Abstract
Paget's disease of the bone (PDB) is an autosomal dominant trait with genetic heterogeneity, characterized by abnormal osteoclastogenesis. Sequestosome 1 (p62) is a scaffold protein that plays an important role in receptor activator of nuclear factor κB (RANK) signaling essential for osteoclast (OCL) differentiation. p62P392L mutation in the ubiquitin-associated (UBA) domain is widely associated with PDB; however, the mechanisms by which p62P392L stimulate OCL differentiation in PDB are not completely understood. Deubiquitinating enzyme cylindromatosis (CYLD) has been shown to negatively regulate RANK ligand-RANK signaling essential for OCL differentiation. Here, we report that CYLD binds with the p62 wild-type (p62WT), non-UBA mutant (p62A381V) but not with the UBA mutant (p62P392L) in OCL progenitor cells. Also, p62P392L induces expression of c-Fos (2.8-fold) and nuclear factor of activated T cells c1 (6.0-fold) transcription factors critical for OCL differentiation. Furthermore, p62P392L expression results in accumulation of polyubiquitinated TNF receptor-associated factor (TRAF)6 and elevated levels of phospho-IκB during OCL differentiation. Retroviral transduction of p62P392L/CYLD short hairpin RNA significantly increased TRAP positive multinucleated OCL formation/bone resorption activity in mouse bone marrow cultures. Thus, the p62P392L mutation abolished CYLD interaction and enhanced OCL development/bone resorption activity in PDB.
Paget's disease of bone (PDB) is a chronic focal skeletal disorder that affects 2–3% of the population over the age of 60 yr. PDB is inherited as an autosomal dominant trait with genetic heterogeneity and characterized by highly localized areas of bone turnover with increased osteoclast (OCL) activity followed by an exaggerated osteoblast response (1). OCL in PDB contain paramyxoviral-nuclear inclusions and nucleocapsid transcripts (2). The nuclear inclusions were present in 20–40% of OCL in all patients with PDB (3). Furthermore, expressions of measles virus nucleocapsid (MVNP) transcripts have been detected in approximately 80% of bone marrow samples from patients with PDB (4). Recently, Merchant et al. (5) have found MVNP expression in pagetic bone and pagetic osteosarcomas. Previously, canine distemper virus nucleocapsid transcripts were also detected in pagetic bone samples (6). However, other workers have been unable to detect expression of paramyxoviral transcripts in PDB (7, 8). Sequestosome 1 (p62) (also known as SQSTM1) is a multifunctional ubiquitin-binding protein, which plays an important role in cell signaling, receptor internalization, and protein turnover (9). Recurrent mutations in p62 ubiquitin-associated (UBA) domain have been identified in about 25% of familial and less than 10% of sporadic cases; however, the P392L amino acid substitution being the most common in PDB (10). Furthermore, a mutation in p62 exon-7 (K378X), which introduces a premature stop codon and thus eliminates entire UBA domain, has been reported (11). Najat et al. (12) have also identified a non-UBA domain mutation, p62A381V associated with PDB. p62 has been shown to physically associate with TNF receptor-associated factor (TRAF)6 and involved in receptor activator of nuclear factor κB (NF-κB) (RANK) signaling critical for OCL differentiation (13). p62 is a scaffold protein that mediates RANK ligand (RANKL) signaling by activating transcription factors such as NF-κB and activator protein 1 (AP-1), which induces the nuclear factor of activated T cells (NFATc1) transcription factor expression essential for osteoclastogenesis (14–16). p62 knockout mice have impaired osteoclastogenesis in response to parathyroid hormone-related peptide (13). It has been shown that p62 mutant (p62P392L) transgenic mice have increased OCL formation but do not develop focal osteolytic lesions with the characteristics of PDB (17). In contrast, recently, p62 UBA domain mutation (p62P394L) has been shown to be sufficient to cause a Paget's disease-like disorder in mice (18). Also, truncation of the p62 UBA domain causes aberrant RANK signaling and increased osteoclastogenesis in RAW 264.7 cell cultures (19). Recently, p62P392L mutation has been shown to alter RANKL signaling and induces activation of human OCL (20). However, the molecular mechanisms by which p62P392L stimulates OCL differentiation in PDB are not completely understood.
Pridgeon et al. (21), using an in vitro expression cloning approach, identified several proteins that interact with the p62 UBA domain. The tumor suppressor cylindromatosis (CYLD) gene was first identified in human affected with familial CYLD, a genetic syndrome in which numerous benign tumors of skin develop, principally on the head and neck region. The disease is inherited in an autosomal manner and is caused by germline mutations in the CYLD gene on chromosome 16q12-q13, which predicts truncation or absence of the encoded protein (22). CYLD protein has been shown to physically interact with p62, and it negatively regulates osteoclastogenesis (23). CYLD is a deubiquitinase that removes the ubiquitin chain from several proteins, particularly TRAF2, TRAF6, and NF-κB essential modulator, and inactivates NF-κB signaling (24–26). The deubiquitination activity of CYLD is highly specific for proteins at lysine-63 (K63)-linked ubiquitin chains in substrate but has been shown to act on K48-linked polyubiquitin chains (27). Polyubiquitination of target protein at K63 influences protein-protein interactions, which play an important role in cell signaling, and ubiquitination at K48 directs to proteosomal degradation via ubiquitin-proteosomal pathway (28). Here, we report that the p62 UBA mutant (p62P392L) abolished the interaction with CYLD, which implicates a potential role in enhanced OCL development in PDB.
Materials and Methods
Reagents and antibodies
Cell culture and DNA transfection reagents were purchased from Invitrogen Corp. (Carlsbad, CA). RANKL and macrophage colony-stimulating factor (M-CSF) were obtained from R&D Systems, Inc. (Minneapolis, MN). Rabbit-anti-CYLD antibody was purchased from Abcam (Cambridge, MA). Rabbit-anti-NFATc1, anti-c-Fos, antihemagglutinin (HA) tag, and peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). pNFAT, pNF-κB, and pAP-1-Luc cis-reporter plasmids were obtained from Stratagene (La Jolla, CA). SuperSignal enhanced chemiluminescence reagent was obtained from Amersham Bioscience (Piscataway, NJ), and nitrocellulose membranes were purchased from Millipore (Bedford, MA). A luciferase reporter assay system was obtained from Promega (Madison, WI).
p62 mutagenesis
A plasmid (pcDNA3-HA-p62) containing the full-length human p62 cDNA (>85% homologous to murine p62) was kindly provided by Jorge Moscat (Sanford-Burnham Medical Research Institute, La Jolla, CA), and the P392L mutation in UBA domain (exon-8) and A381V in non-UBA domain (exon-7) were introduced by site-directed mutagenesis using QuikChange II XL Site-Directed Mutagenesis kit (Stratagene, Inc., La Jolla, CA) as described earlier (29). The mutagenized p62 cDNA was sequence verified for correct introduction of P392L and A381V mutations (Fig. 1A).
