Abstract
Neurons that produce kisspeptin play a critical role in reproduction. However, understanding the molecular physiology of kisspeptin neurons has been limited by the lack of an in vivo marker for those cells. Here, we report the development of a Kiss1-CreGFP knockin mouse, wherein the endogenous Kiss1 promoter directs the expression of a Cre recombinase-enhanced green fluorescent protein (GFP) fusion protein. The pattern of GFP expression in the brain of the knockin recapitulates what has been described earlier for Kiss1 in the male and female mouse, with prominent expression in the arcuate nucleus (ARC) (in both sexes) and the anteroventral periventricular nucleus (in females). Single-cell RT-PCR showed that the Kiss1 transcript is expressed in 100% of GFP-labeled cells, and the CreGFP transcript was regulated by estradiol in the same manner as the Kiss1 gene (i.e. inhibited in the ARC and induced in the anteroventral periventricular nucleus). We used this mouse to evaluate the biophysical properties of kisspeptin (Kiss1) neurons in the ARC of the female mouse. GFP-expressing Kiss1 neurons were identified in hypothalamic slice preparations of the ARC and patch clamped. Whole-cell (and loose attached) recordings revealed that Kiss1 neurons exhibit spontaneous activity and expressed both h- (pacemaker) and T-type calcium currents, and hyperpolarization-activated cyclic nucleotide-regulated 1–4 and CaV3.1 channel subtypes (measured by single cell RT-PCR), respectively. N-methyl-D-aspartate induced bursting activity, characterized by depolarizing/hyperpolarizing oscillations. Therefore, Kiss1 neurons in the ARC share molecular and electrophysiological properties of other CNS pacemaker neurons.
Kisspeptin (encoded by the Kiss1 gene) and its receptor (Kiss1r) are essential for gating the onset of puberty and regulating reproductive function in mammals (1, 2). In the rodent, Kiss1 mRNA is expressed in the hypothalamic arcuate nucleus (ARC) and anteroventral periventricular nucleus (AVPV) (3, 4), where kisspeptin (Kiss1) neurons are thought to serve as conduits for mediating the negative and positive feedback effects of gonadal steroids on GnRH and LH secretion (5–10). Evidence drawn from multiple species suggests that Kiss1 neurons in the ARC may also act as a proximate pacemaker for GnRH neurons. First, GnRH neurons appear to be targets of Kiss1 neurons (4, 11). Virtually all GnRH neurons express Kiss1r (12, 13), and kisspeptin potently stimulates the release of GnRH (3) by depolarizing and inducing the sustained firing of action potentials in GnRH neurons (14–17). Second, multiple unit activity recordings in the region of the goat ARC containing Kiss1 neurons show rhythmic volleys of electrical activity that are temporally correlated with discrete pulses of LH secretion (18). Furthermore, measurement of kisspeptin and GnRH by push-pull cannulae placed near the median eminence of the monkey show that both peptides are secreted in a pulsatile and synchronized fashion (19). Third, kisspeptin antagonists inhibit pulsatile GnRH/LH secretion (20). These observations provide tantalizing, albeit indirect, evidence that Kiss1 neurons in the ARC have the properties of central pacemakers, which are capable of driving pulsatile GnRH secretion.
To study the molecular and biophysical properties of living Kiss1 neurons, we have developed a Kiss1-CreGFP knockin mouse, wherein it is possible to identify Kiss1 neurons in vivo, investigate their behavior, and study their molecular fingerprint. This new mouse bears a fusion cassette of enhanced green fluorescent protein (GFP) and Cre recombinase (Cre) driven by the endogenous Kiss1 promoter, which directs the expression of CreGFP specifically in Kiss1 neurons. Because expression of the fusion protein is controlled by the endogenous Kiss1 promoter, GFP is regulated in precisely the same manner as the normal Kiss1 gene and is expressed only when the endogenous Kiss1 gene is turned on. Cell-specific expression of GFP allows visualization of Kiss1-expressing neurons for purposes of characterizing their molecular properties in slice recordings and conducting single cell RT-PCR to identify the coexpression of important regulatory genes (e.g. ionic channels). The cell-specific expression of Cre also affords the opportunity to create mice with targeted expression of unique reporters or with targeted genetic modifications specifically in cells that express the Kiss1 gene at some point in their lifetime. Here, we report the initial biophysical and molecular characterization of Kiss1 neurons in the ARC of female mice and demonstrate that Kiss1 neurons have unique properties shared by central pacemaker cells.
Materials and Methods
Animals
Female Kiss1-CreGFP mice (F2 generation) were housed at either the University of Washington (UW) or the Oregon Health and Sciences University (OHSU), and surgeries were conducted at each location, according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The UW and OHSU Animal Care and Use Committees approved all of the animal use procedures. At the UW, mice were housed in a light (14-h light, 10-h dark cycle, lights on at 0400)-controlled and temperature (21–23 C)-controlled environment. They were fed standard chow diet and had free access to water. At OHSU, mice were maintained under constant temperature (21–23 C) and 12-h light, 12-h dark cycle schedule (lights on between 0600 and 1800 h), with standard chow and water provided ad libitum.
Generation of the Kiss1-CreGFP mouse (UW)
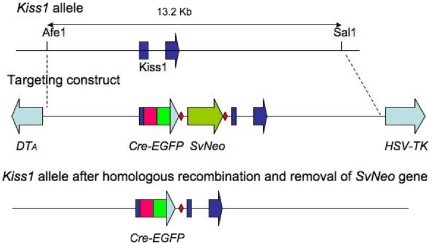
The Kiss1-CreGFP mice were produced by gene targeting technology (21, 22). Briefly, a 13.2-kb Afe1-Sal1 fragment, including approximately 5.0-kb DNA 5′ of the Kiss1 translation start site and approximately 8 kb on the 3′ side, was subcloned from a C57Bl/6 BAC clone (Invitrogen, Carlsbad, CA) into Bluescript. The targeting construct contained a CreGFP fusion protein cassette, followed by an frt-flanked SvNeo gene (for positive selection) inserted just upstream of the normal translation start site for Kiss1. The construct also contained Pgk-DTA and HSV-TK genes for negative selection (Fig. 1). G4 ES cells were electroporated, and 10 positive clones (of 65 analyzed) were identified by digesting DNA with BspH1 and performing Southern blottings using a 350-bp probe located just outside the 5′ boundary of the targeting construct. Positive clones were injected into C57Bl/6 blastocysts, and high percentage chimeras were bred to achieve germline transfer. The frt-Neo gene was removed by breeding the heterozygous mice with FLPer mice (23).
Fig. 1.
