Abstract
Objectives:
The Vitamin Intervention for Stroke Prevention trial found an association between baseline poststroke homocysteine (Hcy) and recurrent stroke. We investigated genes for enzymes and cofactors in the Hcy metabolic pathway for association with Hcy and determined whether associated single nucleotide polymorphisms (SNPs) influenced recurrent stroke risk.
Methods:
Eighty-six SNPs in 9 candidate genes (BHMT1, BHMT2, CBS, CTH, MTHFR, MTR, MTRR, TCN1, and TCN2) were genotyped in 2,206 subjects (83% European American). Associations with Hcy measures were assessed using linear regression models assuming an additive genetic model, adjusting for age, sex, and race and additionally for baseline Hcy when postmethionine load change was assessed. Associations with recurrent stroke were evaluated using survival analyses.
Results:
Five SNPs in the transcobalamin 2 (TCN2) gene were associated with baseline Hcy (false discovery rate [FDR]–adjusted p = 0.049). TCN2 SNP rs731991 was associated with recurrent stroke risk in the low-dose arm of the trial under a recessive model (log-rank test p = 0.009, hazard ratio 0.34). Associations with change in postmethionine load Hcy levels were found with 5 SNPs in the cystathionine β-synthase (CBS) gene (FDR-adjusted p < 0.031).
Conclusions:
TCN2 variants contribute to poststroke Hcy levels, whereas variants in the CBS gene influence Hcy metabolism. Variation in the TCN2 gene also affects recurrent stroke risk in response to cofactor therapy.
Recurrent stroke is the most important modifiable predictor of death or disability at 5 years after first stroke,1 and 54% of those having a recurrent stroke will be disabled.2 The Vitamin Intervention for Stroke Prevention (VISP) trial enrolled patients with cerebral infarction and homocysteine (Hcy) levels in the top quartile for the US population.3 Subjects were randomly assigned to a high or low dose of folic acid, vitamin B6, and vitamin B12. There was a persistent and graded association between baseline Hcy level and vascular outcomes in both treatment groups. Subgroup analyses of the VISP trial showed that baseline fasting Hcy was comparable to postmethionine load test Hcy or change in postmethionine load Hcy in predicting risk of both recurrent stroke and symptomatic coronary heart disease.4,5
A number of studies have found evidence that mild to moderate hyperhomocystinemia may result from genetic variations that alter enzymatic activity in the remethylation or transsulfuration pathways. Associations with Hcy have been reported with genes for methylenetetrahydrofolate reductase (MTHFR), cystathionine β-synthase (CBS), methionine synthase (MTR), methionine synthase reductase (MTRR), and cystathionase (CTH/GCT),6,7 betaine homocysteine methyltransferase (BHMT),8 and transcobalamin 2 (TCN2).8–10 Our objective was to investigate the spectrum of common variations of genes in the Hcy metabolic pathway for contributions to baseline Hcy measures and determine whether associated SNPs also affect recurrent stroke risk.
METHODS
Standard protocol approvals, registrations, and patient consents.
The VISP trial (clinical trial identifier number NCT00004734) was conducted under institutional review board approval at Wake Forest University School of Medicine and each of the clinic sites and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Study design and laboratory measures.
VISP was a multicenter, double-blind, randomized controlled clinical trial that enrolled patients aged 35 or older with a nondisabling cerebral infarction (defined as modified Rankin Scale score ≤3) within 120 days of randomization and Hcy levels greater than the 25th percentile for the general population at screening.3 In August 1997, the eligible Hcy level (25th percentile) was initially >10.5 μmol/L, was modified to >9.5 μmol/L for both sexes on April 7, 1998, and was modified to >9.5 μmol/L (men) and >8.5 μmol/L (women) after April 1, 1999. Subjects were randomly assigned to receive daily doses of the high-dose formulation containing 25 mg pyridoxine (vitamin B6), 0.4 mg cobalamin (vitamin B12), and 2.5 mg folic acid or the low-dose formulation containing 200 μg pyridoxine, 6 μg cobalamin, and 20 μg folic acid. In December 2002, the VISP Data and Safety Monitoring Board recommended to the National Institute of Neurological Disorders and Stroke that the trial be terminated because of the unlikely event of ever demonstrating a treatment difference. At the time of early trial closure, all participants had been in the study at least 1 year. The average follow-up time was 1.7 years (maximum 2 years).
Fasting plasma samples for determination of baseline Hcy were obtained at enrollment. Patients were given a dose of methionine (100 mg/ kg body weight) in fruit juice, and a second blood sample was drawn after 2 hours. Recurrent stroke was defined as an acute ischemic stroke of at least 24 hours duration with focal signs and symptoms, without evidence of primary intracranial hemorrhage or other alternative explanation, together with one of the following: a 1-point increase in the NIH Stroke Scale (NIHSS) in a previously normal section or, lacking this, an appropriate new or extended abnormality seen on CT or MRI.