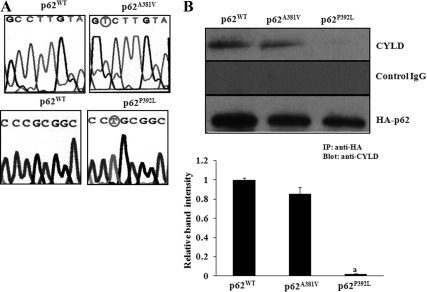
Fig. 1.
p62 interaction with CYLD. A, Site-directed mutagenesis of p62 non-UBA (p62A381V) and UBA (p62P392L) mutations associated with PDB. A plasmid containing the full-length human p62 cDNA was used as template for site-directed mutagenesis of UBA mutant, p62P392L (C-to-T transition), and a non-UBA mutant, p62A381V (C-to-T transition), as described in Materials and Methods. The mutagenized p62 cDNA sequenced to verify correct introduction of the P392L, A381V mutations as circled. B, p62 UBA mutation (p62P392L) abolished interaction with CYLD in pre-OCL cells. Mouse bone marrow-derived nonadherent cells were transduced with retroviral expression plasmids of p62WT, p62A381V, or p62P392L, and cells were stimulated with M-CSF (10 ng/ml) and RANKL (100 ng/ml) for 48 h. Total cell lysates obtained were subjected to immunoprecipitation using rabbit anti-HA antibody. The immunoprecipitants were analyzed by Western blotting using rabbit anti-CYLD antibody. The band intensity was quantified by National Institutes of Health ImageJ program, and CYLD immunoprecipitation was normalized with HA-tagged p62 expressed in these cells. The values are expressed as mean ± sd for three independent experiments (a, P < 0.05).
p62 and CYLD short hairpin RNA (shRNA) retroviral expression
The p62WT and mutant cDNA were excised from the pcDNA3-HA-p62 plasmids by digestion with EcoRI and subcloned into the pLXSN retroviral vector (Clontech Laboratories, Inc., Palo Alto, CA). The resulting plasmid construct transcribes p62 mRNA expression under the control of 5′ long terminal repeat viral promoter elements. The recombinant p62 constructs and CYLD shRNA retroviral plasmid (Open Biosystems, Rockford, IL) were transfected into the PT67 amphotropic packaging cell line using lipofectamine (Invitrogen Corp.). Stable clonal cell lines producing p62 recombinant retrovirus at high titer (1 × 106 virus particles/ml) were established by selecting for resistance to neomycin (600 μg/ml). Similarly, a control retrovirus producer cell line was established by transfecting the cells with the pLXSN empty vector (EV). Producer cell lines were maintained in DMEM containing 10% fetal calf serum (FCS), 100 U/ml each of streptomycin and penicillin, 4 mm l-glutamine, and high glucose (4.5 g/liter). Retroviral supernatants from the producer cell cultures were collected and filtered (0.45 μm pore diameter) for immediate use. Mouse bone marrow-derived nonadherent cells were transduced with p62WT, p62P392L, or p62A381V and CYLD shRNA retroviral supernatants (20%) from the producer cell lines in the presence of polybrene (4 μg/ml) for 24 h at 37 C in a 5% CO2 incubator as described earlier (30).
OCL culture and bone resorption assay
Mouse bone marrow-derived nonadherent cells were dispersed into α-MEM containing 10% FCS and were seeded in 96-well plates at 6 × 105 cells/well in 0.2 ml of medium. Cells were transduced with p62WT, p62A318V, or p62P392L and cultured in the presence of RANKL (100 ng/ml) and M-CSF (10 ng/ml) and were refed twice weekly by semidepletion (half of the medium withdrawn and replaced with fresh medium). At the end of culture period (5 d), the cells were fixed with 2% glutaraldehyde in PBS and stained for tartrate-resistant acid phosphatase (TRAP) activity using a histochemical kit (Sigma, St. Louis, MO). TRAP positive multinucleated cells containing three or more nuclei were scored as OCL cells under a microscope. Bone resorption activity of the OCL was assayed by culturing OCL for 10 d on dentine slices. At the end of the culture period, adherent cells were removed from the dentine disc, using 1 m NaOH and stained with 0.1% toluidine blue. The bone resorption area was quantified using computerized image analysis (Adobe Photoshop and Scion MicroImaging version 4.2). The percentage of the resorbed area was calculated relative to the total dentine disc area.
Coimmunoprecipitation assay
Mouse bone marrow-derived nonadherent cells were seeded in six-well plates (5 × 105 cells/well) were transduced with p62WT, p62P392L, or p62A381V retroviral expression vectors and stimulated with M-CSF (10 ng/ml) and RANKL (100 ng/ml) for 48 h. Total cell lysates were collected in a lysis buffer [50 mm HEPES (pH 7.5), 250 mm NaCl, 0.2 mm EDTA, 10 μm NaF, and 0.5% Nonidet P-40] were immunoprecipitated using anti-HA antibody as described earlier (29). Immunocomplexes were subjected to Western blot analysis for CYLD using rabbit anti-CYLD antibody.
Quantitative real-time RT-PCR
NFATc1 mRNA expression was measured by real-time RT-PCR as described previously (29). Briefly, total RNA was isolated from pre-OCL cells transfected with p62WT, p62A381V, or p62P392L and were stimulated with and without RANKL (100 ng/ml) for 48 h, using RNAzol reagent (Biotecx Laboratories, Houston, TX). A RT reaction was performed using a cDNA synthesis kit (Bio-Rad, Hercules, CA) in a 25-μl reaction volume containing total RNA (2 μg), 1× PCR buffer, and 2 mm MgCl2, at 42 C for 15 min followed by 95 C for 5 min. The quantitative real-time PCR was performed using IQ SYBR Green Supermix in an iCycler (iCycler iQ Single-color Real Time PCR Detection System; Bio-Rad). The primer sequences used to amplify glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were 5′-CCTACCCCCAATGTATCCGTTGTG-3′ (sense) and 5′-GGAGGAATGGGAGTTGCTGTTGAA-3′ (antisense) and for mouse NFATc1 mRNA were 5′-GGCCGCAGAACACTACAGTTA-3′ (sense) and 5′-GAGATACCCGGGGTGGAC-3′ (antisense). Thermal cycling parameters were 94 C for 3 min, followed by 40 cycles of amplifications at 94 C for 30 sec, 60 C for 1 min, 72 C for 1 min, and 72 C for 5 min as the final elongation step. Relative levels of NFATc1 mRNA expression were normalized in all the samples analyzed with respect to GAPDH amplification.
