Abstract
Interactions between brain IGF-I receptors and estrogen receptors regulate female reproductive physiology and behavior. The present study investigated potential mechanisms by which IGF-I receptors in the neuroendocrine hypothalamus regulate GnRH neuronal activation and LH release in young and middle-aged female rats under estradiol (E2) positive feedback conditions. We infused vehicle, IGF-I, or JB-1, a selective antagonist of IGF-I receptors, into the third ventricle of ovariectomized female rats primed with E2 and progesterone or vehicle. In young females, blockade of IGF-I receptors attenuated the steroid hormone-induced LH surge, reduced the percent of GnRH neurons expressing c-fos on the day of the LH surge, and decreased the total number of neurons expressing c-fos in the preoptic area. Middle-aged females had fewer GnRH neurons expressing c-fos during the LH surge than young females, and the LH surge amplitude was attenuated. Infusion of an IGF-I dose previously shown to increase LH surge amplitude did not increase the percent of GnRH neurons expressing c-fos in middle-aged females. Brain IGF-I receptor blockade did not modify E2 induction of progestin receptor-immunoreactive neurons in the preoptic area, arcuate, or ventromedial hypothalamus of young rats. These findings indicate that brain IGF-I receptors are required for E2 activation of GnRH neurons in young rats and for robust GnRH release from axon terminals in middle-aged females. IGF-I likely exerts its effects by actions on E2-sensitive neurons that are upstream of GnRH neurons and terminals.
Estradiol (E2) and progesterone (P) act sequentially in the brain to coordinate female reproductive physiology and behavior. A growing body of evidence indicates that a number of E2-mediated processes in the brain require concomitant signaling by IGF-I receptors. Estrogen receptors (ER) and IGF-I receptors are colocalized in neurons and glia throughout the brain, including the hypothalamus (1, 2). Notably, GnRH neurons express both IGF-I and IGF-I receptors (3, 4). Pharmacological antagonism of brain IGF-I receptors abolishes estrous cyclicity (5), and this action is not attributable to changes in food intake or metabolism. Brain IGF-I receptor blockade also impairs estrogen positive feedback, reducing E2 and P-dependent LH surges. It also abolishes E2 induction of α1B-adrenergic receptor binding in the hypothalamus and preoptic area (POA) (6) and inhibits hormone-dependent reproductive behavior in ovariectomized female rats (7). Intracerebroventricular (icv) infusion of an IGF-I receptor antagonist also prevents estrous cycle-associated fluctuations in synaptic remodeling in the arcuate nucleus (1, 8). These observations support the hypothesis that ongoing signaling by brain IGF-I receptors is necessary for E2 regulation of female reproductive physiology.
Female reproductive aging in rodents is characterized by an attenuated and delayed LH surge (9–11). Age-related LH surge dysfunction likely results from reduced GnRH neuronal activation on the afternoon of the LH surge (12–14). Central and peripheral IGF-I levels decline with aging (15, 16), and we recently demonstrated that icv infusion of IGF-I partially restores LH surge amplitude in middle-aged rats (17). Thus, decreased brain IGF-I signaling may contribute to LH surge dysfunction during early reproductive aging. However, the site(s) and mechanism(s) of IGF-I action in the neuroendocrine brain are unclear. Intracerebroventricular administration of an IGF-I receptor antagonist does not modify GnRH-induced LH release in female rats, suggesting a hypothalamic rather than pituitary site of action (17). Similarly, brain IGF-I receptor blockade has no influence on GnRH content, indicating that IGF-I most likely does not act on GnRH neurons to regulate the amount of GnRH available for release (17).
E2 positive feedback requires induction of hypothalamic progestin receptors (PR) (18) and appropriate activation of ER-expressing afferent neuronal populations that innervate GnRH neurons (6, 19–21). Thus, IGF-I receptors in one or more of these afferent inputs may act together with ER to maintain estrous cyclicity and to mediate E2 positive feedback. We hypothesize that IGF-I signaling is required for GnRH neuron activation, and thus blockade of brain IGF-I receptors reduces GnRH neuronal activation on the day of the LH surge. To test this hypothesis, we quantified expression of the immediate early gene c-fos in GnRH neurons and the induction of PR under E2 positive feedback conditions in young rats. We also assessed whether administration of IGF-I to middle-aged females increases GnRH neuronal activation and expression of PR mRNA. We used continuous icv infusion of IGF-I and JB-1, a specific IGF-I receptor antagonist (22), to modulate hypothalamic IGF-I receptor signaling during steroid hormone priming in female rats.
Materials and Methods
Animals
Young (3–4 months) and middle-aged (retired breeders, 9–11 months) female Sprague Dawley rats (Taconic Farms, Germantown, NY) were housed individually and maintained on a 14-h light, 10-h dark cycle (lights on at 0600 h) with free access to chow and water. Only rats with at least two regular 4- to 5-d estrous cycles were studied. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Rats and were approved by the Institutional Animal Care and Use Committee at the Albert Einstein College of Medicine.
Stereotaxic surgery and osmotic minipump placement
Rats were anesthetized with ketamine/xylazine (80 and 4 mg/kg, respectively, im), ovariohysterectomized (OVX), and placed in a Kopf stereotaxic apparatus. A 22-gauge guide cannula (Plastics One, Roanoke, VA) was placed into the third ventricle (anterior/posterior +0.2 mm; medial/lateral +0.0 mm; dorsal/ventral −9.8 mm relative to Bregma) and attached to an osmotic minipump (Alzet model 2002, flow rate 0.5 μl/h; Durect Corp., Cupertino, CA) connected to a 26-gauge internal cannula that extended 1 mm below the guide (5, 17). Animals recovered for 7 d before further manipulations. Correct guide cannula placement was verified by tracking the cannula path in brain sections.
Intracerebroventricular drug administration
Artificial cerebrospinal fluid (aCSF) [140 mm NaCl, 3 mm KCl, 1.2 mm Na2HPO4, 1 mm MgCl2, 0.27 mm NaH2PO4, 1.2 mm CaCl2, and 7.2 mm dextrose (pH 7.4)] was used to dissolve drugs and infused into all control rats. IGF-I receptors were blocked by infusion of JB-1 (100 μg/ml; Bachem, San Carlos, CA), a peptide corresponding to the recognition motif or D domain within the carboxy terminus of IGF-I that selectively competes for IGF-I binding to IGF-I receptors. JB-1 effectively blocks IGF-I receptor autophosphorylation and cellular proliferation in cultured cells (22), and IGF-I potentiation of norepinephrine stimulated cAMP formation in rat hypothalamic slices (20). Human IGF-I (2 μg/ml; Gropep, Adelaide, Australia) was infused into the third ventricle of some middle-aged rats. The doses of JB-1 and IGF-I were selected based on regimens previously used in our laboratory to attenuate LH surges in young rats and to amplify LH surges in middle-aged rats, respectively (5, 17).
Hormone administration
To induce LH surges, E2 benzoate and P (Steraloids, Inc., Newport, RI) were dissolved in peanut oil and administered sc in a volume of 0.1 ml. At 0900 h on d 7 after the osmotic minipump was placed, rats received the first of two daily injections of 2 μg of E2. At 0900 h 2 d after the first E2 injection, some rats were injected with 500 μg of P. This hormone regimen reliably produces LH surges in 75–80% of OVX female rats (6, 23, 24).