Of the 56 VISP study centers, 46 centers collected blood samples for genetic analyses. DNA was isolated using a Gentra Autopure system (Qiagen, Germantown, MD). Samples underwent whole-genome amplification (WGA) using Repli-g methodology through Molecular Staging Inc. (New Haven, CT) and Qiagen (Hilden, Germany).
SNP selection and genotyping.
Ninety-six single nucleotide polymorphisms (SNPs) were selected in 9 genes involved in Hcy metabolism: BHMT1, BHMT2, CBS, CTH, MTHFR, MTR, MTRR, TCN1, and TCN2 (table e-1 on the Neurology® Web site at www.neurology.org). SNPs were selected in candidate gene loci according to the following criteria: 1) within the proximal and distal 10-kb regions 5′ and 3′ to the given candidate gene (NCBI Build 35); 2) compatibility with the Illumina GoldenGate technology11 as determined by the Assay Design Tool (TechSupport; Illumina, Inc., San Diego, CA); 3) minor allele frequency (MAF) >0.05 or a tag (r2 >0.8) for another SNP with MAF >0.05 as determined by applying the multilocus or “aggressive” Tagger option of Haploview v312 using International HapMap Project data for CEPH and Yoruban populations (release 19).13 For BHMT1, CTH, MTHFR, MTR, MTRR, and TCN2, additional SNPs with prior report of association with a phenotype of interest to the VISP investigators were added (table e-1 a priori candidate SNPs).6–8,10 Genotyping assays using WGA DNA were conducted by the Custom Genotyping Service of Illumina, Inc using the GoldenGate assay. Although the panel included 1,536 SNPs selected largely from vascular candidate genes, the 9 genes analyzed in the present study were selected specifically as a priori biological candidates for associations with baseline Hcy measures in the VISP trial and have therefore been evaluated separately. Eighty-two (85%) of the 96 SNPs selected in the 9 candidate genes were successfully genotyped at a >96% success rate in 2,206 unique subjects. Reproducibility between replicate samples was >99.99%. Four replicates with both WGA and genomic DNA were included in the dataset; all were 100% concordant for all available genotypes.
TCN2 coding SNP rs1801198 (R259P, C776G)8–10 was included in the Illumina GoldenGate genotyping panel but failed to genotype successfully. This SNP, together with TCN2 coding SNPs rs9621049 (S348F) and rs1131603 (L376S) and rs2072195 located in the 3′ untranslated region (3′UTR), were prioritized on the basis of prior association with Hcy or linkage disequilibrium (LD) with SNP rs731991 (associated with recurrent stroke) and subsequently were genotyped using the TaqMan assay.14 Endpoint allelic discrimination was detected using a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Genotyping success rates were >95% for all 4 SNPs.
Statistical analyses.
Associations with Hcy measures were evaluated using linear regression models assuming an additive genetic model, adjusting for age, sex, and race, and, in addition, for baseline Hcy when postmethionine load change was assessed. A dominant genetic model was used in which the minor allele homozygote count was <10. Because there were few individuals per site (from 1 to 127), analyses were not adjusted for collection center. Further adjustments for smoking, levels of folate, vitamins B6, and B12, and stroke severity (measured by the NIHSS) were also explored but did not change the conclusions. A least-squares (LS) mean for each genotype, considered the group mean after adjustment for a covariate, is presented.
False discovery rate (FDR)15,16 was used to adjust for the multiple comparisons for the 86 SNPs. It controls the expected proportion of falsely rejected hypotheses (the FDR). Its threshold is determined from the observed p value distribution and is adaptive to the amount of significant results in the data. It is less conservative than the commonly used Bonferroni adjustment. FDR-adjusted p values were calculated using the procedure multtest in SAS (SAS Institute, Cary, NC).
We assessed associations with recurrent stroke using survival analyses. We initially considered only TCN2 rs731991 and CBS rs234709 and subsequently evaluated the 4 TaqMan-genotyped coding and 3′UTR SNPs rs1801198, rs9621049, rs1131603, and rs2072195. Censoring may occur because of the loss of follow-up or death. First, we used the Kaplan-Meier method to estimate the survival functions for subjects with different genotypes in the 2-year follow-up. The cumulative incidence curve of recurrent stroke was compared using a logrank test. Second, we evaluated whether the selected SNPs were also associated with recurrent stroke using combined and treatment-stratified Cox proportional hazards models. All models were adjusted for age, sex, and race. Note that the interaction between the SNP and treatment was examined before the treatment-stratified models were performed.
RESULTS
Population characteristics.
