Abstract
Clinical evidence that the blockade of IL-1β in type-2 diabetic patients improves glycemia is indicative of an autoinflammatory mechanism that may trigger adiposity-driven pancreatic damage. IL-1β is a key contributor to the obesity-induced inflammation and subsequent insulin resistance, pancreatic β-cell dysfunction, and the onset of type 2 diabetes. Our previous studies demonstrated that the ceramides activate the Nod-like receptor family, pyrin domain containing 3 (Nlrp3) inflammasome to cause the generation of mature IL-1β and ablation of the Nlrp3 inflammasome in diet-induced obesity improves insulin signaling. However, it remains unclear whether the posttranslational processing of active IL-1β in pancreas is regulated by the NLRP3 inflammasome or whether the alternate mechanisms play a dominant role in chronic obesity-induced pancreatic β-cell exhaustion. Here we show that loss of ASC, a critical adaptor required for the assembly of the NLRP3 and absent in melanoma 2 inflammasome substantially improves the insulin action. Surprisingly, despite lower insulin resistance in the chronically obese NLRP3 and ASC knockout mice, the insulin levels were substantially higher when the inflammasome pathway was eliminated. The obesity-induced increase in maturation of pancreatic IL-1β and pancreatic islet fibrosis was dependent on the NLRP3 inflammasome activation. Furthermore, elimination of NLRP3 inflammasome protected the pancreatic β-cells from cell death caused by long-term high-fat feeding during obesity with significant increase in the size of the islets of Langerhans. Collectively, this study provides direct in vivo evidence that activation of the NLRP3 inflammasome in diet-induced obesity is a critical trigger in causing pancreatic damage and is an important mechanism of progression toward type 2 diabetes.
Obesity is associated with self-directed tissue inflammation with the activation of immune cells in absence of definitive pathogenic infection and as-of-yet unidentified autoantigens. Therefore, the obesity-induced inflammation and the development of associated comorbidities like type 2 diabetes (T2D) may qualify the established criterion of autoinflammatory disease cluster described by McGonagle and McDermott (1). It is established that induction of inflammation is an important mechanism in the development of insulin resistance and is a crucial event in the pathogenesis of T2D (2–4). Among a broad array of proinflammatory cytokines and chemokines, the IL-1β and TNFα are considered to be key contributors to the obesity-induced inflammation and subsequent insulin resistance, pancreatic β-cell dysfunction, and the onset of T2D (3, 4). However, the early observations that TNFα induces insulin resistance in vitro and in mouse models have not been supported by successful therapeutic outcomes in several clinical trials in obese T2D patients (5–8). Interestingly, recent randomized clinical trials demonstrate that inhibition of IL-1β signaling leads to sustained reduction in circulating C-reactive protein with an improvement of T2D (9, 10). These data demonstrate that alternate upstream proinflammatory mediators like IL-1β, which trigger the production of other secondary cytokines, may be clinically relevant for management of obesity-induced diseases including T2D. However, the mechanisms that regulate the activation and posttranslational processing of IL-1β during high-fat diet (HFD)-induced pancreatic dysfunction are incompletely understood.
Interestingly, the NOD-like receptors (NLR), innate immune sensors, contribute to chronic inflammatory diseases by sensing danger signals [danger-associated molecular patterns (DAMP)], i.e. endogenous molecules that are produced during organ dysfunction such as ceramides, cholesterol crystals, urate, and β-amyloid leading to atherosclerosis, gout, and Alzheimer's disease (11–14). Among the NLR family, the activation of Nod-like receptor family, pyrin domain containing 3 (NLRP3) (nucleotide-binding domain, leucine-rich-containing family, pyrin domain containing-3) induces recruitment and autocatalytic processing of cysteine protease caspase-1 in a large cytosolic protein complex called inflammasome (15). The activation of caspase-1 is required for the cleavage of stored pro forms of IL-1β into bioactive secreted cytokines (16). The assembly of inflammasomes requires the interaction of pyrin domain of ASC [for apoptosis-associated speck-like protein containing carboxyl-terminal caspase activation recruitment domain (CARD)] with the pyrin domain of NLRP3 and formation of a functional inflammasome complex through CARD-CARD interaction of ASC with procaspase-1 zymogen (12, 15, 17). Recent studies suggest that islet amyloid deposits in pancreas can be sensed by the NLRP3 inflammasome to induce IL-1β activation, and the mice overexpressing human islet amyloid polypeptide (IAPP) produce higher amounts of IL-1β (18). However, there is currently no direct evidence that Nlrp3- or Asc-mediated inflammasome assembly impacts pancreatic function at various stages of obesity. Our data establish that obesity-induced pancreatic dysfunction and insulin levels are regulated by the NLRP3 inflammasome-mediated IL-1β proinflammatory mechanism.
