Abstract
Natriuretic peptide type C (NPPC) and its cognate receptor natriuretic peptide receptor 2 (NPR2) are essential for maintaining meiotic arrest in mouse oocytes residing in Graafian follicles. Cumulus cells, which are associated with the oocyte, express the receptor NPR2, a guanylyl cyclase, whereas mural granulosa cells express ligand NPPC. This study determined the temporal expression of Npr2 and the hormonal factors that participate in regulating its expression and, thereby, in oocyte meiotic arrest. Stimulation of follicular development in vivo with equine chorionic gonadotropin (eCG) promoted expression of Npr2 mRNA by cumulus cells and some periantral mural granulosa cells. However, FSH did not elevate the levels of Npr2 mRNA in cultured cumulus-oocyte complexes (COCs) isolated from mice not stimulated in vivo with eCG. Nevertheless, estradiol elevated expression of this transcript in vitro to the same steady-state level found in COCs isolated from eCG-stimulated follicles in vivo. Expression of Npr2 mRNA was rapidly reduced in COCs in vitro after isolation from eCG-primed mice unless maintained in culture with estradiol. The ability of NPPC to maintain meiotic arrest in cultured COCs was transient unless culture was in estradiol-containing medium. Ability of cumulus cells to produce cyclic GMP, which is required for the maintenance of meiotic arrest, was also lost in the absence of estradiol, indicating that estradiol is required to maintain functional NPR2 receptors on cumulus cells in vitro. It is concluded that estradiol promotes and maintains expression of NPR2 in cumulus cells and participates in NPPC-mediated maintenance of oocyte meiotic arrest in vitro.
The progression of meiosis in oocytes is not continuous as it is in male germ cells but is subject to start and stop signals that are both intrinsic and extrinsic to the oocytes. Prophase I-arrested growing oocytes become competent to resume meiosis after they produce and properly localize the essential intrinsic cell cycle regulatory molecules needed to drive meiotic progress (1, 2). However, factors derived from follicular somatic cells arrest the progression of meiosis despite the readiness of these oocyte-intrinsic meiotic progress pathways. In a normal reproductive cycle, the preovulatory surge of LH from the pituitary stimulates the resumption of meiosis in oocytes in Graafian follicles and ovulation. However, oocytes undergo “spontaneous” resumption of meiosis without hormonal stimulation when removed from ovarian follicles and cultured under simple nutritionally supportive conditions. Germinal vesicle (GV) breakdown (GVB) is the first obvious morphological manifestation of meiotic resumption in oocytes. Spontaneous GVB in rabbit and human oocytes in vitro was first observed in 1935 and led to the suggestion that isolation of the cumulus-oocyte complex (COC) from the remainder of the ovarian follicle separates the complex from meiotic-arresting factors produced by the follicle (3). Spontaneous GVB in culture has been observed in oocytes from other mammalian species (4), and extensive research has focused on identifying the factors participating in maintaining meiotic arrest.
Sustaining elevated levels of cAMP in fully grown oocytes is essential for maintaining meiotic arrest at the GV stage (5–8). Production of cAMP within oocytes is required (9). Although transfer of cAMP produced by mural and/or cumulus granulosa cells via gap junctions from companion cumulus cells to oocytes is possible, it is insufficient, because knockout of the oocyte's ability to produce cAMP results in precocious GVB in vivo (10, 11). Activity of an oocyte-specific phosphodiesterase, phosphodiesterase 3A (PDE3A), degrades cAMP in oocytes to initiate activation of cell cycle-promoting proteins driving GVB (12–14). However, to maintain PDE3A in an inactive state, cyclic GMP (cGMP) produced in cumulus cells and transferred via gap junctions to the oocyte acts as an inhibitor of PDE3A, thus preventing a decrease in oocyte cAMP and GVB (15, 16).
Natriuretic peptide type C (NPPC) (also known as C-type natriuretic peptide or CNP), is expressed in Graafian follicles by mural granulosa cells, which line the follicle wall, and its cognate receptor natriuretic peptide receptor 2 (NPR2) (also known as guanylyl cyclase B or GC-B), a guanylyl cyclase, is expressed by cumulus cells, which surround and associate with oocytes. Some mural granulosa cells, those lining the antral space, often referred to as periantral mural granulosa cells, also express Npr2 mRNA, at levels that appear similar to those of cumulus cells (17). However, expression of Npr2 mRNA by mural granulosa cells decreases dramatically with increasing distance from the oocyte (17). Thus, it is likely that some mural granulosa cells are stimulated by NPPC in an autocrine manner to increase cGMP levels. Thus, NPPC-promoted cGMP may diffuse through the gap junctions that couple mural granulosa cells with cumulus cells (18) and then to the oocyte, or promote other functions within the granulosa cells (19), or both.
Treatment of COCs isolated from Graafian follicles with the low molecular weight form of NPPC composed of 22 amino acids (hereafter referred to as NPPC-22) results in increased levels of cGMP in both cumulus cells and oocytes, of cAMP in oocytes, and in inhibition of GVB. Importantly, precocious resumption of meiosis occurs in oocytes within Graafian follicles in loss-of-function mutants of either Nppc or Npr2, demonstrating the crucial function of NPPC and its cognate receptor NPR2 in maintaining meiotic arrest (17). Here, we begin studies on how this is coordinated with the hormonal milieu of the Graafian follicle primarily using COCs isolated from 22-d-old mice either unstimulated or stimulated with equine chorionic gonadotropin (eCG) in vivo and then cultured.
