Abstract
Proper development of the seminiferous tubules (or testis cords in embryos) is critical for male fertility. Sertoli cells, somatic components of the seminiferous tubules, serve as nurse cells to the male germline, and thus their numbers decide the quantity of sperm output in adulthood. We previously identified activin A, the protein product of the activin βA (Inhba) gene, as a key regulator of murine Sertoli cell proliferation and testis cord expansion during embryogenesis. Although our genetic studies implicated fetal Leydig cells as the primary producers of testicular activin A, gonocytes are another potential source. To investigate the relative contribution of gonocyte-derived activin A to testis morphogenesis, we compared testis development in the Inhba global knockout mouse, which lacks activin A production in all cells (including the gonocytes), and a steroidogenic factor 1 (Sf1)-specific conditional knockout model in which activin A expression in testicular somatic cells is disrupted but gonocyte expression of activin A remains intact. Surprisingly, testis development was comparable in these two models of activin A insufficiency, with similar reductions in Sertoli cell proliferation and minor differences in testis histology. Thus, our findings suggest activin A from male gonocytes is insufficient to promote Sertoli cell proliferation and testis cord expansion in the absence of somatic cell-derived activin A. Evaluation of adult male mice with fetal disruption of activin A revealed reduced testis size, lowered sperm production, altered testicular histology, and elevated plasma FSH levels, defects reminiscent of human cases of androgen-sufficient idiopathic oligozoospermia.
In humans, the increasing incidence of low sperm count and other testicular dysfunctions has led to the hypothesis that these abnormalities together represent a syndrome with common etiology, termed testicular dysgenesis syndrome (TDS) (1–5). The central tenet of the TDS hypothesis is that adult testicular dysfunctions arise as a result of altered development of the testes in utero. However, the rarity of appropriate animal models has limited research into the relationship between fetal testis morphogenesis and adult testicular dysgenesis (5). Although the genetic pathways governing initial establishment of the fetal testes have been largely elucidated in placental mammals, little is known about the signaling pathways controlling further development of the testes before birth. In humans and mice, the embryonic testes contain a series of tubular testis cords that originate as side-by-side transverse tubules separated by a mesenchyme commonly referred to as the testis interstitium (6, 7). Sertoli cells, somatic cells that comprise the testis cord epithelium, produce signaling factors that drive testis cord organization and induce the differentiation of testis-specific cell types within the mesenchyme (8). Between the time of cord formation and birth, testis cords grow from their initial transverse loop shape into highly coiled structures (9, 10). Although Sertoli cell proliferation is undoubtedly critical for the processes of testis cord expansion and coiling, many of the signaling pathways modulating this process remain unknown.
Recently, our group and others have provided genetic evidence indicating that activin A, the protein product of the Inhba gene, is an important regulator of murine testicular cell proliferation during embryogenesis (11, 12). Activin A is a member of the TGFβ superfamily with established roles in the formation of numerous embryonic tissues including the kidneys, pancreas, lungs, and dentition (13, 14). In fetal mouse testes, activin A modulates the relative numbers of Sertoli cells and gonocytes by promoting proliferation of Sertoli cells and inhibiting proliferation of gonocytes (11, 12, 15). We previously uncovered fetal Leydig cells as an important source of testicular activin A; however, observations of activin A expression by gonocytes in a number of species raise the question of whether gonocyte-derived activin A might also positively regulate Sertoli cell proliferation in the mouse (12, 16, 17). Ideally, this question could be addressed by creating a mouse model in which activin A (Inhba) is specifically deleted from gonocytes via the Cre/loxP recombination system. However, current gonocyte-specific Cre transgenic mouse models are unsuitable for our purposes due to either failure to express Cre during embryonic development (i.e. expression is limited to postnatal germ cells) or because expression of Cre is limited to a subset of gonocytes (as few as 55% in some instances) (18–22). To avoid the pitfalls of attempting Cre-mediated deletion of activinA in gonocytes, we sought an alternative approach. We reasoned that by comparing testis development in the Inhba global knockout (KO) mouse model, which lacks expression of activin A in all cell types, with a model in which testicular somatic cell expression of activin A is disrupted but gonocyte-derived activin A remains intact, we could deduce the sufficiency of gonocyte-derived activin A for Sertoli cell proliferation (23). To create the latter model, we used a steroidogenic factor 1-Cre (Sf1-Cre) mouse strain to direct deletion of Inhba to testicular somatic cells, including Sertoli and fetal Leydig cells, by embryonic d 10.5 (E10.5) (24). We hypothesized that if gonocytes are an important source of activin A during fetal testis development, the Inhba global KO model should display testis phenotypes more severe than those in the Sf1-Cre; Inhba conditional KO (cKO) model.
