, Cyclin. Values are expressed as percentage change from controls. Data represent mean ± sem of five values. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
STAT5 overexpression mimics the effects of PRL on FoxO1, p27, p57, and menin
STAT5 is a major downstream mediator of PRL action in target tissues. Indeed, previous studies suggested that STAT5 mediates effects of PRL on cyclin D2 expression in INS-1 cells (34). Here, we compared the effects of constitutive overexpression of STAT5b in 832-13 cells (16) with those of PRL. As shown in Fig. 2A, Stat5b overexpression reduced levels of FoxO1, p57, and p27 mRNAs, mimicking the effects of PRL. STAT5 also reduced expression of menin. In parallel studies, STAT5b overexpression stimulated a 66% increase (P < 0.001) in PRLR mRNA; PRL induced a 2- to 4-fold increase in PRLR mRNA (see below). There were no significant effects of STAT5 on p18; p21 on the other hand was increased 1.9-fold (P < 0.01). However, PRL had no effect on p21 in 832-13 cells overexpressing GFP.
DEX induces FoxO1, p21, and p57 expression and down-regulates the A and B cyclins and PDX-1
Previous studies showed that PRL counteracts the effects of DEX on β-cell replication, survival, and GSIS (16). It is therefore of interest that DEX induced expression of FoxO1, p21, and p57 mRNAs (+167, 179, and 50%, respectively) and suppressed cyclins A2 (−46%), B1 (−32%), and B2 (−21%) as well as PDX-1 (−74%) in rat islets (Fig. 2C). DEX had no effects on p18, p27, D or E cyclins, CDK1 or CDK2, FoxM1, or menin.
Glucose potentiates the effect of PRL on islet DNA synthesis
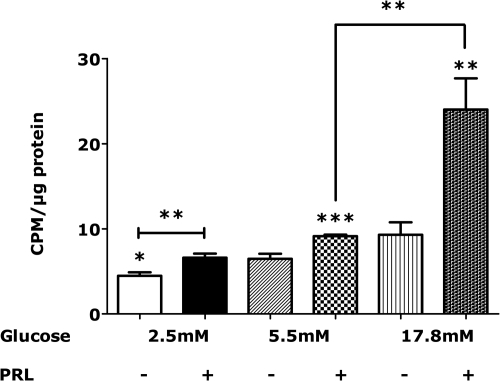
Because PRL promotes β-cell glucose uptake and utilization (16–19), we reasoned that glucose availability might mediate or potentiate the effects of PRL on islet DNA synthesis and gene expression. To test that hypothesis, we incubated rat islets for 48 h in serum-free medium with varying concentrations of glucose in the presence or absence of PRL. [3H]-thymidine was added for the final 4 h. As shown in Fig. 3, [3H]-thymidine incorporation was lower in islets incubated in 2.5 mm glucose than in islets incubated in 5.5 mm glucose. On the other hand, glucose excess (17.8 mm, 324 mg%) alone had no significant effect on islet DNA synthesis. In contrast, PRL increased [3H]-thymidine incorporation 47.8 and 40.9%, respectively, in islets incubated in 2.5 and 5.5 mm glucose. However, PRL stimulated a 158.2% increase in [3H]-thymidine incorporation (P < 0.001 vs. its effects in 2.5 or 5.5 mm glucose) in islets incubated in 17.8 mm glucose. Thus, glucose potentiated the effect of PRL on islet DNA synthesis.
Fig. 3.
Effects of glucose and PRL on [3H]-thymidine incorporation in isolated rat islets. Islets were incubated for 48 h in serum-free RPMI containing 2.5, 5.5, or 17.8 mm glucose in the presence or absence of rat PRL (20 nm). Methyl-[3H]-thymidine was added during the last 4 h of cell culture. [3H]-thymidine incorporated into DNA was normalized to total cellular protein. Values represent the mean + se of 8–12 samples in each group (in a total of three independent experiments). *, P < 0.05; **, P < 0.01; ***, P < 0.001. CPM, Counts per minute.
Glucose and PRL have differential but complementary effects on cell cycle inhibitors and cell cyclins
To explore the mechanisms by which glucose potentiates the effects of PRL on islet DNA synthesis, we first compared the effects of glucose excess and glucose deprivation on gene expression in isolated islets. Gene expression in islets cultured in 17.8 or 2.5 mm glucose (45 mg%) was compared with that in islets cultured in 5.5 mm glucose (100 mg%). We then compared the effects of glucose excess plus PRL in combination with the effects of either glucose excess or PRL alone.
Effect of glucose excess
Relative to islets incubated in 5.5 mm glucose, islets incubated in 17.8 mm glucose had higher expression of cyclins B1, B2, D1 (all mRNAs increased ∼50%), cyclin D3 (+30%), IRS-2 (+70%), and IGF-II (+30%) (Fig. 4, top) at 24 h and slightly lower levels of p21. Levels of p18 (+95%) and Tph1 (+55%) mRNAs were also increased, but there were no significant effects of glucose excess on FoxO1, p27, p57, cyclins A2, D2, or E1, CDK1, PDX-1, menin, or FoxM1 mRNAs. By 48 h (Fig. 4, top), the relative increases in p18 and IRS-2 in glucose-treated (17.8 mm) islets were still apparent, and levels of cyclin D1 (+85%), and Tph1 (+310%) (Fig. 5) mRNAs were higher. In contrast, expression of p21 (−29.2%) and p57 (−25.6%) were reduced. However, the effect of glucose excess on cyclins B1, B2, and D3 and IGF-II had waned, whereas cyclins A2 and B1 were now lower (−44 and −37%, respectively) than in islets incubated in 5.5 mm glucose.
Fig. 4.