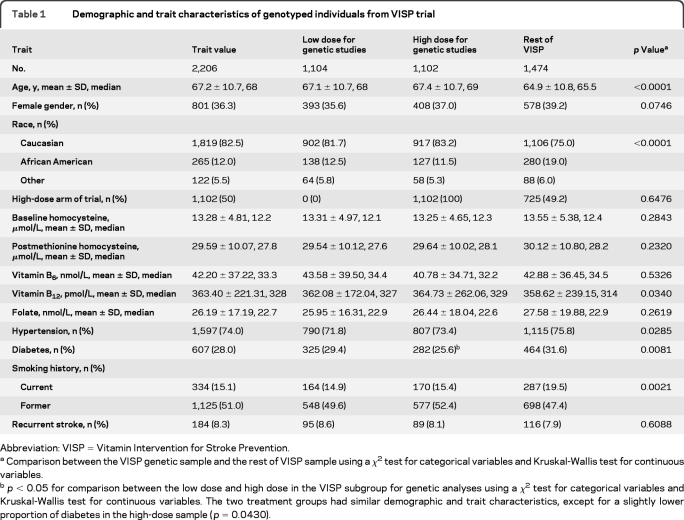
The characteristics of the subpopulation used for this study and the remainder of the VISP population are shown in table 1. The genetic sample is on average 3 years older and more likely to be of European American (EA) origin, compared with participants who did not provide a genetic sample or whose sample was unable to be genotyped. Sites that chose not to collect blood samples for genetic or biomarker studies represented 840 participants from the parent trial, whereas 167 subjects from enrolling sites chose not to participate in the genetic studies. Biases are therefore more likely to reflect nonparticipation in the VISP genetic study by specific sites, rather than individual participation bias. Participants in the 2 arms of the trial investigated in this study are comparable.
Table 1.
Demographic and trait characteristics of genotyped individuals from VISP trial
Abbreviation: VISP = Vitamin Intervention for Stroke Prevention.
Comparison between the VISP genetic sample and the rest of VISP sample using a χ2 test for categorical variables and Kruskal-Wallis test for continuous variables.
p < 0.05 for comparison between the low dose and high dose in the VISP subgroup for genetic analyses using a χ2 test for categorical variables and Kruskal-Wallis test for continuous variables. The two treatment groups had similar demographic and trait characteristics, except for a slightly lower proportion of diabetes in the high-dose sample (p = 0.0430).
Associations with baseline Hcy levels.
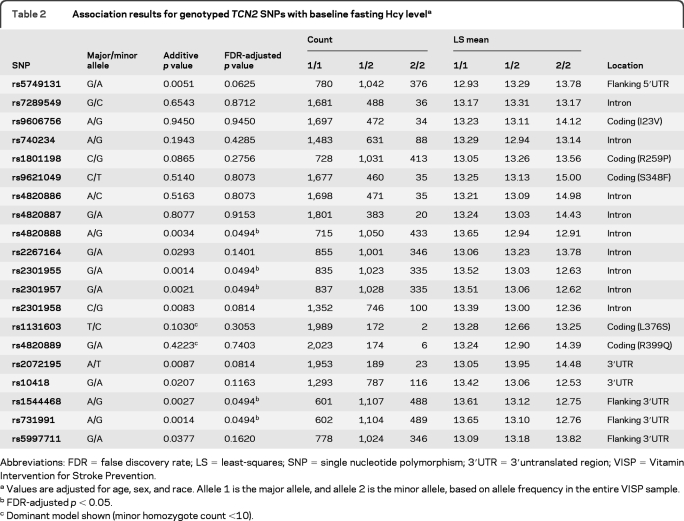
The 5 SNPs significantly associated with baseline fasting Hcy were located in the gene for the vitamin B12 transporter transcobalamin 2 (TCN2) (FDR-adjusted p = 0.0494) (table 2). Associated TCN2 SNPs were consistent with Hardy-Weinberg proportions (p > 0.19). TCN2 coding SNPs rs1801198 (R259P), rs9606756 (I22V), and rs4820889 (R398Q) were not associated with baseline Hcy (table 2). In race-stratified analyses (not shown), TCN2 rs731991 association with Hcy is predominantly driven by EAs (rs731991 A/A genotype LS mean 13.75 μmol/L Hcy, A/G 12.98, G/G 12.76; p = 0.0014), although results trend in the same direction in African Americans (AAs) (rs731991 A/A LS mean 14.26 μmol/L Hcy, A/G 13.92, G/G 13.17; p = 0.31). Differences in significance between racial groups may be influenced by differing allele frequencies (rs731991 MAF: EA 0.49; AA 0.35) as well as differences in power due to contrasting sample sizes (EA n = 1,770 and AA n = 262 genotyped at this SNP). TCN2 LD structure in the VISP sample is shown in figure e-1.
Table 2.
