Aquaporin-4 (AQP4) is the target autoantigen of an immunoglobulin G (IgG) autoantibody that distinguishes a spectrum of inflammatory demyelinating CNS disorders (the neuromyelitis optica [NMO] spectrum) from multiple sclerosis (MS) and other CNS demyelinating disorders.1 Compelling evidence supports this IgG having a central role in the pathogenesis of NMO. AQP4 is concentrated in astrocytic foot processes at interfaces between CNS parenchyma and fluid compartments, both CSF and blood,1 and in areas involved in osmosensitivity and osmoregulation, including supraoptic and paraventricular nuclei of the hypothalamus and sensory circumventricular organs, the subfornical organ, the organum vasculosum of the lamina terminalis, and the area postrema.2 A single case of the syndrome of inappropriate antidiuresis (SIAD) has been described in NMO.3 Here we report the frequency of SIAD in NMO.
Methods.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Institutional Review Board. The study involved retrospective chart review of 160 AQP4-IgG seropositive Mayo Clinic patients identified though the Neuroimmunology Laboratory's NMO database who provided consent to have their records reviewed.
Our inclusion criterion, hyponatremic patients fulfilling modified Bartter and Schwartz criteria for SIAD,4 required that data be available for both serum sodium concentration and blood/urine osmolality, at the onset of NMO or during a relapse of the disease. We excluded patients whose hyponatremia was attributable to carbamazepine or diuretic therapy (3), lymphoma (1), or thyroid dysfunction (1). No patient had signs of cerebral salt wasting syndrome.
Results.
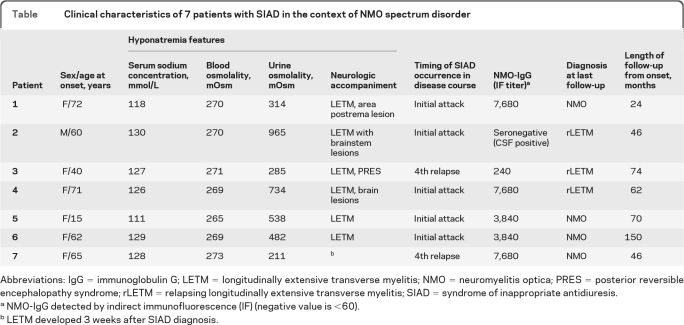
Among 160 patients with NMO or NMO spectrum disorder, 43 had sufficient data for the study. Seven patients (16%) met diagnostic criteria for SIAD (Table). The median age at disease onset was 55 years (range 15–72). The median follow-up interval was 67 months (range 24–150). SIAD was the initial symptom of the attack in 5 of the 43 patients (12%). Hyponatremia was mild (130 mmol/L) in 1 patient, moderate (120–130 mmol/L) in 4, and severe (<120 mmol/L) in 2. Only 1 patient experienced confusion and decreased consciousness attributable to hyponatremia. No information about sodium urinary concentration and plasma vasopressin levels was available. No patient was on any diuretic therapy or had adrenal insufficiency. Creatinine and BUN were unremarkable in all patients. Two patients experienced intractable vomiting, 2 had nausea, and 1 patient developed a syndrome of posterior reversible encephalopathy at the time of documented hyponatremia. Hyponatremia resolved in all patients after fluid intake was restricted to 1 L per day. No patient experienced a recurrence of hyponatremia.
Table.
Clinical characteristics of 7 patients with SIAD in the context of NMO spectrum disorder
Abbreviations: IgG = immunoglobulin G; LETM = longitudinally extensive transverse myelitis; NMO = neuromyelitis optica; PRES = posterior reversible encephalopathy syndrome; rLETM = relapsing longitudinally extensive transverse myelitis; SIAD = syndrome of inappropriate antidiuresis.
NMO-IgG detected by indirect immunofluorescence (IF) (negative value is <60).
LETM developed 3 weeks after SIAD diagnosis.
MRI revealed brain abnormalities in 4 patients; 1 had fluid-attenuated inversion recovery and T2-weighted signal abnormalities extending from the brainstem into the area postrema region. Five patients had radiologic signs compatible with longitudinally extensive transverse myelitis, which in 1 developed 3 weeks after SIAD onset. No patient had evidence of hypothalamic abnormalities on brain MRI.
Discussion.
This study describes SIAD as an accompaniment of an NMO attack in 16% of cases, and identified SIAD at initial NMO attack in 12%. In 1 case (patient 7) SIAD preceded the NMO relapse by 3 weeks, suggesting that SIAD, in some patients with NMO, may herald a relapse. We are aware that the prevalence of SIAD in our cohort may have been overestimated because we selected only patients with documented information about serum sodium concentration, and blood and urine osmolality. However, the relatively high frequency of SIAD in this NMO cohort contrasts with MS, in which SIAD is rare.