A, Effects of glucose excess (17.8 mm) on gene expression in rat islets. Isolated rat islets were incubated for 24 or 48 h in serum-free RPMI containing 17.8 mm glucose. Additional islets were incubated in medium containing 5.5 mm glucose. Values represent mean + se (n = 4–6) percentage change of levels of mRNA relative to controls (islets incubated in 5.5 mm glucose). *, P < 0.05; **, P < 0.01; ***, P < 0.001 relative to controls. Similar results were obtained in three experiments. B, Effects of glucose deprivation (2.5 mm) on gene expression in rat islets. Isolated rat islets were incubated for 24 or 48 h in serum-free RPMI containing 2.5 mm glucose. Additional islets were incubated in medium containing 5.5 mm glucose. Cyc, Cyclin. Values represent mean + se (n = 4–6) percentage change of levels of mRNA relative to controls (islets incubated in 5.5 mm glucose). *, P < 0.05; **, P < 0.01; ***, P < 0.001 relative to controls. Similar results were obtained in two experiments.
Fig. 5.
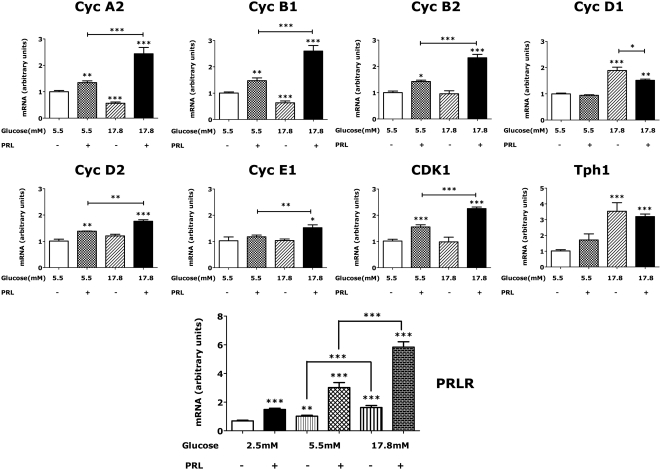
Effects of PRL and glucose in combination on gene expression in rat islets. Isolated rat islets were incubated for 48 h in serum-free RPMI containing varying concentrations of glucose in the presence or absence of rat PRL (20 nm). Values represent mean + se (n = 8–18 in each group in a total of four independent experiments) levels of mRNA relative to controls (islets incubated in 5.5 mm glucose alone). Cyc, Cyclin. Control values were normalized to a mean of 1.0. *, P < 0.05; **, P < 0.01; ***, P < 0.001 relative to controls.
Effect of glucose deprivation
Relative to basal conditions (5.5 mm glucose), glucose deprivation (2.5 mm) reduced acutely (24 h) the expression of cyclins A2, B1, B2, D1, CDK1, IRS-2, IGF-II, PDX-1, and FoxM1 (Fig. 4, bottom). There were also reductions in FoxO1 and p18, but glucose deprivation had no significant effects on cyclins D2, D3, E1, or menin. By 48 h, glucose deprivation increased expression of p27 and menin (Fig. 4, bottom) and reduced the expression of p18.
Effects of glucose excess and PRL in combination
The effects of glucose excess and PRL in combination were compared with the effects of glucose excess or PRL alone. At 24 h, the combination of PRL and glucose excess increased slightly (∼25–30% above that of PRL alone, P < 0.05) the levels of cyclin D2 (data not shown). By 48 h, the levels of cyclins A2, B1, B2, D2, E1, and CDK1 were higher in islets incubated in PRL + 17.8 mm glucose than in islets incubated in either PRL or glucose excess alone (Fig. 5). Addition of PRL blunted the effects of glucose excess on cyclin D1. No additivity or synergism was noted for FoxO1, p21, p27, p57, cyclins D3, PDX-1, FoxM1, menin, or Tph1.
Changes in gene expression during incubation in serum-free medium
Effects of PRL and glucose on gene expression must be assessed in light of changes in gene expression during incubation in serum-free medium. Levels of FoxO1, p27, and IGF-II mRNAs, normalized to actin mRNA, were approximately 50–75% higher (each P < 0.001) in islets incubated in serum-free medium for 48 h than in islets incubated in serum-free medium for 24 h. There were smaller increases in menin (35%), IRS-2 (33%), and cyclin D3 (20%). Conversely, levels of cyclin B1 and CDK1 were 27 and 29% lower at 48 h than at 24 h. There were no significant changes during the 24 to 48 h transition in any of the other proteins studied. Thus, PRL alone or the combination of PRL + glucose excess may defend against the fall in cyclin B1 and CDK1 and/or prevent the increases in FoxO1, p27, and/or menin that occur under serum-free conditions. PRL and glucose-dependent increases in cyclins A2, B2, D2, and E1 expression are more likely stimulatory effects, given that the mRNA levels of these proteins in control islets at 48 h were comparable with those in control islets at 24 h.
Glucose availability modulates β-cell PRLR levels
Rat islet β-cells express both the long and short isoforms of the rat PRLR. The long form mediates the biological actions of lactogenic hormones, whereas the short isoform may, under some conditions, function as a dominant negative. However, long isoform mRNA levels in INS-1 β-cells are 20- to 30-fold greater than short isoform mRNA (data not shown). As shown in Fig. 5, levels of the long isoform of PRLR mRNA in islets incubated in 5.5 or 17.8 mm glucose were 1.48 (P < 0.05)- and 2.35-fold P < 0.001) higher, respectively, than PRLR mRNA levels in islets incubated in 2.5 mm glucose, and glucose potentiated the effect of PRL on PRLR expression.
The effects of PRL and glucose, alone and in combination, on various genes are summarized in Table 2.
Table 2.