NF-κB, AP-1, and NFAT-Luc reporter gene assay
RAW 264.7 cells were cultured in DMEM supplemented with 10% FCS in a humidified atmosphere with 5% CO2 at 37 C. DNA transfections were performed using lipofectamine transfection reagent (Invitrogen Corp.) according to the manufacturer's protocol. RAW 264.7 cells were transfected with NF-κB, AP-1, and NFAT-Luc reporter plasmids and coexpressed with p62WT, p62P392L, or p62A381V. Cells were cultured in the presence or absence of RANKL (100 ng/ml) for 48 h. The cell monolayer was washed twice with PBS and incubated at room temperature for 15 min with 0.3 ml cell lysis buffer. The monolayer was scraped and spun briefly in a microfuge to pellet the debris. Then, a 20-μl aliquot of each sample was mixed with 100 μl of the luciferase assay reagent. Light emission was measured for 10 sec of integrated time using Sirius Luminometer (Promega). The transfection efficiency was normalized by cotransfection with 0.2 μg of pRSV β-gal plasmid and measuring β-galactosidase activity in the cell lysates. LacZ cytochemical activity staining (Invitrogen Corp.) indicated a DNA transfection efficiency (>80%) in RAW 264.7 cells.
TRAF6 ubiquitination assay
Mouse bone marrow-derived nonadherent cells were seeded in six-well plates (5 × 105 cells/well), transduced with p62WT, p62P392L, or p62A381V retroviral expression vectors, and stimulated with M-CSF (10 ng/ml) and RANKL (100 ng/ml). After 48 h, total cell lysates were collected in a lysis buffer [50 mm HEPES (pH 7.5), 250 mm NaCl, 0.2 mm EDTA, 10 μm NaF, and 0.5% Nonidet P-40]. TRAF6 was immunoprecipitated using rabbit-anti-TRAF6 antibody, and the ubiquitin-conjugated TRAF6 was detected by Western blotting using rabbit-antiubiquitin antibody.
Statistical analysis
Results are presented as mean ± sd for three independent experiments and were compared by Student's t test. Values were considered significantly different for a and b, P < 0.05.
Results
p62 UBA mutation (p62P392L) abolished interaction with CYLD in pre-OCL cells
To examine the functional role of p62P392L in OCL differentiation, we developed HA-tagged p62WT, non-UBA mutant (p62A381V), and UBA mutant (p62P392L) retroviral expression vectors as described in Materials and Methods. Mouse bone marrow-derived nonadherent cells were transduced with p62WT, p62A381V, or p62P392L expression vectors and stimulated with M-CSF (10 ng/ml) and RANKL (100 ng/ml) for 48 h. p62 was then immunoprecipitated from the total cell lysates using anti-HA antibody. Western blot analysis of the immunocomplex revealed that CYLD coimmunoprecipitated with p62WT and non-UBA mutant p62A381V but not with UBA mutant p62P392L. In contrast, a control nonspecific rabbit IgG did not immunoprecipitate CYLD (Fig. 1B). These results suggest that the p62 UBA mutation (p62P392L) abolished interaction with CYLD in pre-OCL cells.
p62P392L enhances TRAF6 ubiquitination during OCL differentiation
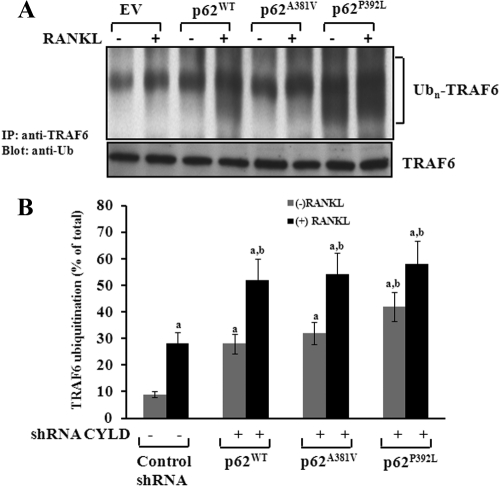
TRAF6 is an adaptor molecule involved in RANK signaling, and polyubiquitination of TRAF6 plays an important role in OCL differentiation. To determine whether the p62P392L mutant modulates TRAF6 ubiquitination during OCL differentiation, nonadherent mouse bone marrow cells were transduced with p62WT, non-UBA mutant (p62A381V), or UBA mutant (p62P392L) and stimulated with RANKL (100 ng/ml) and M-CSF (10 ng/ml) for 48 h. Total cell lysates obtained were subjected to immunoprecipitation of TRAF6 using rabbit anti-TRAF6 antibody. Western blot analysis of the immunocomplexes using antiubiquitin antibody revealed accumulation of polyubiquitinated TRAF6 in p62P392L transduced pre-OCL cells with and without RANKL stimulation compared with p62WT and p62A381V transduced cells. However, a modest increase in TRAF6 ubiquitination was observed in p62WT and p62A381V compared with control EV-transduced pre-OCL cells (Fig. 2A). We next examined whether shRNA suppression of CYLD in the presence of p62WT and mutants modulate TRAF6 ubiquitination in pre-OCL cells. Mouse bone marrow-derived nonadherent cells were transduced with p62 WT, p62A381V, or p62P392L in the presence and absence of CYLD shRNA. Cells were stimulated with RANKL (100 ng/ml) and M-CSF (10 ng/ml) for 48 h, and total cell lysates obtained were subjected to immunoprecipitation of TRAF6. As shown in Fig. 2B, shRNA suppression of CYLD expression significantly increased polyubiquitination of TRAF6 in the presence of p62WT, p62A381V, and p62P392L compared with nonspecific control shRNA transduced pre-OCL cells. These data suggests that p62P392L/CYLD modulates TRAF6 ubiquitination during OCL differentiation.