Materials and Methods
Animals
The Asc−/− and Nlrp3−/− mice have been described previously (13). The mice were fed ad libitum HFD consisting of 60% calories from fat (D12492i; Research Diets Inc., New Brunswick, NJ) starting at 8 wk of age, and control mice were fed a standard chow diet consisting of 4.5% fat (5002; LabDiet, Tallahassee, FL). All experiments and animal use were conducted in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Insulin tolerance test (ITT) and glucose tolerance test (GTT)
The ITT and GTT was conducted as described previously (13).
Insulin and leptin measurement
The serum level of hormones were measured using mouse endocrine Milliplex bead assay as per manufacturer's instructions. In addition, insulin levels reported in Fig. 3B were measured using mouse insulin ELISA kit (Millipore, Billerica, MA).
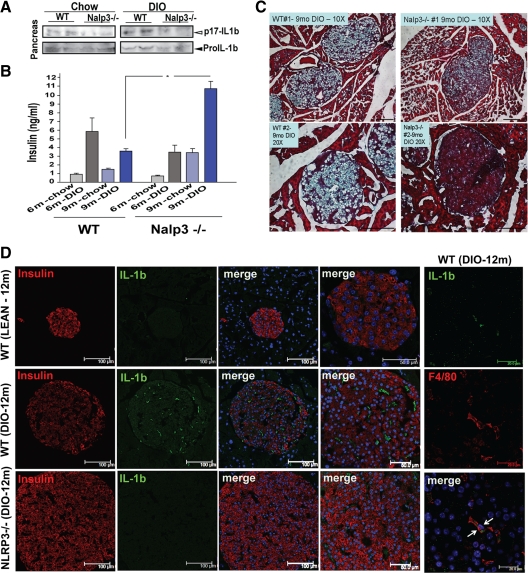
Fig. 3.
Elimination of Nlrp3 inflammasome protects pancreas from obesity-induced IL-1β damage and fibrosis. A, Immunoblotting for active (p17) IL-1β in pancreas tissue lysates of WT and Nlrp3−/− mice maintained on chow and HFD for 9 months. B, Fasting serum insulin levels in a different cohort of WT and Nlrp3−/− mice fed chow and HFD for 6 and 9 months. All data are presented as mean ± sem; n = 6–10 mice. *, P < 0.05. C, The trichrome staining of pancreatic cryosections from 9-month-old WT and Nalp3−/− mice fed 60% HFD. The deposition of collagen and fibrosis can be identified by light blue regions within the pancreatic islets. D, The pancreatic cryosections from 12-month-old WT and Nlrp3−/− mice fed 60% HFD were stained with antiinsulin (red) and anti-IL-1β antibodies. The extreme right panel shows representative image of pancreatic cryosections of 12-month-old DIO mice stained with macrophage marker F4/80 (with membrane staining, in red) and IL-1β (cytoplasmic, in green). The nuclei are counterstained blue with 4′,6-diamidino-2-phenylindole. The confocal images are representative of at least four mice and were repeated thrice.
Western blot analysis
Adipose tissue and pancreas were snap frozen in liquid nitrogen to prepare protein lysates. The immunoblot analysis was performed as described previously (13).
Histology and immunohistochemistry
The pancreas obtained from mice were either fixed in 4% formalin and paraffin embedded or flash frozen and subsequently embedded in Stephens Scientific (Riverdale, NJ) frozen section medium and cut into 5-μm-thick cryostat sections. At least three cryosection serial sections were used for each staining. The formalin-fixed, paraffin-embedded sections were stained with hematoxylin and eosin using autostainer (Dako, Carpinteria, CA), and fibrosis was analyzed by trichrome staining. A total of 250–286 islets from four to six mice of each strain were counted, and the area of each islet was determined by ImageJ software. Six nonserial sections from each mouse were used to count the islets for size measurement. The immunofluorescence confocal microscopy was performed as described previously (13).