Summary of the effects of PRL and glucose on gene expression in male rat islets
| PRL |
High glucose |
Additivity/synergy |
Low glucose |
|||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| PRLR | + | + | + | + | + | + | − | − |
| FoxO1 | − | − | + | − | ||||
| p18 | + | + | − | − | ||||
| p21 | − | − | ||||||
| p27 | − | − | + | |||||
| p57 | − | − | − | − | ||||
| Menin | − | + | ||||||
| Cyclin A2 | + | − | + | − | ||||
| Cyclin B1 | + | + | − | + | − | |||
| Cyclin B2 | + | + | + | − | ||||
| Cyclin D1 | − | + | + | − | − | |||
| Cyclin D2 | + | + | + | |||||
| Cyclin D3 | − | + | ||||||
| Cyclin E1 | + | |||||||
| CDK1 | + | + | + | − | ||||
| CDK2 | ||||||||
| CDK4 | ||||||||
| PDX-1 | − | |||||||
| IRS-2 | + | + | − | |||||
| IGF-II | + | − | ||||||
| FoxM1 | ||||||||
| Tph1 | + | + | ||||||
+, Increase in mRNA; −, decrease in mRNA. A blank space indicates no significant effect.
The effects of PRL and glucose on β-cell [3H]-thymidine incorporation are attenuated by siRNA knockdown of cyclin D2
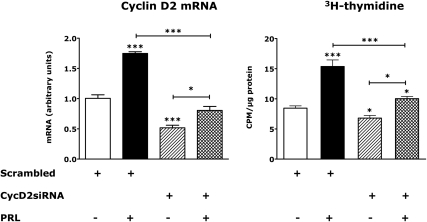
To assess the role of cyclin D2 in PRL and glucose induction of islet DNA synthesis, we treated rat islets with an adenoviral siRNA specific for rat cyclin D2. Control islets were incubated with a scrambled siRNA adenovirus; 24 h later, the islets were washed and incubated for an additional 48 h in serum-free medium containing 17.8 mm glucose ± rat PRL. [3H]-thymidine was added 4 h before processing the cells.
Under these conditions, the cyclin D2 siRNA reduced islet cyclin D2 mRNA levels 55% (P < 0.001). PRL increased cyclin D2 mRNA in control islets (83%, P < 0.05) and increased, but failed to normalize, cyclin D2 mRNA in islets expressing the cyclin D2 siRNA (Fig. 6). In control islets expressing the scrambled siRNA, PRL stimulated an 81.9% increase in [3H]-thymidine incorporation (P < 0.001). This effect of PRL was markedly attenuated by pretreatment with the cyclin D2 siRNA (P < 0.01 vs. PRL effect in scrambled siRNA controls). PRL stimulated a small increase (+18.7%, P < 0.05) in [3H]-thymidine incorporation despite the knockdown of D2 mRNA. Still, the magnitude of induction of [3H]-thymidine incorporation by PRL was lower (P < 0.001) in islets treated with the cyclin D2 siRNA than in islets expressing the scrambled siRNA.
Fig. 6.
Effects of cyclin (Cyc) D2 siRNA on cyclin D2 mRNA and [3H]-thymidine incorporation in rat islets. Rat islets were transfected with adenoviruses encoding a scrambled siRNA or an siRNA encoding cyclin D2. The islets were then incubated for 48 h in serum-free RPMI in the presence or absence or rat PRL (20 nm). [3H]-thymidine was added for the final 4 h of incubation. Cyclin D2 mRNA values represent mean + se of four samples normalized to controls (diluent-treated islets transfected with the scrambled siRNA), which were adjusted so that the mean equaled 1.0. Values for [3H]-thymidine incorporation represent the mean + se of 6–12 samples. *, P < 0.05; ***, P < 0.001 relative to controls. Similar results were obtained in two experiments. CPM, Counts per minute.
Discussion
Changes in the rates of β-cell replication during human and rodent development roughly parallel changes in the circulating concentrations of lactogenic hormones (2, 4–6, 37). For example, the striking increases in human PRL levels after 30 wk gestation (to a peak of 200–450 ng/ml at the time of birth) and the high levels of PRL during the first 2–3 months of life are associated with massive expansion of β-cell mass and increases in insulin production, whereas the fall in PRL levels after infancy is associated temporally with a decline in rates of β-cell replication. Likewise, the up-regulation of β-cell mass and insulin production in the pregnant mother in mid-late gestation (4–6, 37) is associated with dramatic and progressive increases in serum PRL and PL (5, 37).
In the rat, β-cell thymidine and bromodeoxyuridine incorporation are highest in in the fetus on d 20–22 of gestation and decline progressively after postnatal d 2 (38, 39). Thus, in rats and in humans, there is a surge of β-cell replication in late gestation and the immediate perinatal period. Interestingly, PRL levels are very low in the rat fetus and newborn, rising after d 15 to a peak of 19 ng/ml on d 20 (40). Nevertheless, rat PL-II circulates at levels approximating 30 ng/ml in the fetal rat on d 20 of gestation (41). Thus the neonatal surge in β-cell replication in the rat coincides with induction of PL-II, not PRL. Likewise, the surge in β-cell replication in the pregnant rat and mouse between d 9.5 and 15.5 (31, 42, 43) is associated temporally with a dramatic increase in plasma PL-I levels and the emergence of PL-II (44, 45). The twice-daily surges of pituitary PRL in early pregnancy terminate in tandem with the rise in maternal PL-I (46).
The biological actions of rodent PLs, like those of rat PRL, are mediated through binding to rat PRLRs, which are expressed in abundance in β-cells and induced during perinatal development (41, 47, 48) and pregnancy (49). The surge in lactogen levels and induction of PRLR expression in the perinatal period and pregnancy likely promote β-cell expansion and insulin production, because lactogen treatment of human or rodent islets (7–11, 50) or overexpression of PL in pancreatic β-cells (12, 13) stimulates β-cell replication and GSIS and prolongs β-cell survival (51). Conversely, a germline KO of the PRLR, which abolishes the actions of PL as well as PRL, reduces islet density, β-cell mass, GSIS, and glucose tolerance in pregnant and nonpregnant mice (14, 15). In the aggregate, these findings implicate a central role for the lactogens in the control of β-cell mass and insulin production.