Our database previously revealed that 12% of NMO/AQP4-IgG seropositive patients with NMO seen at Mayo Clinic had intractable vomiting as the initial presenting symptom of NMO.5 None of those patients experienced hyponatremia. Nausea and vomiting can be both a symptom and a cause of hyponatremia. Hyponatremia caused by vomiting is hypovolemic which is reflected by an increased blood osmolality. Hyponatremia in the setting of SIAD is euvolemic and hypoosmolar. In 4 of the 7 patients in the present study, vomiting or nausea coincided with SIAD, suggesting a potential role of the area postrema in SIAD. Neurons in the area postrema are osmosensitive and regulate vasopressin secretion.6 The 3 patients with SIAD without nausea and vomiting (patients 3, 6, and 7) may have had lesions involving the hypothalamic supraoptic and paraventricular nuclei or other AQP4-enriched circumventricular organs serving osmosensitive functions.
In the mammalian CNS, AQP4 interacts with the transient receptor potential channel, vanilloid subfamily (TRPV4), an osmotically activated ion channel expressed in circumventricular organs. This interaction is essential for TRPV4 to function as an astroglial osmosensor.7 Thus SIAD secondary to impairment of an AQP4-coupled TRPV4 channel may represent another pathogenic outcome of IgG interacting with AQP4 in NMO.
Acknowledgment:
The authors thank other members of the Mayo Clinic NMO Study Group (Drs. Dean Wingerchuk, Istvan Pirko, and Andrew Mckeon) for enrolling their patients in the Mayo Clinic Study Specific NMO Repository.
Footnotes
Author contributions: Study concept and design: R.I., S.J.P. Acquisition, analysis, and interpretation of data: R.I., C.F.L., C.C., B.G.W., S.J.P. Drafting of manuscript: R.I., S.J.P. Revising the manuscript for important intellectual content: R.I., S.H., V.A.L., S.J.P.
Disclosure: Dr. Iorio reports no disclosures. Dr. Lucchinetti receives royalties from the publication of Blue Books of Neurology: Multiple Sclerosis 3 (Saunders Elsevier, 2010); receives research support from the NIH, the National MS Society, and the Guthy Jackson Charitable Foundation; and receives license royalties for a patent relating to aquaporin-4 antibodies for diagnosis of neuromyelitis optica. Dr. Lennon is a named inventor on a patent (#7101679 issued 2006) relating to aquaporin-4 antibodies for diagnosis of neuromyelitis optica and receives royalties for this technology; is a named inventor on patents (#12/678,350 filed 2010 and #12/573,942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; and receives research support from the Guthy Jackson Charitable Foundation. Dr. Costanzi reports no disclosures. Dr. Hinson is a named inventor on a patent (#12/573,942 filed 2008) that relates to functional AQP4/NMO-IgG assays. Dr. Weinshenker serves on data safety monitoring boards for Novartis and Biogen Idec; serves on the editorial boards of the Canadian Journal of Neurological Sciences and the Turkish Journal of Neurology; has received research support from Genzyme Corporation and the Guthy Jackson Charitable Foundation; and receives license royalties for a patent relating to aquaporin-4 antibodies for diagnosis of neuromyelitis optica. Dr. Pittock is a named inventor on patents (#12/678,350 filed 2010 and #12/573,942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; receives research support from Alexion Pharmaceuticals, Inc., the Guthy Jackson Charitable Foundation, and the NIH.
References
- 1. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815 [DOI] [PubMed] [Google Scholar]
- 2. Mesbah-Benmessaoud O, Benabdesselam R, Hardin-Pouzet H, Dorbani-Mamine L, Grange-Messent V. Cellular and subcellular aquaporin-4 distribution in the mouse neurohypophysis and the effects of osmotic stimulation. J Histochem Cytochem 2011;59:88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. You XF, Qin W, Hu WL. Aquaporin-4 antibody positive neuromyelitis optica with syndrome of inappropriate antidiuretic hormone secretion. Neurosciences 2011;16:68–71 [PubMed] [Google Scholar]
- 4. Ellison DH, Berl T. Clinical practice: the syndrome of inappropriate antidiuresis. N Engl J Med 2007;356:2064–2072 [DOI] [PubMed] [Google Scholar]
- 5. Apiwattanakul M, Popescu BF, Matiello M, et al. Intractable vomiting as the initial presentation of neuromyelitis optica. Ann Neurol 2010;68:757–761 [DOI] [PubMed] [Google Scholar]
- 6. Curtis KS, Huang W, Sved AF, Verbalis JG, Stricker EM. Impaired osmoregulatory responses in rats with area postrema lesions. Am J Physiol 1999;277:R209–R219 [DOI] [PubMed] [Google Scholar]
- 7. Benfenati V, Caprini M, Dovizio M, et al. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci USA 2011;108:2563–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]