Kiss1-CreGFP targeting construct. A 13.2-kb Afe1-Sal1 fragment, including approximately 5.0-kb DNA 5′of the Kiss1 translation start site and approximately 8 kb on the 3′ side (depicted as broken dark blue arrow with a line through it in the top panel), was subcloned from a C57Bl/6 BAC clone into Bluescript. The targeting construct (depicted in the middle panel) contained a CreGFP fusion protein cassette (red and green arrow inserted in the middle of the first segment of the dark blue broken arrow from the top panel), followed by an frt-flanked SvNeo gene [for positive selection, illustrated by red diamonds (frt sites) surrounding a green arrow (SvNeo)] inserted just upstream of the normal start site for Kiss1. The construct also contained Pgk-DTA and HSV-TK (light blue arrows) genes for negative selection. The frt-Neo gene was removed by breeding the heterozygous mice with FLPer mice. The final construct is depicted in the bottom panel, illustrating a new Kiss1 allele that contains a Cre-eGFP cassette inserted before the native Kiss1 start site.
Ovariectomies (OVX) and estradiol (E2) replacement (UW)
OVX was performed on adult female mice through bilateral lumbar incisions while maintaining the animals under isoflurane inhalation anesthesia (Abbott Laboratory, Abbott Park, IL) delivered by vaporizer (VetEquip, Pleasanton, CA). Vasculature to the ovary and body wall were sutured, and wound clips were used to close the incision. Immediately after OVX, oil-filled capsules (sham) or E2 plus oil-filled capsules were implanted sc via a small midscapular incision at the base of the neck; wound clips were used to close the incision. For E2 implants, SILASTIC tubing (inner diameter, 1.47 mm; outer diameter, 1.95 mm; Dow Corning Corp., Midland, MI) was cut to 1.5 cm; one end was sealed with silicone cement (0.25 cm) and allowed to cure overnight. Crystalline E2 (Sigma, St. Louis, MO) at a dose of 1 mg/ml was dissolved in safflower oil based on previous studies (5, 8). After capsules were filled with E2 in oil, the end of the capsule was sealed with silicone cement (0.25 cm) and allowed to cure overnight. The day before the surgery, implants were washed in two changes of 100% ethanol for 10 min and then placed in sterile physiological saline overnight. All animals were given ketoprofen analgesia before surgery and for 48 h after surgery.
Castration of adult males (UW)
Testes were removed from adult males through a midline ventral incision while maintaining the animals under isoflurane inhalation anesthesia (Abbott Laboratory) delivered by vaporizer (VetEquip). Vasculature to the testes was sutured to prevent internal bleeding, the body wall was sutured, and wound clips were used to close the incision. Gonadectomized males were given a sham implant and compared with gonad-intact males. All animals were given ketoprofen analgesia before surgery and for 48 h after surgery.
Validation of GFP expression and regulation in Kiss1-CreGFP mice (UW)
To establish that the pattern of GFP expression in the brain of Kiss1-CreGFP mice recapitulates that reported earlier for the distribution of Kiss1 mRNA and determine whether gonadal steroids control the expression of GFP, we analyzed the pattern of GFP expression in male and female Kiss1-CreGFP mice. Males were either left intact or gonadectomized, and females were OVX and treated with either sham or E2 (as described above). On d 7 after gonadectomized, mice were anesthetized with ketamine/xylazine cocktail and perfused with 4% paraformaldehyde (PFA) + 0.1 m PBS. Brains were extracted and placed into 4% PFA + 0.1 m PBS overnight in a dark environment. Once the brains sank, they were transferred into a 1:1 solution of 4% PFA + 0.1 m PBS: 30% sucrose + 0.1% sodium azide (NaN3) in 0.1 m PBS, and upon sinking, the brains were transferred to 30% sucrose + 0.1% NaN3 in 0.1 m PBS. Sections were cut on a cryostat at 30 μm and mounted on slides. Twelve series were collected into 0.1 m PBS/0.1% NaN3 and stored at 4 C. Sections were processed by immunohistochemistry for GFP. Briefly, 30-μm free-floating sections were blocked in PBS with 10% normal goat serum (NGS) and 0.2% Triton X-100 for 1 h at room temperature followed by an overnight incubation with primary antibody solution containing rabbit anti-GFP (1:1000; Invitrogen) in PBS with 1% NGS and 0.2% Triton X-100. After three washes in PBS with 0.2% Triton X-100, a secondary antibody solution containing antirabbit antibody conjugated to Alexa Fluor 488 (1:500; Invitrogen) was added to the sections and incubated for 2 h at room temperature. After the incubation, sections were washed three times for 5 min in PBS with 0.2% Triton X-100, mounted onto slides, and analyzed with confocal microscopy.
Generation of Kiss1-CreGFP x lacZ mice (UW)
To determine the pattern of Cre expression in the Kiss1-CreGFP mice and develop a line of mice that express the β-galactosidase (β-Gal) reporter in cells that either produce the Kiss1 transcript in adulthood or once expressed Kiss1 during development but no longer do as adults, we crossed the Kiss1-CreGFP mice to the Cre reporter strain B6.129S4Gt(ROSA)26Sortm1Sor/J (stock no. 003474; The Jackson Laboratory, Bar Harbor, ME). In these mice, the expression of the reporter gene (lacZ) is prevented by a loxP-flanked STOP sequence. Crossing the Kiss1-CreGFP mouse to this reporter mouse results in the deletion of the floxed STOP sequence and allows the expression of lacZ in the cell types wherein Cre is expressed. Once recombination has occurred, lacZ is continuously expressed in that cell. While generating the Kiss1-CreGFP:lacZRep mice, we noticed that some animals (three out of 15) showed early Cre expression, which produced a nonspecific widespread β-Gal staining detected by immunofluorescence. To identify this early recombination event, a standard PCR of tail DNA using primers across the loxP sites (forward, 5′-AAAGTCGCTCTGAGTTGTTAT-3′; reverse, 5′-ATTAAGTTGGGTAACGCC-3′) was performed. The presence of recombination results in a PCR product of 500–600 bp, and animals showing this recombination product in the tail DNA were excluded from the study.
Double-labeling for GFP and β-Gal (UW)
To map and compare the distribution of β-Gal and GFP in Kiss1-CreGFP x lacZ female mice, we double-labeled GFP and β-Gal in these mice and analyzed their distribution in various areas of the brain, focusing on the ARC, where our biophysical studies were conducted. Cells that were single- and double-labeled with GFP and β-Gal were counted, and their anatomical distributions were qualitatively assessed. For double-labeling of GFP and β-Gal, adult OVX females (n = 3) were transcardiacally perfused 7 d after OVX with PBS containing 4% PFA. Brains were removed, postfixed overnight, and cryo-protected with 30% sucrose in PBS. For cryo-sectioning, brains were frozen for 5 min on dry ice and sectioned in a freezing microtome. For immunofluorescence, 30-μm free-floating sections were blocked in PBS with 10% NGS and 0.2% Triton X-100 for 1 h at room temperature followed by an overnight incubation with primary antibody solution containing chicken anti-β-Gal antibody (1:5000; Abcam, Cambridge, MA) and rabbit anti-GFP antibody (1:1000; Invitrogen) in PBS with 1% NGS and 0.2% Triton X-100. After three washes in PBS with 0.2% Triton X-100, a secondary antibody solution containing antichicken antibody conjugated to Alexa Fluor 568 and antirabbit antibody conjugated to Alexa Fluor 488 (both 1:500; Invitrogen) was added to the sections and incubated for 1 h at room temperature. After the incubation, sections were washed three times for 5 min in PBS with 0.2% Triton X-100 and mounted onto slides before imaging with an epi-fluorescent microscope (Eclipse E600; Nikon, Melville, NY). To determine the percentage of colocalization of β-Gal and GFP, the total number of β-Gal-positive cells in the ARC was counted, and the percentage double-labeling for GFP (i.e. β-Gal + GFP) was calculated in one of every six sections (180 μm apart, eight sections per mouse) for the three different animals.