Materials and Methods
Generation of transgenic mice
Inhba+/− mice (kindly provided by Dr. Martin Matzuk) were intercrossed to produce Inhba−/− (global KO) embryos (23). Inhba+/− mice were also mated to Sf1cre/+ transgenic mice and resulting Sf1cre/+;Inhba+/− animals were crossed to Inhbafl/fl mice to produce Sf1cre/+;Inhbafl/− cKO animals (somatic cKO) (24, 25). Timed matings were produced by housing female mice with males overnight and checking for vaginal plugs the next morning (E0.5 is 1200 h on the day when a vaginal plug was found). Fetal tissue was collected from E12.5–E19.5. For fetal analysis, no differences were observed between Inhba+/− and Sflcre/+;Inhba+/fl control genotypes; therefore, only data from Inhba+/− embryos are presented. For adult analysis, tissues from somatic cKO and control males were collected at 12–16 wk of age. All procedures described were reviewed and approved by the Institutional Animal Care and Use Committee and were performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
Immunohistochemistry and histology of murine testes
For immunohistochemistry, testes were fixed in 4% paraformaldehyde in PBS at 4 C overnight, dehydrated through a sucrose gradient, embedded, and cryosectioned. An antibody specific to laminin (1:200; Sigma Chemical Co., St. Louis, MO) was used to mark the testis cord boundaries. For histological analysis, newborn and adult samples were fixed in Bouin's fixative, dehydrated through an ethanol gradient, and embedded in paraffin wax. Sections were stained with hematoxylin and eosin.
Analysis of murine Sertoli cell proliferation
Ki67 immunohistochemistry was performed on 10-μm frozen sections of E19.5 testes following the manufacturer's instructions included with the TSA Fluorescein System Kit (PerkinElmer, Waltham, MA). Ki67 antibody (Abcam, Cambridge, MA) was diluted 1:1500 in the Tris-NaCl blocking buffer included with the TSA Fluorescein System Kit. A minimum of three animals per genotype were analyzed. Per animal, at least three alternating testis cross-sections a minimum of 40 μm apart were photographed. Testis cross-sections were photographed at ×20 magnification, and all visible Sertoli cells within the field were included in the counts. Sertoli cells were identified by their location at the periphery of the testis cords and characteristic triangular/rectangular nuclear shape (as opposed to the round nuclei of germ cells located within the lumen of the testis cords). The percentage of proliferation was calculated as the number of Ki67-positive Sertoli cells divided by the total number of Sertoli cell nuclei. Data are presented as the percentage of Ki67-positive Sertoli cells, rather than absolute number, to minimize sampling error. Because values did not statistically differ among control genotypes, only data from Inhba+/− testes are shown.
Human fetal testis culture and analysis of Sertoli cell proliferation
Human fetal testes (retrieved from morphologically normal fetuses at 14–19 wk gestational age after medical termination of pregnancy with informed consent) were dissected in sterile PBS and cut into fragments of approximately 1 mm3. Fragments were cultured in MEMα with phenol red (GibcoBRL, Life Technologies, Carlsbad, CA) supplemented with 2 mm pyruvate, 2 mm glutamine, 1× ITS (insulin, transferrin, selenium) supplement (Lonza, Basel, Switzerland), and 3 mg/ml BSA, penicillin, streptomycin, and amphotericin. For each fetus, six to 10 testis fragments were cultured in medium only and six to 10 fragments were cultured in medium containing 100 ng/ml recombinant human activin A protein (R&D Systems, Minneapolis, MN). Because a dose-response curve could not be explored due to the rarity of samples, the 100 ng/ml dose of recombinant human activin A protein was selected due to its ability to elicit biological responses of human tissues in a number of in vitro experiments (26–28). Testis fragments were cultured for 24 h in a humidified incubator at 37 C and 5% CO2 at an air-liquid interface on 24-well plate permeable tissue culture inserts (Griner Bio-One, Frickenhausen, Germany). In each well, 450 μl medium was placed below the membrane and 50 μl above to prevent drying. After culture, fragments were retrieved, washed briefly in PBS, and fixed in Bouin's solution for 1 h before processing into paraffin blocks. This study received approval from the Lothian Research Ethics Committee.
Ki67 immunohistochemistry was performed on 5-μm paraffin sections of cultured testis fragments after antigen retrieval. Sections were incubated in Ki67 primary antibody (BD PharMingen, Franklin Lakes, NJ) diluted 1:500 in Tris-NaCl blocking buffer provided in the TSA Fluorescein System Kit (PerkinElmer). Signal was amplified after the manufacturer's instructions included with the TSA Fluorescein System Kit. For Sertoli cell counting, testis cross-sections were photographed at ×20 magnification, and all visible Sertoli cells within the field were included in the counts. Sertoli cells were distinguished from germ cells by their smaller, more oval nuclear shape (as opposed to the large, round nuclear shape of germ cells). At least seven testis fragment cross-sections from five independent experiments were analyzed per treatment group. The percentage of proliferation was calculated as the number of Ki67-positive Sertoli (or germ) cells divided by the total number of Sertoli (or germ) cell nuclei. Data are presented as the percentage of Ki67-positive cells, rather than absolute number, to minimize sampling error.
Daily sperm production
Analysis and calculation of daily sperm production followed the procedure of Joyce et al. (29) with slight modifications. After removal of the tunica, testes were immersed in PBS containing 0.01% Triton X-100 and homogenized for 30 sec using a Polytron homogenizer. Spermatid heads in testis homogenate were then counted on a hemacytometer.
Hormone analysis
Plasma FSH levels were measured in duplicate by RIA according to instructions with kits from the National Hormone and Pituitary Distribution Program (Torrance, CA). The sensitivity of the FSH assay was 1.0 ng/ml, and intra-assay coefficient of variation was 8.69%. The RIA results were calculated by four-parameter logistic analysis using AssayZap (BioSoft).
Statistical analysis
Statistical differences were determined via two-tailed t test comparisons.
