Abstract
Objective:
We investigated whether crossed legs are a prognostic marker in patients with severe stroke.
Methods:
In this controlled prospective observational study, we observed patients with severe stroke who crossed their legs during their hospital stay and matched them with randomly selected severe stroke patients who did not cross their legs. The patients were evaluated upon admission, on the day of leg crossing, upon discharge, and at 1 year after discharge. The Glasgow Coma Scale, the NIH Stroke Scale (NIHSS), the modified Rankin Scale (mRS), and the Barthel Index (BI) were obtained.
Results:
Patients who crossed their legs (n = 34) and matched controls (n = 34) did not differ in any scale upon admission. At the time of discharge, the GCS did not differ, but the NIHSS was better in crossed legs patients (6.5 vs 10.6; p = 0.0026), as was the mRS (3.4 vs 5.1, p < 0.001), and the BI (34.0 vs 21.1; p = 0.0073). At 1-year follow-up, mRS (2.9 vs 5.1, p < 0.001) and the BI (71.3 vs 49.2; p = 0.045) were also better in the crossed leg group. The mortality between the groups differed grossly; only 1 patient died in the crossing group compared to 18 in the noncrossing group (p < 0.001).
Conclusion:
Leg crossing is an easily obtained clinical sign and is independent of additional technical examinations. Leg crossing within the first 15 days after severe stroke indicates a favorable outcome which includes less neurologic deficits, better independence in daily life, and lower rates of death.
Stroke is the third most frequent cause of death and the most frequent cause of permanent disability in developed countries.1 Several prognostic models have been developed to predict functional recovery and survival.2–4 However, the contributing factors for these models are difficult to assess and may not be applicable in patients with severe stroke who require ventilation or circulatory support.5
In our neurologic intensive care unit (NICU) early crossing of legs was observed in patients with severe stroke despite markedly reduced consciousness, paresis of the crossed leg, need of ventilation, or need of circulatory support. To evaluate the prognostic significance of this sign we performed a prospective study in patients with severe stroke.
METHODS
Patients.
We included patients with severe stroke (intracranial CNS infarction or bleeding) who were admitted to our NICU from May 2005 to September 2006. We arbitrarily defined “severe stroke” as a cerebral infarction or bleeding where the patient had to be referred to our NICU because of severely impaired consciousness, need of mechanical ventilation, need of circulatory support, need of extraventricular drainage, or need for a high level of neurologic monitoring. All medical staff (physicians, nurses) were instructed to report leg crossing, which was then noted in the patient chart.
The control group consisted of patients after severe stroke that did not cross their legs during their stay at the NICU. They were matched based on age, Glasgow Coma Scale (GCS) score, and severity of neurologic impairment (NIH Stroke Scale [NIHSS]) upon admission. Matching was performed without knowledge of the scales at discharge. Patients were only included in the study when the rating scales at admission and discharge were obtained without the influence of sedative medications. Sedation was allowed on the crossing day. In the crossing group, 8 patients were lost to 1-year follow-up, compared to 6 controls.
Standard protocol approvals, registrations, and patient consents.
The ethics committee of the University of Munich approved this study, and patients or next of kin gave informed consent to the use of their data. One patient withdrew consent at the time of 1-year follow-up; those data were omitted.
Rating scales.
At admission, crossing day, discharge, and follow-up, the Glasgow Coma Scale (GCS6), Barthel index (BI,7 ranking a patient's level of independence in daily activities from 0 [fully dependent] to 100 [fully independent]), NIHSS8 (evaluating severity of neurologic impairment from 0 [no impairment] to 42 [severe impairment]), and modified Rankin scale (mRS,9 disability score, ranging from 0 [full health] to 6 [death]) were documented. Furthermore, the mRS before onset of symptoms was documented. The 1-year follow-up was performed by a phone interview. Therefore, the NIHSS was not assessed because it requires clinical examination.
Statistical analysis.
The differences between the 2 groups were compared using the Mann-Whitney U test. Differences in categorical data were tested with Fisher exact test. Results were considered statistically significant when p < 0.05.
RESULTS
Patients.
Of 120 patients with severe stroke, the crossing leg sign was observed in 34 patients. They were matched to 34 patients who did not cross their legs. The underlying syndromes and comorbidities did not differ (table e-1 on the Neurology® Web site at www.neurology.org). The first time of leg crossing was observed after an average of 10.5 days (median 7 days, SD 12.6 days, minimum 0 days, maximum 59 days) after intensive care unit admission. Twenty patients crossed their right leg over their left (58.8%), 13 crossed their left over the right (38.2%), and 1 patient crossed both ways (2.9%). Six patients crossed with the leg contralateral to their lesion.
Outcome results.