Electrophysiological characterization of GFP-expressing Kiss1 neurons in female mice (OHSU)
Animals were OVX under isofluorane anesthesia 7 d before experimentation and were given a dose of 4 mg/kg carprofen (Rimadyl, Pfizer Animal Health, NY) immediately after surgery for analgesia. On the 7th d after OVX, animals were killed by decapitation. The brain was quickly removed, and a block containing the basal hypothalamus (BH) was immediately dissected. The BH block was submerged in cold (4 C) oxygenated (95% O2, 5% CO2) high sucrose artificial cerebrospinal fluid (aCSF): 208 mm sucrose, 2 mm KCl, 26 mm NaHCO3, 10 mm glucose, 1.25 mm NaH2PO4, 2 mm MgSO4, 1 mm CaCl2, and 10 mm HEPES (pH 7.4). Coronal slices (∼250 μm) were cut on a vibratome, during which time (10 min) the slices were bathed in high sucrose aCSF at 4 C. The slices were then transferred to an auxiliary chamber where they were kept at room temperature (25 C) in aCSF consisting of 124 mm NaCl, 5 mm KCl, 2.6 mm NaH2PO4, 2 mm MgSO4, 2 mm CaCl2, 26 mm NaHCO3, 10 mm HEPES, and 10 mm glucose (pH 7.4) until recording (recovery for 2 h). A single slice from the ARC was transferred to the recording chamber one at a time and was kept viable by continually perfusing with warm (35 C) oxygenated aCSF at 1.5 ml/min.
Visualized whole-cell patch recording (OHSU)
Whole-cell patch recordings were made in ARC-GFP-tagged Kiss1 neurons using an Olympus BX51 fixed-stage scope out-fitted with IR-DIC video imaging (Olympus, Center Valley, PA), as described (24). Patch pipettes (1.5 mm outer diameter borosilicate glass; A-M Systems, Seattle, WA) were pulled on a Flaming/Brown puller (Model P-97; Sutter Instrument Co., Novato, CA) and filled with the following solution: 128 mm potassium gluconate, 10 mm NaCl, 1 mm MgCl2, 11 mm EGTA, 10 mm HEPES, 2 mm ATP, and 0.25 mm GTP; adjusted to pH 7.3 with KOH; 295 mOsm. Pipette resistances ranged from 3–5 mΩ. In whole-cell configuration, access resistance was less than 20 mΩ; the access resistance was 80% compensated. The input resistance was calculated by measuring the slope of the current-voltage relationship curve between −70 and −50 mV. Standard whole-cell patch recording procedures and pharmacological testing were followed, as described (16, 25, 26). Electrophysiological signals were amplified with an Axopatch 200B amplifier and digitized with a Digidata 1440A (Molecular Devices, Foster City, CA), and the data were analyzed with p-Clamp software (version 10.0; Molecular Devices). Steady-state current/voltage (I/V) plots were constructed, with step command potentials from −140 to −50 mV at an increment of 10 mV (with holding potential at −60 mV) and duration of 1 sec. The liquid junction potential was corrected for all analyses. For the loose-patch cell recordings, the patch pipettes were filled with aCSF, and a loose seal (20- to 60-mΩ seal resistance) was formed on the Kiss1 neurons to measure spontaneous action currents in voltage clamp mode with the voltage clamped to 0 mV relative to the bath potential (27). Consecutive current traces were filtered at 2 kHz and acquired at a sampling rate of 50 kHz.
Molecular characterization of GFP-expressing Kiss1 neurons by single-cell RT-PCR (scRT-PCR) (OHSU)
Tissue preparation
On the day of experimentation, a 1-wk-OVX mouse was given a dose of ketamine (15 mg ip). The animal was then rapidly decapitated, its brain removed from the skull, and a diencephalic block was dissected. The resultant block was mounted on a cutting platform that was then secured in a vibratome well filled with ice-cold, oxygenated (95% O2, 5% CO2) high sucrose aCSF. Four coronal slices (240 μm) were cut through the hypothalamus. The slices were transferred to a multiwell auxiliary chamber containing oxygenated aCSF: 124 mm NaCl, 5 mm KCl, 1.44 mm NaH2PO4, 5 mm HEPES, 10 mm dextrose, 26 mm NaHCO3, and 2 mm CaCl2 and kept there until recovery (1–2 h).
Neuronal harvesting in the ARC and RT
The method used for neuronal harvesting was according to established procedures (28) with some modifications as follows. Briefly, the ARC was dissected and exposed to protease and gentle trituration to disperse the neurons. The dispersed cells were visualized using a Leitz inverted microscope, patched, and then harvested with gentle suction using the XenoWorks microinjector system (Sutter Instrument Co.). Cells were harvested as single cells or as pools of five individual cells. The contents of the pipette were expelled into a test tube containing a solution with 1× Invitrogen Superscript III buffer, 15 or 18 U of RNasin (Promega, Madison, WI) and 10 mm of dithiothreitol in 5 or 8 μl total volume for single cells and pools, respectively. Each harvested cell (or pool of cells) was reverse transcribed as described previously (28), with modifications in which both random primers (100 ng/cell; Promega) and anchored oligo(dT)20 primer (400 ng/cell; Invitrogen), and Superscript III reverse-transcriptase (100 U/cell, Invitrogen) was used. In addition, harvested aCSF in the vicinity of the dispersed cells also underwent RT and was used as a control. Cells and tissue RNA used as negative controls were processed as described above but without RT (−RT). Each cell or pool of cells was analyzed by using either PCR or real-time PCR.
Single-cell RT-PCR
The PCR was performed using 2–3 μl of cDNA template from each RT reaction in a 30-μl PCR mix. Forty to 50 cycles of amplification were performed using a Bio-Rad C1000 Thermal Cycler (Bio-Rad, Hercules, CA) according to established protocols (28, 29). PCR products were visualized with ethidium bromide on a 2% agarose gel.