Immunohistochemistry for GnRH and c-fos
These protocols were adapted from the published work of Gloria Hoffman (13, 25–27). OVX animals primed with oil or hormones were perfused between 1500 and 1730 h with 4% paraformaldehyde in phosphate buffer (pH 6.8), approximately 6–8.5 h after the last oil or P injection. The brains were postfixed in 4% paraformaldehyde overnight at 4 C and then placed in 30% sucrose until they sank. Six sets of coronal sections (30 μm) starting at the level of the organum vasclosum of the lamina terminalis (Bregma +0.48 mm) and continuing through the medial POA (Bregma −0.72 mm) were collected from each animal, with each set containing every sixth section. Sections were stored in cryoprotectant (28) at −20 C until processed for immunolabeling.
Tissue sections were rinsed in potassium PBS (KPBS) [0.05 m (pH 7.4)] to remove cryoprotectant, incubated in 3% H2O2 for 10 min to block endogenous peroxidase activity, and incubated in KPBS plus 0.04% Triton X-100 (KPBS-Tx) and 1% BSA for 1 h at room temperature. Sections were then incubated in goat anti-c-fos antibody (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in KPBS-Tx and 1% BSA for 48 h at 4 C. The sections were then incubated in biotinylated antigoat IgG (1:600; Vector Laboratories, Burlingame, CA) in KPBS-Tx for 1 h at room temperature, rinsed, and incubated for 1 h in avidin biotin complex (Elite ABC kit; Vector Laboratories). After rinsing in KPBS and 0.175 m sodium acetate, the sections were stained in nickel sulfate (25 mg/ml) and diaminobenzidine-HCl (0.2 mg/ml) in 0.175 m sodium acetate containing 30% H2O2 for 10 min followed by a final rinse in KPBS and sodium acetate. c-fos immunoreactivity was visualized as blue/black in the nuclei of neurons. For colocalization of c-fos in GnRH neurons, the sequence of reactions was then repeated, substituting rabbit-anti-GnRH antiserum as the primary antibody (1:5000, LR-5, a generous gift from R. Benoit, McGill University, Montreal, Canada) for 24 h at 4 C. After rinsing, sections were incubated in biotinylated antirabbit IgG (Vector Laboratories) diluted (1:600) in KPBS-Tx for 1 h at room temperature, rinsed, and reacted with the avidin biotin complex as described above. A mixture of H2O2 and diaminobenzidine-HCl in Tris [0.05 m (pH 7.2)] (Sigma-Aldrich, Inc., St. Louis, MO) was used as the chromogen to yield a brown staining in the cytoplasm. Sections were mounted onto Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). After drying overnight, the sections were dehydrated with ascending alcohol concentrations, cleared with xylenes, and coverslipped. A series of tissue sections was treated identically except primary antibody was omitted from the incubation to control for antibody specificity.
Immunohistochemistry for PR
Separate OVX young females, primed with E2 and P as described above, were used to determine whether IGF-I receptor blockade influences E2 induction of hypothalamic PR. Forty-eight hours after the first E2 or oil injection, and between 4 and 5.5 h after P or oil injection, animals were perfused with 2.5% acrolein and 4% paraformaldehyde. The brains were postfixed overnight in 4% paraformaldehyde and then placed in 30% sucrose until they sank. Sections (25 μm) through the POA (Bregma 0.00 mm) and arcuate/median eminence (Bregma −2.76 mm) were cut and stored in cryoprotectant at −20 C. Sections were rinsed several times in Tris-buffered saline (TBS), incubated for 20 min in 0.1% sodium borohydride, rinsed again, and incubated in 1% H2O2 plus 20% normal goat serum (Vector Laboratories) for 1 h. Sections were incubated with rabbit anti-PR antibody (1:500; Dako, Carpinteria, CA) in TBS with 0.3% Triton X-100, 2% normal goat serum, and 0.5% gelatin overnight at room temperature. Rinsed sections were incubated in biotinylated goat antirabbit IgG (1:500) at room temperature for 90 min, rinsed, and incubated for 1 h in Elite ABC kit. Finally, sections were rinsed in TBS, developed using a Vector SG substrate kit (Vector Laboratories), mounted onto Superfrost Plus slides, dried overnight, dehydrated with ascending alcohol concentrations, cleared with xylenes, and coverslipped. A series of tissue sections was treated identically except without primary antibody to control for antibody specificity.
Cell counting
GnRH and c-fos immunopositive cells
Previous studies reported that GnRH neurons express c-fos in the periventricular POA during LH surges (26, 27). To quantify GnRH immunoreactive (GnRH-ir) and c-fos-ir neurons, five sections of POA in the 1-in-6 series were viewed under a microscope (Zeiss Axioversion; Carl Zeiss, Thornwood, NY). POA sections corresponded to plates 30–35 of the Paxinos and Watson atlas (29). Cells were considered c-fos-ir if they had blue/black nuclear staining with distinct nuclear boundaries. GnRH-ir cells were counted if they had brown cytoplasmic staining. GnRH neurons expressing c-fos were counted at ×40 magnification; if cells had both brown cytoplasmic and blue/black nuclear staining, they were considered double labeled (Figs. 1 and 2). Cell counting was performed by two counters blinded to treatment (interrater variation < 10.5%) and average cell counts reported. Total GnRH and c-fos neurons as well as the percent of GnRH neurons expressing c-fos were calculated. When using the previously described immunostaining conditions, we did not observe c-fos labeling in GnRH neurons of OVX females primed with oil (data not shown).
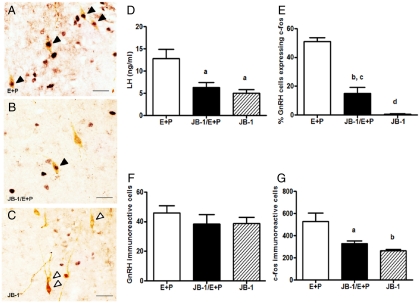
Fig. 1.
Blockade of brain IGF-I receptor reduces the percent of GnRH neurons expressing c-fos and decreases LH release in young OVX, hormone-treated females. Rats were given two daily doses of E2 benzoate (E) (2 μg) and P (500 μg) or oil (vehicle) and perfused 6–8 h (between 1500 and 1700 h) after the final injection of oil or P. Young rats were infused icv with aCSF (vehicle) or JB-1 (100 μg/ml) beginning at the time of OVX. Representative sections of double-label immunohistochemistry (magnification, ×40) showing GnRH neurons (brown cytoplasm) with and without c-fos-ir nuclei (black) in OVX hormone or oil-injected rats infused with (A) aCSF/E + P, (B) JB-1/E + P, or (C) JB-1/oil. Solid arrowheads indicate double-labeled neurons. Open arrowheads indicate GnRH neurons without c-fos. Scale bars, 10 μm. D, Serum LH (ng/ml, mean ± sem) at the time of perfusion in OVX females treated as described above. E, Percentage of GnRH neurons expressing c-fos in young OVX females treated as described above. F, Total number of GnRH neurons (mean ± sem) in the POA of young OVX females treated as described above. G, Total number of c-fos-ir neurons (mean ± sem) in the POA of OVX females treated as described above. a, P < 0.05 vs. E + P treated and infused with aCSF; b, P < 0.01 vs. E + P treated and infused with aCSF; c, P < 0.05 vs. oil treated and infused with JB-1; d, P < 0.0001 vs. E + P treated and infused with aCSF (n = 4–6).