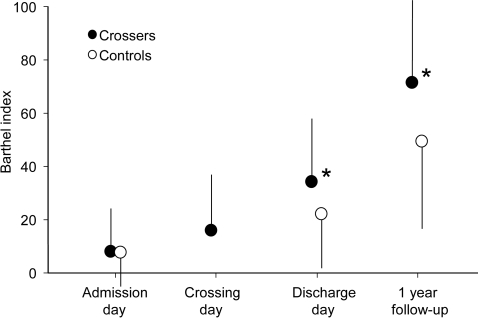
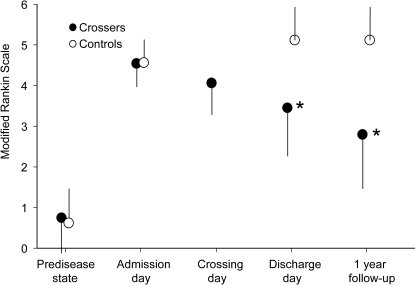
At admission, no differences between “crossers” or “noncrossers” were observed for the GCS, the NIHSS, the mRS, or the BI (table 1). However, over time, the other scores demonstrated significant differences between the 2 groups. Upon discharge, the NIHSS was lower for crossers, indicating less severe neurologic deficits, and the BI was higher, demonstrating higher functional independence of the patient (figure 1). These results improved even further at the 1-year follow-up. The mRS was lower in the crossing group at discharge and follow-up, indicating less disability (figure 2). The OR for attaining a mRS of at least 2 (able to look after own affairs without assistance) at the 1-year follow-up was 8.1 for crossers (10/26) compared to noncrossers (2/28).
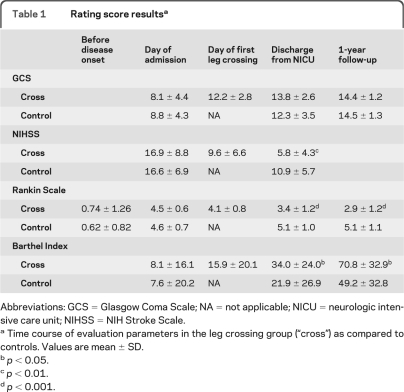
Table 1.
Rating score resultsa
Abbreviations: GCS = Glasgow Coma Scale; NA = not applicable; NICU = neurologic intensive care unit; NIHSS = NIH Stroke Scale.
Time course of evaluation parameters in the leg crossing group (“cross”) as compared to controls. Values are mean ± SD.
p < 0.05.
p < 0.01.
p < 0.001.
Figure 1. Barthel Index.
Barthel Index scores of level of independence in the crossed leg (“crossers,” full circles) and control groups (empty circles). High values represent high level of independence. Error bars indicate the SD. *p < 0.05.
Figure 2. Modified Rankin Scale.
Modified Rankin Scale scores, indicating level of disability, in the crossed leg (“crossers,” full circles) and control groups (empty circles). High values represent high disability. Error bars indicate the SD. *p < 0.001.
When patients crossed their legs after day 15, no BI outcome over 15 was attained (figure e-1). The 6 patients who crossed the leg contralateral to the CNS lesion showed no difference in outcome. The mortality rate between the 2 groups differed grossly: in the crossed leg group only 1 patient died compared to 18 fatalities in the control group (odds ratio 37.1; p < 0.0001).
DISCUSSION
Consistent with our hypothesis, we found that leg crossing early after severe stroke was a favorable prognostic indicator of outcome both at hospital discharge and up to 1 year after admission. Moreover, this marker conveyed not only statistical relevance but also substantial clinical improvement. For example, based on the modified mRS at 1 year after discharge, the average leg crosser was moderately disabled, but could walk unassisted, while average noncrossers were severely disabled and required constant attention.9 Next to this gradual outcome scale, the difference in mortality between the leg-crossing group (8.8%) and the noncrossers (52.9%) was striking. Compared with commonly used predictive metrics, which require neurologic assessment by a trained health care provider and may only provide information about recovery up to 3 months,2–4 leg crossing is easily assessed, and provides prognostic information up to 1 year. From an epistemologic view, leg-crossing translates a gradual improvement of state of health into a categorical marker. That allows easier categorization compared to judging from gradual parameters like size of lesion or amount of paresis2–4 where a threshold has to be defined rather than being observed.
In general, people have preferences of which leg to cross over which, which refers to the asymmetric use of bilateral limbs or sense organs, like handedness. Similar to the preference of handedness, right over left leg crossing is more common (62.4%) than left over right leg crossing (25.7%).10 In stroke patients, these preferences may become important because of the accompanying hemiparesis, considering that more than 50 N of force have to be applied for leg crossing (appendix e-1). On our patients, though, the side of leg crossing did not differ from the literature.10
The limitations of our study may be that leg crossing was missed by the staff, but that would bias toward the null hypothesis. Additionally, as this study could not be blinded, a selection bias may be present. We believe this bias to be small, because on one hand this was a pilot study and it was unknown whether crossed legs indicate a favorable outcome. Conversely, we lack prognostic factors that are easily obtained from clinical observation, so we believe that the expectations of the medical staff did not severely impact the outcome of this study.