Association results for genotyped TCN2 SNPs with baseline fasting Hcy levela
Abbreviations: FDR = false discovery rate; LS = least-squares; SNP = single nucleotide polymorphism; 3′UTR = 3′untranslated region; VISP = Vitamin Intervention for Stroke Prevention.
Values are adjusted for age, sex, and race. Allele 1 is the major allele, and allele 2 is the minor allele, based on allele frequency in the entire VISP sample.
FDR-adjusted p < 0.05.
Dominant model shown (minor homozygote count <10).
Associations with postmethionine load Hcy levels.
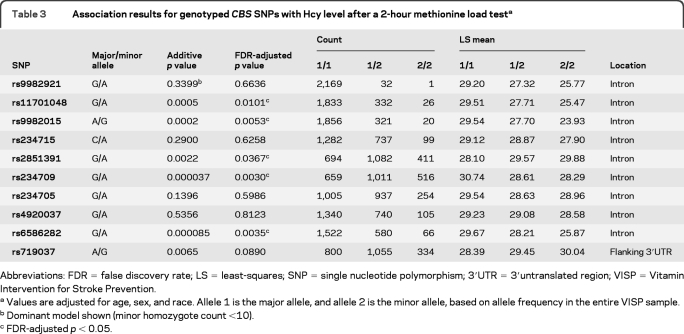
The strongest associations with postmethionine load Hcy levels were with 5 SNPs in the CBS gene (FDR-adjusted p < 0.031) (table 3). Four of these CBS SNPs plus one additional SNP were also associated with change in postmethionine load Hcy level (table e-2). All associated CBS SNPs were consistent with Hardy-Weinberg proportions (p > 0.02), except for rs234709 (p = 0.001).
Table 3.
Association results for genotyped CBS SNPs with Hcy level after a 2-hour methionine load testa
Abbreviations: FDR = false discovery rate; LS = least-squares; SNP = single nucleotide polymorphism; 3′UTR = 3′untranslated region; VISP = Vitamin Intervention for Stroke Prevention.
Values are adjusted for age, sex, and race. Allele 1 is the major allele, and allele 2 is the minor allele, based on allele frequency in the entire VISP sample.
Dominant model shown (minor homozygote count <10).
FDR-adjusted p < 0.05.
Associations with recurrent stroke.
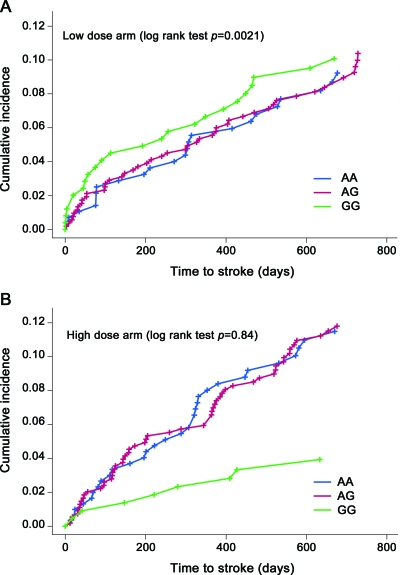
Given that both baseline fasting Hcy and the methionine load test predicted risk of recurrent stroke,4,5 we evaluated whether the SNPs most strongly associated with baseline Hcy level, rs731991 (table 2) and rs234709 (table 3), were also associated with recurrent stroke using combined (not shown) and treatment-stratified Cox proportional hazards models. All models were adjusted for age, sex, and race. Survival analysis of TCN2 rs731991 showed a significant association between AA/AG genotypes and increased risk of recurrent stroke in the low-dose arm of the study. There were 52 recurrent strokes in subjects with the rs731991 AA genotype, 101 in those with the AG genotype, and 30 in those with the GG genotype. Although the additive model was initially tested, subsequent examination of results (figure 1) demonstrated that a recessive model best fits the data (logrank test p = 0.009, hazard ratio 0.34). No significant difference was seen in the high-dose arm (p = 0.92). The p value for interaction between rs731991 (AA/AG vs GG) and treatment (high vs low) was 0.0087. Forty-four subjects with a B12 level of less than 150 pmol/L at baseline, 6 months, or 18 months received supplemental B12 during the course of the trial to reduce the likelihood of neurologic consequences. However, only 2 of these subjects experienced recurrent stroke during the course of the trial. Both received supplemental B12 only at baseline. Exclusion of these 2 subjects (who carry rs731991 AG and GG genotypes) from survival analyses did not change our conclusions.
Figure 1. Cumulative incidence curves of recurrent stroke for TCN2 SNP rs731991, stratified by (A) low-dose and (B) high-dose arms of the trial.
Blue line, AA genotype, red line, AG genotype; green line, GG genotype.