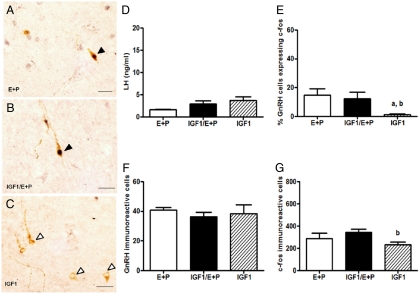
Fig. 2.
Intracerebroventricular infusion of IGF-I does not alter the percent of GnRH neurons expressing c-fos in POA of middle-aged, steroid-treated females. Rats were given two daily doses of E2 benzoate (E) (2 μg) and P (500 μg) or oil (vehicle) and perfused 6–8.0 h (between 1500 and 1700 h) after the final injection of oil or P. Rats were infused icv with aCSF (vehicle) or IGF-I (2 μg/ml) beginning at the time of OVX. Representative sections of double-label immunohistochemistry (magnification, ×40) showing GnRH neurons (brown cytoplasm) with and without c-fos-ir nuclei (black) in OVX hormone or oil-injected middle-aged rats infused with (A) aCSF/E + P, (B) IGF/E + P, or (C) IGF-I/oil. Solid arrowheads indicate double-labeled cells. Open arrowheads indicate GnRH neurons without c-fos. Scale bars, 10 μm. D, Serum LH (ng/ml, mean ± sem) at the time of perfusion in OVX middle-aged females treated as described above. E, Percentage of GnRH neurons expressing c-fos in OVX middle-aged females treated as described above. F, Total GnRH neuron numbers (mean ± sem) in the POA of OVX middle-aged females treated as described above. G, Total number of c-fos-ir neurons (mean ± sem) in the POA of OVX middle-aged females treated as described above. a, P < 0.01 vs. E + P infused with aCSF; b, P < 0.05 vs. E + P infused with IGF-I (n = 4–6).
PR immunopositive cells
Cells were considered PR-ir if they had blue/black nuclear staining with distinct nuclear boundaries. PR-ir cells were counted on an Olympus BX60 microscope (Olympus, New York, NY). Cells were counted bilaterally in the POA (Fig. 3A), the arcuate nucleus, and the ventrolateral portion of the ventromedial hypothalamic nucleus (VMH) (Fig. 3B). POA sections corresponded to plate 34, and sections of the arcuate and VMH corresponded to plate 54 of the Paxinos and Watson atlas (29). POA sections were matched based on the structure of the anterior commissure, and arcuate/VMH sections were matched based on the location of the fornix and the shape of the third ventricle. Two matched, nonadjacent sections of each region were counted per brain. Cells were counted by two counters (interrater variation <10%), both of whom were blind to treatment, and average cell numbers reported. The total cells per brain region were summed and analyzed.
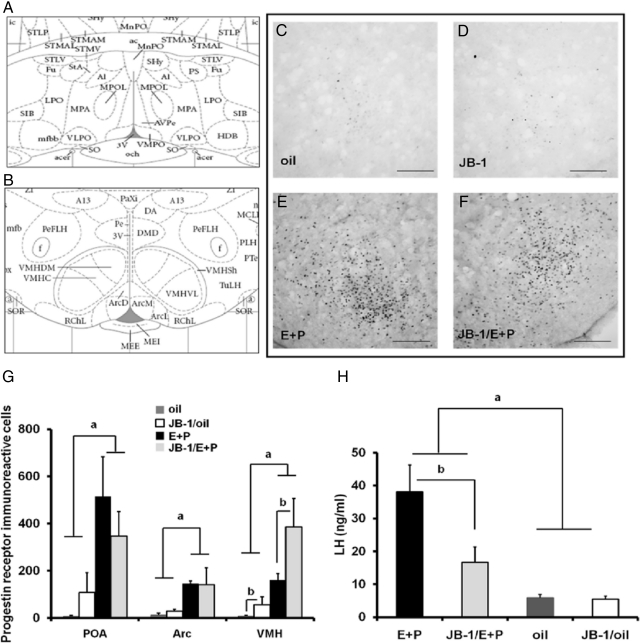
Fig. 3.
Independent of hormone priming icv infusion of JB-1 increased the number of PR-ir cells in the VMH but not in the POA or arcuate (Arc) nucleus of OVX young females. Rats were given two daily doses of E2 benzoate (E) (2 μg) and P (500 μg) or oil (vehicle) and perfused 4–5.5 h (1300–1430 h) after the final injection of oil or P. A, The level (Paxinos and Watson plate 34 [Ref. 29]) at which PR-ir cells were counted in the POA. B, The level (Paxinos and Watson plate 54 [Ref. 29]) at which PR-ir cells were counted in the Arc nucleus and the VMH. ac, Anterior commissure; f, fornix; 3V, third ventricle. Representative sections show few PR-ir cells in the VMH of OVX rats injected with oil and infused with (C) aCSF or (D) JB-1 (magnification, ×20). Scale bars, 100 μm. Representative sections show many PR-ir cells in the VMH of OVX rats injected with E + P and infused with (E) aCSF or (F) JB-1 (magnification, ×20). a, P < 0.05 vs. E + P; b, P < 0.05 vs. JB-1 (n = 5–6). Scale bars, 100 μm. G, Total PR-ir cells counted in each of the brain regions (mean ± sem). A main effect of hormone (F = 41.7, P < 0.0001) and main effect of JB-1 to increase the number of PR-ir cell numbers (F = 10.9, P < 0.01) was observed in the VMH. H, Serum LH (ng/ml, mean ± sem) in OVX females treated as described above. a, P < 0.0001 vs. oil and oil/JB-1; b, P < 0.01 vs. JB-1/E + P.
Hypothalamic dissection, RNA purification, RT, and real-time PCR for PR
Independent groups of OVX, young and middle-aged rats were primed with oil or E2 and P as described above. Rats were killed 4 h after the P or last oil injection. The entire hypothalamus and POA were dissected and transected just posterior to the optic chiasm as described (23). The anterior hypothalamus, which includes the POA, and the posterior hypothalamus, which includes the arcuate and VMH, were snap frozen on dry ice and stored at −80 C until quantitation of PR mRNA.