Leg crossing is an inexpensive, easily obtained clinical sign. Therefore, it can be used widely in daily practice by an intensive care unit team; possibly it may be used even by families or other nonmedical caregivers, which should be evaluated in future studies. Leg crossing within the first 15 days after severe stroke indicates a favorable outcome which includes less neurologic deficits, better independence in daily life, and lower rates of death. Based on this small study, larger future studies could confirm the prognostic value of leg crossing in general stroke, or evaluate its prognostic value in other intensive care unit settings like septic or metabolic coma.
ACKNOWLEDGMENT
The authors thank Professor Jenni Carbaugh Cook and Eric Cook of Nottingham, NH, for copyediting the manuscript and all nurses and doctors of the Neurological Intensive Care Unit of the University of Munich for careful observation of crossed legs.
GLOSSARY
- BI
Barthel Index
- GCS
Glasgow Coma Scale
- mRS
modified Rankin Scale
- NICU
neurologic intensive care unit
- NIHSS
NIH Stroke Scale
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Rémi: data and statistical analysis, figure production, manuscript draft and revision. Dr. Pfefferkorn: study design, follow-up, manuscript revision. Dr. Owens: critical manuscript revisions. Dr. Schankin: data collection, manuscript revision. Dr. Dehning: follow-up, manuscript revision. Dr. Birnbaum: data collection, manuscript revision. Dr. Bender: data collection, manuscript revision. Dr. Klein: data collection, manuscript revision. Dr. Adamec: force calculations, manuscript revision. Dr. Pfister: critical manuscript revisions. Dr. Straube: study design, manuscript revision. Dr. Feddersen: study idea, study design, data collection, follow-up, manuscript draft and revision.
DISCLOSURE
Dr. Rémi has received speaker honoraria from UCB and Pfizer Inc. Dr. Pfefferkorn reports no disclosures. Dr. Owens receives research support from the NIH/NHBLI. Dr. Schankin received research support from Merck Sharp & Dohme and Deutsche Forschungsgemeinschaft (German Research Council). Dr. Dehning, Dr. Birnbaum, Dr. Bender, Dr. Klein, and Dr. Adamec report no disclosures. Dr. Pfister received speaker honoraria from Novartis; serves on the editorial board of Journal of Neurology; and has received research support from Deutsche Forschungsgemeinschaft (German Research Council). Dr. Straube serves on the scientific advisory boards for Desitin Pharmaceuticals, GmbH, Allergan, Inc., and Merck Sharp & Dohme; has received speaker honoraria from Desitin Pharmaceuticals, GmbH, Allergan, Inc., Pfizer Inc, and Merck Sharp & Dohme; serves on the editorial board of the Journal of Headache and Pain; and receives research support from Deutsche Forschungsgemeinschaft (German Research Council). and University of Munich. Dr. Feddersen received speaker honoraria from UCB and Pfizer Inc.
REFERENCES
- 1. Truelsen T, Piechowski-Jozwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol 2006; 13: 581– 598 [DOI] [PubMed] [Google Scholar]
- 2. Counsell C, Dennis M, Lewis S. Prediction of outcome after stroke. Lancet 2001; 358: 1553– 1554 [DOI] [PubMed] [Google Scholar]
- 3. Konig IR, Ziegler A, Bluhmki E, et al. Predicting long-term outcome after acute ischemic stroke: a simple index works in patients from controlled clinical trials. Stroke 2008; 39: 1821– 1826 [DOI] [PubMed] [Google Scholar]
- 4. Weimar C, Ziegler A, Sacco RL, Diener HC, Konig IR. Predicting recovery after intracerebral hemorrhage: an external validation in patients from controlled clinical trials. J Neurol 2009; 256: 464– 469 [DOI] [PubMed] [Google Scholar]
- 5. Holloway RG, Benesch CG, Burgin WS, Zentner JB. Prognosis and decision making in severe stroke. JAMA 2005; 294: 725– 733 [DOI] [PubMed] [Google Scholar]
- 6. Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet 1974; 2: 81– 84 [DOI] [PubMed] [Google Scholar]
- 7. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007; 38: 1091– 1096 [DOI] [PubMed] [Google Scholar]
- 8. Brott T, Marler JR, Olinger CP, et al. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke 1989; 20: 871– 875 [DOI] [PubMed] [Google Scholar]
- 9. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604– 607 [DOI] [PubMed] [Google Scholar]
- 10. Reiss M. Leg-crossing: incidence and inheritance. Neuropsychologia 1994; 32: 747– 750 [DOI] [PubMed] [Google Scholar]