Fig. 2.
p62P392L enhance TRAF6 polyubiquitination during OCL differentiation. A, Mouse bone marrow-derived nonadherent cells were transduced with p62WT, p62A381V, or p62P392L, and cells were stimulated with M-CSF (10 ng/ml) and RANKL (100 ng/ml) for 48 h. Cells transduced with EV served as control. B, shRNA knockdown of CYLD expression enhances TRAF6 polyubiquitination (Ubn) in pre-OCL cells. Cells were transduced retroviral expression plasmids of p62WT, p62A381V, or p62P392L with a nonspecific control shRNA and CYLD shRNA and stimulated with M-CSF (10 ng/ml) and RANKL (100 ng/ml) for 48 h. Total cell lysates obtained were subjected to immunoprecipitation using anti-TRAF6 antibody, and TRAF6 ubiquitination was analyzed by Western blotting using rabbit-antiubiquitin antibody. The band intensity was quantified by National Institutes of Health ImageJ program. The values are expressed as mean ± sd for three independent experiments (a and b, P < 0.05; compared with EV with and without RANKL stimulation, respectively).
p62P392L modulation of downstream effectors of RANK signaling
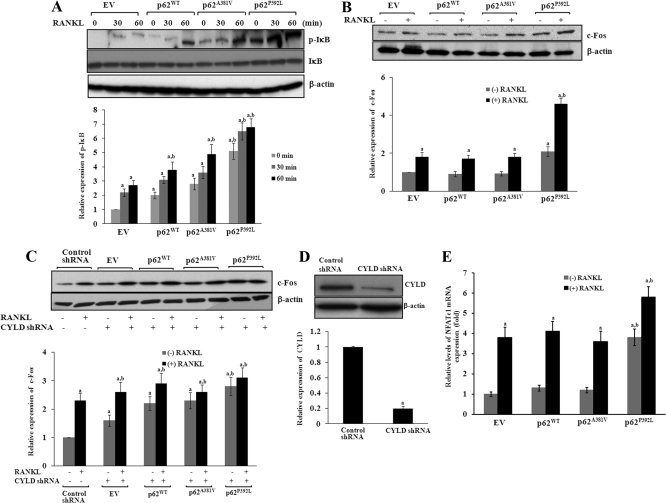
p62P392L has been shown to increase OCL differentiation (17, 20). Therefore, we examined whether p62P392L/CYLD modulates downstream effectors of RANK signaling during OCL differentiation. Mouse bone marrow-derived nonadherent cells were transduced with p62WT, p62A381V, or p62P392L and stimulated with M-CSF (10 ng/ml) and RANKL (100 ng/ml) for different time points (0–60 min). Western blot analysis of total cell lysates obtained from pre-OCL cells revealed that p62P392L induced high level expression of phospho-IκB with and without RANKL stimulation compared with p62WT and p62P381V transduced cells. However, we observed a modest increase in p-IκB expression in p62WT and p62A381V compared with EV transduced cells (Fig. 3A). Furthermore, pre-OCL cells stimulated with RANKL and M-CSF for 48 h had increased (2.8-fold) c-Fos expression in p62P392L compared with p62WT or p62A381V transduced cells (Fig. 3B). To further examine the role of p62P392L/CYLD on c-Fos expression, mouse bone marrow-derived nonadherent cells were transduced with CYLD shRNA coexpressed with p62WT, p62A381V, or p62P392L and stimulated with RANKL and M-CSF for 48 h. Western blot analysis of total cell lysates identified that CYLD knockdown significantly increased c-Fos expression in p62WT, p62A381V, or p62P392L mutant transduced cells without RANKL stimulation compared with control shRNA transduced cells (Fig. 3C). shRNA suppression (∼80%) of CYLD expression was confirmed by Western blot analysis (Fig. 3D). These results further suggest that p62P392L enhanced c-Fos gene expression during OCL differentiation. c-Fos plays an important role in expression of NFATc1, a critical transcription factor essential for OCL differentiation and bone resorption. Therefore, we next examined the functional impact of p62P392L in NFATc1 expression during OCL differentiation. Real-time PCR analysis of total RNA isolated from pre-OCL cells showed that p62P392L significantly increased (4.0-fold) NFATc1 mRNA expression without RANKL stimulation compared with p62WT or p62A381V transduced cells. In contrast, no significant changes in NFATc1 expression were observed in p62WT and p62A381V transduced cells compared with EV transduced cells (Fig. 3E).
Fig. 3.
p62P392L modulation of RANK signaling. Mouse bone marrow-derived nonadherent cells were transduced with p62 WT, p62A381V, or p62P392L and stimulated with M-CSF (10 ng/ml) and RANKL (100 ng/ml) for different time points (0–60 min). Cells transduced with EV served as control. A, Total cell lysates obtained from the pre-OCL cells were analyzed by Western blotting for p-IκB expression. B, Total cell lysates obtained from pre-OCL cells stimulated with M-CSF and RANKL for 48 h were analyzed by Western blotting for c-Fos expression. C, Mouse bone marrow-derived nonadherent cells were transduced with p62WT, p62A381V, or p62P392L with presence of CYLD shRNA. Cells transduced with a nonspecific shRNA served as control. Cells were stimulated with M-CSF (10 ng/ml) and RANKL (100 ng/ml) for 48 h, and total cell lysates obtained were subjected to Western blot analysis of c-Fos expression. D, Western blot analysis of shRNA suppression of CYLD expression compared with nonspecific control shRNA. E, Total RNA isolated from pre-OCL cells transduced with p62WT, p62A381V, or p62P392L and stimulated with RANKL and M-CSF for 48 h was subjected to real-time PCR analysis for NFATc1 mRNA expression and normalized with GAPDH mRNA amplification in these cells. The band intensity was quantified by National Institutes of Health ImageJ program, and values are expressed as mean ± sd for three independent experiments (a and, b P < 0.05; compared with EV with and without RANKL stimulation, respectively).
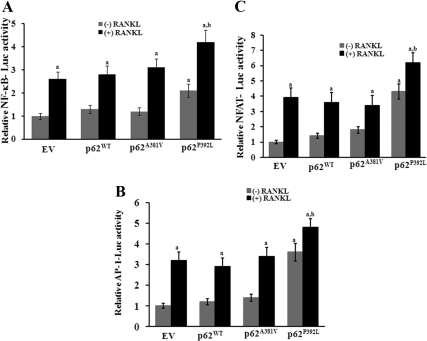
To further confirm that p62 mutant modulates NF-κB, AP-1, and NFAT transcriptional activity, pNF-κB- Luc, AP-1-Luc, and NFAT-Luc reporter gene plasmids were transfected with p62WT, p62A381V, or p62P392L into RAW 264.7 cells and stimulated with RANKL (100 ng/ml) for 48 h. Total cell lysates obtained from these pre-OCL cells were analyzed for luciferase activity as described in Materials and Methods. Mutant p62P392L significantly increased NF-κB, AP-1, and NFAT reporter gene activity with and without RANKL stimulation compared with p62WT and p62A381V transfected cells. However, there was no significant change in the reporter gene activity with p62WT and p62A381V mutant compared with control EV transfected cells (Fig. 4, A–C). These results suggest that the p62 UBA mutant (p62P392L) stimulates downstream effectors of the RANK signaling pathway during OCL differentiation.