No associations with recurrent stroke were observed with putative functional TCN2 coding SNPs (rs1801198, rs9621049, and rs1131603) or rs2072195. We did not find any relationship between CBS SNP rs234709 and recurrent stroke (p = 0.95 in the low-dose arm and p = 0.86 in the high-dose arm).
DISCUSSION
Our results are consistent with previous suggestions that plasma levels of fasting Hcy reflect cobalamin (vitamin B12)- and folate-dependent remethylation, whereas levels postmethionine loading reflect pyridoxal 5′-phosphate (vitamin B6)–dependent transsulfuration.17
Transcobalamin 2 is the primary plasma facilitator of cellular uptake of B12.18 The widely studied TCN2 C776G (rs1801198, or R259P) mutation9,10 and A67G (rs9606756, I22V)10 have been associated with plasma Hcy levels in other studies and influence the proportion of B12 bound to transcobalamin.19 In contrast, in our study, neither SNP was associated with baseline poststroke Hcy measures (table 2). However, 5 other SNPs in the TCN2 gene, located in intron 7 and flanking the 3′UTR, were associated with baseline fasting Hcy (table 2). Interestingly, rs731991 was not associated with the baseline plasma B12 level (p = 0.83), although 10 TCN2 SNPs (rs731991, rs2267164, rs1544468, rs1801198, rs482088, rs4820887, rs2301957, rs2301955, rs9606756, and rs740234) showed a significant interaction with B12 in determining baseline Hcy (FDR-adjusted p < 0.05). The most significant interactions were with rs731991 and rs2267164 (FDR-adjusted p = 0.00213). Baseline B12 levels may reflect the complex combination of dietary intake, turnover, and excretion rates, whereas interactions may relate to B12 plasma transport and bioavailability for participation as a cofactor in the one-carbon metabolic pathway.
The TCN2 genotype appears to influence the recurrent stroke event rate only in the setting of inadequate B12 supplementation (figure 1). The recurrent stroke rate differed by the rs731991 genotype in the low-dose arm despite subjects receiving 3 times the recommended daily allowance for B12 (6 μg) and slightly higher B12 levels in the genetic substudy compared with the rest of the VISP sample (table 1) and the mean Hcy difference between the 2 homozygote extremes (AA vs GG) at baseline being only 0.89 μmol/L (table 2). In the high-dose arm, supplementation with 400 μg B12 potentially overwhelms any putative influence of TCN2 rs731991 on B12 bioavailability because recurrent stroke rates are the same across all groups (p = 0.84) (figure 1). An alternative explanation is that, although the majority of subjects (rs731991 A/A and A/G genotypes) see a modest decrease in recurrent stroke risk with B vitamin supplementation, rs731991 G/G carriers' risk for recurrent stroke increases (or they lose their relative protection) with B vitamin supplementation (figure 1). If, for example, this group is more efficient at transporting B12, there are reports of increased vascular disease with high B12 levels.20–22 Further analyses will be required to localize possibly independent regions of association and determine their function.
Methionine loading may expose a latent abnormality of Hcy metabolism in up to 40% of people with a fasting plasma Hcy level in the normal range.17,23 CBS deficiency is the most common cause of homocystinuria.24 A common 68-bp insertion at the intron 7-exon 8 boundary of the CBS gene (844ins68) present in the heterozygous state in approximately 12% of Caucasians25 has been associated with lower postmethionine load Hcy levels,26,27 especially in the presence of low vitamin B6. A 31-bp variable number tandem repeat (VNTR) that spans the exon 13–intron 13 boundary of the CBS gene has also been associated with Hcy and postmethionine load levels via alternative splicing at the exon 13–intron 13 splice junction site, with an increasing number of repeat elements showing a significant increase in plasma Hcy concentrations, especially after methionine loading.19,28 How associated CBS SNPs (table 3) relate to the 68-bp insertion and 31-bp VNTR in terms of LD remains to be explored. Although associated SNP rs6586282 is within intron 12, 244 bp from the VNTR, the most associated SNP, rs234709, is located in intron 2 and a separate LD block (figure e-2).
The modest reduction in total Hcy observed in the VISP trial may be due in part to the addition of folic acid to enriched cereal grain foods in the United States (initiated in 1996 and mandated in January 1998) that coincided with the initiation of the VISP trial,4 probably reducing the number of participants with high total Hcy who might be most likely to benefit from the intervention.29 The VISP trial investigators4 concluded that determinants of Hcy other than folate may be more important in this setting, which would be consistent with our findings.