Results
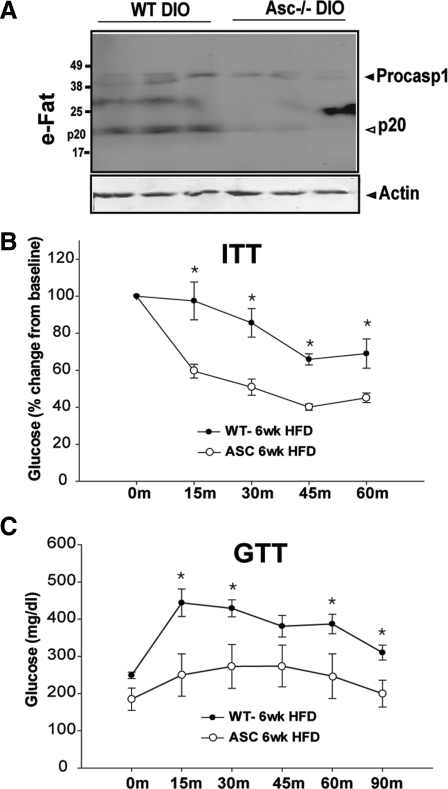
Given the CARD domain of ASC protein binds to the CARD domain of procaspase-1 for the assembly of NLRP3 and absent in melanoma 2 (AIM2) inflammasomes and autoactivation of caspase-1 (15), we determined the role of ASC in caspase-1 processing and insulin action in diet-induced obesity. We found that obesity induces the cleavage of the inactive p45 form of procaspase-1 into enzymatically active p20 heterodimer. Compared with wild-type (WT) diet-induced obese (DIO) mice, the ablation of ASC prevented the HFD-induced caspase-1 activation (Fig. 1A). Interestingly, the elimination of ASC in DIO mice led to a significant improvement in insulin action as measured by the ITT and GTT (Fig. 1, B and C).
Fig. 1.
Ablation of ASC lowers caspase-1 activation and improves insulin action in DIO. A, Western blot analysis of caspase-1 in epididymal fat (eFat) of C57/B6 mice. The activated p20 isoform of caspase-1 increases during obesity and ablation of ASC, a critical adaptor for NLRP3 inflammasome prevents autocatalytic activation of caspase-1. B, The percent change in glucose levels after the ITT in WT and ASC-deficient mice fed 60% HFD for 6 wk. The data are presented as mean (sem) with n = 6. The drop in glucose levels after insulin injection was significant at each time point (*, P < 0.01). C, The GTT in WT and ASC-deficient mice fed 60% HFD for 6 wk. The ASC mice displayed significant reduction in glucose values after the ip glucose injection (n = 6).