Fig. 4.
A, p62P392L enhances NF-κB, AP-1 (B), and NFAT-Luc reporter gene activity (C). RAW 264.7 cells were transfected with NF-κB, AP-1, and NFAT- Luc reporter plasmids and coexpressed with p62WT, p62A381V, or p62P392 and stimulated with and without RANKL (100 ng/ml) for 48 h. Total cell lysates prepared were assayed for luciferase activity. The transfection efficiency was normalized by β-galactosidase activity coexpressed in these cells. Values are expressed as mean ± sd for three independent experiments (a and b, P < 0.05; compared with EV with and without RANKL stimulation, respectively).
p62P392L/CYLD shRNA increase OCL differentiation/bone resorption activity
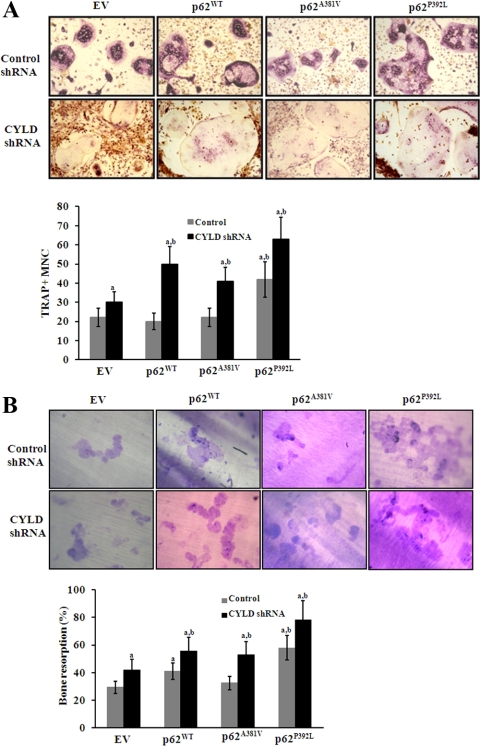
To determine the potential of p62P392L/CYLD shRNA to stimulate OCL formation, mouse bone marrow-derived nonadherent cells were transduced with CYLD shRNA coexpressed with p62WT, p62P392L, or p62A381V and cultured for OCL differentiation in the presence of RANKL (100 ng/ml) and M-CSF (10 ng/ml) for 5 d. Cells transduced with nonspecific shRNA served as control. The number of TRAP positive multinucleated OCL formed in these cultures was scored. p62P392L significantly increased OCL formation compared with control EV transduced cells. In contrast, no significant change in the rate of OCL formation was observed in p62WT and p62A381V transduced cells (Fig. 5A, upper panel). Furthermore, shRNA suppression of CYLD expression significantly increased OCL formation with p62WT and p62A381V similar to p62P392L compared with EV transduced cells (Fig. 5A, lower panel). shRNA knockdown of CYLD significantly increased OCL formation compared with nonspecific control shRNA transduced cells, which confirms that CYLD negatively regulates osteoclastogenesis (23). In addition, bone resorption activity of OCL was significantly increased with mutant p62P392L compared with p62WT and p62A381V transduced cells (Fig. 5B, upper panel). Also, shRNA suppression of CYLD expression significantly increased bone resorption activity in p62WT and p62A381V similar to mutant p62 P392L compared with EV transduced cells (Fig. 5B, lower panel). Collectively, these results suggest that the p62 UBA mutation (p62P392L) associated with PDB abolished interaction with CYLD, which promotes RANK signaling and increased OCL formation/bone resorption activity (Fig. 6).
Fig. 5.
p62P392L/CYLD shRNA stimulation of OCL differentiation in mouse bone marrow cultures. A, Mouse bone marrow-derived nonadherent cells were transduced with CYLD shRNA and coexpressed with p62WT, p62A381V, or p62P392L. Cells were stimulated with RANKL (100 ng/ml) and M-CSF (10 ng/ml) for 5 d, and the TRAP (+) multinucleated OCL formed in these cultures were scored. B, Bone resorption activity of OCL on dentine. The percentage of resorbed area on dentine was quantified as described in Materials and Methods. The results represent quadruplicate cultures of three independent experiments (a and b, P < 0.05; compared with EV with and without RANKL stimulation, respectively).
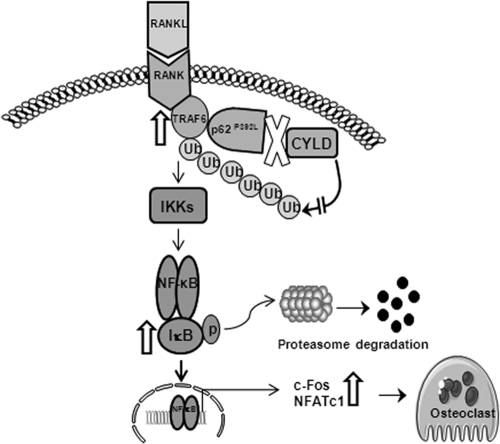
Fig. 6.
Schematic illustration of p62P392L mutant regulation of RANK signaling during OCL differentiation. p62P392L mutation widely associated with PDB abolished interaction with CYLD, which promotes RANK signaling through enhanced TRAF6 ubiquitination, c-Fos, and NFATc1 gene expression (as indicated by open arrows), and thereby increases the OCL formation/bone resorption activity.
Discussion
p62 has been shown to interact with the atypical protein kinase C and TRAF6 to modulate RANK signaling and osteoclastogenesis (13). Ubiquitination of RANK adaptor protein, TRAF6, plays an important role in activation of NF-κB during OCL differentiation (31). The UBA domain truncated p62 has been shown to increase RANKL induced NFAT expression and ERK phosphorylation during osteoclastogenesis of RAW 264.7 cells (19). Deubiquitinating enzyme, CYLD, has been shown to inhibit NF-κB activation through deubiquitinatin of TRAF2 and TRAF6 (24). Furthermore, CYLD interacts with the p62 UBA domain to inhibit TRAF6 ubiquitination and negatively regulates RANK signaling and osteoclastogenesis (23). In the present study, we identify that in contrast to p62WT and non-UBA mutant (p62A381V), UBA mutant (p62P392L) abolished CYLD interaction, and this indicates that RANK signaling essential for osteoclastogenesis is modulated in PDB. We consistently observed accumulation of polyubiquitinated TRAF6 in p62P392L transduced pre-OCL cells.