The extensively studied “thermolabile” MTHFR C677T SNP (rs1801133) with reduced enzymatic activity30 was not associated with Hcy (p = 0.22), postmethionine load test Hcy (p = 0.0104), or change in postmethionine load Hcy (p = 0.031). Associations between MTHFR 677TT genotype and mild hyperhomocysteinemia have been detected primarily in the presence of low folate status.31–33 However, there was no significant interaction between any of the candidate gene SNPs and baseline folate in determining Hcy (FDR-adjusted p > 0.58), including MTHFR C677T (FDR-adjusted p = 0.99). It is possible that folate fortification and the VISP intervention treatments negated the impact of MTHFR variation on Hcy in the majority of VISP trial patients.
There are a number of limitations to the present study. Prospective serial analyses of Hcy after ischemic stroke have shown that Hcy increases in the subacute stage and remains stable during the convalescent period.34–37 Results may not be generalizable to an event-free population or the full spectrum of Hcy values. However, 75% of those screened qualified for the study, and the effect of hyperhomocysteinemia is believed to be linear,38,39 so results should be generalizable to the population at greatest risk for recurrent stroke. The 4 genomic and WGA replicates had consistent genotypes across all 82 SNPs, suggesting that there was no amplification bias based on SNP location. However, this does not represent a broad sampling of DNAs of differing quality; thus, we cannot rule out possible artifacts introduced by WGA in a subset of samples.
If we had used the conservative Bonferroni correction for the 82 SNP tests (p < 0.0006), only 4 associations with postmethionine load (rs11701048, rs9982015, rs234709, and rs6586282) (table 3) and Hcy change (rs2851391, rs234709, rs6586282, and rs719037) (table e-2) would be considered significant. Using the FDR approach, we were able to additionally detect significant associations with 5 TCN2 SNPs (rs4820888, rs2301955, rs2301957, rs1544468, and rs731991) associated with baseline fasting Hcy level, CBS SNP rs2851391 associated with postmethionine load, and CBS SNP rs9982015 associated with Hcy change.
Varying combinations and doses of folate, vitamin B6, and vitamin B12 have been studied in high-risk populations in large-scale trials with conflicting results, and the exact role of Hcy and the impact of vitamin supplementation on vascular events remains a controversial topic.40 A multifactorial approach that takes into account genotypes that influence individual responses to enzymatic cofactor therapy may need to be considered to reconcile the results of vitamin supplementation trials. Genetic factors that determine bioavailability of cobalamin, such as TCN2, may be important confounders of the effectiveness of cofactor supplementation on recurrent stroke risk.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients for their participation; recruiting teams at each of the VISP clinic sites; Stephen Campbell, Lily Wang, PhD, and Lloyd Chambless, PhD, from the VISP Statistical Coordinating Center at the University of North Carolina at Chapel Hill; M. René Malinow, MD, OR Regional Primate Research Center, for his pivotal role as one of the instigators of the VISP trial and as an important consultant throughout the trial; technicians Charlotte Hollingsworth, Candace Gordon, Lindsay Holder, Michael Gehring, and Andrew Hniat, programmer Min Zhong, Mark Hansen, and colleagues at Illumina Inc.; and Tim Howard, PhD, Wake Forest University School of Medicine, for helpful discussions.
GLOSSARY
- AA
African American
- EA
European American
- FDR
false discovery rate
- Hcy
homocysteine
- LD
linkage disequilibrium
- LS
least-squares
- MAF
minor allele frequency
- NIHSS
NIH Stroke Scale
- SNP
single nucleotide polymorphism
- 3′UTR
3′untranslated region
- VISP
Vitamin Intervention for Stroke Prevention
- VNTR
variable number tandem repeat
- WGA
whole-genome amplification
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Hsu: drafting/revising the manuscript, analysis or interpretation of data, and statistical analysis. E.G. Sides: drafting/revising the manuscript and acquisition of data. Dr. Mychaleckyj: study concept or design and analysis or interpretation of data. Dr. Worrall: drafting/revising the manuscript, acquisition of data, and study supervision. G. Elias: analysis or interpretation of data, acquisition of data, and statistical analysis. Dr. Liu: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, and study supervision. Dr. Chen: analysis or interpretation of data and statistical analysis. Dr. Coull: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, and study supervision. Dr. Toole: drafting/revising the manuscript, study concept or design, study supervision, and obtaining funding. Dr. Rich: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, and obtaining funding. Dr. Furie: drafting/revising the manuscript, study concept or design, and analysis or interpretation of data. Dr. Sale: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, and obtaining funding.