PRL induction of β-cell replication has been ascribed to direct induction of cyclin D2 expression (34), increases in FoxM1 (29) and Tph1 expression (31, 32), and/or suppression of menin (30). Given that the lactogens promote β-cell glucose uptake and utilization (16–19), we hypothesized that glucose availability might mediate or modulate the effects of PRL on β-cell mass and function. To that end, we explored the interactions of glucose and PRL in the control of DNA synthesis and cell cycle gene expression in isolated rat islets.
Our initial studies showed time-dependent changes in expression of islet cell cyclins and cell cycle inhibitors in association with PRL induction of [3H]-thymidine incorporation. During the first 24 h of incubation in serum-free medium, PRL down-regulated the cell cycle inhibitors FoxO1, p27, p57, and, to a limited and variable extent, menin. Cyclins D1 and D3 were also reduced, but PRL stimulated a small increase in expression of CDK1. Suppression of cell cycle inhibitors was followed (at 48 h) by induction of cyclins A2, B1, B2, and D2, and further increases in CDK1, whereas p57 levels remained suppressed. These findings suggest that PRL induction of cell cyclins is preceded by down-regulation of cell cycle inhibitors. PRL had no consistent effects on Tph1, although it should be noted that the levels of expression of the gene in these islets of these male rats were quite low (baseline CT, ∼31).
The effects of PRL contrast with those of the glucocorticoid, DEX. DEX induced FoxO1, p21, and p57 mRNAs and suppressed cyclins A2, B1, B2, and PDX-1 in rat islets; In contrast, PRL suppressed FoxO1, p57, and p27 and induced cyclins A2, B1, B2, and D2. These findings may explain in part the opposing effects of PRL and DEX on β-cell replication as well as GSIS (16, 19). A previous study showing up-regulation of p21 in islets that overexpress mouse PL in vivo (52) is difficult to interpret, because the mice had fasting and postprandial hypoglycemia. Our studies here show that glucocorticoids, which would likely increase in response to hypoglycemia, increase islet p21 mRNA levels, whereas prolonged (48 h) exposure to glucose excess reduces islet p21 expression.
We recently showed that PRL reduces nuclear FoxO1 protein levels as well as FoxO1 mRNA in isolated rat islets (16). Extensive follow-up studies will be required to assess the effects of PRL and DEX on the protein levels of all of the genes described in the current investigation. It should be acknowledged that changes in mRNA levels may not be reflected in changes in cellular protein levels. Nevertheless, the identification of FoxO1, p27, p57, and cyclins A2, B1, B2, and D2 as direct or indirect PRL target genes has interesting physiological and clinical implications. FoxO1, p27, and p57 are functional inhibitors of cell cycle progression. FoxO1 reduces β-cell replication and increases β-cell apoptosis (22–24) and can promote (53–55) the expression of p27 and p57, which inhibit β-cell CDKs (most potently in the neonatal period) (56, 57). Down-regulation of FoxO1 is essential for β-cell compensation to insulin resistance (24). FoxO1 is overexpressed in the islets of diabetic men and women (58), and FoxO1 polymorphisms are associated with diabetes risk (59). Conversely, KO mutations in p27 increase β-cell proliferation in IRS-2 KO mice (60) and are associated with development of human insulin secreting tumors (61, 62), whereas deletions of p57 are associated with neonatal hyperinsulinism (63). Thus, down-regulation of FoxO1, p27, and p57 by PRL may facilitate induction of β-cell replication and increases in β-cell mass.
Induction of islet DNA synthesis and β-cell replication are mediated by D-type cyclins, which associate with CDKs 4 and 6. The cyclin/CDK complexes inactivate (through phosphoryation) the retinoblastoma tumor suppressor protein and promote progression through the G1 phase of the cell cycle. Progression in late G1 and S phase is promoted by association of cyclins A and E with CDK2. Cyclin D2, which is expressed exclusively in islet β-cells in the postnatal period (64, 65), appears to play a role in PRL action in the rodent pancreas: knockdown of cyclin D2 expression in isolated rat islets attenuates PRL induction of β-cell [3H]-thymidine incorporation. Nevertheless, cyclin D2 levels rise only marginally during rodent pregnancy (31, 32), when PL levels rise dramatically, and the cyclin D2 siRNA did not abolish the effect of PRL on DNA synthesis in islets. This latter finding might reflect incomplete (55%) knockdown of cyclin D2 but may also implicate roles for other cyclins, including the A and B cyclins, in PRL action. Indeed, cyclins A2, B1, and B2 are up-regulated (increases in mRNAs for A2, B1, B2 ≫ D2) in islets of pregnant mice (32) and are higher in PRL-treated than in control rat islets (this study). The up-regulation of A and B cyclins in pregnancy may therefore be mediated in part by PL and/or PRL.
The precise mechanisms by which PRL regulates the various cell cycle inhibitors and cyclins are unclear, but overexpression studies suggest that STAT5 serves as a proximate mediator of PRL suppression of FoxO1, p27, p57, and menin mRNAs (this study) and induction of cyclin D2 (34). PRL's down-regulation of FoxO1 and menin may contribute to suppression of p27 and p57 (30, 53–55) and induction of cyclin D2 (22–24), whereas PRL's up-regulation of islet Tph1 may promote induction of A and B cyclins in pregnancy (31, 32). Alternatively, PRL treatment may defend (directly or indirectly) against induction of FoxO1, p27, p57, and menin and/or loss of A and B cyclins under stress, as suggested by our studies of the effects of DEX and glucose deprivation on cell cycle genes and our analysis of changes in gene expression under serum-free conditions.