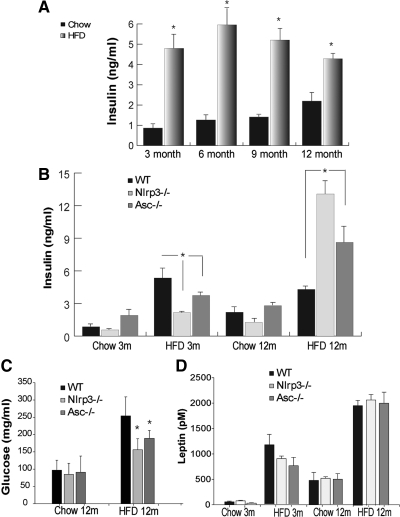
To further understand the impact of the inflammasome-mediated proinflammatory state on the kinetics of insulin levels, we aged the mice on chow and 60% HFD until 1 yr and evaluated them at 3, 6, 9, and 12 months of age. The HFD-fed mice had profound insulin resistance as revealed by higher fasting insulin levels. Interestingly, in 1-yr-old DIO mice, the insulin levels tended to be lower than 6-month-old DIO mice, suggesting potential defects in β-cells to compensate for the increasing degree of insulin resistance (Fig. 2A). We next aged the WT, Nlrp3−/− and Asc−/− mice cohorts on chow and 60% HFD until 1 yr to determine whether the inflammasome-dependent mechanism plays a predominant role in regulation of insulin levels, IL-1β activation, and β-cell mass in obesity. In chow-fed mice, ablation of NLRP3 and ASC did not affect the fasting insulin levels (Fig. 2B). Consistent with our previous data that the loss of NLRP3 (13) and current data that ablation of ASC improves insulin sensitivity, we found that compared with 3-month-old WT DIO control animals, the Nlrp3−/− and Asc−/− mice on 60% HFD had significantly lower insulin levels (Fig. 2B). In chow-fed 1-yr-old mice, we did not observe any significant differences between WT, Nlrp3−/−, and Asc−/− mice (Fig. 2B). Strikingly, compared with 12-month-old WT DIO mice, age-matched Nlrp3−/− and Asc−/− DIO animals had significantly higher fasting insulin levels (Fig. 2B) and lower fasting glucose levels (Fig. 2C). Ablation of NLRP3 and ASC had no impact on serum leptin levels in either chow- or HFD-fed mice at 3 and 12 months of age (Fig. 3D).
Fig. 2.
Ablation of Nlrp3 inflammasome improves pancreatic insulin production in chronic obesity. A, The kinetics of fasting serum insulin levels in male C57/B6 mice fed the normal chow diet and 60% HFD. The data are presented as mean (sem) with n = 5–8 per age group. B, The kinetics of fasting serum insulin levels in WT, NLRP3−/−, and ASC−/− mice fed normal chow diet and 60% HFD up to 12 months. The data are presented as mean (sem) with n = 5–8 (*, P < 0.01). C and D, Fasting glucose levels (C) and kinetics (D) of fasting serum leptin levels in in WT, NLRP3−/−, and ASC−/− mice fed normal chow diet and 60% HFD up to 12 months. The data are presented as mean (sem) with n = 5–8.
Our previous studies identified that ceramides, which are known to be elevated in obesity and T2D (19, 20), cause the activation of IL-1β via the NLRP3 inflammasome-dependent pathway (13). Therefore, we next investigated whether the NLRP3 inflammasome pathway regulates the IL-1β processing into the active p17 form within the pancreas of chronically obese mice. Compared with 9-month-old chow-fed WT mice, the 9-month-old DIO mice displayed a substantial increase in IL-1β activation, whereas the ablation of NLRP3 prevented an obesity-induced increase in the active p17 cleaved form of IL-1β (Fig. 3A). Consistent with our earlier findings, examination of fasting insulin levels also revealed significant increase in serum insulin concentration in a separate cohort of 9-month-old chronically DIO Nlrp3−/− mice but not in 6-month-old Nlrp3-deficient animals (Fig. 3B). Interestingly, consistent with the role of IL-1β-driven inflammation in instigation of fibrosis, we found that pancreatic islets of 9-month-old Nlrp3−/− obese mice were protected from extracellular matrix deposition and fibrosis.
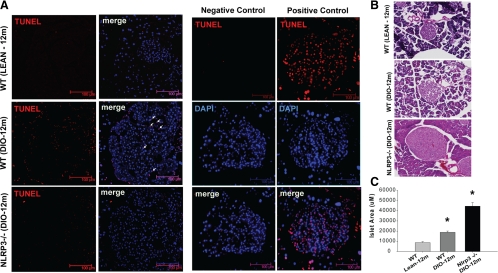
Initial immunofluorescence confocal microscopy revealed that compared with 1-yr-old chow-fed mice, the insulin-stained pancreatic islets were substantially larger in age-matched DIO mice (Fig. 3D). Of note, there was an increase in IL-1β expression within the pancreatic islets of DIO mice that was substantially lower upon ablation of NLRP3 (Fig. 3D). Also, IL-1β was not colocalized within the insulin-expressing β-cells in the pancreatic islets, and cytoplasmic IL-1β was expressed in F4/80-expressing pancreatic macrophages (Fig. 3D). Further analysis revealed that obesity-induced increase in β-cell death was reduced in the 1-yr-old Nlrp3−/− obese mice (Fig. 4A). To quantify whether the loss of Nlrp3 inflammasome-mediated signaling in advanced obesity protects pancreatic islets and to determine the size of the islets, we conducted morphometric analysis of hematoxylin- and eosin-stained pancreatic section from 1-yr-old WT and Nlrp3−/− obese mice. This analysis revealed that compared with WT DIO mice, the elimination of NLRP3 led to a significant increase in the size of pancreatic islets (Fig. 4, B and C). These results establish that NLRP3 inflammasome activation is a key mechanism that causes HFD-induced pancreatic damage and may participate in promoting the progression of an obesity-induced insulin-resistant state toward the onset of T2D.