Ubiquitination of signaling molecules by E3 ubiquitin ligases has been shown to modulate NF-κB signaling (32). Because CYLD negatively regulates different signaling pathways by deubiquitination of K63-linked polyubiquitin chains from several substrates, it is possible that lack of p62P392L interaction with CYLD may affect the ubiquitination status of other RANK signaling molecules that may also play a role in enhanced osteoclastogenesis. Elevated p-IκB expression in p62P392L mutant transduced pre-OCL cells suggests p62P392L activation of NF-κB in these cells. However, a modest increase observed in TRAF6 ubiquitination and p-IκB expression in p62WT and p62A381V compared with control EV transduced cells is more likely due to overexpression of p62 in these cells. Therefore, the mutant p62P392L in contrast to p62WT and p62A381V stimulates NF-κB activity in pre-OCL cells, and this suggests that lack of p62P392L interaction with CYLD may have a specific functional role in enhanced osteoclastogenesis in PDB. Previously, p62 has been shown to be involved in NF-κB activation (13). Further, p62P392L mutation has been shown to elevate NF-κB activation as well as p38 MAPK and ERK1/2 signaling in OCL precursors stimulated with RANKL (17). However, Rea et al. (11) have shown that p62 wild type reduces and p62P392L mutant increases NF-κB activation in HEK293 and Cos-1 cells. Similarly, both p62 non-UBA (A381V) and UBA mutant (P392L) have been shown to increase NF-κB reporter gene activity in HEK293 cells (12). The variability in p62 regulation of NF-κB activity could be due to cell specificity and response to RANKL stimulation. p62 (440 amino acids) contains various domains that mediate protein-protein interactions (33). Several proteins that interact with p62 UBA domain, which includes calmodulin kinase II, nuclear receptor co-receptor I, heat shock protein 70, FK506 binding protein 14, homeobox protein Meis2, and Unc51 like kinase II, have been identified (21). In addition, p62 protein has been shown to interact with several signaling proteins, such as mitogen-activated protein kinases, atypical protein kinase C, p56lck, receptor-interacting protein, TRAF6, ubiquitin, ubiquitinating, and deubiquitnating enzymes (33). Therefore, it is possible that p62A381V may affect interaction with signaling molecules other than CYLD and modulates NF-κB activation in a cell-specific manner. Furthermore, our results that p62P392L expression/shRNA knockdown of CYLD increased NF-κB, AP-1, and NFAT reporter gene activity indicate that mutant p62P392L modulates NF-κB target gene expression required for enhanced osteoclastogenesis in PDB. This is further confirmed by the results that CYLD knockdown significantly increased c-Fos expression in p62WT, p62A381V, and p62P392L mutant transduced cells without RANKL stimulation. Consistently, up-regulation of c-Fos gene expression in pagetic OCL and osteoblast cells has been reported (34). NFATc1 is a critical transcription factor for osteoclastogenesis (14), and our findings that p62P392L up-regulated NFATc1 expression in pre-OCL cells favors increased osteoclastogenesis associated with PDB. Gene expression profiles in OCL revealed no significant change in p62 and CYLD expression in PDB (35). Inhibition of CYLD resulted in NF-κB activation and apoptotic resistance in hepatocellular carcinoma cells (36). Therefore, it is possible that the p62P392L mutant may have a functional role in proliferation/survival of OCL progenitor cells, which have been shown to be increased in PDB (37). Also B-cell lymphoma 2, antiapoptotic gene expression has been shown to be up-regulated in pagetic bone (38). OCL formed in pagetic bone marrow cultures have high levels of IL-6 and TRAP activity (39). We identified that p62P392L expression increased TRAP mRNA expression in pre-OCL cells (data not shown). p62P392L modulation of c-Fos and NFATc1 expression implicated a potential role in enhanced osteoclatogenesis in PDB. Confirming this, we found that p62P392L expression or shRNA knockdown of CYLD resulted in increased OCL formation and bone resorption activity in mouse bone marrow cultures. Therefore, lack of p62 UBA mutant interaction with CYLD results in increased OCL formation. Mutations in valosin-containing protein, which also contains the UBA domain, have been linked to inclusion body myopathy that is associated with PDB and frontotemporal dementia (IBMFD) (40). Valosin-containing protein is a multiubiquitin chain targeting factor for proteosome degradation, and it also plays an important role in regulating the NF-κB signaling cascade (41). Therefore, identification of p62P392L regulated gene expression profiling is important to better understand the pathogenesis of PDB. Transgenic mice harboring p62P394L mutation (equivalent to human p62P392L) showed increase osteoclastogenic potential due to increased RANKL expression in marrow stromal cells in the bone microenvironment. The OCL precursors from these mice also demonstrated increased sensitivity to RANKL but not to 1,25 (OH)2D3 (42). Furthermore, p62 UBA mutant (p62P394L) and MVNP coexpression in mice developed OCL with pagetic phenotype and increased IL-6 production (43). In contrast, others have recently shown that mice with p62P394L mutation have focal lesions with increased OCL number, size, and some nuclear inclusions (18). Consistently, the OCL precursors from these mice are hypersensitive to RANKL. These studies have also suggested that p62P394L mutation up-regulates autophagy, a cellular process for lysosomal degradation of damaged/dysfunctional organelles and protein aggregates (44). However, p62 null mice have a normal skeletal phenotype with no alterations were found in the trabecular size and number of OCL, suggesting that basal osteoclastogenesis is not affected by the loss of p62 (13). Therefore, p62 mutant protein-protein interactions play an important role in enhanced OCL development in PDB.
PDB patients with p62 mutations displayed polyostotic involvement, indicating severity of the disease (45, 46). However, the familial history of PDB is about 15–40% patients with a first degree relative and of which only 20–30% have a p62 mutation. Thus, p62 mutations occur in 5–10% of patients with PDB. Therefore, p62 mutant stimulation of OCL formation/bone resorption is associated with a very limited patient population. Recently, genome-wide association studies in individuals without p62 mutations have further identified genetic variants CSF1, OPTN, and TNFRSF11A as risk factors that predispose to PDB; however,, their functional role in pathogenesis of PDB is yet to be elucidated (47). Presence of nuclear inclusions in pagetic OCL suggested a viral etiology for PDB; however, no infectious virus is isolated. Despite the controversy about the identity of paramyxoviral nuclear inclusions and MVNP expression in pagetic OCL, it has been shown that targeted expression of MVNP to OCL lineage develops pagetic-like bone lesions in mice (48). Therefore, both genetic and environmental factors, such as paramyxoviruses, play an important role in pathogenesis of PDB. In conclusions, p62 UBA mutation (p62P392L) abolished interaction with CYLD and contributed to enhanced OCL development and excess bone resorption associated with PDB.