Quantitative PCR (qPCR)
qPCR was performed on an Applied Biosystems 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA) with the SybrGreen master-mix method according to established protocols (28). A standard curve was prepared for each primer pair [hyperpolarization-activated cyclic nucleotide-regulated (HCN)1, HCN2, HCN3, HCN4, and β-actin] with mediobasal hypothalamus (MBH) cDNA serial dilutions, and Kiss1 neuronal pools were analyzed. In all cases, real-time PCR assays were tested to determine and compare the efficiencies of the target and control gene amplifications. Serially diluted cDNA from mouse BH, assayed in triplicate, were used to construct standard curves (see figure 7), and the amplification efficiency for each primer pair was calculated as follows: E = 10(−1/m) − 1, where m is slope (30, 31). Amplification efficiencies were 100% for all primer sets, including β-actin. The Applied Biosystems sequence detection software system (version 1.3) was used to generate the standard curves. The comparative ΔΔ cycle threshold (CT) method was used to calculate the individual values for each sample as described previously (28). Briefly, cDNA samples (4 μl for HCN transcripts and 2 μl for β-actin) from Kiss1 pools were run in duplicates, and the mean ΔCT of the HCN1 values for Kiss1 pools was used as a calibrator when comparing the mRNA quantities of HCN1, HCN2, and HCN3 (HCN4 was not detectable in 4-μl samples of the Kiss1 pools). The relative linear quantity of the target gene was calculated using the formula 2−ΔΔCT (30). Therefore, the data were expressed as an n-fold change in gene expression normalized to a reference gene (β-actin) and relative to the HCN1 values. The data are reported as relative mRNA expression. For statistical analysis, a one-way ANOVA with Dunnett's post hoc test was performed with GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA).
Primer design and development
Primer pairs for scRT-PCR and qPCR were developed by using the mRNA sequence of the respective genes from the National Center for Biotechnology Information (see Table 1). Primers were designed using the Clone Manager software (Sci Ed Software, Cary, NC) based on the mRNA sequences. The primers were designed to cross introns to preclude any genomic DNA amplification, and the PCR product from single cells was sequenced to confirm the correct product.
Table 1.
Primers used for generating probes for scRT-PCR in GFP-expressing neurons in the ARC of Kiss1-CreGFP female mice
| Name | Product length (bp) | Primer sequence | bp no. | Accession no. |
|---|---|---|---|---|
| Kiss1 | 132 | TGCTGCTTCTCCTCTGT | 64–80 | NM_178260 |
| ACCGCGATTCCTTTTCC | 195–179 | |||
| HCN1 | 136 | TTGCTGGCGTTATCACCAAG | 1527–1546 | NM_010408 |
| AGGGAGTAAAGACGACAGTAGG | 1662–1641 | |||
| HCN2 | 97 | ATGCTGCAAGACTTCCCCAGCG | 1423–1444 | NM_008226 |
| TGGCCTTGAAGAGCGCGAAC | 1591–1572 | |||
| HCN3 | 118 | TGCGGTGCTTGAGGAGTTCC | 1664–1682 | NM_008227 |
| ACTCGGCTCAGAGCGTTTC | 1781–1763 | |||
| HCN4 | 123 | CACTAAGGGCAACAAAGAGACC | 1929–1948 | NM_001081192 |
| AGTGAGTAGAGGCGGCAATAAG | 2051–2030 | |||
| CaV3.1 | 144 | TACTTCATCGCCCTCATGAC | 2935–2954 | NM_009783 |
| GTTGACAGGCAGCTGAATAC | 3059–3078 | |||
| CaV3.2 | 284 | CTCTGGGCTTCCTTTAGTAG | 2640–2659 | NM_021415 |
| ATCTCCCAGACGCTTATG | 2906–2923 | |||
| CaV3.3 | 128 | TGGGCATTTTTGGCAAGAA | 965–973 | NM_001044308 |
| CAGTGCGGATGGCTGACA | 1093–1110 | |||
| POMC | 242 | CCTCCTGCTTCAGACCTCCAT | 45–65 | NM_008895 |
| CAGCACTGCTGCTGTTCCT | 286–304 | |||
| β-Actin | 110 | AAGGCCAACCGTGAAAAGAT | 416–435 | NM_007393 |
| GTGGTACGACCAGAGGCATAC | 505–525 |
POMC, Proopiomelanocortin.
Results
Validation of the Kiss1-CreGFP knockin mouse
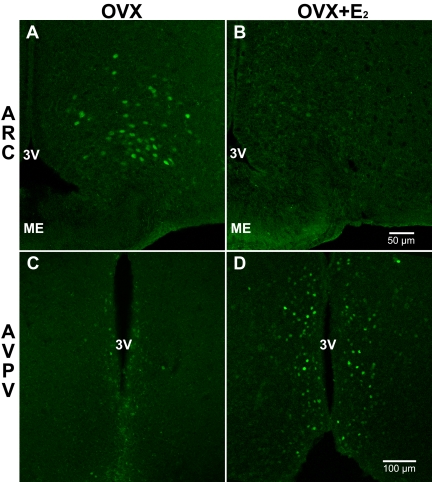
Cells expressing GFP were clearly observed in the ARC and the AVPV/periventricular nucleus, areas previously shown to comprise Kiss1- and kisspeptin-expressing cells in the brain of the female (Fig. 2) (3, 7), as well as the male mouse (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) (6). Furthermore, analysis by RT-PCR of 160 cells from six animals revealed that all GFP-expressing cells were positive for Kiss1, whereas none of the 13 nonfluorescent cells from those same animals had detectable levels of Kiss1 mRNA (13 cells from six animals). We also tested whether any of the GFP-expressing neurons expressed Pomc mRNA (n = 43 cells from two different animals) and found that none of the GFP-labeled cells expressed detectable levels of Pomc mRNA. To confirm that expression of GFP in the Kiss1-CreGFP mice is under the control of the endogenous Kiss1 promoter (as expected), we compared the relative expression of GFP in the presence and absence of E2 and found that E2 regulates CreGFP in precisely the same manner as Kiss1, inducing its expression in the AVPV and inhibiting its expression in the ARC (Fig. 2). Thus, based on their anatomical distribution, confirmation of Kiss1 expression in the GFP-labeled cells by scRT-PCR, and evidence of regulation by the endogenous promoter, we deduce that GFP-expressing cells visualized in Kiss1-CreGFP knockin mice represent Kiss1 neurons.
Fig. 2.
Photomicrographs of GFP-expressing cells in the ARC and the AVPV of OVX Kiss1-CreGFP knockin mice with and without E2 treatment. The distributions of GFP expression in the ARC (A and B) and the AVPV (C and D) are similar to the known distributions of Kiss1 mRNA in those same regions. Furthermore, E2 treatment reduces GFP expression in the ARC (B) compared with controls (A) but increases GFP expression in the AVPV (D) compared with controls (C), just as E2 has been shown to inhibit Kiss1 mRNA expression in the ARC and induce its expression in the AVPV (5). A and B show endogenous GFP fluorescence. In C and D, fluorescent immunohistochemistry was used to improve the visualization of GFP. ME, Median eminence; 3V, third ventricle.