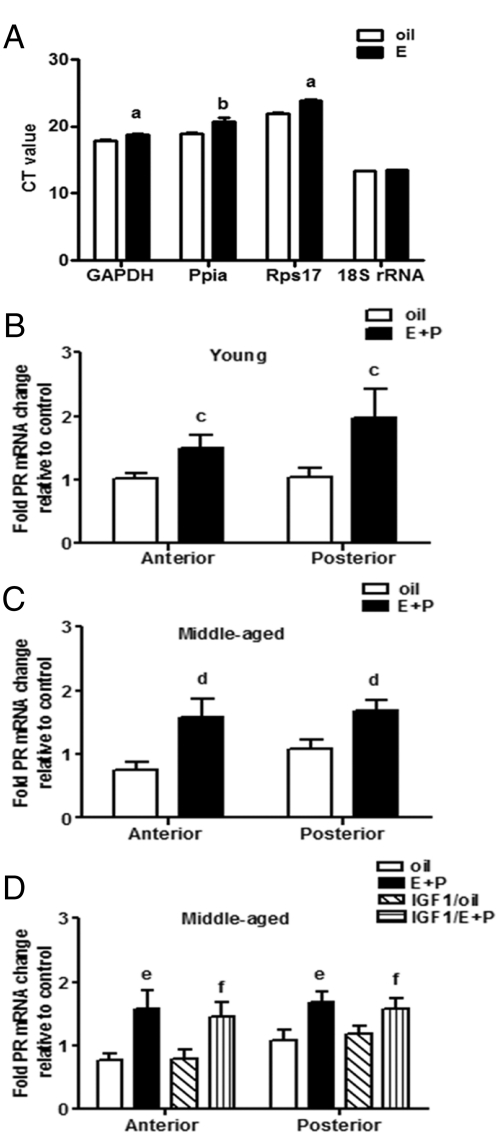
DNA-free total RNA was purified using the RNeasy lipid minikit (QIAGEN, Valencia, CA), including a deoxyribonuclease step. RT was performed using the high-capacity cDNA RT kit with ribonuclease inhibitor (Applied Biosystems, Foster City, CA) using 500 ng of RNA. Gene expression was measured by real-time PCR using TaqMan gene expression assays and master mix (Applied Biosystems) according to the manufacturer's instructions. To identify an appropriate housekeeping gene for normalization of PR mRNA expression, we compared four different candidates: rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (VIC probe, reference sequence Rn01775763_g1; context sequence NM017008.3), rat cyclophilin A (Ppia) (Fam probe, reference sequence Rn00690933_m1; context sequence NM017101.1), rat ribosomal protein S17 (Rps17) (Fam probe, reference sequence Rn00820807_g1; context sequence K0293303.1), and 18S ribosomal RNA (18S rRNA) (Fam probe, reference sequence Hs99999901_s1, context sequence X03205.1). GAPDH, Ppia, and Rps17 mRNA levels were modestly but significantly increased by E2, but 18S rRNA was not (Fig. 4A). Therefore, the final reaction mix contained proprietary TaqMan probes and primers for 18S rRNA as the endogenous control and for PR (Fam probe, reference sequence Rn00575662_m1; context sequence NM022847.1)]. Real-time PCR was performed using an ABI PRISM 7900HT (Applied Biosystems) in simplex conditions using 50 ng of cDNA.
Fig. 4.
IGF-I does not regulate hypothalamic PR mRNA expression. Female rats received two daily doses of E2 benzoate (E) (2 μg), P (500 μg), or vehicle (oil) and were killed 52 h after the first injection of oil or E. A, Hypothalamic GAPDH, Ppia, and Rps17 but not 18S rRNA gene expression was significantly increased by E in young OVX females. E + P treatment significantly increased PR mRNA expression in the anterior (F = 10.98, P < 0.01) and posterior (F = 11.67, P < 0.01) hypothalamus of young (B) and middle-aged (C and D) females. Age did not affect PR mRNA expression in the anterior or posterior hypothalamus of oil or E + P-treated females (B and C). E + P-treated OVX middle-aged rats infused with icv IGF-I (2 μg/ml) or aCSF (vehicle) expressed similar levels of PR mRNA in the anterior and posterior hypothalamus (D). a, P < 0.001 vs. oil (n = 4); b, P < 0.0001 vs. oil (n = 4); c, P < 0.05 vs. oil (n = 8); d, P < 0.05 vs. oil (n = 8); e and f, P < 0.05 vs. oil and IGF-I/oil, respectively (n = 8).
Amplified transcripts for PR were quantified using the comparative threshold cycle method and 18S rRNA as normalizer. The fold change in PR expression was calculated as 2−ΔΔCT, where CT, threshold cycle; ΔCT = CT (PR) − CT (18S rRNA), ΔΔCT = ΔCT (experimental) − ΔCT (reference). ΔCT (reference) was calculated using the mean of the ΔCT for the anterior or posterior hypothalamus of OVX controls treated with aCSF plus oil.
LH radioimmunoassay
Blood was collected by cardiac puncture at the time of perfusion. Serum LH concentrations were measured in duplicate with rat double-antibody assay reagents provided by the Reproduction Ligand Assay and Analysis Core, General Clinical Research Center, University of Virginia (Charlottesville, VA) (Figs. 1D and 2D) and Northwestern University Assay Core (Evanston, IL) (Fig. 3H). The lower limit of the LH assay, and the intra- and interassay coefficients of variation, were 0.04 ng/ml, 3.9 and 5.7% (University of Virginia) and 0.2 ng/ml, 2.9 and 8.7% (Northwestern University), respectively.
Statistical analysis
Data are expressed as mean ± sem. ANOVA was used to evaluate between-group differences in GnRH and c-fos cell numbers and percent of GnRH neurons expressing c-fos. Student's t test was used to analyze housekeeping gene expression in E2-treated rats and controls. Two-way ANOVA (age × treatment) was used to determine differences in PR mRNA. PR-ir cell counts were transformed via a rank transformation to permit two-way ANOVA of nonparametric data. P < 0.05 was considered statistically significant. Post hoc Bonferroni or Turkey's test was used for multiple comparisons of different groups.
Results
Effects of brain IGF-I receptor blockade on the percent of GnRH neurons expressing c-fos and the number of c-fos expressing neurons in young rats
To determine whether IGF-I receptor blockade affects GnRH neuronal activation, we analyzed the percent of GnRH neurons expressing c-fos in young, OVX steroid-primed females after chronic infusion of aCSF or the IGF-I receptor antagonist JB-1. Rats were killed between 1500 and 1730 h, when c-fos expression in GnRH neurons is maximal in young and middle-aged females (14). There were significant differences (F = 71.9, P < 0.0001) (Fig. 1) in the percent of GnRH neurons expressing c-fos. Few GnRH neurons in oil-treated (data not shown) or oil-treated and JB-1-infused young females expressed c-fos (Fig. 1, C and E). As expected, E2 + P treatment increased the percent of GnRH neurons expressing c-fos to approximately 50% (Fig. 1, A and E) (12, 14, 26, 27). Blockade of IGF-I receptors with JB-1 in E2 + P-treated females significantly reduced the percent of GnRH neurons expressing c-fos to approximately 15% (P < 0.01) (Fig. 1, B, E, and G). Neither JB-1 nor hormone treatment altered the total number of GnRH-ir neurons (Fig. 1F).
After E2 (30) and P (27, 31) treatment, neurons along the periventricular zone of the POA strongly express fos-like proteins coincident with the LH surge. E2 + P treatment significantly increased the number of c-fos-ir cells (F = 11.4, P < 0.01) in the POA (Fig. 1G). Blockade of IGF-I receptors in hormone-treated young females significantly (P < 0.05) reduced c-fos-ir neuron numbers by approximately 33% (Fig. 1G).
Effects of increasing brain IGF-I on the percent of GnRH neurons expressing c-fos and the number of c-fos-expressing neurons in middle-aged females
To determine whether IGF-I augmentation of the LH surge amplitude in middle-aged females reflects an increase in GnRH neuronal activation, we infused a dose of IGF-I into the third ventricle that significantly increases steroid-dependent LH surges (17). IGF-I infusion did not affect the number of GnRH neurons expressing c-fos in oil-treated middle-aged females (Fig. 2, C and E). As reported previously (12–14), the percent of GnRH neurons expressing c-fos was significantly lower in E2 + P-primed, middle-aged females (∼15%, P < 0.01) (Fig. 2E) than in young hormone-treated rats (∼50%) (Fig. 1E). However, the dose of IGF-I (2.0 μg/ml) that significantly increased the LH surge amplitude in hormone-treated middle-aged rats (17) did not change the proportion of GnRH neurons expressing c-fos (Fig. 2, B and E). Middle-aged rats had equivalent numbers of GnRH-ir neurons as young rats, and infusion of IGF-1 did not alter the number of GnRH neurons (Fig. 2F).