Results
Inhba global KO mouse embryos develop stunted testis cords
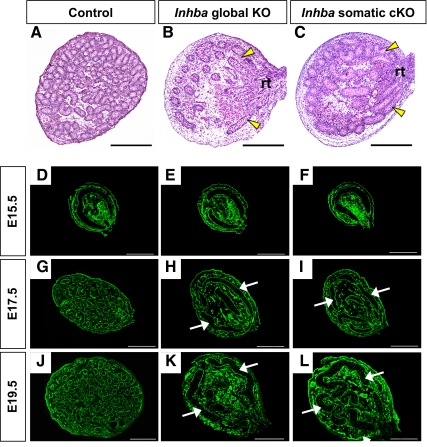
To investigate the general function of activin A during testis morphogenesis, we analyzed histological sections from E19.5/newborn control (wild-type and heterozygote; only heterozygote is shown here) and Inhba global KO testes (23). Whereas numerous testis cord cross-sections were observed in sections from control testes (Fig. 1A), the testis cords of E19.5 Inhba global KO embryos were visibly stunted (Fig. 1B). The extension of linear sections of testis cords from the rete testis was easily identified in global KO testes but not in controls. In addition, a general decrease in the number of testis cord cross-sections was apparent in global KO testes, as was a general increase in cord cross-sectional diameter. We next performed a time-course analysis of global KO testes to determine when testis cord dysgenesis starts. Cross-sections of fetal testes were immunostained for laminin to demarcate the basal lamina of the testis cords (Fig. 1, D–L). From E12.5 through E15.5, the testis cords of both control and global KO embryos appeared as simple transverse circular loops, and no obvious differences were observed (only E15.5 is shown in Fig. 1, D and E). After E15.5 in control testes, testis cord coiling became apparent, eventually leading to numerous circular sections by birth (Fig. 1, G and J). In contrast, testis cords of global KO embryos were underdeveloped, and only minimal coiling was present by E17.5 (Fig. 1H). Even by E19.5, the circular loop typical of early-stage testis cords was still evident in the global KO testes (Fig. 1K). Taken together, although not essential for initial formation of the testis cords, activin A was required for coiling and expansion of the testis cords late in fetal development.
Fig. 1.
Morphogenesis of the testis cords in control, Inhba global KO, and Inhba somatic cKO (Sf1cre/+;Inhbafl/−) mouse embryos. A–C, Histological testis sections from E19.5 control (A), Inhba global KO (B), and Inhba somatic cKO (C) embryos stained with hematoxylin and eosin. Yellow arrowheads indicate straight lengths of testis cords emerging from the rete testis. Scale bar, 250 μm. rt, Rete testis. D–L, Time-course analysis of testis cord morphogenesis in control (D, G, and J), Inhba global KO (E, H, and K), and Inhba somatic cKO (F, I, and L) mouse embryos. Laminin staining (green) was used to outline the testis cords. White arrows indicate abnormal testis cords. Scale bar, 100 μm.
Conditional ablation of Inhba in somatic cells of the fetal testis results in testis phenotypes similar to those in the Inhba global KO testes
Analysis of testis development in Inhba global KO embryos confirmed the critical role of activin A in normal testis morphogenesis. To infer the relative role of gonocyte-derived activin A, we next evaluated testis development in Inhba somatic cKO mice (24, 25). In this somatic cKO model, Inhba is deleted from testicular somatic cells but not from gonocytes. Upon histological analysis, the appearance of testis cords in E19.5 somatic cKO mice (Fig. 1C) significantly differed from those of controls (Fig. 1A). Fewer testis cord cross-sections were evident in somatic cKO testes compared with controls, as was a general increase in the diameter of cord cross-sections. The testis cord dysgenesis phenotype in somatic cKO mice was reminiscent of to that of global KO embryos (Fig. 1B).
We next compared spatiotemporal development of the testis cord loops in Inhba somatic cKO embryos with that of Inhba global KO testes. We observed no obvious differences in testis cord morphogenesis between E12.5 and E15.5 in control (Fig. 1D, only E15.5 results are shown), global KO (Fig. 1E), and somatic cKO testes (Fig. 1F). By E17.5, testis cords of global KO (Fig. 1H) and somatic cKO (Fig. 1I) embryos were underdeveloped and retained their circular shape. The similarities between global KO (Fig. 1K) and somatic cKO (Fig. 1L) testes were still visible at E19.5, with a general failure of testis cord coiling and elongation evident in both genotypes. The gross recapitulation of the Inhba global KO testis cord dysgenesis phenotype in mice lacking Inhba expression in solely the SF1-positive somatic cell population indicates these somatic cells, rather than gonocytes, are the major source of functional activin A in the fetal testes.