Injection of immature rats with either eCG or the synthetic estrogen diethylstilbesterol (DES) resulted in increased Npr2 mRNA levels and NPPC binding by granulosa cells isolated after injection (20). These were mostly mural granulosa cells, which are readily extruded from follicles by the isolation methods used (21). It was concluded that gonadotropins and estrogens regulate the NPR2/NPPC system in rat granulosa cells (20). Estrogens also play an important role in cumulus cell function. The expansion of the cumulus oophorus in estrogen receptor β (Esr2) null mice is abnormal after human CG (hCG) stimulation (22, 23). Moreover, maintenance of isolated mouse COCs for 48 h in vitro results in loss of the ability to undergo cumulus expansion in response to epidermal growth factor. However, culture of COC in medium containing estradiol sustains this ability (24). We report here that estradiol promotes and maintains expression of Npr2 mRNA by mouse cumulus cells in vitro, thereby augmenting their ability to produce cGMP in response to NPPC-22 and the maintenance of meiotic arrest by NPPC-22. Therefore, estradiol promotes and maintains expression of NPR2 in cumulus cells in vitro and may participate in mechanisms maintaining oocyte meiotic arrest in Graafian follicles.
Materials and Methods
Animals
(C57BL/6J X SJL/J)F1 mice raised in the colonies of the investigators were used in these studies. Mice were used between the ages of 20 and 22 d, some having been injected with 5 IU of eCG 44 h before use. All animal protocols were approved by the Administrative Panel on Laboratory Animal Care at The Jackson Laboratory, and all experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Recombinant human FSH was obtained from the EMD-Serono Research Institute (Billerica, MA).
Isolation and culture of COCs
Ovaries from either unstimulated or eCG-stimulated mice were immersed in culture medium supplemented with either 10 μm milrinone or milrinone plus 100 nm NPPC-22. The culture medium was Waymouth MB752/1 supplemented with 75 μg/ml penicillin G, 50 μg/ml streptomycin sulfate, 0.23 mm pyruvate, and 3 mg/ml BSA. After isolation of COCs, as described previously (25), they were washed in the medium of final incubation and cultured for 4 or 24 h as described in Results. At the termination of culture, cumulus cells were stripped from the oocytes by repeated drawing in and out of glass pipets whose diameter was slightly smaller than that of the oocytes. Oocytes were then examined for the presence or absence of a GV using a stereo microscope. Presence of a GV indicated that the oocyte had been maintained in meiotic arrest. Cultures were maintained in an atmosphere of 5% O2, 5% CO2, and 90% N2 at 37 C in a modular incubation chamber (Billups Rothenberg, Del Mar, CA).
In situ hybridization of Npr2 mRNA
Ovaries isolated from 21-d-old unstimulated, eCG (48 h)-stimulated, or eCG (48 h) + hCG (3 h)-stimulated mice were fixed in in 4% paraformaldehyde, dehydrated, embedded in paraffin, and sectioned for in situ hybridization. In situ hybridization of Npr2 mRNA was carried out according to the procedure as described previously (26) using the same 33P-labeled riboprobes as shown in our previous study (17).
Quantitative RT-PCR (qRT-PCR) analysis
Cumulus cells of freshly isolated or cultured COCs were stripped from the oocytes by passing through a fine-bore glass pipette with the inner diameter slightly narrower than the oocyte and collected in 350 μl of RLT buffer for RNA extraction. Total RNA isolation and purification, in vitro transcription, and real-time PCR analyses were carried out as described previously (27, 28) to quantify the steady-state mRNA levels of Npr2 and the housekeeping gene Rpl19 (internal control). The same primers for Npr2 and Rpl19 as shown in our previous study (17) were used for real-time PCR. The levels of Npr2 mRNA were presented as relative to a control group whose expression level was set at 1. Each experiment was repeated independently at least three times.
Determination of cGMP levels in cumulus cells and oocytes
COCs, either freshly isolated or after 24 h of culture, were incubated for 1 h with or without 30 nm NPPC-20; cumulus cells and oocytes from groups of 100–200 COCs were manually dissociated by pipetting with a small fine-bore pipette in culture medium containing 0.2 mm 3-isobutyl-1-methylxanthine and collected for cGMP assay. The levels of cGMP were then determined using the same protocol as described in our previous study (17) using cGMP-EIA kits obtained from Cayman Chemicals (Ann Arbor, MI) (17).
Statistical analysis
JMP 7 software was used to conduct t tests or the Tukey-Kramer honestly significant difference test for paired or multiple comparisons, respectively (SAS Institute, Inc., Cary, NC). A P value of less than 0.05 was considered statistically significant.