Acknowledgments
We thank Danielle Mumford for technical assistance.
This work was supported by the Department of Defense Medical Research Award PR080480.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP-1
- Activator protein 1
- CYLD
- cylindromatosis
- EV
- empty vector
- FCS
- fetal calf serum
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- HA
- hemagglutinin
- K63
- lysine-63
- M-CSF
- macrophage colony-stimulating factor
- MVNP
- measles virus nucleocapsid
- NFATc1
- nuclear factor of activated T cells c1
- NF-κB
- nuclear factor κB
- OCL
- osteoclast
- p62
- sequestosome 1
- PDB
- Paget's disease of bone
- RANK
- receptor activator of NF-κB
- RANKL
- RANK ligand
- shRNA
- short hairpin RNA
- TRAF
- TNF receptor-associated factor
- TRAP
- tartrate-resistant acid phosphatase
- UBA
- ubiquitin associated.
References
- 1. Morales-Piga AA, Rey-Rey JS, Corres-González J, García-Sagredo JM, López-Abente G. 1995. Frequency and characteristics of familial aggregation of Paget's disease of bone. J Bone Miner Res 10:663–670 [DOI] [PubMed] [Google Scholar]
- 2. Reddy SV. 2004. Etiology of Paget's disease and osteoclast abnormalities. J Cell Biochem 93:688–696 [DOI] [PubMed] [Google Scholar]
- 3. Mills BG, Singer FR. 1976. Nuclear inclusions in Paget's disease of bone. Science 194:201–202 [DOI] [PubMed] [Google Scholar]
- 4. Friedrichs WE, Reddy SV, Bruder JM, Cundy T, Cornish J, Singer FR, Roodman GD. 2002. Sequence analysis of measles virus nucleocapsid transcripts in patients with Paget's disease. J Bone Miner Res 17:145–151 [DOI] [PubMed] [Google Scholar]
- 5. Merchant A, Smielewska M, Patel N, Akunowicz JD, Saria EA, Delaney JD, Leach RJ, Seton M, Hansen MF. 2009. Somatic mutations in SQSTM1 detected in affected tissues from patients with sporadic Paget's disease of bone. J Bone Miner Res 24:484–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mee AP, Dixon JA, Hoyland JA, Davies M, Selby PL, Mawer EB. 1998. Detection of canine distemper virus in 100% of Paget's disease samples by in situ-reverse transcriptase-polymerase chain reaction. Bone 23:171–175 [DOI] [PubMed] [Google Scholar]
- 7. Helfrich MH, Hobson RP, Grabowski PS, Zurbriggen A, Cosby SL, Dickson GR, Fraser WD, Ooi CG, Selby PL, Crisp AJ, Wallace RG, Kahn S, Ralston SH. 2000. A negative search for a paramyxoviral etiology of Paget's disease of bone: molecular, immunological, and ultrastructural studies in UK patients. J Bone Miner Res 15:2315–2329 [DOI] [PubMed] [Google Scholar]
- 8. Matthews BG, Afzal MA, Minor PD, Bava U, Callon KE, Pitto RP, Cundy T, Cornish J, Reid IR, Naot D. 2008. Failure to detect measles virus ribonucleic acid in bone cells from patients with Paget's disease. J Clin Endocrinol Metab 93:1398–1401 [DOI] [PubMed] [Google Scholar]
- 9. Seibenhener ML, Geetha T, Wooten MW. 2007. Sequestosome 1/p62—more than just a scaffold. FEBS Lett 581:175–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hocking LJ, Lucas GJ, Daroszewska A, Mangion J, Olavesen M, Cundy T, Nicholson GC, Ward L, Bennett ST, Wuyts W, Van Hul W, Ralston SH. 2002. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget's disease. Hum Mol Genet 11:2735–2739 [DOI] [PubMed] [Google Scholar]
- 11. Rea SL, Walsh JP, Ward L, Yip K, Ward BK, Kent GN, Steer JH, Xu J, Ratajczak T. 2006. A novel mutation (K378X) in the sequestosome 1 gene associated with increased NF-κB signaling and Paget's disease of bone with a severe phenotype. J Bone Miner Res 21:1136–1145 [DOI] [PubMed] [Google Scholar]
- 12. Najat D, Garner T, Hagen T, Shaw B, Sheppard PW, Falchetti A, Marini F, Brandi ML, Long JE, Cavey JR, Searle MS, Layfield R. 2009. Characterization of a non-UBA domain missense mutation of sequestosome 1 (SQSTM1) in Paget's disease of bone. J Bone Miner Res 24:632–642 [DOI] [PubMed] [Google Scholar]
- 13. Durán A, Serrano M, Leitges M, Flores JM, Picard S, Brown JP, Moscat J, Diaz-Meco MT. 2004. The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell 6:303–309 [DOI] [PubMed] [Google Scholar]
- 14. Takayanagi H. 2007. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7:292–304 [DOI] [PubMed] [Google Scholar]
- 15. Yamashita T, Yao Z, Li F, Zhang Q, Badell IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, Xing L, Boyce BF. 2007. NF-κB p50 and p52 regulate receptor activator of NF-κB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem 282:18245–18253 [DOI] [PubMed] [Google Scholar]
- 16. Teitelbaum SL, Ross FP. 2003. Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638–649 [DOI] [PubMed] [Google Scholar]
- 17. Kurihara N, Hiruma Y, Zhou H, Subler MA, Dempster DW, Singer FR, Reddy SV, Gruber HE, Windle JJ, Roodman GD. 2007. Mutation of the sequestosome 1 (p62) gene increases osteoclastogenesis but does not induce Paget disease. J Clin Invest 117:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daroszewska A, van 't Hof RJ, Rojas JA, Layfield R, Landao-Basonga E, Rose L, Rose K, Ralston SH. 2011. A point mutation in the ubiquitin associated domain of SQSMT1 is sufficient to cause a Paget's disease like disorder in mice. Hum Mol Genet 20:2734–2744 [DOI] [PubMed] [Google Scholar]
- 19. Yip KH, Feng H, Pavlos NJ, Zheng MH, Xu J. 2006. p62 ubiquitin binding-associated domain mediated the receptor activator of nuclear factor-κB ligand-induced osteoclast formation: a new insight into the pathogenesis of Paget's disease of bone. Am J Pathol 169:503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chamoux E, Couture J, Bisson M, Morissette J, Brown JP, Roux S. 2009. The p62 P392L mutation linked to Paget's disease induces activation of human osteoclasts. Mol Endocrinol 23:1668–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pridgeon JW, Geetha T, Wooten MW. 2003. A method to identify p62's UBA domain interacting proteins. Biol Proced Online 5:228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, Jones C, Hansen J, Blair E, Hofmann B, Siebert R, Turner G, Evans DG, Schrander-Stumpel C, Beemer FA, van Den Ouweland A, Halley D, Delpech B, Cleveland MG, Leigh I, Leisti J, Rasmussen S. 2000. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet 25:160–165 [DOI] [PubMed] [Google Scholar]