The role of FoxM1 in PRL signaling is unclear. We found no significant effects of PRL on FoxM1 expression in male rat islets cultured in serum-free medium during 48 h of incubation. In contrast, Zhang et al. (29) found that 4 d of treatment with human PL increased FoxM1 mRNA in islets of female mice cultured in 10% horse serum. FoxM1 is up-regulated in rodent islets during pregnancy in association with high rates of β-cell replication, and whole pancreatic deletion of FoxM1 causes maternal glucose intolerance and reverses the induction of β-cell bromodeoxyuridine uptake in mice that overexpress mouse PL (29). However, postpartum females with pancreatic FoxM1 deletions have normal β-cell mass, owing to increases in β-cell size and, possibly, induction of β-cell neogenesis. Interestingly, overexpression of FoxM1 in mouse or human islets (66) had no effect on expression of D cyclins, CDKs 4 or 6, p18, p21, p27, or p57, suggesting that the effects of PRL on islet DNA synthesis and β-cell replication are not mediated exclusively through induction of FoxM1.
In contrast to PRL, glucose excess (17.8 mm) had no significant effect on islet [3H]-thymidine incorporation in male rat islets during the time periods studied. Yet glucose excess up-regulated acutely (24 h) the expression of cyclins B1, B2, D1, D3, IRS-2, IGF-II, and Tph1, findings consistent with previous studies that demonstrated glucose induction of cyclin D1, IRS-2, and IGF-II in human mesenchymal cells (67), pancreatic islets (68, 69), and INS-1 cells (70), respectively.
What differences in gene expression might explain the differential effects of PRL and glucose on [3H[-thymidine incorporation? First, although PRL reduced acutely the expression of FoxO1, p27, p57, and, to a lesser and variable extent, menin in isolated islets, glucose had no effect on FoxO1, p27, or menin and reduced p57 only slightly at 48 h. Conversely, glucose stimulated a sharp increase in the expression of p18. p18 is an inhibitor of β-cell CDKs; deletion of the protein causes hyperplasia of pancreatic islets and, in combination with p27 deletions, can promote the development of insulinomas (71, 72). Thus, induction of p18 by glucose excess might counteract its induction of cell cyclins, IRS-2, IGF-II, and Tph1 and thereby restrain β-cell replication.
Second, in contrast to PRL, glucose alone had no effect on islet cyclin D2, which is required for rodent β-cell replication (64, 65). Finally, by 48 h, the effect of glucose on cyclins A2, B1, B2, D3, and IGF-II had waned. In contrast, cyclins A2, B1, B2, and CDK1 were higher in PRL-treated islets.
Nevertheless, glucose excess potentiated the effect of PRL on [3H]-thymidine incorporation during the 48-h incubation. In part, this additivity or synergy of effects may reflect the confluence of disparate streams that converge at the level of the cell cycle. Although PRL treatment up-regulates (or defends against a fall in) cyclins A2, B1, B2, D2, and CDK1 and down-regulates (or defends against a rise in) FoxO1, p27, p57, and menin, glucose up-regulates cyclins D1, D3, IRS-2, IGF-II, and Tph1. The differential but complementary effects of PRL and glucose may facilitate greater induction of β-cell DNA synthesis than either PRL or glucose alone. In this regard, it is of interest that cyclins D2 and D1 may act in concert to maintain β-cell mass and function, at least under certain conditions. In C57Bl6/129 hybrid mice, a KO of cyclin D2 reduces β-cell replication by 3 months of age and leads to β-cell hypoplasia, relative insulinopenia, and glucose intolerance by 9 months (64). However, combined deletion of cyclin D1 (+/−) and cyclin D2 (−/−) reduces β-cell proliferation markedly by postnatal d 16 and leads to frank diabetes by 3 months of age. Cyclin D2 is absolutely required for postnatal β-cell replication in nonhybrid C57Bl6 mice: cyclin D2 KO abolishes β-cell replication by postnatal d 4 and decreases β-cell mass by d 14 and glucose tolerance by 4 wk of age (65).
In contrast to their differential effects on D cyclins, the combination of PRL and glucose excess had additive or synergistic effects on cyclins A2, B1, B2, D2, E1, and CDK1. The mechanisms explaining this synergy are currently unclear. However, glucose up-regulates PRLR mRNA levels and potentiates the effects of PRL on PRLR expression, whereas PRL increases β-cell glucose uptake and utilization. This interplay of hormones and nutrients may foster β-cell expansion and promote β-cell function.
Our studies suggest a new paradigm for the control of β-cell mass and insulin production by hormones and nutrients. PRL up-regulates β-cell glucose transporter 2, glucokinase, and pyruvate dehydrogenase activity and thereby facilitates glucose uptake and utilization. At the same time, glucose increases islet PRLR expression and potentiates the effects of PRL on cell cycle gene expression and DNA synthesis. These findings suggest novel targets for prevention of neonatal glucose intolerance and gestational diabetes and may provide new insight into the pathogenesis of β-cell hyperplasia in infants of diabetic mothers and obese subjects with insulin resistance.
Acknowledgments
We thank Dr. Sam Stephens and Dr. Christopher Newgard of the Sarah W. Stedman Nutrition Center for reviewing our manuscript and the National Hormone and Peptide program and Dr. A. F. Parlow, Scientific Director, for providing rat PRL.
This work was supported by the National Institute of Child Health and Human Development Grant HD024192, American Diabetes Association Grant 7-08-RA-46), and grants from the Pfizer Corp. (M.F.) and the Duke Children's Miracle Network (R.A.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CDK1
- Cyclin-dependent kinase 1
- CT
- threshold cycle
- DEX
- dexamethasone
- FBS
- fetal bovine serum
- Fox
- forkhead box protein
- GFP
- green fluorescent protein
- GSIS
- glucose-stimulated insulin secretion
- HSA
- human serum albumin
- IRS-2
- insulin receptor substrate 2
- KO
- knockout
- PDX-1
- pancreatic and duodenal homeobox 1
- PL
- placental lactogen
- PRL
- prolactin
- PRLR
- PRL receptor
- qRT-PCR
- quantitative real-time PCR
- siRNA
- small interfering RNA
- Stat
- signal transducer and activator of transcription
- Tph1
- tryptophan hydroxylase 1.