Activin A promotes proliferation of the Sertoli cells during fetal life in both mice and humans
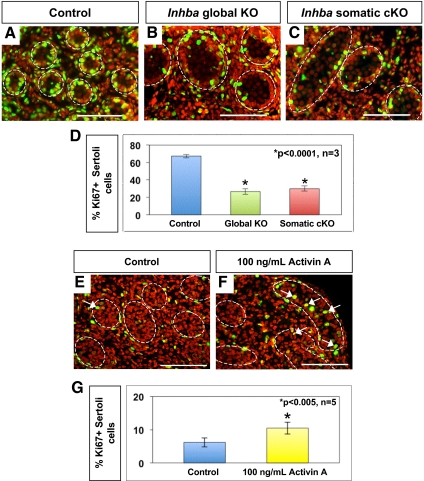
To identify the mechanism behind the failure of testis cord expansion in global and somatic Inhba KO testes, we analyzed Sertoli cell proliferation and apoptosis. Immunohistochemistry for the proliferation marker Ki67 revealed that numerous Sertoli cells were proliferating at E19.5 in control testes (Fig. 2A). In both global KO and somatic cKO testes, a reduction in Sertoli cell proliferation was observed (Fig. 2, B and C). Quantitative analysis confirmed the percentage of Ki67-positive Sertoli cells was significantly decreased, and to a similar magnitude, in both global KO (Fig. 2D, 26.60 ± 3.17%) and somatic cKO (29.93 ± 2.96%) testes compared with controls (67.36 ± 2.05%, P < 0.0001; n = 3 animals per genotype). We then evaluated cell death and found apoptotic Sertoli cells to be rare in the testes of all genotypes based on terminal deoxynucleotidyl transferase dUTP nick end labeling analysis or TUNEL (data not shown). We concluded that defects in testis cord expansion in Inhba global KO and somatic cKO embryos were the result of reduced Sertoli cell proliferation. Our observation of a reduced rate of Sertoli cell proliferation at E19.5 would suggest an eventual decrease in Sertoli cell number in Inhba global KO and somatic cKO mice compared with controls, corroborating the recent report by Mendis et al. (12).
Fig. 2.
Effects of activin A on Sertoli cell proliferation in mouse and human fetal testes. A–C, Double-immunofluorescent staining for Ki67 (green) and DAPI nuclear dye (red) in E19.5 control (A), Inhba global KO (B), and Inhba somatic cKO (C) testes. Merged image results in yellow color of proliferating cell nuclei. Scale bar, 100 μm. White dotted lines outline the testis cords. D, Percentage of Ki67-positive Sertoli cells in newborn control, Inhba global KO, and Inhba somatic cKO testes compared with control, (n = 3 for each genotype). *, Statistical difference from the control (P < 0.0001). E and F, Double-immunofluorescent staining for Ki67 (green) and DAPI nuclear dye (red) in 14- to 19-wk gestational age human testes cultured for 24 h in the absence (E, control) or presence (F) of 100 ng/ml recombinant human activin A protein. Scale bar, 100 μm. White dashed line indicates outline of testis cords. G, Percentage of Ki67-positive Sertoli cells in human control fetal testes and fetal testes cultured with recombinant human activin A protein. *, Statistical difference from the control (P < 0.005; n = 5).
Activin βA has also been detected in human fetal testes, suggesting the stimulatory effect of activin A upon Sertoli cell proliferation could be conserved between mice and humans (30). To test this hypothesis, we cultured 14- to 19-wk gestational age human testes for 24 h in the presence or absence of exogenous human recombinant activin A protein (100 ng/ml) (26–28). After culture, testis sections were immunostained for the proliferation marker Ki67 (Fig. 2, E and F), and the proliferating Sertoli cells were counted. Sertoli cells were distinguished from germ cells based upon differences in nuclear size and shape [using 4′,6-diamidino-2-phenylindole (DAPI) nuclear dye staining]. The percentage of proliferating Sertoli cells was significantly higher in human fetal testes cultured in the presence of activin A (Fig. 2G, 10.49 ± 1.80%) compared with untreated control testes (6.20 ± 1.39%, P < 0.005, n = 5). This result, coupled with the mouse genetic data, suggests activin A is a conserved stimulatory factor to fetal Sertoli cells of the testis cord epithelium in mice and humans. Interestingly, although activin A has been reported to inhibit gonocyte proliferation in mouse testes, we observed no statistical difference in germ cell proliferation between activin A-treated and control human testes at this time point (data not shown).
Adult Inhba cKO mice have reduced testis size, lower sperm production, and abnormal seminiferous tubule histology
Because a lack of somatic cell-derived activin βA led to fetal testis cord dysgenesis, we wondered whether this fetal defect could impact adult testicular function. Global Inhba KO mice die shortly after birth; therefore, we evaluated testis development in control (Sf1cre/+;Inhba+/fl) and Inhba somatic cKO adult males at 12–16 wk of age. The somatic cKO mice exhibited normal growth patterns and survival (Table 1) (24). The grossly normal appearance of somatic cKO adult male mice suggested Inhba expression within nongonadal SF1-positive cell populations (adrenal, hypothalamus, and pancreas) is not critical for the function of these organs or for overall health.
Table 1.
Measurement of body weight, anogenital distance, and seminal vesicle weight in 12- to 16-wk-old control and Inhba somatic cKO male mice
| Genotype | n | Body weight (g) | Anogenital distance (mm) | Seminal vesicle weight (mg) |
|---|---|---|---|---|
| Control | 9 | 29.74 ± 3.35 | 15.15 ± 1.03 | 270.26 ± 48.60 |
| Inhba somatic cKO | 10 | 26.71 ± 1.51 | 14.99 ± 0.81 | 258.44 ± 43.67 |
Values are shown as average ± sd. No statistical differences were observed between control and somatic cKO for any parameters (P > 0.01).