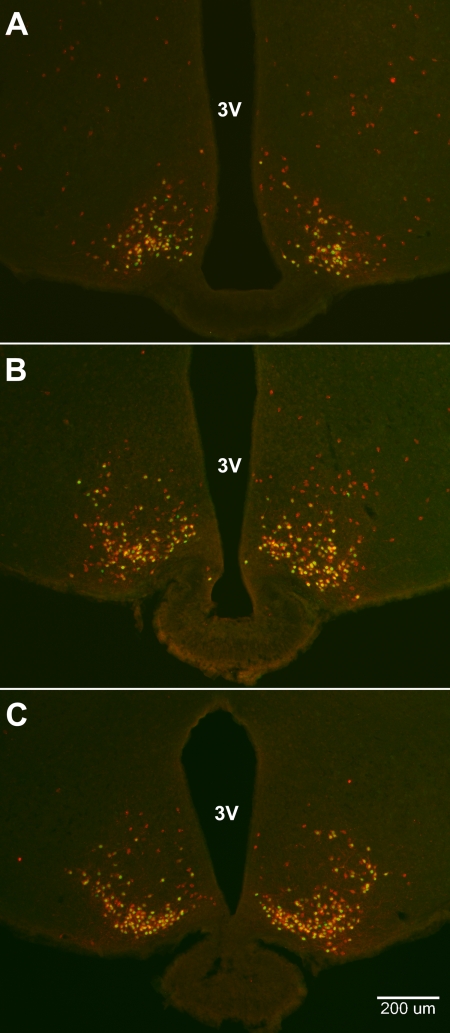
Kiss1-CreGFP mice crossed with lacZ reporter mice produced F1 mice in which β-Gal is expressed in cells that either currently (in adulthood) or earlier in development expressed the CreGFP fusion protein driven by the endogenous Kiss1 promoter. Thus, β-Gal marks every cell that ever expressed the Kiss1 gene at any time in its history. First, we found that the anatomical distribution of GFP-expressing cells in the cross was indistinguishable from that observed in the Kiss1-CreGFP mouse (e.g. prominent expression in the ARC and AVPV). Second, the number of cells showing GFP staining alone was insignificant (Fig. 3, A–C). Third, the distribution of cells stained for β-Gal only in the MBH extended well beyond the distribution of cells that were double-labeled for both markers, (Fig. 3, A–C) and outside of the boundary previously reported for Kiss1 mRNA in the ARC (5, 6), extending into the dorsomedial and ventrolateral parts of the ventromedial hypothalamic nucleus. Analysis revealed that approximately 75% (74 ± 5%) of the cells comprised within the arcuate were colabeled, whereas the remaining cells were immunopositive for β-Gal only. This implies that a significant number of β-Gal-labeled cells once expressed Kiss1 during development but no longer do so as adults, whereas cells that express both β-Gal and GFP represent the population that currently express Kiss1 and would thus constitute “Kiss1 neurons.”
Fig. 3.
Photomicrographs of GFP (green)- and β-Gal (red)-stained neurons in the ARC of OVX female Kiss1-CreGFP:LacZRep mice. Neurons labeled for both GFP and β-Gal appear yellow. Panels show sections from the rostral (A), middle (B), and caudal (C) arcuate. 3V, Third ventricle.
Biophysical properties of GFP-labeled Kiss1 neurons in the ARC
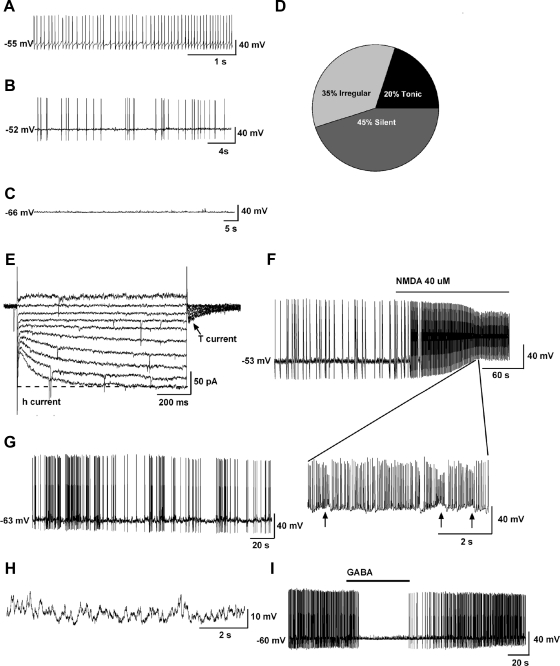
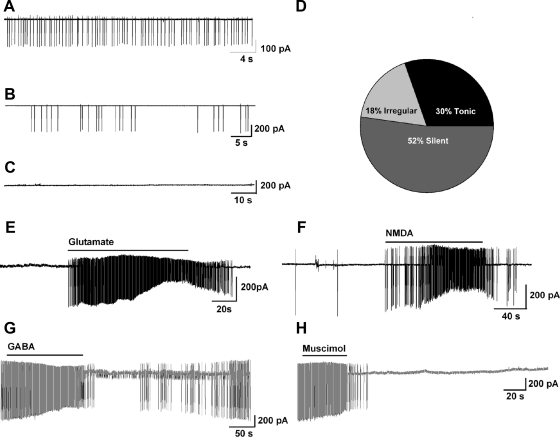
Using whole-cell patch recording in oil-treated OVX females, we found that Kiss1 neurons in the ARC exhibited a resting membrane potential of −63.8 ± 2.3 mV (n = 20). Kiss1 neurons in the ARC exhibited tonic, irregular, and silent firing patterns (Fig. 4, A–D). Under loose-patch, recording conditions in which the cell's internal milieu is undisturbed, Kiss1 neurons in the ARC exhibited virtually the same tonic, irregular, and silent firing patterns (Fig. 5, A–D). Moreover, the majority (>80%) of Kiss1 neurons in the ARC expressed both pacemaker (h-current) and T-type Ca2+ currents, as revealed by measuring the response to hyperpolarizing steps from −65 to −140 mV, where Vhold= −60 mV (n = 49) (Fig. 4E). Because the expression of h-current and T-type calcium currents is critical for generating burst firing in hypothalamic and thalamic neurons (32–34), we determined whether or not Kiss1 cells in the ARC exhibit burst-firing behavior. To evaluate this possibility, we applied the glutamate receptor agonist N-methyl-D-aspartate (NMDA) (40 μm) to Kiss1 neurons in the ARC and found that these cells exhibited clear burst firing in both whole-cell patch (Fig. 4, F–H) and loose-patch (Fig. 5, E and F) cell recordings, similar to that described for Kiss1 neurons in the guinea pig (24). Moreover, in the presence of tetrodotoxin (applied to block fast Na+ spikes), NMDA-dependent membrane oscillations (up and down states) were clearly evident (Fig. 4H). Therefore, Kiss1 neurons in the ARC exhibit NMDA-driven and presumably glutamate-driven burst firing. Moreover, it appears that Kiss1 neurons in the ARC of the female mouse express two endogenous pacemaker conductances (T-type calcium and h-currents), which can be activated through glutamatergic inputs to generate burst firing. In addition, the application of γ-aminobutyric acid (GABA) (100 μm) to the bath silenced the firing of Kiss1 neurons in the ARC (Figs. 4I and 5, G and H).