Results
Hormonal control of steady-state Npr2 mRNA levels in vivo
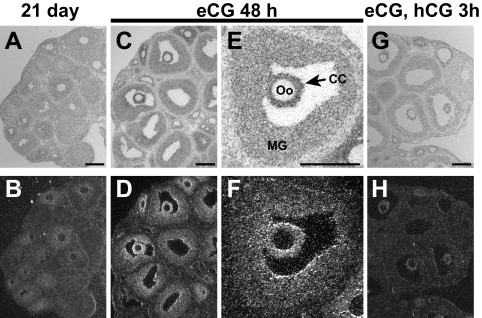
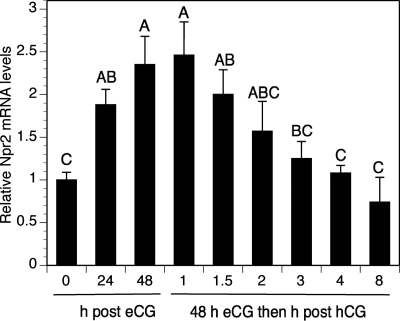
It was demonstrated previously by in situ hybridization that Npr2 mRNA in Graafian follicles of eCG-primed mice is distributed in a gradient from highest levels in cumulus cells, and some periantral mural cells, to lowest levels in mural granulosa cells with increasing distance from the oocytes (17). This cellular distribution of Npr2 mRNA expression is confirmed here in Fig. 1, C–F. In addition, Fig. 1 shows lower expression of Npr2 mRNA in all follicular cell types, including cumulus cells before stimulation with eCG (Fig. 1, A and B). Expression is reduced in the cumulus cells 3 h after treatment of eCG-stimulated mice with hCG (Fig. 1, G and H). This regulation of Npr2 mRNA steady-state levels in cumulus cells was confirmed by qRT-PCR. Relative levels approximately doubled by 24 h and reached a peak by 48 h after eCG then declined to pre-eCG levels by 3 h after hCG (Fig. 2). Thus, expression of Npr2 mRNA in the small to midsize antral follicles of 21-d-old mice not stimulated with eCG in vivo is relatively low but increases dramatically in cumulus cells and periantral mural granulosa cells after eCG-stimulation of follicular development.
Fig. 1.
Localization of Npr2 mRNA in ovarian follicles shown by in situ hybridization. Bright field micrographs are shown in the top panels (A, C, E, and G) and dark field in the bottom (B, D, F, and H). CC and arrows, Cumulus cells; MG, mural granulosa cells; Oo, oocyte. Scale bars, 200 μm.
Fig. 2.
Gonadotropin control of Npr2 mRNA expression in cumulus cells in vivo by qRT-PCR. The mean value in the control (0-h eCG treatment) was set at a value of 1, and expression levels in other samples are shown relative to the control. Bars show the mean ± sem of four independent samples. Relative expression levels not connected by the same letter are significantly different (P < 0.05).
Effects of FSH and estradiol on Npr2 mRNA levels in cumulus cells in vitro
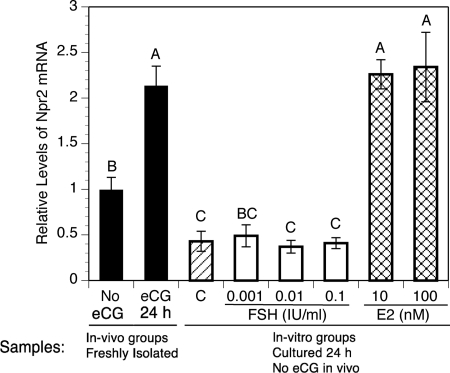
The increased levels of Npr2 mRNA stimulated by eCG suggested that levels of this transcript may be directly regulated by FSH. To test this, COCs isolated from the ovaries of mice not stimulated with eCG were cultured for 24 h with no FSH (control) and a wide range of FSH concentrations (0.001–0.1 IU/ml) recombinant human FSH. In a previous study, a concentration of 0.005 IU/ml of the same preparation of FSH was sufficiently active to promote the development of competence by cumulus cells to undergo expansion in response to a high concentration of FSH (0.5 IU) (29). Levels of Npr2 mRNA in control cultures were reduced to about half those measured in COCs freshly isolated from mice not stimulated with eCG. Surprisingly, FSH failed to increase Npr2 mRNA levels in culture (Fig. 3). COCs were also cultured with 10 or 100 nm estradiol, because 1) the synthetic estrogen DES promoted Npr2 expression in rat granulosa cells in vivo (20), 2) estradiol was found to benefit cumulus cell function in vitro (24), and 3) deletion of Esr2 (encoding the estrogen receptor β) negatively affects cumulus cell function (22, 23). Both concentrations of estradiol elevated expression of Npr2 mRNA in vitro to levels equivalent to that found in COCs freshly isolated 24-h post-eCG injection but not maintained in culture (Fig. 3).
Fig. 3.
Effect of FSH and estradiol in vitro on levels of Npr2 mRNA in cumulus cells of COCs isolated from mice that were not stimulated with eCG. The mean value in COCs freshly isolated from mice not stimulated by eCG was set at 1, and other levels are shown relative to it. Bars show the mean ± sem of three independent samples. The solid bars compare levels in COCs freshly isolated from unstimulated mice to those taken from mice 24 h after eCG. The single hatched bar (C) shows the relative level in control COCs cultured 24 h without either FSH or estradiol (E2). White bars show relative levels in COCs cultured in various concentrations of FSH for 24 h. Cross hatched bars show relative levels in COCs cultured 24 h in 10 or 100 nm estradiol. Relative expression levels not connected by the same letter are significantly different (P < 0.05).