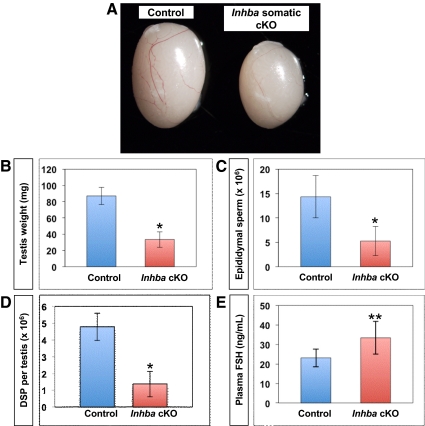
Body weight did not significantly differ between 12- to 16-wk-old Inhba cKO and age-matched control males (Table 1). However, a significant reduction in testis weight was evident in Inhba cKO males (Fig. 3, A and B, P < 0.0001; n = 9 for control; n = 10 for Inhba cKO). Despite this dramatic difference in testis size, no significant differences in androgen-sensitive endpoints such as anogenital distance and seminal vesicle weight were observed between age-matched control and Inhba cKO males (Table 1). These results complement our previous finding that newborn Inhba global KO testes produced normal levels of testosterone (31).
Fig. 3.
Testis development and sperm production in 12- to 16-wk-old control (Sf1cre/+;Inhbafl/+) and Inhba somatic cKO adult mice. A, Image of representative testes from control (left) and Inhba somatic cKO (right) mice. B, Testis weight (in milligrams) of somatic cKO and control male mice. *, Statistical difference of P < 0.0001 for cKO compared with controls (n = 9 for control, and n = 10 for cKO). C, Cauda epididymal sperm concentration (in millions) in somatic cKO and control male mice. *, Statistical difference of P < 0.0001 for cKO vs. controls (n = 9 for control, and n = 10 for cKO). D, Average daily sperm production (DSP) per testis in somatic cKO and control male mice. Asterisk indicates statistical difference of P < 0.0001 for cKO compared with control (n = 9 for control, and n = 10 for cKO). E, Average plasma FSH levels (in nanograms per milliliter of plasma) in somatic cKO and control male mice. **, Statistical difference of P < 0.006 for cKO compared with control (n = 9 for both genotypes).
We next evaluated sperm output to determine whether Inhba cKO male mice produced fewer sperm than controls. Cauda epididymal sperm concentration was significantly reduced in 12- to 16-wk-old Inhba cKO males compared with age-matched controls (Fig. 3C, P < 0.0001; n = 9 for control; n = 10 for Inhba cKO). Daily sperm production was also significantly decreased in Inhba cKO males compared with controls (Fig. 3D, P < 0.05; n = 9 for control; n = 10 for Inhba cKO). Taken together, these results indicated Inhba cKO males are capable of both producing and storing sperm at 12–16 wk of age. However, the testes of Inhba somatic cKO male mice produce fewer sperm than controls even when differences in testis size are taken into account. Despite these testicular defects, limited-scale breeding studies of Inhba somatic cKO adult male mice revealed these animals to be capable of siring pups. This result was not surprising given estimations that reductions in fertility are not apparent in the male mouse until sperm production falls below 10% of normal values (32).
In the mouse as well as in humans, spermatogenesis is modulated by both androgens and FSH (33–36). Pituitary-derived activins are known to stimulate FSH synthesis; therefore, we sought to ensure the reduction in sperm production in Inhba somatic cKO males was not due to suppressed FSH (37). We found plasma FSH levels in 12- to 16-wk-old Inhba somatic cKO males were significantly elevated compared with controls (Fig. 3E, P < 0.01; n = 9). This result indicated low sperm production in Inhba somatic cKO males did not result from FSH insufficiency.
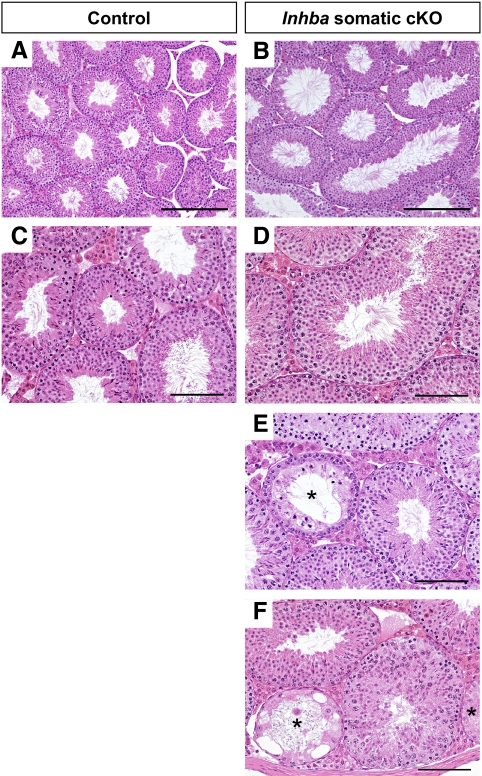
To further investigate the cause of low sperm counts in Inhba somatic cKO male mice, we evaluated testis histology and observed generally enlarged seminiferous tubules compared with controls (Fig. 4, A and B). Spermatogenesis was grossly normal in cKO testes at 12–16 wk (Fig. 4, B and D); however, focal dysgenic tubules characterized by abnormal or absent spermatogenesis were found in cKO testes (Fig. 4, E and F) but not in controls (Fig. 4, A and C). Additional abnormalities observed in tubules from Inhba cKO testes included the sporadic presence of meiotic abnormalities, mixed-stage tubules, failures of spermiation, and loss of spermatogonia. It is likely the decreased sperm production in Inhba somatic cKO male results from these spermatogenic defects.