Fig. 4.
Electrophysiological characteristics of arcuate Kiss1 neurons in oil-treated OVX Kiss1-CreGFP mice using whole-cell patch recording. Kiss1 neurons in the ARC (of the female) rest at −63.8 ± 2.3 mV (n = 20). A–C, Representative traces of action potentials recorded from arcuate Kiss1 neurons showing tonic (A), irregular (B), and silent (C) firing patterns. D, Summary pie chart of the firing pattern distribution in ARC Kiss1 neurons (n = 20). E–H, Endogenous conductances and NMDA-induced burst firing of an ARC Kiss1 neuron. E, Ensemble of currents in response to depolarizing/hyperpolarizing steps from −50 to −140 mV illustrating the expression of a hyperpolarization-activated cation current (h-current) and a T-type Ca2+ current (arrow) in a representative Kiss1 neuron. Vhold = −60 mV. F, Current clamp recording in an ARC Kiss1 neuron showing the response to NMDA (40 μm). The spiking in F was expanded to illustrate the pronounced effects of NMDA on burst firing activity of Kiss1 neurons with an ensemble of spikes riding on top of low-threshold spikes (arrows). G, In a current clamped state close to the resting membrane potential, one can see the bursting activity in the presence of NMDA. H, In the presence of tetrodotoxin to block the fast Na+ spikes, one can clearly see the membrane oscillations (up and down states) induced by NMDA. Eighty percent of ARC Kiss1 neurons expressed the endogenous conductances (h-current, T-current) that are critical for generating burst firing activity. Although ARC Kiss1 neurons did not exhibit spontaneous burst firing activity, glutamate (NMDA) was able to induce burst firing activity in all of the cells. I, GABA (100 μm) inhibited firing in another arcuate kisspeptin neuron. Drugs were rapidly perfused into the bath as a 100-μl bolus.
Fig. 5.
Cellular characterization of arcuate Kiss1 neurons in oil-treated, OVX Kiss1-CreGFP mice in loose-patch cell recordings. A–C, Representative traces of action potentials recorded from Kiss1 neurons showing tonic (A), irregular (B), and silent (C) firing patterns. D, Summary pie chart of the firing pattern distribution in arcuate Kiss1 neurons (n = 23). E and F, Glutamate-induced (100 μm, n = 4) (E) and NMDA-induced (40 μm, n = 4) (F) burst firing in arcuate kisspeptin neurons. G and H, GABA-inhibited (100 μm, n = 5) (G) and muscimol-inhibited (10 μm, n = 4) (H) firing in arcuate Kis1 neurons. Drugs were rapidly perfused into the bath as a 100-μl bolus.
scRT-PCR for channel subunits
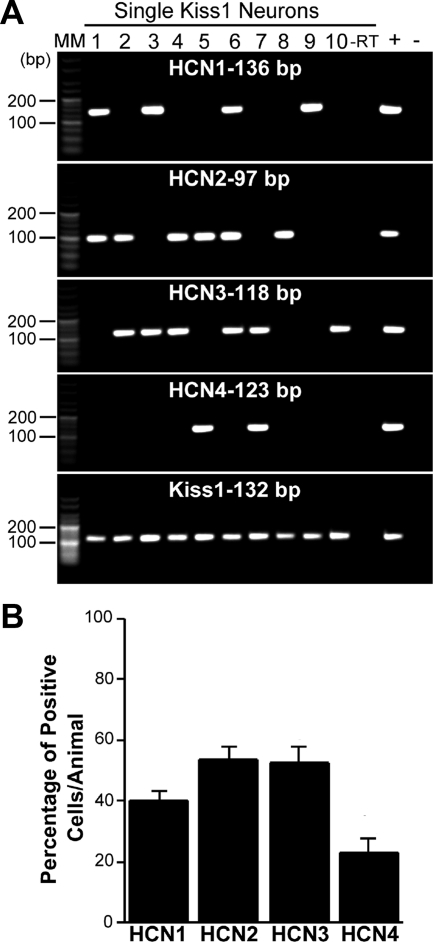
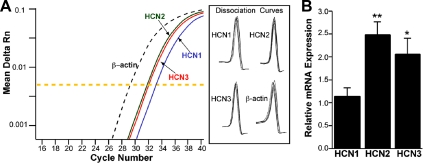
The whole-cell analysis revealed that the pacemaker (hyperpolarization-activated, nonselective cation) current (Ih) was prominent in Kiss1 neurons in the ARC. Because hyperpolarization-activated cyclic nucleotide-gated (HCN) channels underlie the h-current, we used scRT-PCR to determine which of the HCN channel subtypes might be expressed in these Kiss1 cells. We found HCN1, HCN2, and HCN3 transcript in 40, 54, and 53%, of Kiss1 cells, respectively, whereas HCN4 was only found in 23% of Kiss1 neurons (an average of 25 cells/animal from four animals) (Fig. 6, A and B). For a more quantitative analysis, we measured HCN channel subtypes in pools of Kiss1 neurons in the ARC by using real-time PCR (an average of three pools per animal from three animals) (Fig. 7A). This analysis revealed that HCN2 and HCN3 were more highly expressed than HCN1, corroborating the scRT-PCR findings. The amount of the HCN1 channel subtype mRNA was about 50% of that found for the HCN2 and HCN3 channel subunit mRNA (Fig. 7B), and HCN4 mRNA was undetectable at the cDNA level used.
Fig. 6.
Expression of HCN channels in single Kiss1 neurons from the ARC of OVX Kiss1-CreGFP mice. A, Representative gels illustrating the mRNA expression of HCN channel subtypes 1–4. The expected sizes for the PCR products are as follows: for HCN1, 136 bp; for HCN2, 97 bp; for HCN3, 118 bp; for HCN4; 123 bp; and for Kiss1, 132 bp. As a negative control, a cell reacted −RT did not express any of the transcripts. MBH tissue RNA was also included as a positive control (+, With RT) and negative control (−, Without RT). MM, Molecular markers. B, Summary bar graphs of the percentage expression of HCN1, HCN2, HCN3, and HCN4. An average of 25 cells/animal from six animals was analyzed by scRT-PCR, and the mean number of neurons expressing HCN channel subtypes from each animal was determined. Bar graphs represent the mean ± sem of the percentage of Kiss1 neurons expressing each HCN subtype per animal.
Fig. 7.