The number of c-fos-ir cells in middle-aged females was about 2-fold less (289.7 ± 48.7) (Fig. 2G) than in young females (527.4 ± 77.4) (see Fig. 1G) under E2 positive feedback conditions. The number of c-fos positive neurons in the POA of middle-aged rats was significantly higher in hormone-treated than in IGF-I/oil-treated females (231.0 ± 26.8, P < 0.05). However, there was no effect of IGF-I on c-fos positive neurons in the POA of hormone-treated, middle-aged females (346.0 ± 26.5) (Fig. 2G).
Effects of IGF-I receptor blockade on hypothalamic PR induction in young rats
To determine whether IGF-I receptor signaling affects LH release by modulating E2-dependent PR expression, we assessed the effect of IGF-I receptor blockade on E2-dependent increases in the number of PR-ir neurons in brain regions that control reproductive function (Fig. 3). In the POA (F = 25.7, P < 0.0001) and the arcuate (F = 21.4, P < 0.0005) of young rats, E2 treatment significantly increased PR-ir cell number. The effect of E2 on PR-ir was not affected by JB-1 in the POA or arcuate. In the VMH (Fig. 3, E–G), E2 significantly increased PR-ir cell numbers (F = 41.7, P < 0.0001). Interestingly, JB-1 significantly increased PR-ir expression in the VMH of hormone and oil-treated females (F = 10.9, P < 0.01) and tended to increase PR-ir expression in the POA of oil-treated females (Fig. 3G).
Hypothalamic PR mRNA expression in young and middle-aged rats
Age did not affect PR mRNA levels in the anterior or posterior hypothalamus of oil or E2-primed females of either age. E2 significantly increased PR mRNA levels in the anterior (F = 11.0, P < 0.01) (Fig. 4, B and C) and posterior hypothalamus (F = 8.1, P < 0.01) (Fig. 4, B and C) of both age groups. Elevations of PR mRNA by E2 are in good agreement with previous reports (32–34). We also infused IGF-I in middle-aged females to determine whether IGF-I affects LH release by increasing PR mRNA expression. However, IGF-I did not alter PR mRNA expression in either hypothalamic region of oil or hormone-treated, middle-aged rats (Fig. 4D).
Circulating LH levels after blockade of brain IGF-I receptors in young rats and icv infusion of IGF-I in middle-aged rats
To confirm that animals used for GnRH and c-fos immunolabeling responded to treatment, we measured serum LH in a blood sample collected just before perfusion. As expected, hormone treatment increased serum LH, and JB-1 infusion significantly reduced LH by about 50% in young animals (Fig. 1D). In middle-aged rats, serum LH levels did not differ in hormone-treated females infused with aCSF and an IGF-I dose previously shown to potentiate peak LH release in this age group (17) (Fig. 2D). This likely reflects the fact that the perfusion time, a time interval that reflects when maximal numbers of GnRH neurons express c-fos, was earlier than the LH surge, which is characteristically delayed in middle-aged females (17).
We also measured serum LH in young animals used for PR immunostaining. As expected, hormone treatment significantly increased LH release (F = 25.4, P < 0.0001) (Fig. 3H), and JB-1 attenuated hormone-induced LH release (F = 6.36, P < 0.05; interaction between hormone and JB-1: F = 5.94, P < 0.05).
Discussion
The present data provide strong evidence that hypothalamic IGF-I receptors are essential for E2-dependent activation of GnRH neurons on the day of the LH surge in young rats. Because we previously showed that IGF-I does not regulate GnRH peptide content (17), our findings suggest that IGF-I action in GnRH neurons is not necessary to generate an LH surge. This conclusion is consistent with a recent report that female mice with targeted deletion of IGF-I receptors in GnRH neurons can reproduce normally once they go through puberty (35). Therefore, we conclude that ongoing brain IGF-I receptor signaling is necessary for E2 to generate the afferent signals that normally activate GnRH neurons under conditions of positive feedback. Thus, the ability of JB-1 to attenuate estrous cycles in ovary-intact females (5) most likely reflects disruption of E2 positive feedback. However, brain IGF-I receptor signaling is not required for E2 induction of hypothalamic PR, suggesting that PR may be necessary but not sufficient for generation of the LH surge. This finding differs from our earlier report that icv infusion of JB-1 blocks E2 induction of hypothalamic α1B-adrenergic receptors, which are important mediators of both the LH surge and female reproductive behaviors (6). We also demonstrate that doses of IGF-I that increase LH surge amplitude in middle-aged females (17) do not reverse the age-associated decline in GnRH neuronal activation observed on the day of the LH surge. Thus, IGF-I may also act on GnRH nerve terminals in the arcuate/median eminence and/or afferent input to these terminals to increase GnRH release and hence LH surge amplitude. This interpretation is consistent with reports that E2 and IGF-I work together to remodel synaptic architecture in the arcuate nucleus (1).
IGF-I receptors are necessary for GnRH neuronal activation during the steroid-induced LH surge
The expression of c-fos has been used to explore activation of GnRH neurons during spontaneous (26, 27) and steroid-induced (36, 37) LH surges in rats and mice. As expected (12, 14, 36, 37), treatment of OVX young rats with E2 and P increased the percent of GnRH neurons expressing c-fos to about 50%, in association with the LH surge. The percent of GnRH neurons expressing c-fos after IGF-I receptor blockade was decreased (∼15%), and this correlated with a significantly reduced LH release. Although GnRH neurons express both IGF-I and IGF-I receptors (3, 4), hypothalamic IGF-I receptors are not essential for GnRH peptide synthesis in rats (17). Similarly, female mice lacking IGF-I receptors in GnRH neurons are fertile (35). Lastly, brain IGF-I receptor blockade does not affect pituitary responsiveness to exogenous GnRH (17). Taken together, our findings indicate that IGF-I acts on E2-sensitive neurons in the hypothalamus of young rats that drive GnRH neuronal activation on the day of the LH surge.
IGF-I-independent decline of GnRH neuronal activation in middle-aged rats
The percent of GnRH neurons expressing c-fos on the day of the LH surge is much lower in middle-aged than in young female rats, suggesting that impaired GnRH neuronal activation under E2 positive feedback conditions is responsible for the characteristically delayed and attenuated LH surge (12–14). Circulating and tissue levels of IGF-I decline with age (15, 16). We recently reported that E2 fails to increase circulating IGF-I, and icv infusion of IGF-I partially restores LH surge amplitude in middle-aged females (17). Therefore, we hypothesized that increasing hypothalamic levels of IGF-I increases GnRH neuronal activation, reflected in greater expression of c-fos in GnRH neurons. However, IGF-I did not increase c-fos expression in GnRH neurons of middle-aged females. Serum collected when IGF-I-treated rats were killed for immunohistochemistry did not exhibit elevated levels of LH. This likely reflects the time that animals were killed (between 1500 and 1730 h), a time that reflects when c-fos expression is maximal in GnRH neurons of middle-aged rats (12, 14). The LH surge is typically delayed until 1800–2000 h in middle-aged rats (24) even when they are infused with IGF-I (17).
Tanycytes transport IGF-I from the blood into the median eminence, and uptake of IGF-I at this site increases markedly at the time of the preovulatory LH surge (8, 38, 39). These observations combined with our finding that IGF-I infusion does not increase c-fos expression in GnRH neurons from middle-aged rats suggest that the median eminence, where GnRH is secreted into the pituitary portal vasculature, may be a critical location for IGF-I facilitation of LH release, especially in middle-aged females. This interpretation is consistent with reports that E2 significantly increases IGF-I levels and decreases the number of axo-somatic synapses in the arcuate nucleus of young female rats (8). Administration of JB-1 prevents the decrease in axo-somatic synapses (1, 8). Taken together, these findings suggest that IGF-I also modulates E2 regulation of afferent signals that influence GnRH release from nerve terminals.