Fig. 4.
Testis histology in 12- to 16-wk-old control and Inhba somatic cKO adult mice. A and B, Hematoxylin- and eosin-stained sections from control (A) and somatic cKO (B) testes shown at ×10 magnification. Scale bar, 250 μm. C–F, At higher magnification (×20), tubules undergoing normal spermatogenesis are evident in both control (C) and somatic cKO (D) testes. However, focal dysgenic tubules are also present in testes from somatic cKO (E and F) mice. *, Dysgenic tubules. Scale bar, 100 μm.
Discussion
Somatic cells in the fetal testis are the major source of activin A that regulates testis cord expansion
Our analysis of testis cord development in Inhba global KO and somatic cell cKO embryos indicates the contribution of germ cell-derived activin A protein is likely minimal compared with activin A coming from the SF1-expressing somatic cells. Activin A is globally absent in Inhba KO embryos, leading to a failure of testis cord expansion due to decreased Sertoli cell proliferation. In the Inhba somatic cKO mouse model, production of activin A in gonocytes should remain intact, yet the testis cord dysgenesis phenotype is similar to that of the Inhba global KO model. If gonocyte-derived activin A was in fact critical, we would anticipate a less severe or lack of testis phenotypes in the Inhba somatic cKO model compared with the global KO model. However, our results do not support this hypothesis.
Our results implicate SF1-positive somatic cells as the primary source of activin A protein during murine testis morphogenesis (Fig. 5). The SF1-positive cell populations of the fetal testes include Sertoli cells, fetal Leydig cells, and unidentified mesenchymal somatic cells (24). Previously, we demonstrated that fetal Leydig cells are an important source of activin A during murine fetal testis development (11). Based on our current findings, Sertoli cells appear unlikely to be a source of activin A in murine fetal testes. Additionally, testes of Sertoli cell-specific Inhba cKO were found to develop similarly to controls (personal communication with Martin Matzuk, Baylor College of Medicine, Houston, TX). Fetal Leydig cells and/or unknown SF1-positive cell populations within the testis mesenchyme are thus the best candidates for the cellular source of testicular activin A (11). Interestingly, activin βA also localizes to the fetal Leydig cells in human fetal testes, raising the possibility that fetal Leydig cell regulation of Sertoli cell proliferation via activin A is a conserved mechanism (30).
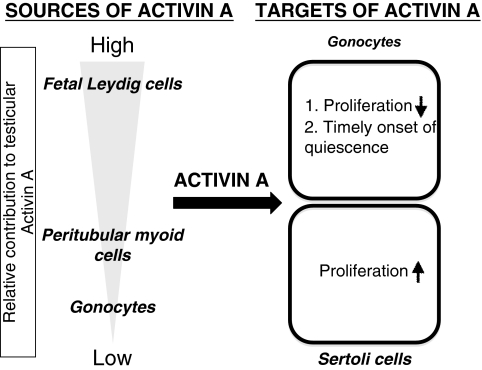
Fig. 5.
Model of the current understanding of activin A function during fetal testis development. The primary cellular sources of activin A are the fetal Leydig cells, with peritubular myoid cells and gonocytes also contributing. Activin A signals directly to the gonocytes to inhibit proliferation and to modulate the appropriate timing of quiescence. Activin A also targets Sertoli cells and stimulates the proliferation necessary for expansion of the testis cords (embryonic precursors to the seminiferous tubules).
Activin A expression by Sertoli cells changes over time
At first glance, our conclusion that Sertoli cells are not a likely source of activin A may appear to contrast with a number of publications that specifically detail activin A secretion by Sertoli cells (17, 38–42). However, upon closer examination it becomes evident that Sertoli cell production of activin A is highly dependent upon the age of the animal. Several studies conducted in the rat have indicated early postnatal (d 3–9) proliferative-phase Sertoli cells are unlikely to secrete activin A (42, 43). However, production of activin βA subunits by adult Sertoli cells has been reported in a number of different species including humans (44–49). Testicular activin A levels are reported to be dramatically lower in mature rat or mouse testes compared with early postnatal testes, suggesting adult Sertoli cells do not secrete large amounts of activin A (40, 41). The switch to a low-activin A environment within postnatal testes coincides with, and is thought to influence, the differentiation and maturation of both Sertoli and germ cells (48). Although the roles of Sertoli cell-derived activin A in mature testes are not fully understood, observations of regulated production of activin A at various stages of the seminiferous epithelium cycle implicate activin A as a local modulator of spermatogenesis (49). The spermatogenic defects we observed in adult Inhba somatic cKO mice may therefore be related not only to abnormal development of the testes during fetal life but also to disrupted activin A paracrine signaling between Sertoli cells and spermatogenic cells in adulthood.