Relative HCN subtype expression in Kiss1 neurons in the ARC of Kiss1-CreGFP mice. A, Real-time PCR amplification and dissociation curves for HCN1–3 and β-actin. The HCN1 (blue line), HCN2 (green line), and HCN3 (red line) depict the mean CT values for each of the three channel subtypes. The expression values were normalized to β-actin (black dotted line). The yellow dotted line is the midpoint of the exponential phase of amplification at which the CT was determined. B, qPCR measurements of HCN1, HCN2, and HCN3 mRNA in Kiss1 neuronal pools (three to six pools per animal) from OVX mice (n = 3 animals). HCN2 and HCN3 had higher expression relative to HCN1. The expression values were calculated via the ΔΔCT method and normalized to the mean ΔCT of HCN1 expression levels. Bar graphs represent the mean ± sem. *, P < 0.05, HCN3 vs. HCN1 (ANOVA); **, P < 0.01, HCN2 vs. HCN1 (ANOVA).
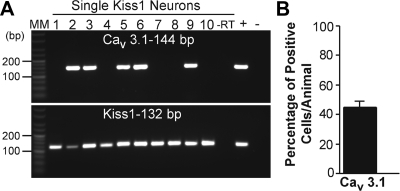
Because the T-type calcium channels also play a prominent role in burst firing activity [McCormick and Huguenard (34), Erickson et al. (32), and Kelly and co-workers (33)], we sought evidence for their expression in Kiss1 neurons in the ARC. Again, by scRT-PCR, we found that T-type subunits were expressed in Kiss1 neurons in the ARC, with CaV3.1 mRNA being coexpressed in 44% of Kiss1 neurons (an average of 20 cells/animal from six animals) (Fig. 8). The CaV3.2 subunit was also detectable, but CaV3.3 was nondetectable in Kiss1 neurons (data not shown).
Fig. 8.
Expression the T-type Ca2+ channel CaV3.1 subtype in single Kiss1 neurons of female Kiss1-CreGFP OVX female mice. A, A representative gel illustrating the expression of CaV 3.1 and Kiss1 mRNA in Kiss1 neurons of the ARC. As a negative control, a cell reacted −RT did not express any of the transcripts. BH tissue was also included as a positive control (+, With RT) and negative control (−, Without RT). MM, Molecular markers. B, Summary bar graph of percentage expression of CaV3.1 in Kiss1 ARC neurons. An average of 20 cells/animal were harvested from six mice and were analyzed for CaV3.1 expression by scRT-PCR. Bar graphs represent the mean ± sem of the percentage of Kiss1 neurons expressing CaV3.1 per animal.
Discussion
We describe the initial characterization of a mouse that expresses a Cre-GFP fusion protein, knocked into the endogenous Kiss1 locus. This mouse expresses GFP and Cre in neurons that are actively transcribing the Kiss1 gene. We verified that GFP-expressing neurons in these mice are in fact Kiss1 neurons by confirming that such cells express Kiss1 mRNA by scRT-PCR of GFP-expressing cells and documenting that GFP-expressing cells show a distribution profile comparable with the expression of Kiss1 mRNA as mapped by in situ hybridization (3, 5, 6). Because the construct was knocked into the endogenous Kiss1 locus, we anticipated that GFP would be regulated by factors known to regulate the expression of Kiss1 mRNA, including sex steroids. We affirmed this supposition by demonstrating that E2 regulates the expression of GFP in the same manner as Kiss1 in both males and females (i.e. inhibits GFP expression in the ARC and induces its expression in the AVPV) (5, 6).
The Kiss1-CreGFP mice described here differ in several respects from two other previously reported models (35, 36). We used a targeted knockin strategy to direct the expression of both GFP and Cre under the endogenous Kiss1 promoter, whereas Cravo et al. (35) used a BAC transgenic to produce their model, and Mayer et al. (36) inserted an IRES-Cre downstream of the Kiss1 coding region to produce their mice. Second, our mice express a GFP-Cre fusion cassette under the transcriptional control of the endogenous Kiss1 promoter. Thus, in the Kiss1-CreGFP knockin mouse, GFP is expressed by Kiss1 neurons (and can be visualized in vivo), without having to be crossed with a reporter mouse. This is important, because we have observed that when the Kiss1-CreGFP mouse is crossed with the lac Z/β-Gal mouse, we see a greater number and wider distribution of β-Gal-positive cells in the Kiss1-CreGFP x reporter cross than can be seen by either GFP-labeling in the Kiss1-CreGFP mouse or single label in situ hybridization for Kiss1 mRNA in wild-type mice (Fig. 3) (5, 6). The distribution of β-Gal in the Kiss1-Cre transgenic x lacZ cross described by Cravo et al. (35) shows a similar extended expression pattern. Therefore, when any Kiss1-Cre model is bred to a reporter mouse, one cannot be certain that the cells labeled with the reporter in the adult are, in fact, contemporaneously expressing the Kiss1 gene, without independent corroboration of the identity of those cells (e.g. scRT-PCR). This phenomenon may reflect the complex lineage of Kiss1 neurons during embryonic development, wherein some Kiss1-expressing progenitor cells differentiate into a non-Kiss1 lineage, as has been reported for Pomc neurons (37). It may also be the case that Kiss1 is turned on transiently (at low levels) in many cells in the brain (and elsewhere) during embryonic development, only to be silenced in adulthood. This fact has no bearing on our recordings (or scRT-PCR) results, because our mice have GFP knocked into the endogenous Kiss1 locus (under the Kiss1 promoter). However, care must be exercised when interpreting results from either electrophysiological measurements or targeted gene disruption studies based on mice produced by crossing any Kiss1-Cre mouse with a mouse bearing either a reporter or a conditional allele to create cell-specific knockouts (e.g. estrogen receptor α) (36, 38). This is because Kiss1 promoter-dependent Cre recombination during development is likely to be more extensive and more variable than predicted simply based on the pattern of Kiss1 expression in the adult.
Although GnRH neurons themselves exhibit spontaneous tonic and burst firing activity (39), growing evidence suggests that Kiss1 neurons in the ARC provide the rhythmic drive to GnRH neurons that leads to pulsatile GnRH and LH secretion (18–20, 40–43). Indeed, we argued that if Kiss1 neurons in the ARC were the source of the rhythmic drive to pulsatile GnRH secretion, they would exhibit biophysical properties shared by other pacemaker-type cells in the hypothalamus, thalamus, and subthalamic nucleus (24, 32–34, 44). Using whole-cell patch recording techniques, we targeted GFP-expressing cells in the ARC of Kiss1-CreGFP mice and studied their electrophysiological properties. First, we demonstrated the expression of a hyperpolarization-induced cation current (h-current) and a transient (T-type) Ca2+ current, both of which are critical conductances exhibited by pacemaker neurons (34, 45). Second, we found that Kiss1 neurons in the ARC express the requisite transcripts, HCN and CaV3.1 channels, that underlie these pacemaker currents. Third, we observed that Kiss1 neurons exhibit NMDA-induced burst firing activity, characterized by membrane oscillations. Finally, we learned that GABA profoundly inhibits Kiss1 neurons and could thus serve as one arc in a feedback circuit that governs rhythmic burst firing of Kiss1 neurons (34, 44). These observations in the female mouse extend and corroborate earlier findings in “native” Kiss1 neurons in the ARC of the guinea pig, which also express h- and T-currents and exhibit burst firing in response to NMDA and may thus represent shared features among these neurons across species (29).