Effect of estradiol on NPPC-22-mediated meiotic arrest
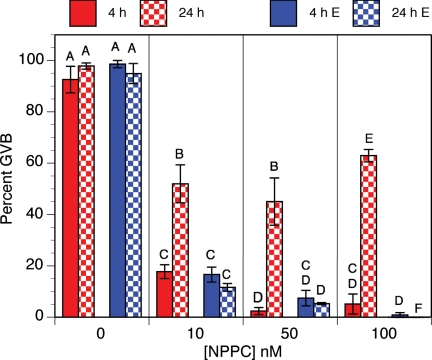
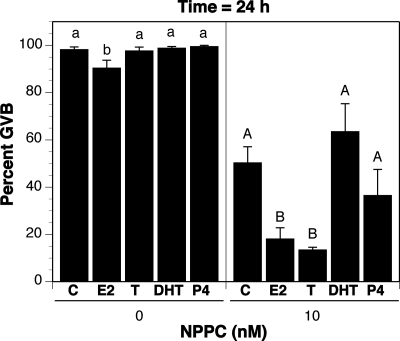
When assessed after 4 h of culture, NPPC-22 (but not the related peptides NPPA or NPPB) inhibited GVB in COCs isolated from the Graafian follicles of eCG-stimulated mice (17). This effect of NPPC-22 was confirmed here with maximum inhibition at 50 nm NPPC-22 (Fig. 4). However, when assessed after 24 h of culture, it was clear that NPPC-22-mediated meiotic arrest had been transient, because about half of the oocytes underwent GVB (Fig. 4). Nevertheless, estradiol (100 nm) stabilized NPPC-22-mediated meiotic arrest for at least 24 h; treatment with 10 nm NPPC-22 and 100 nm estradiol resulted in 90% inhibition of GVB (Fig. 4). To determine the specificity of steroid hormone stabilization of NPPC-22-mediated meiotic arrest, COCs isolated from eCG-primed mice were cultured for 24 h in medium containing no steroid hormones or no NPPC or 100 nm of various other steroid hormones plus 10 nm NPPC-22. Estradiol without NPPC had a slight effect in inhibiting GVB, but no effect was seen with testosterone, dihydrotestosterone (DHT), or progesterone. As in the previous experiment, estradiol stabilized NPPC-22-mediated meiotic arrest in COCs isolated from eCG-stimulated mice, and testosterone had the same effect as estradiol. However, neither DHT nor progesterone had any significant effect (Fig. 5).
Fig. 4.
Effect of estradiol on NPPC-mediated meiotic arrest in vitro. The proportion of oocytes having undergone GVB was determined at 4 and 24 h of culture. Bars indicate the mean ± sem of four independent experiments with at least 25 cumulus cell-enclosed oocytes assessed at each point in each experiment. Red bars, No estradiol; blue bars, 100 nm estradiol (E); solid bars, GVB assessed at 4 h; checkered bars, GVB assessed at 24 h. Percentages not connected by the same letter are significantly different (P < 0.05).
Fig. 5.
Effect of various steroid hormones on NPPC-mediated meiotic arrest in vitro. The proportion of oocytes having undergone GVB was determined at 24 h culture with or without (C) various steroid hormones. Bars indicated the mean ± sem of four independent experiments with at least 25 COCs assessed per group in each experiment. All steroid hormones were used at a concentration of 100 nm. Left panel, No NPPC; right panel, 10 nm NPPC; E2, estradiol; T, testosterone; P4, progesterone. Percentages not connected by the same letter are significantly different (P < 0.05).
Effect of estradiol on Npr2 mRNA levels in cumulus cells
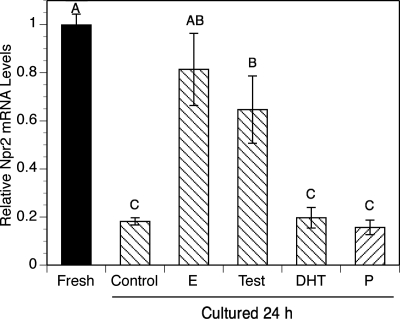
Because estradiol promoted the expression of Npr2 mRNA in cumulus cells of COCs isolated from mice that were not stimulated with eCG (Fig. 2), we hypothesized that estradiol sustains the levels of Npr2 mRNA of COCs isolated from eCG-primed mice when levels of Npr2 mRNA were already high at the time of COC isolation. To test this hypothesis, and the potential effects of other steroid hormones, COCs were isolated from ovaries 44 h after stimulating mice with eCG and were then cultured 24 h in medium supplemented with the PDE3A inhibitor 10 μm milrinone to maintain all oocytes in all groups in meiotic arrest throughout the culture period. Because oocyte-cumulus cell communication has a significant impact on cumulus cell function, and oocytes alone are able to affect the steady-state levels of Npr2 mRNA in cumulus cells (17), the presence of milrinone prevents a potentially confounding effect caused by having oocytes resume meiosis and possibly affecting cumulus cell function differently from meiosis-arrested oocytes. Milrinone was used in this experiment to maintain meiotic arrest rather than NPPC-22, because, as shown above, NPPC-22 does not sustain meiotic arrest for 24 h in the absence of estradiol or testosterone. After a 24-h culture, the relative levels of Npr2 mRNA were measured by qRT-PCR. Culture in control medium without steroid hormones dramatically decreased levels of Npr2 mRNA compared with COCs freshly isolated from eCG-primed mice (Fig. 6). However, estradiol and testosterone sustained expression of Npr2 mRNA at the same level as that in COCs freshly isolated from eCG-primed mice. Neither DHT nor progesterone sustained Npr2 mRNA expression (Fig. 6).