The study of mice with disrupted testicular activin A signaling highlights the different requirements for activin A in fetal and postnatal testicular functions
Nearly a decade ago, the TDS hypothesis was put forth, postulating that adult testicular dysfunction in humans may originate during embryonic life (5). Given the difficulties of investigating this relationship in humans, research into the fetal origins of testicular dysgenesis has relied upon the use of animal models. The argument for a fetal origin of hypospadias and cryptorchidism is obvious, because these dysfunctions are readily apparent perinatally in humans and many animal species. However, a fetal origin of low sperm count is more difficult to comprehend given the dramatic changes the testes undergo between fetal life and adult spermatogenesis. Results in this study demonstrate that during fetal development, testicular activin A promotes Sertoli cell proliferation and subsequent expansion of the testis cords before birth. In mice, this proliferation sets the stage for postnatal testicular growth and ultimately normal spermatogenesis in adulthood. Conversely, disruption of activin A in the somatic cells of fetal testes leads to underdevelopment of the testis cords. This fetal testicular dysgenesis persists into adulthood, resulting in focal seminiferous tubule dysfunction, elevated plasma FSH, and decreased sperm production. It is important to note that in our Inhba somatic cKO mouse model, activin βA is permanently removed from the SF1-positive somatic cells of the testis early in fetal testis development (by E10.5) (24). Therefore, whereas this model provides an opportunity to track the effects of fetal loss of testicular activin A throughout life, it does not allow us to study the specific role of testicular activin A during critical time points between fetal life and adulthood. Currently, testicular activin A is not thought to have a direct impact on spermatogenesis in rodents. Although activin A is essential for the production of FSH by the pituitary gland, the activin that stimulates FSH production comes from pituitary gonadotrophs rather than the testes (37). In addition, testicular activin A protein levels are much lower in the testes of sexually mature mice compared with newborn mice, suggesting activin A may not be a critical regulator of adult testicular function (40).
Disruption of testicular activin A signaling during fetal development creates a murine model of testicular cord dysgenesis
The study of animal models, particularly rodents, has provided valuable insight into the pathways related to some TDS symptoms. Historically, the preferred animal model for TDS has been the phthalate-treated rat (50). In utero exposure to phthalates induces cryptorchidism, hypospadias, reduced Sertoli cell proliferation, and focal testis cord dysgenesis, thus recapitulating several key aspects of TDS (51). However, many of the TDS-related symptoms in the phthalate-exposed rat model are related to decreased androgen production and/or signaling during both fetal and perinatal life (51–54). Therefore, although the phthalate-exposed rat model is useful for the study of TDS symptoms arising from insufficient androgen signaling, it is not an ideal model in which to study the presumably more common situation of androgen-sufficient testicular dysfunction. Recently, fetal testis dysgenesis has been also been reported in a mouse model lacking betaglycan, a coreceptor for TGFβ superfamily ligands (55). As is the case in the phthalate-exposed rat model, testicular dysgenesis in the betaglycan-null mouse model is associated with abnormal fetal Leydig cell function and impairment of steroidogenic pathways.
In this study, we present a mouse model that recapitulates what is thought to be the most common presentation of TDS in the human population, i.e. low sperm count in normally masculinized males (5). In general, idiopathic oligozoospermia in humans has not been associated with decreased serum or local testosterone levels (56–58). Instead, elevated FSH levels are frequently regarded as a hallmark of low sperm production in men (56–62). We found Inhba somatic cKO mice to present with decreased sperm production, altered testicular histology, and elevated FSH levels in adulthood. Although the Sf1-Cre transgene used in this study is also known to target regions of the pituitary and hypothalamus, we do not think the increased FSH in Inhba somatic cKO mice is due to this localized loss of pituitary activin A because we observed a similar increase in serum FSH in fetal Leydig cell-specific Inhba cKO mice (11, 24). Additionally, we would anticipate deletion of Inhba in the pituitary to decrease serum FSH levels because activins classically function to stimulate FSH production. It is also important to note that Inhba cKO mice are normally masculinized as evidenced by the proper development of all androgen-sensitive parameters (Table 1); therefore, the low sperm count, elevated FSH levels, and testicular dysgenesis present in this model are not likely to result from insufficient androgen production. In our previous study, we found loss of activin A signaling did not decrease testosterone production (31). In addition to reduced sperm production and elevated FSH levels, Inhba cKO males present with changes in testicular histology similar to clinical observations of men with idiopathic infertility, including regions of arrested spermatogenesis and increased Sertoli cell vacuolization (62–64). The similarities between Inhba somatic cKO mice and men with idiopathic oligozoospermia suggest these KO mice could serve as a useful model for the study of molecular aspects of androgen-sufficient oligozoospermia.
Acknowledgments
We thank Dr. Martin Matzuk (Baylor College of Medicine) for the Inhba+/− and Inhbafl/fl mouse strains, Dr. Rex Hess (University of Illinois) for providing histological expertise, and Sam Marion and Dr. Patricia Hoyer (University of Arizona, Tucson, AZ) for FSH measurement. We also appreciate all of the Yao lab members for their assistance and support.
This study was funded by the National Institutes of Health (HD046861 and Intramural Research Fund to H.H.-C.Y; Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant Predoctoral Traineeships in Endocrine, Developmental, and Reproductive Toxicology or T32 ES07326 to D.R.A.), the United Kingdom MRC (WBS U. 1276.00.002.00001 to R.A.A.), and Medical Research Scotland (354 Germany to A.J.C.).
We dedicate this study to our collaborator and friend Dr. Keith Parker, who passed away on December 13, 2008.