Although Kiss1 neurons in the ARC sit at a relatively negative resting membrane potential (−63.8 mV in OVX mice), this potential is depolarized relative to the range necessary to recruit a critical fraction of T-type calcium channels (16). In addition, the vast majority of the h-current is activated at even more negative potentials (46). Thus, inhibitory synaptic inputs (e.g. GABA via GABA-B receptors and dynorphin via κ opioid receptors) may be necessary for reaching these hyperpolarized states to recruit more T-type calcium channels and activate a critical fraction of the h-current. GABAergic neurons are abundant in the ARC (47), and dynorphin is colocalized in Kiss1 neurons (42) Thus, it is conceivable that either (or both) GABA and dynorphin produced by Kiss1 neurons themselves could act autosynaptically to hyperpolarized Kiss1 neurons between bouts of activity, as has been previously suggested (18, 42). Therefore, we conclude that Kiss1 neurons in the ARC of the mouse contain the requisite molecular components and elicit electrophysiological behaviors that would allow them to act in a pacemaker network and serve as a proximate source of the stimulatory input to GnRH neurons.
Supplementary Material
Acknowledgments
We thank Amy E. Oakley, Paul Amieux, Megan McClean, Victor M. Navarro, Jodi Downs, Nicole Filipek, Katey Feng, Ali Yasrebi, and Sam Medina for their assistance in performing this work and preparing this manuscript.
This work was supported by National Institutes of Health (NIH) Grants R01 HD049651 (to R.A.S.), R01 NS43330 (to O.K.R.), R01 NS38809 (to M.J.K.), R01 DK68098 (to M.J.K. and O.K.R.), and MH086386 (to G.S.M.) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through cooperative agreements U54 HD12629 (to the University of Washington Center for Research in Reproduction and Contraception). This work was also supported by the Developmental Biology Training Grant at the University of Washington NIH T32HD007183 and the Reproductive Biology Training Grant at Oregon Health and Sciences University NIH T32HD007133.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- Artificial cerebrospinal fluid
- ARC
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- BH
- basal hypothalamus
- Cre
- Cre recombinase
- CT
- cycle threshold
- E2
- estradiol
- GABA
- γ-aminobutyric acid
- β-Gal
- β-galactosidase
- GFP
- green fluorescent protein
- HCN
- hyperpolarization-activated cyclic nucleotide-regulated
- MBH
- mediobasal hypothalamus
- NMDA
- N-methyl-D-aspartate
- NGS
- normal goat serum
- OHSU
- Oregon Health and Sciences University
- OVX
- ovariectomy
- PFA
- paraformaldehyde
- qPCR
- quantitative PCR
- scRT-PCR
- single-cell RT-PCR
- UW
- University of Washington.
References
- 1. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 3. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 4. Mikkelsen JD, Simonneaux V. 2009. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides 30:26–33 [DOI] [PubMed] [Google Scholar]
- 5. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 6. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 7. Clarkson J, Herbison AE. 2009. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol 21:305–311 [DOI] [PubMed] [Google Scholar]
- 8. Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. 2007. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 27:12088–12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glidewell-Kenney C, Weiss J, Hurley LA, Levine JE, Jameson JL. 2008. Estrogen receptor α signaling pathways differentially regulate gonadotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse. Endocrinology 149:4168–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oakley AE, Clifton DK, Steiner RA. 2009. Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. 2008. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. 2004. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- 13. Parhar IS. 2005. GnRH and gpcr: laser-captured single cell gene profiling. Fish Physiol Biochem 31:153–156 [DOI] [PubMed] [Google Scholar]
- 14. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pielecka-Fortuna J, Chu Z, Moenter SM. 2008. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. 2008. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28:4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu X, Lee K, Herbison AE. 2008. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149:4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. 2010. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. 2009. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas KR, Capecchi MR. 1987. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51:503–512 [DOI] [PubMed] [Google Scholar]
- 22. Thomas KR, Deng C, Capecchi MR. 1992. High-fidelity gene targeting in embryonic stem cells by using sequence replacement vectors. Mol Cell Biol 12:2919–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farley FW, Soriano P, Steffen LS, Dymecki SM. 2000. Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28:106–110 [PubMed] [Google Scholar]
- 24. Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. 2010. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 30:1560–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. 2003. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 144:1331–1340 [DOI] [PubMed] [Google Scholar]
- 26. Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. 2007. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 27:10153–10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nunemaker CS, DeFazio RA, Moenter SM. 2003. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online 5:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 2009. 17Beta-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 29:10552–10562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. 2011. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology 152:1503–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 31. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erickson KR, Ronnekleiv OK, Kelly MJ. 1993. Role of a T-type calcium current in supporting a depolarizing potential, damped oscillations, and phasic firing in vasopressinergic guinea pig supraoptic neurons. Neuroendocrinology 57:789–800 [DOI] [PubMed] [Google Scholar]
- 33. Erickson KR, Ronnekleiv OK, Kelly MJ. 1993. Electrophysiology of guinea-pig supraoptic neurones: role of a hyperpolarization-activated cation current in phasic firing. J Physiol 460:407–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCormick DA, Huguenard JR. 1992. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol 68:1384–1400 [DOI] [PubMed] [Google Scholar]
- 35. Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. 2011. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. 2010. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc Natl Acad Sci USA 107:22693–22698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Padilla SL, Carmody JS, Zeltser LM. 2010. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med 16:403–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donato J, Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. 2011. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest 121:355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ronnekleiv OK, Bosch MA, Xhang C. 18. May 2011 17β-Estradiol regulation of GnRH neuronal excitability. J Neuroendocrinol 10.1111/j.1365-2826.2011.02160.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li XF, Kinsey-Jones JS, Cheng Y., Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O'Byrne KT. 2009. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One 4:e8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. 2010. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bevan MD, Wilson CJ. 1999. Mechanisms underlying spontaneous oscillation and rhythmic firing in rat subthalamic neurons. J Neurosci 19:7617–7628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santoro B, Tibbs GR. 1999. The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann NY Acad Sci 868:741–764 [DOI] [PubMed] [Google Scholar]
- 46. Chu Z, Takagi H, Moenter SM. 2010. Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. J Neurosci 30:13373–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wagner EJ, Bosch MA, Kelly MJ, Rønnekleiv OK. 1999. A powerful GABA(B) receptor-mediated inhibition of GABAergic neurons in arcuate nucleus. Neuroreport 10:2681–2687 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.