Fig. 6.
Effect of various steroid hormones on relative levels of Npr2 mRNA in cultured COCs. COCs isolated from the ovaries of eCG-primed mice were cultured in milrinone (10 μm)-containing medium and treated without (control) or with 100 nm various steroid hormones for 24 h. Cumulus cells were collected at the end of culture, and the levels of Npr2 mRNA were measured. The mean value in COCs freshly isolated from eCG-primed mice was set at 1, and other levels are shown relative to the fresh group. Bars show the mean ± sem of three independent experiments. C, Control without any steroids; E, estradiol; Test, testosterone; P, progesterone. Relative levels not connected by the same letter are significantly different (P < 0.05).
Effect of estradiol on maintaining functional NPR2 receptors
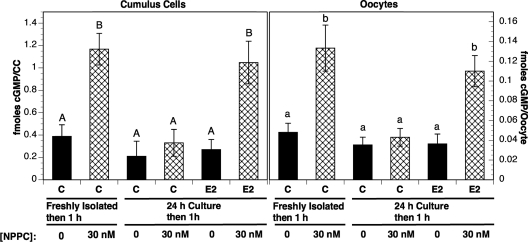
Because NPR2 is a guanylyl cyclase, elevated cGMP levels after NPPC-22-treatment are indicative, at least in part, of functional NPR2 receptors. To determine whether estradiol maintains the ability of COCs to respond to NPPC by cGMP production, COCs isolated from eCG-primed mice were cultured in medium supplemented with milrinone to maintain oocytes in meiotic arrest (as above) plus or minus estradiol and then incubated with NPPC-22 for 1 h. The cumulus cells were then removed from the oocytes, and the levels of cGMP were measured in both cumulus cells and oocytes. In both cumulus cells and oocytes of COCs freshly isolated from eCG-stimulated mice, the cGMP levels were increased by NPPC-22-treatment as described previously (17). However, the levels of cGMP in both cell types of COCs cultured for 24 h in absence of estradiol remained at the same level after incubation with NPPC-22 as fresh COCs that were not stimulated with NPPC-22 (Fig. 7). In contrast, cGMP levels were elevated by NPPC-22-stimulation in both cumulus cells and oocytes when COCs were cultured for 24 h with estradiol (Fig. 7).
Fig. 7.
cGMP levels in cumulus cells and oocytes after stimulation of freshly isolated or cultured COCs with NPPC; effect of culture with estradiol. COCs were isolated from eCG-stimulated mice. They were incubated with or without 30 nm NPPC for 1 h either immediately (freshly isolated) or after culture for 24 h (24-h culture) in medium with or without (C) estradiol (E2) (100 nm). Solid bars indicated groups not incubated with NPPC, whereas cross hatched bars show groups treated for 1 h with 30 nm NPPC (17). The left panel shows cGMP levels in the cumulus cells (CC) (fmoles per CC of one COC). The right panel shows cGMP levels in oocytes (fmoles per oocyte). Values indicate the mean ± sem of four independent experiments with at least 100 COCs assessed at each point in each experiment. Levels of cGMP not connected by the same letter are significantly different (P < 0.05).
Discussion
In the absence of estradiol in vitro, the ability of NPPC-22 to maintain meiotic arrest in COCs isolated from eCG-stimulated follicles was transient. However, estradiol stabilized NPPC-22-mediated inhibition of meiotic arrest for at least 24 h. This stabilization appears due to the maintenance of Npr2 mRNA levels and functional NPR2 receptors. Estradiol maintained the ability of NPPC-22 to stimulate cGMP production by cumulus cells in vitro to the same level as that stimulated in COCs freshly isolated from eCG-primed ovaries. The levels of cGMP were elevated in both cumulus cells and oocytes. Oocytes do not express Npr2 mRNA. Therefore, cGMP, production of which is promoted by NPPC-22, is probably transferred from the cumulus cells to the oocytes, where it functions to inhibit PDE3A and maintain meiotic arrest (15, 16).
Levels of Npr2 mRNA in cumulus cells of unstimulated follicles are relatively low when compared with those of eCG-stimulated follicles. However, these levels were increased in vivo by stimulation with eCG, and then they declined rapidly after administration of hCG. However, FSH did not increase Npr2 transcripts in cultured COCs isolated from mice not stimulated with eCG in vivo. Nevertheless, estradiol increased expression in vitro similarly to eCG stimulation in vivo. When COCs were isolated from ovaries 44 h after administration of eCG, a time when expression of Npr2 mRNA was high, the levels rapidly decreased in vitro unless COCs were cultured in medium supplemented with estradiol. Thus estradiol directly promotes the elevation of steady-state Npr2 mRNA levels in cumulus cells in vitro and is also required to maintain these high levels in vitro even after they have been elevated by eCG-stimulation in vivo. FSH failed to promote elevation of Npr2 mRNA levels in cumulus cells in vitro. This could result from the loss of cumulus cell responsiveness to FSH in vitro (24). Alternatively (or additionally), elevated levels of Npr2 mRNA may not be a direct effect of FSH on cumulus cells in vivo. Stimulation of follicular development in vivo with eCG is probably mediated, at least in part, by elevated estradiol levels. Thus, stimulation of Npr2 mRNA levels in cumulus cells by eCG in vivo is probably an indirect result of the FSH activity of eCG (30) elevating estradiol levels (for review, see Ref. 31). This is consistent with the findings that the synthetic estrogen DES stimulated Npr2 mRNA levels and NPPC (C-type natriuretic peptide)-induced cGMP production by rat granulosa cells in vivo (20). Thus, the failure of FSH to promote elevated Npr2 mRNA levels by isolated COCs in vitro is probably due to the absence of androgen precursor for the production of estrogens.