References
- 1. Hellerstrom C, Swenne I. 1991. Functional maturation and proliferation of fetal pancreatic β-cells. Diabetes 40(Suppl 2):89–93 [DOI] [PubMed] [Google Scholar]
- 2. Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. 2008. β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaijser M, Bonamy AK, Akre O, Cnattingius S, Granath F, Norman M, Ekbom A. 2009. Perinatal risk factors for diabetes in later life. Diabetes 58:523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, Butler PC. 2010. Adaptive changes in pancreatic β cell fractional area and β cell turnover in human pregnancy. Diabetologia 53:2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freemark M. 2010. Placental hormones and the control of fetal growth. J Clin Endocrinol Metab 95:2054–2057 [DOI] [PubMed] [Google Scholar]
- 6. Van Assche FA, Aerts L, De Prins F. 1978. A morphological study of the endocrine pancreas in human pregnancy. Br J Obstet Gynaecol 85:818–820 [DOI] [PubMed] [Google Scholar]
- 7. Brelje TC, Sorenson RL. 1991. Role of prolactin versus growth hormone on islet B-cell proliferation in vitro: implications for pregnancy. Endocrinology 128:45–57 [DOI] [PubMed] [Google Scholar]
- 8. Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, Friesen HG, Sorenson RL. 1993. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 132:879–887 [DOI] [PubMed] [Google Scholar]
- 9. Brelje TC, Parsons JA, Sorenson RL. 1994. Regulation of islet β-cell proliferation by prolactin in rat islets. Diabetes 43:263–273 [DOI] [PubMed] [Google Scholar]
- 10. Nielsen JH. 1982. Effects of growth hormone, prolactin, and placental lactogen on insulin content and release, and deoxyribonucleic acid synthesis in cultured pancreatic islets. Endocrinology 110:600–606 [DOI] [PubMed] [Google Scholar]
- 11. Swenne I, Hill DJ, Strain AJ, Milner RD. 1987. Effects of human placental lactogen and growth hormone on the production of insulin and somatomedin C/insulin-like growth factor I by human fetal pancreas in tissue culture. J Endocrinol 113:297–303 [DOI] [PubMed] [Google Scholar]
- 12. Fleenor D, Petryk A, Driscoll P, Freemark M. 2000. Constitutive expression of placental lactogen in pancreatic β cells: effects on cell morphology, growth, and gene expression. Pediatr Res 47:136–142 [DOI] [PubMed] [Google Scholar]
- 13. Vasavada RC, Garcia-Ocaña A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF. 2000. Targeted expression of placental lactogen in the β cells of transgenic mice results in β cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 275:15399–15406 [DOI] [PubMed] [Google Scholar]
- 14. Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, Kendall W, Oden J, Bridges S, Binart N, Breant B, Kelly PA. 2002. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology 143:1378–1385 [DOI] [PubMed] [Google Scholar]
- 15. Huang C, Snider F, Cross JC. 2009. Prolactin receptor is required for normal glucose homeostasis and modulation of β-cell mass during pregnancy. Endocrinology 150:1618–1626 [DOI] [PubMed] [Google Scholar]
- 16. Arumugam R, Horowitz E, Lu D, Collier JJ, Ronnebaum S, Fleenor D, Freemark M. 2008. The interplay of prolactin and the glucocorticoids in the regulation of β-cell gene expression, fatty acid oxidation, and glucose-stimulated insulin secretion: implications for carbohydrate metabolism in pregnancy. Endocrinology 149:5401–5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petryk A, Fleenor D, Driscoll P, Freemark M. 2000. Prolactin induction of insulin gene expression: the roles of glucose and glucose transporter-2. J Endocrinol 164:277–286 [DOI] [PubMed] [Google Scholar]
- 18. Weinhaus AJ, Stout LE, Bhagroo NV, Brelje TC, Sorenson RL. 2007. Regulation of glucokinase in pancreatic islets by prolactin: a mechanism for increasing glucose-stimulated insulin secretion during pregnancy. J Endocrinol 193:367–381 [DOI] [PubMed] [Google Scholar]
- 19. Arumugam R, Horowitz E, Noland RC, Lu D, Fleenor D, Freemark M. 2010. Regulation of islet β-cell pyruvate metabolism: interactions of prolactin, glucose, and dexamethasone. Endocrinology 151:3074–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez SC, Cras-Méneur C, Bernal-Mizrachi E, Permutt MA. 2006. Glucose regulates Foxo1 through insulin receptor signaling in the pancreatic islet β-cell. Diabetes 55:1581–1591 [DOI] [PubMed] [Google Scholar]
- 21. Buteau J, Spatz ML, Accili D. 2006. Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic β-cell mass. Diabetes 55:1190–1196 [DOI] [PubMed] [Google Scholar]
- 22. Buteau J, Shlien A, Foisy S, Accili D. 2007. Metabolic diapause in pancreatic β-cells expressing a gain-of-function mutant of the forkhead protein Foxo1. J Biol Chem 282:287–293 [DOI] [PubMed] [Google Scholar]
- 23. Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, 3rd, Wright CV, White MF, Arden KC, Accili D. 2002. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest 110:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okamoto H, Hribal ML, Lin HV, Bennett WR, Ward A, Accili D. 2006. Role of the forkhead protein FoxO1 in β cell compensation to insulin resistance. J Clin Invest 116:775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kushner JA, Ye J, Schubert M, Burks DJ, Dow MA, Flint CL, Dutta S, Wright CV, Montminy MR, White MF. 2002. Pdx1 restores β cell function in Irs2 knockout mice. J Clin Invest 109:1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF. 1998. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904 [DOI] [PubMed] [Google Scholar]