IGF-I action on ER target genes
Cross talk between brain ER and IGF-I receptors modulates many aspects of female reproductive function, such as synaptic remodeling in the arcuate nucleus, estrous cyclicity, LH release, and lordosis behavior (5, 6, 8). We previously identified functional interactions between hypothalamic ER, IGF-I receptors, and α1B-adrenoceptors, a genomic target of E2 that mediates the facilitatory effects of norepinephrine on both lordosis behavior and LH surges (6, 20, 40). Blockade of IGF-I receptors with JB-1 abolished E2-induced increases in hypothalamic α1B-adrenoceptor density (6), suggesting that IGF-I receptors are required for some genomic responses mediated by ERα signaling. This could represent a general mechanism by which IGF-I influences E2 regulation of the reproductive axis. Therefore, we assessed another well-known genomic response to E2, hypothalamic PR expression. P modulates the E2-dependent LH surge, enhancing both its amplitude and duration (41). Similarly, the time course of E2 induction of hypothalamic PR correlates with expression of lordosis behavior (42, 43).
As expected, E2 priming consistently increased hypothalamic PR mRNA expression (32, 43–45), and the magnitude of the increase was similar in young and middle-aged females (46, 47). In middle-aged rats, exogenous IGF-I did not increase PR mRNA levels. In young rats, E2 also increased PR-ir cells in the POA, arcuate, and VMH; this increase was not prevented by infusion of JB-1 in a dose that attenuates the LH surge (5, 17). LH measurements confirm that animals responded as expected to both hormone treatment and IGF-I receptor blockade. Serum LH levels were significantly higher in hormone-treated females compared with females receiving no hormones, and LH levels were significantly reduced in hormone-treated females infused with JB-1. Thus, brain IGF-I receptor blockade does not interfere with E2 positive feedback by preventing PR induction.
Interestingly, in the VMH, JB-1 significantly increased PR-ir cell number in oil and hormone-treated females. This suggests that under normal conditions, endogenous IGF-I may restrict PR expression in this region. Upon binding of agonists, PR are phosphorylated by MAPK, which targets PR for proteasomal degradation (48). Because IGF-I activates MAPK, blockade of IGF-I receptors may reduce MAPK activity and thus decrease degradation of PR. This could prolong the half-life of PR, increasing PR-ir cell numbers in the VMH. Present data also suggest that IGF-I receptors are not required for all genomic responses mediated by ERα in the hypothalamus. Perhaps IGF-I receptors are not necessary for genomic responses to E2 that involve binding of ER dimers to classical estrogen response elements (such as PR induction). Instead, they may be critical for other reproductively relevant genomic responses to ERα that involve other intracellular signaling pathways and promoter elements.
Interactions of IGF-I with hypothalamic neurotransmitters and neuropeptides
We hypothesize that IGF-I regulates the preovulatory GnRH/LH surge by modulating E2-regulated afferent systems that innervate GnRH neurons and axon terminals. A recent study of mice, in which IGF-I receptors were ablated from GnRH neurons, showed that loss of IGF-I receptors in GnRH neurons did not affect estrous cycles, fertility, or gonadotropin levels (35). As noted above, we reported that one of the functional interactions between E2 and IGF-I is the regulation of hypothalamic α1B-adrenoceptors (6, 49). Neurotransmitters such as γ-aminobutyric acid (GABA) and Glu are other likely targets. GABA- and Glu-immunopositive terminals provide most of the synaptic contacts onto GnRH neurons (50). Pubertal maturation of GnRH neuronal function is advanced by IGF-I (51) and is influenced by excitatory input from N-methyl-D-aspartate receptors (52–54), which respond to endogenous Glu, and inhibitory input from GABAA receptors (55, 56). The steroid-induced LH surge is preceded by a rise in Glu and a decline in GABA release in the POA (57, 58). Thus, GABA and Glu neurons are poised mechanistically and spatially to be targets of IGF-I regulation of the neuroendocrine axis. Moreover, reduced hypothalamic Glu and increased GABAA neurotransmission under estrogen positive feedback conditions contribute to the attenuated LH surges in middle-aged rats (23, 24). Whether IGF-I restores the imbalance of GABA and Glu release in the POA in middle-aged females is an interesting question.
Recently, kisspeptin has emerged as a key regulator of GnRH/LH release (59–61). Kisspeptin neurons in the anteroventral periventricular (AVPV) area express ERα (62, 63) and innervate GnRH neurons, which express the kisspeptin receptor. Kisspeptin neurons in the AVPV express c-fos during the LH surge (64), and there is a positive correlation between the extent of c-fos expression in kisspeptin and in GnRH neurons. Kisspeptins are potent elicitors of GnRH secretion (60, 65), and kisspeptin antagonists prevent preovulatory surges of LH and FSH (66). Female reproductive aging is characterized by reduced E2 induction of kisspeptin expression in the AVPV (23, 67), and IGF-I given either systemically or centrally increases Kiss1 mRNA expression (68, 69). These results could suggest that IGF-I regulates central networks controlling reproductive function by affecting E2-regulated kisspeptin expression. Moreover, age-related changes in kisspeptin signaling might reflect, in part, a failure of IGF-I and E2 to appropriately regulate kisspeptin.
Conclusions
The present findings provide compelling evidence that brain IGF-I receptor signaling is necessary for GnRH neuronal activation under estrogen positive feedback conditions in young rats. The most parsimonious interpretation is that IGF-I acts on ER-expressing neuronal populations that provide afferent input to GnRH neurons. Brain IGF-I receptor blockade did not modify the E2-dependent induction in hypothalamic PR-ir in young rats, and IGF-I did not modify E2 induction of hypothalamic PR mRNA levels in middle-aged females. Therefore, IGF-I does not act by interfering with progestin responsiveness. Infusion of an IGF-I dose that amplifies the LH surge did not increase GnRH neuronal activation in middle-aged rats. Thus, in middle-aged rats, exogenous IGF-I may act primarily on E2-responsive inputs onto GnRH axon terminals to partially restore the LH surge.
Acknowledgments
We thank Kwame Kyei for assistance with counting neurons immunoreactive for GnRH and c-fos; Nina Mikkilineni for assistance with PR immunostaining and cell counting; Dr. Gloria Hoffman and Dr. Jun Shu for assistance with GnRH and c-fos immunohistochemistry; Dr. Diane Lebesgue for assistance with RT-PCR; and Dr. R. Benoit for providing the GnRH primary antibody.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54 HD058155 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and by the Department of Obstetrics and Gynecology and Women's Health, Albert Einstein College of Medicine.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- Artificial cerebrospinal fluid
- AVPV
- anteroventral periventricular
- E2
- estradiol
- ER
- estrogen receptor
- GABA
- γ-aminobutyric acid
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GnRH-ir
- GnRH immunoreactive
- icv
- intracerebroventricular
- KPBS
- potassium PBS
- KPBS-Tx
- KPBS plus 0.04% Triton X-100
- OVX
- ovariohysterectomized
- P
- progesterone
- POA
- preoptic area
- Ppia
- cyclophilin A
- PR
- progestin receptor
- Rps17
- ribosomal protein S17
- 18S rRNA
- 18S ribosomal RNA
- TBS
- Tris-buffered saline
- VMH
- ventromedial hypothalamic nucleus.