- 23. Jin W, Chang M, Paul EM, Babu G, Lee AJ, Reiley W, Wright A, Zhang M, You J, Sun SC. 2008. Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. J Clin Invest 118:1858–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. 2003. CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members. Nature 424:793–796 [DOI] [PubMed] [Google Scholar]
- 25. Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. 2003. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424:797–801 [DOI] [PubMed] [Google Scholar]
- 26. Kovalenko A, Chable-Bessia C, Cantarella G, Israël A, Wallach D, Courtois G. 2003. The tumour suppressor CYLD negatively regulates NF-κB signalling by deubiquitination. Nature 424:801–805 [DOI] [PubMed] [Google Scholar]
- 27. Blake PW, Toro JR. 2009. Update of cylindromatosis gene (CYLD) mutations in Brooke-Spiegler syndrome: novel insights into the role of deubiquitination in cell signaling. Hum Mutat 30:1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song L, Rape M. 2008. Reverse the curse—the role of deubiquitination in cell cycle control. Curr Opin Cell Biol 20:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sundaram K, Senn J, Yuvaraj S, Rao DS, Reddy SV. 2009. FGF-2 stimulation of RANK ligand expression in Paget's disease of bone. Mol Endocrinol 23:1445–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shanmugarajan S, Youssef RF, Pati P, Ries WL, Rao DS, Reddy SV. 2008. Osteoclast inhibitory peptide-1 (OIP-1) inhibits measles virus nucleocapsid protein stimulated osteoclast formation/activity. J Cell Biochem 104:1500–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J. 2001. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J 20:1271–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harhaj EW, Dixit VM. 2011. Deubiquitinases in the regulation of NF-κB signaling. Cell Res 21:22–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roodman GD, Windle JJ. 2005. Paget disease of bone. J Clin Invest 115:200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoyland J, Sharpe PT. 1994. Upregulation of c-fos protooncogene expression in pagetic osteoclasts. J Bone Miner Res 9:1191–1194 [DOI] [PubMed] [Google Scholar]
- 35. Michou L, Chamoux E, Couture J, Morissette J, Brown JP, Roux S. 2010. Gene expression profile in osteoclasts from patients with Paget's disease of bone. Bone 46:598–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Urbanik T, Köhler BC, Boger RJ, Wörns MA, Heeger S, Otto G, Hövelmeyer N, Galle PR, Schuchmann M, Waisman A, Schulze-Bergkamen H. 2011. Down-regulation of CYLD as a trigger for NF-κB activation and a mechanism of apoptotic resistance in hepatocellular carcinoma cells. Int J Oncol 38:121–131 [PubMed] [Google Scholar]
- 37. Demulder A, Takahashi S, Singer FR, Hosking DJ, Roodman GD. 1993. Abnormalities in osteoclast precursors and marrow accessory cells in Paget's disease. Endocrinology 133:1978–1982 [DOI] [PubMed] [Google Scholar]
- 38. Brandwood CP, Hoyland JA, Hillarby MC, Berry JL, Davies M, Selby PL, Mee AP. 2003. Apoptotic gene expression in Paget's disease: a possible role for Bcl-2. J Pathol 201:504–512 [DOI] [PubMed] [Google Scholar]
- 39. Roodman GD, Kurihara N, Ohsaki Y, Kukita A, Hosking D, Demulder A, Smith JF, Singer FR. 1992. Interleukin 6. A potential autocrine/paracrine factor in Paget's disease of bone. J Clin Invest 89:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weihl CC, Pestronk A, Kimonis VE. 2009. Valosin-containing protein disease: inclusion body myopathy with Paget's disease of the bone and fronto-temporal dementia. Neuromuscul Disord 19:308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Custer SK, Neumann M, Lu H, Wright AC, Taylor JP. 2010. Transgenic mice expressing mutant forms VCP/p97 recapitulate the full spectrum of IBMPFD including degeneration in muscle, brain and bone. Hum Mol Genet 19:1741–1755 [DOI] [PubMed] [Google Scholar]
- 42. Hiruma Y, Kurihara N, Subler MA, Zhou H, Boykin CS, Zhang H, Ishizuka S, Dempster DW, Roodman GD, Windle JJ. 2008. A SQSTM1/p62 mutation linked to Paget's disease increases the osteoclastogenic potential of the bone microenvironment. Hum Mol Genet 17:3708–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kurihara N, Hiruma Y, Yamana K, Michou L, Rousseau C, Morissette J, Galson DL, Teramachi J, Zhou H, Dempster DW, Windle JJ, Brown JP, Roodman GD. 2011. Contributions of the measles virus nucleocapsid gene and the SQSTM1/p62(P392L) mutation to Paget's disease. Cell Metab 13:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Behrends C, Sowa ME, Gygi SP, Harper JW. 2010. Network organization of the human autophagy system. Nature 466:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Michou L, Morissette J, Gagnon ER, Marquis A, Dellabadia M, Brown JP, Siris ES. 2011. Novel SQSTM1 mutations in patients with Paget's disease of bone in an unrelated multiethnic American population. Bone 48:456–460 [DOI] [PubMed] [Google Scholar]
- 46. Visconti MR, Langston AL, Alonso N, Goodman K, Selby PL, Fraser WD, Ralston SH. 2010. Mutations of SQSTM1 are associated with severity and clinical outcome in paget disease of bone. J Bone Miner Res 25:2368–2373 [DOI] [PubMed] [Google Scholar]
- 47. Albagha OM, Visconti MR, Alonso N, Langston AL, Cundy T, Dargie R, Dunlop MG, Fraser WD, Hooper MJ, Isaia G, Nicholson GC, del Pino Montes J, Gonzalez-Sarmiento R, di Stefano M, Tenesa A, Walsh JP, Ralston SH. 2010. Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget's disease of bone. Nat Genet 42:520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kurihara N, Zhou H, Reddy SV, Garcia Palacios V, Subler MA, Dempster DW, Windle JJ, Roodman GD. 2006. Expression of measles virus nucleocapsid protein in osteoclasts induces Paget's disease-like bone lesions in mice. J Bone Miner Res 21:446–455 [DOI] [PubMed] [Google Scholar]