Fig. 4.
Ablation of Nlrp3 inflammasome protects β-cell death and increases islet size in chronic mouse model of middle-age obesity. A, The confocal immunofluorescence microcopy of pancreatic islets stained for terminal transferase dUTP nick end labeling (TUNEL) (in red) in 12-month-old chow-fed WT and 60% HFD-fed WT and NLRP3−/− mice. TUNEL-positive cells are marked by arrowheads. The negative control of pancreatic cryosections was prepared by omitting terminal transferase, and positive control includes permeabilization of cryosections with micrococcal nuclease or deoxyribonuclease 1 to induce DNA strand breaks. B, The pancreatic islet morphometry revealed that compared with 12-month-old WT obese mice, in age-matched NLRP3−/− mice, the pancreatic islet size was significantly larger. C, A total of 250–286 islets from four to six mice of each strain were counted, and the area of each islet was determined by ImageJ software. Six nonserial sections from each mouse were used to count the islets. The ablation of NLRP3 causes significant increase in islet size (*, P < 0.01).
Discussion
It is established that IL-1β plays an important role in the development of T2D (3, 4, 9, 10). IL-1β interacts with the type 1 IL-1 receptor followed by recruitment of coreceptor chain called the IL-1 receptor accessory protein to initiate a proinflammatory cascade (21). Examination of several organs including adipose tissue and lymphoid organs show that the highest expression of type 1 IL-1 receptor is observed in pancreatic islets (22). Several lines of evidence indicate that the local IL-1β production in pancreatic islets causes the cell death of β-cells and compromises their ability to produce insulin (3, 4). Consistent with these data, blocking IL-1β signaling in humans lowers the severity of T2D (9, 10). However, the basic mechanism of posttranslational processing through which IL-1β is cleaved and activated and affects the pancreas during various stages of obesity in not fully understood. Notably, posttranslational processing of IL-1β is complex and can be regulated through several inflammasomes, e.g. NLRP1 inflammasome, NLRC4 inflammasome, NLRP3 inflammasome, and AIM2 inflammasome (17). Furthermore, pro-IL-1β can be processed through caspase-1- and inflammasome-independent mechanisms. For example, several neutrophil- and macrophage-derived serine proteases such as proteinase-3, elastase, and cathepsin G can also cleave the pro-form of IL-1β (23, 24). Our data demonstrate a specific role of NLRP3 inflammasome in the activation of IL-1β during chronic obesity-associated pancreatic damage.