DISCLOSURE
Dr. Hsu and E.G. Sides report no disclosures. Dr. Mychaleckyj receives research support from Abbott, the NIH, and the Bill and Melinda Gates Foundation. Dr. Worrall serves as an Associate Editor of Neurology® and on the editorial board of Seminars in Neurology; receives royalties from the publication of Merritt's Neurology, 10th, 11th, and 12th eds. (chapter author); receives/has received research support from the NIH (NHGRI, NHLBI, NINDS) and from the University of Virginia–CTSA Pilot Project. G. Elias, Dr. Liu, and Dr. Chen report no disclosures. Dr. Coull serves on data safety monitoring boards for the NIH/NINDS and on a scientific advisory board for Neurobiological Technologies; and serves on the editorial boards of Stroke and Journal of Stroke and Cerebrovascular Diseases. Dr. Toole reports no disclosures. Dr. Rich receives research support from the NIH and Wake Forest University. Dr. Furie serves on a data safety monitoring board for the NIH/NINDS; serves as Vice Editor of Stroke; receives publishing royalties from UpToDate, Inc.; and receives research support from the NIH/NINDS, the American Heart Association, and the Deane Institute. Dr. Sale receives research support from the NIH.
REFERENCES
- 1. Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989–1990. Stroke 2002;33:1034–1040 [DOI] [PubMed] [Google Scholar]
- 2. Hankey GJ, Spiesser J, Hakimi Z, Carita P, Gabriel S. Time frame and predictors of recovery from disability following recurrent ischemic stroke. Neurology 2007;68:202–205 [DOI] [PubMed] [Google Scholar]
- 3. Toole JF. Vitamin intervention for stroke prevention. J Neurol Sci 2002;203–204:121–124 [DOI] [PubMed] [Google Scholar]
- 4. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004;291:565–575 [DOI] [PubMed] [Google Scholar]
- 5. Pettigrew LC, Bang H, Chambless LE, Howard VJ, Toole JF. Assessment of pre- and post-methionine load homocysteine for prediction of recurrent stroke and coronary artery disease in the Vitamin Intervention for Stroke Prevention Trial. Atherosclerosis 2008;200:345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma P, Senthilkumar RD, Brahmachari V, et al. Mining literature for a comprehensive pathway analysis: a case study for retrieval of homocysteine related genes for genetic and epigenetic studies. Lipids Health Dis 2006;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang J, Huff AM, Spence JD, Hegele RA. Single nucleotide polymorphism in CTH associated with variation in plasma homocysteine concentration. Clin Genet 2004;65:483–486 [DOI] [PubMed] [Google Scholar]
- 8. Fredriksen A, Meyer K, Ueland PM, Vollset SE, Grotmol T, Schneede J. Large-scale population-based metabolic phenotyping of thirteen genetic polymorphisms related to one-carbon metabolism. Hum Mutat 2007;28:856–865 [DOI] [PubMed] [Google Scholar]
- 9. Namour F, Olivier J, Abdelmouttaleb I, et al. Transcobalamin codon 259 polymorphism in HT-29 and Caco-2 cells and in Caucasians: relation to transcobalamin and homocysteine concentration in blood. Blood 2001;97:1092–1098 [DOI] [PubMed] [Google Scholar]
- 10. Lievers KJ, Afman LA, Kluijtmans LA, et al. Polymorphisms in the transcobalamin gene: association with plasma homocysteine in healthy individuals and vascular disease patients. Clin Chem 2002;48:1383–1389 [PubMed] [Google Scholar]
- 11. Gunderson KL, Kruglyak S, Graige MS, et al. Decoding randomly ordered DNA arrays. Genome Res 2004;14:870–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005;21:263–265 [DOI] [PubMed] [Google Scholar]
- 13. International HapMap Consortium The International HapMap Project. Nature 2003;426:789–796 [DOI] [PubMed] [Google Scholar]
- 14. Livak KJ, Marmaro J, Todd JA. Towards fully automated genome-wide polymorphism screening. Nat Genet 1995;9:341–342 [DOI] [PubMed] [Google Scholar]
- 15. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300 [Google Scholar]
- 16. Benjamini Y, Yekateuli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat 2001;29:1165–1188 [Google Scholar]
- 17. Bostom AG, Shemin D, Lapane KL, et al. Folate status is the major determinant of fasting total plasma homocysteine levels in maintenance dialysis patients. Atherosclerosis 1996;123:193–202 [DOI] [PubMed] [Google Scholar]
- 18. Seetharam B, Bose S, Li N. Cellular import of cobalamin (vitamin B-12). J Nutr 1999;129:1761–1764 [DOI] [PubMed] [Google Scholar]