References
- 1. Cardona-Gómez GP, Trejo JL, Fernandez AM, Garcia-Segura LM. 2000. Estrogen receptors and insulin-like growth factor-I receptors mediate estrogen-dependent synaptic plasticity. Neuroreport 11:1735–1738 [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Segura LM, Rodriguez JR, Torres-Aleman I. 1997. Localization of the insulin-like growth factor I receptor in the cerebellum and hypothalamus of adult rats: an electron microscopic study. J Neurocytol 26:479–490 [DOI] [PubMed] [Google Scholar]
- 3. Daftary SS, Gore AC. 2003. Developmental changes in hypothalamic insulin-like growth factor-1: relationship to gonadotropin-releasing hormone neurons. Endocrinology 144:2034–2045 [DOI] [PubMed] [Google Scholar]
- 4. Daftary SS, Gore AC. 2004. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormones neurones during postnatal development. J Neuroendocrinol 16:160–169 [DOI] [PubMed] [Google Scholar]
- 5. Todd BJ, Fraley GS, Peck AC, Schwartz GJ, Etgen AM. 2007. Central insulin-like growth factor 1 receptors play distinct roles in the control of reproduction, food intake, and body weight in female rats. Biol Reprod 77:492–503 [DOI] [PubMed] [Google Scholar]
- 6. Quesada A, Etgen AM. 2002. Functional interactions between estrogen and insulin-like growth factor-I in the regulation of α1B-adrenoceptors and female reproductive function. J Neurosci 22:2401–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Etgen AM, Acosta-Martinez M. 2003. Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology 144:3828–3835 [DOI] [PubMed] [Google Scholar]
- 8. Fernandez-Galaz MC, Naftolin F, Garcia-Segura LM. 1999. Phasic synaptic remodeling of the rat arcuate nucleus during the estrous cycle depends on insulin-like growth factor-I receptor activation. J Neurosci Res 55:286–292 [DOI] [PubMed] [Google Scholar]
- 9. Cooper RL, Conn PM, Walker RF. 1980. Characterization of the LH surge in middle-aged female rats. Biol Reprod 23:611–615 [DOI] [PubMed] [Google Scholar]
- 10. Downs JL, Wise PM. 2009. The role of the brain in female reproductive aging. Mol Cell Endocrinol 299:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wise PM. 1982. Alterations in the proestrous pattern of median eminence LHRH, serum LH, FSH, estradiol and progesterone concentrations in middle-aged rats. Life Sci 31:165–173 [DOI] [PubMed] [Google Scholar]
- 12. Krajnak K, Rosewell KL, Wise PM. 2001. Fos-induction in gonadotropin-releasing hormone neurons receiving vasoactive intestinal polypeptide innervation is reduced in middle-aged female rats. Biol Reprod 64:1160–1164 [DOI] [PubMed] [Google Scholar]
- 13. Le WW, Wise PM, Murphy AZ, Coolen LM, Hoffman GE. 2001. Parallel declines in Fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology 142:4976–4982 [DOI] [PubMed] [Google Scholar]
- 14. Rubin BS, Lee CE, King JC. 1994. A reduced proportion of luteinizing hormone (LH)-releasing hormone neurons express Fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biol Reprod 51:1264–1272 [DOI] [PubMed] [Google Scholar]
- 15. Bartke A, Chandrashekar V, Dominici F, Turyn D, Kinney B, Steger R, Kopchick JJ. 2003. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology 4:1–8 [DOI] [PubMed] [Google Scholar]
- 16. Miller BH, Gore AC. 2001. Alterations in hypothalamic insulin-like growth factor-I and its associations with gonadotropin releasing hormone neurones during reproductive development and ageing. J Neuroendocrinol 13:728–736 [DOI] [PubMed] [Google Scholar]
- 17. Todd BJ, Merhi ZO, Shu J, Etgen AM, Neal-Perry GS. 2010. Hypothalamic insulin-like growth factor-I receptors are necessary for hormone-dependent luteinizing hormone surges: implications for female reproductive aging. Endocrinology 151:1356–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bauer-Dantoin AC, Tabesh B, Norgle JR, Levine JE. 1993. RU486 administration blocks neuropeptide Y potentiation of luteinizing hormone (LH)-releasing hormone-induced LH surges in proestrous rats. Endocrinology 133:2418–2423 [DOI] [PubMed] [Google Scholar]
- 19. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 20. Quesada A, Etgen AM. 2001. Insulin-like growth factor-1 regulation of α(1)-adrenergic receptor signaling is estradiol dependent in the preoptic area and hypothalamus of female rats. Endocrinology 142:599–607 [DOI] [PubMed] [Google Scholar]
- 21. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 22. Pietrzkowski Z, Wernicke D, Porcu P, Jameson BA, Baserga R. 1992. Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor 1. Cancer Res 52:6447–6451 [PubMed] [Google Scholar]
- 23. Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. 2009. The excitatory peptide kisspeptin restores the luteinizing hormone surge and modulates amino acid neurotransmission in the medial preoptic area of middle-aged rats. Endocrinology 150:3699–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. 2005. Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology 146:4331–4339 [DOI] [PubMed] [Google Scholar]
- 25. Hoffman GE, Le WW, Sita LV. 2008. The importance of titrating antibodies for immunocytochemical methods. Curr Protoc Neurosci Chapter 2:Unit 2.12 [DOI] [PubMed] [Google Scholar]
- 26. Lee WS, Smith MS, Hoffman GE. 1990. Progesterone enhances the surge of luteinizing hormone by increasing the activation of luteinizing hormone-releasing hormone neurons. Endocrinology 127:2604–2606 [DOI] [PubMed] [Google Scholar]
- 27. Lee WS, Smith MS, Hoffman GE. 1990. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci USA 87:5163–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoffman GE, Le WW. 2004. Just cool it! Cryoprotectant anti-freeze in immunocytochemistry and in situ hybridization. Peptides 25:425–431 [DOI] [PubMed] [Google Scholar]
- 29. Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates. 6th ed San Diego: Academic Press [Google Scholar]
- 30. Insel TR. 1990. Regional induction of c-fos-like protein in rat brain after estradiol administration. Endocrinology 126:1849–1853 [DOI] [PubMed] [Google Scholar]
- 31. Coolen LM, Peters HJ, Veening JG. 1996. Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res 738:67–82 [DOI] [PubMed] [Google Scholar]
- 32. Lauber AH, Romano GJ, Pfaff DW. 1991. Sex difference in estradiol regulation of progestin receptor mRNA in rat mediobasal hypothalamus as demonstrated by in situ hybridization. Neuroendocrinology 53:608–613 [DOI] [PubMed] [Google Scholar]
- 33. Romano GJ, Krust A, Pfaff DW. 1989. Expression and estrogen regulation of progesterone receptor mRNA in neurons of the mediobasal hypothalamus: an in situ hybridization study. Mol Endocrinol 3:1295–1300 [DOI] [PubMed] [Google Scholar]
- 34. Simerly RB, Carr AM, Zee MC, Lorang D. 1996. Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol 8:45–56 [DOI] [PubMed] [Google Scholar]
- 35. Divall SA, Williams TR, Carver SE, Koch L, Brüning JC, Kahn CR, Wondisford F, Radovick S, Wolfe A. 2010. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest 120:2900–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoffman GE, Lee WS, Attardi B, Yann V, Fitzsimmons MD. 1990. Luteinizing hormone-releasing hormone neurons express c-fos antigen after steroid activation. Endocrinology 126:1736–1741 [DOI] [PubMed] [Google Scholar]