The myeloid cell-expressed NLRP3 inflammasome has recently been implicated in sensing obesity-associated danger signals to cause IL-1β- and IL-18-driven proinflammatory state that impairs insulin sensitivity (13, 25). Given the expanded adipose tissue with infiltrated macrophages is a major site of origin of inflammation in obesity (2, 13), we asked whether the lack of ASC, which is required for the assembly of NLRP3 and AIM2 inflammasome, regulates caspase-1 activation and insulin action. These data revealed that within 6 wk of HFD feeding, the inflammasome is activated in the adipose tissue, suggesting that caspase-1 activation is an early event that triggers the origin of inflammation. Furthermore, the loss of ASC afforded substantial protection from insulin resistance in early-stage obesity, suggesting that in addition to NLRP3, the AIM2 inflammasome may also participate in obesity-associated inflammation. We have previously shown that elimination of NLRP3 can improve insulin action in mice that are maintained on 60% HFD until 3, 6, and 9 months of age (13). These previous studies showed that the 9-month-old Nlrp3−/− obese mice had significantly lower fasting glucose values with significant reduction in blood glucose levels in response to glucose challenge (13). These data prompted us to investigate the fasting insulin levels in ASC- and NLRP3-null mice until 1 yr of HFD feeding. As expected, the fasting insulin levels were significantly reduced concomitant with improved insulin sensitivity in early stages of obesity. In chronically obese 1 year old mice, absence of Nlrp3 inflammasome lowers the severity of insulin-resistance (IR) (13). Interestingly, despite relatively lower IR, we observed 2- to 3-fold higher insulin levels in chronically obese Nlrp3 knockout mice. These data suggest that pancreatic Beta cells in obese Nlrp3 null mice are protected from HFD diet-induced inflammatory damage and are able to compensate by increasing insulin levels in face of above normal glucose levels. Consistent with these data, IL-1β has been shown to compromise β-cell secretory function (26). However, additional studies in chronically obese mice will be required to definitively address whether the lack of inflammasome activation improves the insulin secretion capacity of pancreatic β-cells. Furthermore, given that mitochondrial reactive oxygen species are increased during obesity and reactive oxygen species are implicated in Nlrp3 inflammasome assembly (27), it is possible that mitochondrial dysfunction could impact Nlrp3 inflammasome activation and lead to pancreatic damage in obesity.
The infiltration of activated immune cells in pancreas is associated with the development of fibrosis as well as amyloid deposition in the pancreas of T2D patients (3, 4). Recent studies demonstrate that along with ceramides (13) and fatty acids (25), IAPP also activates the NLRP3 inflammasome, and overexpression of human IAPP in pancreatic islets causes IL-1β activation (18). Our data demonstrate that the activation of IL-1β in pancreas is dependent in part on the NLRP3 inflammasome-dependent mechanism. Consistent with relationship between IL-1β and development of fibrosis (3, 4), we found that the elimination of NLRP3 inflammasome-dependent IL-1β production protects the development of islet fibrosis in chronically obese mice. Furthermore, obesity-induced pancreatic β-cell death is regulated by the NLRP3 inflammasome. Consistent with reduced β-cell death and higher insulin levels in NLRP3-deficient mice in late-stage obesity, data showing that ablation of NLRP3 increases pancreatic islet size provide new information that inhibition of NLRP3 may protect from T2D. These data also suggest that as obesity progresses into an insulin-dependent stage when β-cells undergo exhaustion, the absence of NLRP3 inflammasome-mediated IL1β processing maintains β-cells, which then successfully compensates for persistent above-normal glucose levels by producing more insulin. Recent evidence suggests that the widely used sulfonylurea class of hypoglycemic drug glyburide also blocks NLRP3 inflammasome (28). Furthermore, glyburide affords substantial protection from gram-negative sepsis in diabetic patients (29). Taken together, these findings suggest that inhibition of NLRP3 inflammasome may offer new therapeutic approaches against the development of T2D in dietary obesity. Our data suggest that the NLRP3 inflammasome-dependent innate immune-sensing pathway is an important regulator of pancreatic posttranslational processing of IL-1β in DIO.
Acknowledgments
We thank Vishva M. Dixit at Genentech Inc. for providing the anti-caspase-1 antibody and Asc−/− and Nlrp3−/− mice. We also thank Eric Ravussin for helpful discussions and Comparative Biology Core staff for animal care.
The research in Dixit Laboratory is supported in part by the National Institutes of Health (NIH) (AG31797 and DK090556), Coypu Foundation, and Pennington Biomedical Research Foundation. The present work used the facilities of the Genomics and Cell Biology and Bioimaging Core facilities supported by NIH Grant 1 P20 RR02/1945 and P30 DK072476.
A.A. and Y.-H.Y. contributed equally in generating the data, participating in discussion, and manuscript preparation. B.V. performed the Milliplex hormone assay and maintained the knockout mouse colony. D.B. participated in pancreatic morphometry and confocal microscopic analyses. A.R. participated in animal husbandry and ITT and GTT analyses and researched the data. V.D.D. conceived the project, designed the experiments, participated in conducting the experiments, directed the project, and wrote the manuscript.
Disclosure Summary: Authors have nothing to declare.