- 27. Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T. 2000. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory β-cell hyperplasia. Diabetes 49:1880–1889 [DOI] [PubMed] [Google Scholar]
- 28. Petrik J, Pell JM, Arany E, McDonald TJ, Dean WL, Reik W, Hill DJ. 1999. Overexpression of insulin-like growth factor-II in transgenic mice is associated with pancreatic islet cell hyperplasia. Endocrinology 140:2353–2363 [DOI] [PubMed] [Google Scholar]
- 29. Zhang H, Zhang J, Pope CF, Crawford LA, Vasavada RC, Jagasia SM, Gannon M. 2010. Gestational diabetes mellitus resulting from impaired β-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes 59:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK. 2007. Menin controls growth of pancreatic β-cells in pregnant mice and promotes gestational diabetes mellitus. Science 318:806–809 [DOI] [PubMed] [Google Scholar]
- 31. Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, Yang K, Honig G, van der Hart M, Kishimoto N, Wang J, Yagihashi S, Tecott LH, Watada H, German MS. 2010. Serotonin regulates pancreatic β cell mass during pregnancy. Nat Med 16:804–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rieck S, White P, Schug J, Fox AJ, Smirnova O, Gao N, Gupta RK, Wang ZV, Scherer PE, Keller MP, Attie AD, Kaestner KH. 2009. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol 23:1702–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Milburn JL, Jr, Hirose H, Lee YH, Nagasawa Y, Ogawa A, Ohneda M, BeltrandelRio H, Newgard CB, Johnson JH, Unger RH. 1995. Pancreatic β-cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J Biol Chem 270:1295–1299 [DOI] [PubMed] [Google Scholar]
- 34. Friedrichsen BN, Richter HE, Hansen JA, Rhodes CJ, Nielsen JH, Billestrup N, Møldrup A. 2003. Signal transducer and activator of transcription 5 activation is sufficient to drive transcriptional induction of cyclin D2 gene and proliferation of rat pancreatic β-cells. Mol Endocrinol 17:945–958 [DOI] [PubMed] [Google Scholar]
- 35. Bain JR, Schisler JC, Takeuchi K, Newgard CB, Becker TC. 2004. An adenovirus vector for efficient RNA interference-mediated suppression of target genes in insulinoma cells and pancreatic islets of langerhans. Diabetes 53:2190–2194 [DOI] [PubMed] [Google Scholar]
- 36. Becker TC, Noel RJ, Coats WS, Gomez-Foix AM, Alam T, Gerard RD, Newgard CB. 1994. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol 43(Pt A):161–189 [DOI] [PubMed] [Google Scholar]
- 37. Freemark M. 2006. Regulation of maternal metabolism by pituitary and placental hormones: roles in fetal development and metabolic programming. Horm Res 65(Suppl 3):41–49 [DOI] [PubMed] [Google Scholar]
- 38. Kaung HL. 1994. Growth dynamics of pancreatic islet cell populations during fetal and neonatal development of the rat. Dev Dyn 200:163–175 [DOI] [PubMed] [Google Scholar]
- 39. Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. 1997. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 138:1736–1741 [DOI] [PubMed] [Google Scholar]
- 40. Yamanouchi H, Kitauchi S, Shiino M. 1997. Changes in prolactin secretion in postnatal rats and effect of neonatal thyroidectomy. Mol Cell Endocrinol 134:101–107 [DOI] [PubMed] [Google Scholar]
- 41. Freemark M, Kirk K, Pihoker C, Robertson MC, Shiu RP, Driscoll P. 1993. Pregnancy lactogens in the rat conceptus and fetus: circulating levels, distribution of binding, and expression of receptor messenger ribonucleic acid. Endocrinology 133:1830–1842 [DOI] [PubMed] [Google Scholar]
- 42. Xue Y, Liu C, Xu Y, Yuan Q, Xu K, Mao X, Chen G, Wu X, Brendel MD, Liu C. 2010. Study on pancreatic islet adaptation and gene expression during pregnancy in rats. Endocrine 37:83–97 [DOI] [PubMed] [Google Scholar]
- 43. Schraenen A, Lemaire K, de Faudeur G, Hendrickx N, Granvik M, Van Lommel L, Mallet J, Vodjdani G, Gilon P, Binart N, in't Veld P, Schuit F. 2010. Placental lactogens induce serotonin biosynthesis in a subset of mouse β cells during pregnancy. Diabetologia 53:2589–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Robertson MC, Schroedter IC, Friesen HG. 1991. Molecular cloning and expression of rat placental lactogen-Iv, a variant of rPL-I present in late pregnant rat placenta. Endocrinology 129:2746–2756 [DOI] [PubMed] [Google Scholar]
- 45. Soares MJ, Colosi P, Talamantes F. 1982. The development and characterization of a homologous radioimmunoassay for mouse placental lactogen. Endocrinology 110:668–670 [DOI] [PubMed] [Google Scholar]
- 46. Voogt J, Robertson M, Friesen H. 1982. Inverse relationship of prolactin and rat placental lactogen during pregnancy. Biol Reprod 26:800–805 [DOI] [PubMed] [Google Scholar]
- 47. Royster M, Driscoll P, Kelly PA, Freemark M. 1995. The prolactin receptor in the fetal rat: cellular localization of messenger ribonucleic acid, immunoreactive protein, and ligand-binding activity and induction of expression in late gestation. Endocrinology 136:3892–3900 [DOI] [PubMed] [Google Scholar]
- 48. Freemark M, Driscoll P, Maaskant R, Petryk A, Kelly PA. 1997. Ontogenesis of prolactin receptors in the human fetus in early gestation. Implications for tissue differentiation and development. J Clin Invest 99:1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Møldrup A, Petersen ED, Nielsen JH. 1993. Effects of sex and pregnancy hormones on growth hormone and prolactin receptor gene expression in insulin-producing cells. Endocrinology 133:1165–1172 [DOI] [PubMed] [Google Scholar]