- 19. Afman LA, Lievers KJ, Kluijtmans LA, Trijbels FJ, Blom HJ. Gene-gene interaction between the cystathionine β-synthase 31 base pair variable number of tandem repeats and the methylenetetrahydrofolate reductase 677C>T polymorphism on homocysteine levels and risk for neural tube defects. Mol Genet Metab 2003;78:211–215 [DOI] [PubMed] [Google Scholar]
- 20. House AA, Eliasziw M, Cattran DC, et al. Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA 2010;303:1603–1609 [DOI] [PubMed] [Google Scholar]
- 21. Pancharuniti N, Lewis CA, Sauberlich HE, et al. Plasma homocyst(e)ine, folate, and vitamin B-12 concentrations and risk for early-onset coronary artery disease. Am J Clin Nutr 1994;59:940–948 [DOI] [PubMed] [Google Scholar]
- 22. Zeitlin A, Frishman WH, Chang CJ. The association of vitamin B 12 and folate blood levels with mortality and cardiovascular morbidity incidence in the old old: the Bronx aging study. Am J Ther 1997;4:275–281 [DOI] [PubMed] [Google Scholar]
- 23. Graham IM, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project JAMA 1997;277:1775–1781 [DOI] [PubMed] [Google Scholar]
- 24. Kluijtmans LA, Boers GH, Stevens EM, et al. Defective cystathionine β-synthase regulation by S-adenosylmethionine in a partially pyridoxine responsive homocystinuria patient. J Clin Invest 1996;98:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsai MY, Bignell M, Schwichtenberg K, Hanson NQ. High prevalence of a mutation in the cystathionine β-synthase gene. Am J Hum Genet 1996;59:1262–1267 [PMC free article] [PubMed] [Google Scholar]
- 26. De Stefano V, Dekou V, Nicaud V, et al. Linkage disequilibrium at the cystathionine β synthase (CBS) locus and the association between genetic variation at the CBS locus and plasma levels of homocysteine: the Ears II Group: European Atherosclerosis Research Study. Ann Hum Genet 1998;62:481–490 [DOI] [PubMed] [Google Scholar]
- 27. Tsai MY, Yang F, Bignell M, Aras O, Hanson NQ. Relation between plasma homocysteine concentration, the 844ins68 variant of the cystathionine β-synthase gene, and pyridoxal-5′-phosphate concentration. Mol Genet Metab 1999;67:352–356 [DOI] [PubMed] [Google Scholar]
- 28. Lievers KJ, Kluijtmans LA, Heil SG, et al. A 31 bp VNTR in the cystathionine β-synthase (CBS) gene is associated with reduced CBS activity and elevated post-load homocysteine levels. Eur J Hum Genet 2001;9:583–589 [DOI] [PubMed] [Google Scholar]
- 29. Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA 1992;268:877–881 [PubMed] [Google Scholar]
- 30. Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995;10:111–113 [DOI] [PubMed] [Google Scholar]
- 31. Harmon DL, Woodside JV, Yarnell JW, et al. The common ‘thermolabile’ variant of methylene tetrahydrofolate reductase is a major determinant of mild hyperhomocysteinaemia. QJM 1996;89:571–577 [DOI] [PubMed] [Google Scholar]
- 32. Jacques PF, Bostom AG, Williams RR, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation 1996;93:7–9 [DOI] [PubMed] [Google Scholar]
- 33. Malinow MR, Nieto FJ, Kruger WD, et al. The effects of folic acid supplementation on plasma total homocysteine are modulated by multivitamin use and methylenetetrahydrofolate reductase genotypes. Arterioscler Thromb Vasc Biol 1997;17:1157–1162 [DOI] [PubMed] [Google Scholar]
- 34. Haapaniemi E, Helenius J, Soinne L, Syrjala M, Kaste M, Tatlisumak T. Serial measurements of plasma homocysteine levels in early and late phases of ischemic stroke. Eur J Neurol 2007;14:12–17 [DOI] [PubMed] [Google Scholar]
- 35. Howard VJ, Sides EG, Newman GC, et al. Changes in plasma homocyst(e)ine in the acute phase after stroke. Stroke 2002;33:473–478 [DOI] [PubMed] [Google Scholar]
- 36. Lindgren A, Brattstrom L, Norrving B, Hultberg B, Andersson A, Johansson BB. Plasma homocysteine in the acute and convalescent phases after stroke. Stroke 1995;26:795–800 [DOI] [PubMed] [Google Scholar]
- 37. Meiklejohn DJ, Vickers MA, Dijkhuisen R, Greaves M. Plasma homocysteine concentrations in the acute and convalescent periods of atherothrombotic stroke. Stroke 2001;32:57–62 [DOI] [PubMed] [Google Scholar]
- 38. Bostom AG, Silbershatz H, Rosenberg IH, et al. Nonfasting plasma total homocysteine levels and all-cause and cardiovascular disease mortality in elderly Framingham men and women. Arch Intern Med 1999;159:1077–1080 [DOI] [PubMed] [Google Scholar]
- 39. Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 1997;337:230–236 [DOI] [PubMed] [Google Scholar]
- 40. Ntaios GC, Savopoulos CG, Chatzinikolaou AC, Kaiafa GD, Hatzitolios A. Vitamins and stroke: the homocysteine hypothesis still in doubt. Neurologist 2008;14:2–4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.