For editorial see page 4005
- AIM2
- Absent in melanoma 2
- ASC
- apoptosis-associated speck-like protein containing carboxyl-terminal CARD
- CARD
- caspase activation recruitment domain
- DIO
- diet-induced obese
- GTT
- glucose tolerance test
- HFD
- high-fat diet
- IAPP
- islet amyloid polypeptide
- ITT
- insulin tolerance test
- NLR
- NOD-like receptor
- NLRP3
- Nod-like receptor family, pyrin domain containing 3
- T2D
- type 2 diabetes
- WT
- wild type.
References
- 1. McGonagle D, McDermott MF. 2006. A proposed classification of the immunological diseases. PLoS Med 3:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hotamisligil GS, Erbay E. 2008. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 8:923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donath MY, Shoelson SE. 2011. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11:8–107 [DOI] [PubMed] [Google Scholar]
- 4. Dinarello CA, Donath MY, Mandrup-Poulsen T. 2010. Role of IL-1β in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 17:314–321 [DOI] [PubMed] [Google Scholar]
- 5. Dominguez H, Storgaard H, Rask-Madsen C, Steffen Hermann T, Ihlemann N, Baunbjerg Nielsen D, Spohr C, Kober L, Vaag A, Torp-Pedersen C. 2005. Metabolic and vascular effects of tumor necrosis factor-α blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res 42:517–525 [DOI] [PubMed] [Google Scholar]
- 6. Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. 2006. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med 24:902–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lo J, Bernstein LE, Canavan B, Torriani M, Jackson MB, Ahima RS, Grinspoon SK. 2007. Effects of TNF-α neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. Am J Physiol Endocrinol Metab 293:E102–E109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. 1996. Effects of an engineered human anti-TNF-α antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 45:881–885 [DOI] [PubMed] [Google Scholar]
- 9. Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. 2007. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 10. Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T. 2008. Treatment with an interleukin 1β antibody improves glycemic control in diet-induced obesity. Cytokine 44:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. 2008. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol 9:857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schroder K, Zhou R, Tschopp J. 2010. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327:296–300 [DOI] [PubMed] [Google Scholar]
- 13. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. 2011. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. 2010. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464:1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinon F, Burns K, Tschopp J. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 10:417–426 [DOI] [PubMed] [Google Scholar]
- 16. Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. 1995. Altered cytokine export and apoptosis in mice deficient in interleukin-1 β converting enzyme. Science 267:2000–2003 [DOI] [PubMed] [Google Scholar]
- 17. Lamkanfi M, Dixit VM. 2009. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev 227:95–105 [DOI] [PubMed] [Google Scholar]
- 18. Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nuñez G, Yodoi J, Kahn SE, Lavelle EC, O'Neill LA. 2010. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 11:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Major CD, Gao ZY, Wolf BA. 1999. Activation of the sphingomyelinase/ceramide signal transduction pathway in insulin-secreting β-cells: role in cytokine-induced β-cell death. Diabetes 48:1372–1380 [DOI] [PubMed] [Google Scholar]
- 20. Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. 2003. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem 278:30015–30021 [DOI] [PubMed] [Google Scholar]
- 21. Dinarello CA. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550 [DOI] [PubMed] [Google Scholar]
- 22. Böni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, Kerr-Conte J, Pattou F, Ehses JA, Schuit FC, Donath MY. 2009. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 150:5218–5229 [DOI] [PubMed] [Google Scholar]
- 23. Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, Pham CT, van der Meer JW, Dinarello CA, van den Berg WB. 2009. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1β. Arthritis Rheum 60:3651–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. 2010. IL-1β processing in host defense: beyond the inflammasomes. PLoS Pathog 6:e1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. 2011. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. 2002. Glucose-induced β-cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou R, Yazdi AS, Menu P, Tschopp J. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221–225 [DOI] [PubMed] [Google Scholar]
- 28. Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. 2009. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol 187:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koh GC, Maude RR, Schreiber MF, Limmathurotsakul D, Wiersinga WJ, Wuthiekanun V, Lee SJ, Mahavanakul W, Chaowagul W, Chierakul W, White NJ, van der Poll T, Day NP, Dougan G, Peacock SJ. 2011. Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis 15:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]