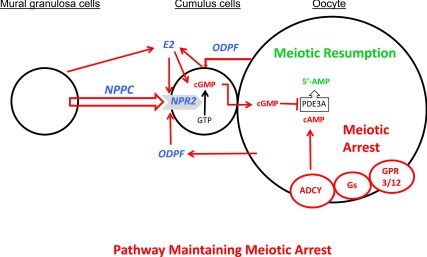
In light of the findings reported here, we can now add molecular participants to the working model put forward previously (17) for the maintenance of meiotic arrest in Graafian follicles (Fig. 8). Maintaining oocyte meiotic arrest requires sustaining a critical level of cAMP (5–8, 11). Oocyte cAMP is generated by adenylyl cyclase (9), whose function is sustained by the constitutive activity of G protein-coupled receptors 3 and 12 via guanine nucleotide binding protein, α stimulating complex (10, 32, 33). However, without suppression of cAMP phosphodiesterase PDE3A activity in oocytes, concentrations of cAMP would decrease to levels insufficient to maintain oocytes at the GV stage. cGMP inhibits the activation of PDE3A (16) after transfer from cumulus cells to oocytes via the gap junctions that metabolically couple these cells (15). Thus, the mechanisms for generating cGMP in cumulus cells are crucial for maintaining oocyte meiotic arrest in Graafian follicles. A small peptide, NPPC, produced primarily by mural granulosa cells of Graafian follicles binds to its cognate receptor, NPR2, expressed on cumulus cells. NPR2 is a guanylyl cyclase, and the NPPC/NPR2 ligand/receptor interaction results in elevation of cumulus cell cGMP, which is transferred to the oocyte. Once in the oocyte, cumulus cell-derived cGMP suppresses PDE3A to maintain the critical level of cAMP in the oocyte that sustains meiotic arrest. Thus, regulation of NPPC and NPR2 levels in granulosa cells is an integral aspect of the system that maintains oocyte meiotic arrest in Graafian follicles. The results presented previously and here reveal that both oocyte-derived paracrine factors (ODPFs) (17) and estradiol participate to promote functional NPR2 receptors. The model depicted in Fig. 8 posits that both mural granulosa cells and cumulus cells produce the estradiol that promotes expression of NPR2 receptors, as well other aspects of cumulus cell development and function. In addition to the production of estradiol by mural granulosa cells through FSH-stimulated aromatization of testosterone (see Ref. 31), ODPFs also promote the production of estradiol by cumulus cells (34, 35).
Fig. 8.
A working model depicting the participation of NPPC, NPR2, and estradiol (E2) in the mechanism(s) for maintaining mammalian oocyte meiotic arrest that is consistent with results of in vitro studies presented here. See text for details. ADCY, Adenylyl cyclase; GPR3/12, G protein-coupled receptors 3 and 12; Gs, GNAS (guanine nucleotide binding protein, α stimulating) complex.
ODPFs promote Npr2 mRNA expression by cumulus cells. Although the ODPF bone morphogenetic protein 15 alone did not promote expression to the same levels as oocyte coculture, it was required in combination with other ODPFs, either growth differentiation factor 9 or fibroblast growth factor 8 (17). The mechanisms for the action of ODPFs in promoting Npr2 mRNA expression are not clear. Promoting estradiol production as described above may be one function, but this is insufficient for maintaining meiotic arrest in the absence of aromatizable androgen. Therefore, ODPFs must promote other cumulus cell activities required for NPR2 production.
Testosterone, as well as estradiol, sustained the ability of NPPC to maintain meiotic arrest in vitro. This action was likely due to aromatization of testosterone to estradiol, rather than direct androgen action, because the nonaromatizable androgen dihydrotesterone was unable to sustain responses to NPPC. ODPFs promote estradiol production by cumulus cells only in the presence of testosterone (34, 35). Therefore, exogenous testosterone probably provided substrate for estradiol production sufficient to sustain cumulus cell responses to NPPC. Nonetheless, some direct androgen participation in this pathway, although insufficient to maintain responsiveness, cannot be excluded by our results. Moreover, whether ODPFs can promote sufficient expression of NPR2 by cumulus cells to maintain meiotic arrest even in the absence of estrogens or androgens remains to be tested.
Other factors may also participate in oocyte meiotic arrest. The purine hypoxanthine is present in high (mm) concentrations in preparations of follicular fluid and, at these concentrations, maintains a high proportion of cumulus cell-enclosed mouse oocytes in meiotic arrest (36, 37). This proportion is increased by addition of adenosine, which is also present in preparations of follicular fluid (37). However, the effect of these purines alone is insufficient to maintain meiotic arrest in Graafian follicles, because precocious GVB occurs in these follicles in Npr2 and Nppc mutants (17). Whether the pathways of hypoxanthine-mediated meiotic arrest interact with the NPPC/NPR2/cGMP pathways remains to be determined. However, inhibition of inosine monophosphate dehydrogenase activity, which is important for the generation of both guanylyl and adenylyl compounds, reverses meiotic arrest either in vivo or in hypoxanthine-arrested oocytes in vitro (38, 39).