- 37. Wu TJ, Segal AZ, Miller GM, Gibson MJ, Silverman AJ. 1992. FOS expression in gonadotropin-releasing hormone neurons: enhancement by steroid treatment and mating. Endocrinology 131:2045–2050 [DOI] [PubMed] [Google Scholar]
- 38. Fernandez-Galaz MC, Torres-Aleman I, Garcia-Segura LM. 1996. Endocrine-dependent accumulation of IGF-I by hypothalamic glia. Neuroreport 8:373–377 [DOI] [PubMed] [Google Scholar]
- 39. Rodríguez EM, Blázquez JL, Pastor FE, Peláez B, Peña P, Peruzzo B, Amat P. 2005. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol 247:89–164 [DOI] [PubMed] [Google Scholar]
- 40. Weesner GD, Krey LC, Pfaff DW. 1993. α1Adrenergic regulation of estrogen-induced increases in luteinizing hormone-releasing hormone mRNA levels and release. Brain Res Mol Brain Res 17:77–82 [DOI] [PubMed] [Google Scholar]
- 41. Evans NP, Richter TA, Skinner DC, Robinson JE. 2002. Neuroendocrine mechanisms underlying the effects of progesterone on the oestradiol-induced GnRH/LH surge. Reprod Suppl 59:57–66 [PubMed] [Google Scholar]
- 42. DonCarlos LL, Greene GL, Morrell JI. 1989. Estrogen plus progesterone increases progestin receptor immunoreactivity in the brain of ovariectomized guinea pigs. Neuroendocrinology 50:613–623 [DOI] [PubMed] [Google Scholar]
- 43. Parsons B, Rainbow TC, Snyder L, McEwen BS. 1984. Progesterone-like effects of estradiol on reproductive behavior and hypothalamic progestin receptors in the female rat. Neuroendocrinology 39:25–30 [DOI] [PubMed] [Google Scholar]
- 44. Brown TJ, Clark AS, MacLusky NJ. 1987. Regional sex differences in progestin receptor induction in the rat hypothalamus: effects of various doses of estradiol benzoate. J Neurosci 7:2529–2536 [PMC free article] [PubMed] [Google Scholar]
- 45. Shughrue PJ, Lane MV, Merchenthaler I. 1997. Regulation of progesterone receptor messenger ribonucleic acid in the rat medial preoptic nucleus by estrogenic and antiestrogenic compounds: an in situ hybridization study. Endocrinology 138:5476–5484 [DOI] [PubMed] [Google Scholar]
- 46. Brown TJ, MacLusky NJ, Shanabrough M, Naftolin F. 1990. Comparison of age- and sex-related changes in cell nuclear estrogen-binding capacity and progestin receptor induction in the rat brain. Endocrinology 126:2965–2972 [DOI] [PubMed] [Google Scholar]
- 47. Wise PM, Parsons B. 1984. Nuclear estradiol and cytosol progestin receptor concentrations in the brain and the pituitary gland and sexual behavior in ovariectomized estradiol-treated middle-aged rats. Endocrinology 115:810–816 [DOI] [PubMed] [Google Scholar]
- 48. Lange CA, Shen T, Horwitz KB. 2000. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA 97:1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Etgen AM, González-Flores O, Todd BJ. 2006. The role of insulin-like growth factor-I and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Front Neuroendocrinol 27:363–375 [DOI] [PubMed] [Google Scholar]
- 50. Mahesh VB, Brann DW. 2005. Regulatory role of excitatory amino acids in reproduction. Endocrine 28:271–280 [DOI] [PubMed] [Google Scholar]
- 51. Hiney JK, Srivastava V, Dearth RK, Dees WL. 2004. Influence of estradiol on insulin-like growth factor-1-induced luteinizing hormone secretion. Brain Res 1013:91–97 [DOI] [PubMed] [Google Scholar]
- 52. Adams MM, Flagg RA, Gore AC. 1999. Perinatal changes in hypothalamic N-methyl-D-aspartate receptors and their relationship to gonadotropin-releasing hormone neurons. Endocrinology 140:2288–2296 [DOI] [PubMed] [Google Scholar]
- 53. Clarkson J, Herbison AE. 2006. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol Cell Endocrinol 254- 255:32–38 [DOI] [PubMed] [Google Scholar]
- 54. Iremonger KJ, Constantin S, Liu X, Herbison AE. 2010. Glutamate regulation of GnRH neuron excitability. Brain Res 1364:35–43 [DOI] [PubMed] [Google Scholar]
- 55. Han SK, Abraham IM, Herbison AE. 2002. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 143:1459–1466 [DOI] [PubMed] [Google Scholar]
- 56. Maffucci JA, Gore AC. 2009. Chapter 2: hypothalamic neural systems controlling the female reproductive life cycle gonadotropin-releasing hormone, glutamate, and GABA. Int Rev Cell Mol Biol 274:69–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jarry H, Leonhardt S, Schwarze T, Wuttke W. 1995. Preoptic rather than mediobasal hypothalamic amino acid neurotransmitter release regulates GnRH secretion during the estrogen-induced LH surge in the ovariectomized rat. Neuroendocrinology 62:479–486 [DOI] [PubMed] [Google Scholar]
- 58. Ping L, Mahesh VB, Wiedmeier VT, Brann DW. 1994. Release of glutamate and aspartate from the preoptic area during the progesterone-induced LH surge: in vivo microdialysis studies. Neuroendocrinology 59:318–324 [DOI] [PubMed] [Google Scholar]
- 59. Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2008. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol 29:48–69 [DOI] [PubMed] [Google Scholar]
- 60. Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M. 2008. Opposite roles of estrogen receptor (ER)-α and ERβ in the modulation of luteinizing hormone responses to kisspeptin in the female rat: implications for the generation of the preovulatory surge. Endocrinology 149:1627–1637 [DOI] [PubMed] [Google Scholar]
- 61. Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. 2009. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. 2007. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378 [DOI] [PubMed] [Google Scholar]
- 63. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. 2006. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neurosci Lett 401:225–230 [DOI] [PubMed] [Google Scholar]
- 64. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. 2008. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Castellano JM, Navarro VM, Fernandez-Fernandez R, Castano JP, Malagon MM, Aguilar E, Dieguez C, Magni P, Pinilla L, Tena-Sempere M. 2006. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol Cell Endocrinol 257- 258:75–83 [DOI] [PubMed] [Google Scholar]
- 66. Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M. 2010. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology 151:722–730 [DOI] [PubMed] [Google Scholar]
- 67. Lederman MA, Lebesgue D, Gonzalez VV, Shu J, Merhi ZO, Etgen AM, Neal-Perry G. 2010. Age-related LH surge dysfunction correlates with reduced responsiveness of hypothalamic anteroventral periventricular nucleus kisspeptin neurons to estradiol positive feedback in middle-aged rats. Neuropharmacology 58:314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hiney JK, Srivastava VK, Les Dees W. 2010. Insulin-like growth factor-1 stimulation of hypothalamic KiSS-1 gene expression is mediated by Akt: effect of alcohol. Neuroscience 166:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hiney JK, Srivastava VK, Pine MD, Les Dees W. 2009. Insulin-like growth factor-I activates KiSS-1 gene expression in the brain of the prepubertal female rat. Endocrinology 150:376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]