- 50. Labriola L, Montor WR, Krogh K, Lojudice FH, Genzini T, Goldberg AC, Eliaschewitz FG, Sogayar MC. 2007. Beneficial effects of prolactin and laminin on human pancreatic islet-cell cultures. Mol Cell Endocrinol 263:120–133 [DOI] [PubMed] [Google Scholar]
- 51. Fujinaka Y, Takane K, Yamashita H, Vasavada RC. 2007. Lactogens promote β cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J Biol Chem 282:30707–30717 [DOI] [PubMed] [Google Scholar]
- 52. Cozar-Castellano I, Weinstock M, Haught M, Velázquez-Garcia S, Sipula D, Stewart AF. 2006. Evaluation of β-cell replication in mice transgenic for hepatocyte growth factor and placental lactogen: comprehensive characterization of the G1/S regulatory proteins reveals unique involvement of p21cip. Diabetes 55:70–77 [PubMed] [Google Scholar]
- 53. Lees SJ, Childs TE, Booth FW. 2008. Age-dependent FOXO regulation of p27Kip1 expression via a conserved binding motif in rat muscle precursor cells. Am J Physiol Cell Physiol 295:C1238–C1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV, Ishihara O, Lam EW, Unterman TG, Brosens JJ, Kim JJ. 2007. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 21:2334–2349 [DOI] [PubMed] [Google Scholar]
- 55. Evans-Anderson HJ, Alfieri CM, Yutzey KE. 2008. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res 102:686–694 [DOI] [PubMed] [Google Scholar]
- 56. Georgia S, Bhushan A. 2006. p27 Regulates the transition of β-cells from quiescence to proliferation. Diabetes 55:2950–2956 [DOI] [PubMed] [Google Scholar]
- 57. Rachdi L, Balcazar N, Elghazi L, Barker DJ, Krits I, Kiyokawa H, Bernal-Mizrachi E. 2006. Differential effects of p27 in regulation of β-cell mass during development, neonatal period, and adult life. Diabetes 55:3520–3528 [DOI] [PubMed] [Google Scholar]
- 58. Del Guerra S, Lupi R, Marselli L, Masini M, Bugliani M, Sbrana S, Torri S, Pollera M, Boggi U, Mosca F, Del Prato S, Marchetti P. 2005. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 54:727–735 [DOI] [PubMed] [Google Scholar]
- 59. Müssig K, Staiger H, Machicao F, Kirchhoff K, Guthoff M, Schäfer SA, Kantartzis K, Silbernagel G, Stefan N, Holst JJ, Gallwitz B, Häring HU, Fritsche A. 2009. Association of type 2 diabetes candidate polymorphisms in KCNQ1 with incretin and insulin secretion. Diabetes 58:1715–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, Kido Y, Hayashi Y, Nakayama KI, White MF, Kasuga M. 2005. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 11:175–182 [DOI] [PubMed] [Google Scholar]
- 61. Fontanière S, Tost J, Wierinckx A, Lachuer J, Lu J, Hussein N, Busato F, Gut I, Wang ZQ, Zhang CX. 2006. Gene expression profiling in insulinomas of Men1 β-cell mutant mice reveals early genetic and epigenetic events involved in pancreatic β-cell tumorigenesis. Endocr Relat Cancer 13:1223–1236 [DOI] [PubMed] [Google Scholar]
- 62. Agarwal SK, Mateo CM, Marx SJ. 2009. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab 94:1826–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kassem SA, Ariel I, Thornton PS, Hussain K, Smith V, Lindley KJ, Aynsley-Green A, Glaser B. 2001. p57(KIP2) expression in normal islet cells and in hyperinsulinism of infancy. Diabetes 50:2763–2769 [DOI] [PubMed] [Google Scholar]
- 64. Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF. 2005. Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Mol Cell Biol 25:3752–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Georgia S, Bhushan A. 2004. β Cell replication is the primary mechanism for maintaining postnatal β cell mass. J Clin Invest 114:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Davis DB, Lavine JA, Suhonen JI, Krautkramer KA, Rabaglia ME, Sperger JM, Fernandez LA, Yandell BS, Keller MP, Wang IM, Schadt EE, Attie AD. 2010. FoxM1 is up-regulated by obesity and stimulates β-cell proliferation. Mol Endocrinol 24:1822–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ryu JM, Lee MY, Yun SP, Han HJ. 2010. High glucose regulates cyclin D1/E of human mesenchymal stem cells through TGF-β1 expression via Ca2+/PKC/MAPKs and PI3K/Akt/mTOR signal pathways. J Cell Physiol 224:59–70 [DOI] [PubMed] [Google Scholar]
- 68. Lingohr MK, Briaud I, Dickson LM, McCuaig JF, Alárcon C, Wicksteed BL, Rhodes CJ. 2006. Specific regulation of IRS-2 expression by glucose in rat primary pancreatic islet β-cells. J Biol Chem 281:15884–15892 [DOI] [PubMed] [Google Scholar]
- 69. Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. 2009. Glucose effects on β-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol 29:3219–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Asfari M, De W, Nöel M, Holthuizen PE, Czernichow P. 1995. Insulin-like growth factor-II gene expression in a rat insulin-producing β-cell line (INS-1) is regulated by glucose. Diabetologia 38:927–935 [DOI] [PubMed] [Google Scholar]
- 71. Franklin DS, Godfrey VL, O'Brien DA, Deng C, Xiong Y. 2000. Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol Cell Biol 20:6147–6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK. 2005. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci USA 102:14659–14664 [DOI] [PMC free article] [PubMed] [Google Scholar]