An oocyte maturation inhibitor (OMI) found in porcine follicular fluid was reported to be a low molecular weight peptide of approximately 2000 (40). The molecular weight of NPPC-22 is 2197. The sequences of mouse, pig, and rat NPPC-22 are identical. Whether OMI is NPPC-22 remains to be determined. Suggestively, mural granulosa cells produce both OMI and NPPC-22, both act to maintain meiotic arrest via cumulus cells, and neither have arresting activity on denuded oocytes in vitro (40). Also, in contrast to mural granulosa cells, cumulus cells did not produce OMI activity (40), an observation consistent with low Nppc expression by cumulus cells (17).
The interaction and cross talk among cell types (mural granulosa cells, cumulus cells, and oocytes) and among signaling pathways (NPPC/NPR2, ODPFs, estradiol, and hypoxanthine) comprise a complex interactive system focused on maintaining mammalian oocytes in meiotic arrest. Optimal fertility requires synchrony in the regulation of oocyte meiotic events and ovulation (10, 33, 41). It would not be surprising, therefore, if compensatory or redundant mechanisms evolved into interacting pathways that maintain meiotic arrest and assure this essential synchrony. It is concluded from the results presented here that estradiol promotes and maintains expression of NPR2 in cumulus cells in vitro, and it is suggested that estrogens participate in the mechanisms maintaining oocyte meiotic arrest in Graafian follicles. However, whether estrogens function in the pathways maintaining meiotic arrest in vivo remains to be demonstrated.
Acknowledgments
We thank Dr. Joanne Fortune, Dr. Mary Ann Handel, and Dr. Laurinda Jaffe for valuable discussions and suggestions in the preparation of this manuscript.
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants HD23839 and HD21970 (to J.J.E., K.S., K.W., Y.-Q.S.) and by the National Basic Research Program of China no. 2012CB944401 and the State Key Laboratory for Agrobiotechnology of China Grant 2011SKLAB04-2 (to M.Z. and G.X.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- cGMP
- Cyclic GMP
- COC
- cumulus-oocyte complex
- DES
- diethylstilbesterol
- DHT
- dihydrotestosterone
- eCG
- equine chorionic gonadotropin
- GV
- germinal vesicle
- GVB
- GV breakdown
- hCG
- human CG
- NPR2
- natriuretic peptide receptor 2
- NPPC
- natriuretic peptide type C
- NPPC-22
- NPPC composed of 22 amino acids
- ODPF
- oocyte-derived paracrine factor
- OMI
- oocyte maturation inhibitor
- PDE3A
- phosphodiesterase 3A
- qRT-PCR
- quantitative RT-PCR.
References
- 1. Chesnel F, Eppig JJ. 1995. Synthesis and accumulation of p34cdc2 and cyclin B in mouse oocytes during acquisition of competence to resume meiosis. Mol Reprod Dev 40:503–508 [DOI] [PubMed] [Google Scholar]
- 2. Mitra J, Schultz RM. 1996. Regulation of the acquisition of meiotic competence in the mouse: changes in the subcellular localization of cdc2, cyclin b1, cdc25C and wee1, and in the concentration of these proteins and their transcripts. J Cell Sci 109:2407–2415 [DOI] [PubMed] [Google Scholar]
- 3. Pincus G, Enzmann EV. 1935. The comparative behavior of mammalian eggs in vivo and in vitro. I. The activation of ovarian eggs. J Exp Med 62:665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edwards RG. 1965. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature 208:349–351 [DOI] [PubMed] [Google Scholar]
- 5. Cho WK, Stern S, Biggers JD. 1974. Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J Exp Zool 187:383–386 [DOI] [PubMed] [Google Scholar]
- 6. Magnusson C, Hillensjo T. 1977. Inhibition of maturation and metabolism of rat oocytes by cyclic AMP. J Exp Zool 201:138–147 [DOI] [PubMed] [Google Scholar]
- 7. Vivarelli E, Conti M, DeFelici M, Siracusa G. 1983. Meiotic resumption and intracellular cAMP levels in mouse oocytes with compounds which act on cAMP metabolism. Cell Diff 12:1170–1172 [DOI] [PubMed] [Google Scholar]
- 8. Aberdam E, Hanski E, Dekel N. 1987. Maintenance of meiotic arrest in isolated rat oocytes by the invasive adenylate cyclase of Bordetella pertussis. Biol Reprod 36:530–535 [DOI] [PubMed] [Google Scholar]
- 9. Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M. 2003. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol 258:385–396 [DOI] [PubMed] [Google Scholar]
- 10. Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. 2004. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 306:1947–1950 [DOI] [PubMed] [Google Scholar]
- 11. Vaccari S, Horner K, Mehlmann LM, Conti M. 2008. Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest. Dev Biol 316:124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiersma A, Hirsch B, Tsafriri A, Hanssen RG, Van de Kant M, Kloosterboer HJ, Conti M, Hsueh AJ. 1998. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J Clin Invest 102:532–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conti M, Andersen CB, Richard FJ, Shitsukawa K, Tsafriri A. 1998. Role of cyclic nucleotide phosphodiesterases in resumption of meiosis. Mol Cell Endocrinol 145:9–14 [DOI] [PubMed] [Google Scholar]
- 14. Richard FJ, Tsafriri A, Conti M. 2001. Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod 65:1444–1451 [DOI] [PubMed] [Google Scholar]
- 15. Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. 2009. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136:1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaccari S, Weeks JL, 2nd, Hsieh M, Menniti FS, Conti M. 2009. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod 81:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. 2010. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330:366–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. 2008. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135:3229–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. LaPolt PS, Leung K, Ishimaru R, Tafoya MA, You-hsin Chen J. 2003. Roles of cyclic GMP in modulating ovarian functions. Reprod Biomed Online 6:15–23 [DOI] [PubMed] [Google Scholar]
- 20. Noubani A, Farookhi R, Gutkowska J. 2000. B-type natriuretic peptide receptor expression and activity are hormonally regulated in rat ovarian cells. Endocrinology 141:551–559 [DOI] [PubMed] [Google Scholar]
- 21. Farookhi R, Keyes PL, Kahn LE. 1982. A method for transplantation of luteinizing granulosa cells: evidence for progesterone secretion. Biol Reprod 27:1261–1266 [DOI] [PubMed] [Google Scholar]
- 22. Couse JF, Yates MM, Deroo BJ, Korach KS. 2005. Estrogen receptor-β is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 146:3247–3262 [DOI] [PubMed] [Google Scholar]
- 23. Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS. 2005. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER)α and ERβ null mice indicate a role for ERβ in follicular maturation. Endocrinology 146:2817–2826 [DOI] [PubMed] [Google Scholar]
- 24. Sugiura K, Su YQ, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. 2010. Estrogen promotes the development of mouse cumulus cells in coordination with oocyte-derived GDF9 and BMP15. Mol Endocrinol 24:2303–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O'Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ. 2007. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 134:2593–2603 [DOI] [PubMed] [Google Scholar]
- 26. Eppig JJ, Wigglesworth K, Pendola FL. 2002. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci USA 99:2890–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ. 2007. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol 302:104–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Su YQ, Sugiura K, Wigglesworth K, O'Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ. 2008. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135:111–121 [DOI] [PubMed] [Google Scholar]
- 29. Diaz FJ, Wigglesworth K, Eppig JJ. 2007. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol 305:300–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Licht P, Gallo AB, Aggarwal BB, Farmer SW, Castelino JB, Papkoff H. 1979. Biological and binding activities of equine pituitary gonadotrophins and pregnant mare serum gonadotrophin. J Endocrinol 83:311–322 [DOI] [PubMed] [Google Scholar]
- 31. Richards JS. 2001. Perspective: the ovarian follicle—a perspective in 2001. Endocrinology 142:2184–2193 [DOI] [PubMed] [Google Scholar]
- 32. Mehlmann LM, Jones TL, Jaffe LA. 2002. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science 297:1343–1345 [DOI] [PubMed] [Google Scholar]
- 33. Hinckley M, Vaccari S, Horner K, Chen R, Conti M. 2005. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol 287:249–261 [DOI] [PubMed] [Google Scholar]
- 34. Vanderhyden BC, Cohen JN, Morley P. 1993. Mouse oocytes regulate granulosa cell steroidogenesis. Endocrinology 133:423–426 [DOI] [PubMed] [Google Scholar]
- 35. Vanderhyden BC, Macdonald EA. 1998. Mouse oocytes regulate granulosa cell steroidogenesis throughout follicular development. Biol Reprod 59:1296–1301 [DOI] [PubMed] [Google Scholar]
- 36. Downs SM, Coleman DL, Ward-Bailey PF, Eppig JJ. 1985. Hypoxanthine is the principal inhibitor of murine oocyte maturation in a low molecular weight fraction of porcine follicular fluid. Proc Natl Acad Sci USA 82:454–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eppig JJ, Ward-Bailey PF, Coleman DL. 1985. Hypoxanthine and adenosine in murine ovarian follicular fluid: concentrations and activity in maintaining oocyte meiotic arrest. Biol Reprod 33:1041–1049 [DOI] [PubMed] [Google Scholar]
- 38. Downs SM, Coleman DL, Eppig JJ. 1986. Maintenance of murine oocyte meiotic arrest: uptake and metabolism of hypoxanthine and adenosine by cumulus cell-enclosed and denuded oocytes. Dev Biol 117:174–183 [DOI] [PubMed] [Google Scholar]
- 39. Downs SM, Eppig JJ. 1987. Induction of mouse oocyte maturation in vivo by perturbants of purine metabolism. Biol Reprod 36:431–437 [DOI] [PubMed] [Google Scholar]
- 40. Tsafriri A, Dekel N, Bar-Ami S. 1982. The role of oocyte maturation inhibitor in follicular regulation of oocyte maturation. J Reprod Fert 64:541–551 [DOI] [PubMed] [Google Scholar]
- 41. Ledent C, Demeestere I, Blum D, Petermans J, Hämäläinen T, Smits G, Vassart G. 2005. Premature ovarian aging in mice deficient for Gpr3. Proc Natl Acad Sci USA 102:8922–8926 [DOI] [PMC free article] [PubMed] [Google Scholar]