Disclosure Summary: D.R.A., J.T., A.J.C., and H.H.-C.Y. have nothing to declare. R.A.A. received grant support from a Medical Research Council (MRC) Research Grant.
Footnotes
- cKO
- Conditional KO
- DAPI
- 4′,6-diamidino-2-phenylindole
- E10.5
- embryonic d 10.5
- KO
- knockout
- SF1
- steroidogenic factor 1
- TDS
- testicular dysgenesis syndrome.
References
- 1. Andersen AG, Jensen TK, Carlsen E, Jørgensen N, Andersson AM, Krarup T, Keiding N, Skakkebaek NE. 2000. High frequency of sub-optimal semen quality in an unselected population of young men. Hum Reprod 15:366–372 [DOI] [PubMed] [Google Scholar]
- 2. Auger J, Kunstmann JM, Czyglik F, Jouannet P. 1995. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med 332:281–285 [DOI] [PubMed] [Google Scholar]
- 3. Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. 1992. Evidence for decreasing quality of semen during past 50 years. BMJ 305:609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Irvine S, Cawood E, Richardson D, MacDonald E, Aitken J. 1996. Evidence of deteriorating semen quality in the United Kingdom: birth cohort study in 577 men in Scotland over 11 years. BMJ 312:467–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skakkebaek NE, Rajpert-De Meyts E, Main KM. 2001. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16:972–978 [DOI] [PubMed] [Google Scholar]
- 6. Clermont Y, Huckins C. 1961. Microscopic anatomy of the sex cords and seminiferous tubules in growing and adult albino rats. Am J Anat 108:79–98 [Google Scholar]
- 7. Roosen-Runge EC. 1961. Rudimental “genital canals” of the gonad in rat embryos. Acta Anat 44:1–11 [PubMed] [Google Scholar]
- 8. Brennan J, Capel B. 2004. One tissue, two fates: Molecular genetic events that underlie testis versus ovary development. Nat Rev Genet 5:509–521 [DOI] [PubMed] [Google Scholar]
- 9. Combes AN, Lesieur E, Harley VR, Sinclair AH, Little MH, Wilhelm D, Koopman P. 2009. Three-dimensional visualization of testis cord morphogenesis, a novel tubulogenic mechanism in development. Dev Dyn 238:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nel-Themaat L, Vadakkan TJ, Wang Y, Dickinson ME, Akiyama H, Behringer RR. 2009. Morphometric analysis of testis cord formation in Sox9-EGFP mice. Dev Dyn 238:1100–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Archambeault DR, Yao HH. 2010. Activin A, a product of fetal Leydig cells, is a unique paracrine regulator of Sertoli cell proliferation and fetal testis cord expansion. Proc Natl Acad Sci USA 107:10526–10531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mendis SH, Meachem SJ, Sarraj MA, Loveland KL. 2011. Activin A balances Sertoli and germ cell proliferation in the fetal mouse testis. Biol Reprod 84:379–391 [DOI] [PubMed] [Google Scholar]
- 13. Ritvos O, Tuuri T, Erämaa M, Sainio K, Hildén K, Saxén L, Gilbert SF. 1995. Activin disrupts epithelial branching morphogenesis in the developing glandular organs of the mouse. Mech Dev 50:229–245 [DOI] [PubMed] [Google Scholar]
- 14. Ferguson CA, Tucker AS, Christensen L, Lau AL, Matzuk MM, Sharpe PT. 1998. Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev 12:2636–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richards AJ, Enders GC, Resnick JL. 1999. Activin and TGFβ limit murine primordial germ cell proliferation. 207:470–475 [DOI] [PubMed] [Google Scholar]
- 16. Jarred RA, Cancilla B, Richards M, Groome NP, McNatty KP, Risbridger GP. 1999. Differential localization of inhibin subunit proteins in the ovine testis during fetal gonadal development. Endocrinology 140:979–986 [DOI] [PubMed] [Google Scholar]
- 17. Meehan T, Schlatt S, O'Bryan MK, de Kretser DM, Loveland KL. 2000. Regulation of germ cell and Sertoli cell development by activin, follistatin, and FSH. Dev Biol 220:225–237 [DOI] [PubMed] [Google Scholar]
- 18. Lomelí H, Ramos-Mejía V, Gertsenstein M, Lobe CG, Nagy A. 2000. Targeted insertion of Cre recombinase into the TNAP gene: excision in primordial germ cells. Genesis 26:116–117 [PubMed] [Google Scholar]
- 19. Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, Saitou M, Surani MA. 2005. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436:207–213 [DOI] [PubMed] [Google Scholar]
- 20. Gallardo T, Shirley L, John GB, Castrillon DH. 2007. Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis 45:413–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inselman AL, Nakamura N, Brown PR, Willis WD, Goulding EH, Eddy EM. 2010. Heat shock protein 2 promoter drives Cre expression in spermatocytes of transgenic mice. Genesis 48:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lei Z, Lin J, Li X, Li S, Zhou H, Araki Y, Lan ZJ. 2010. Postnatal male germ-cell expression of cre recombinase in Tex101-iCre transgenic mice. Genesis 48:717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matzuk MM, Kumar TR, Vassalli A, Bickenbach JR, Roop DR, Jaenisch R, Bradley A. 1995. Functional analysis of activins during mammalian development. Nature 374:354–356 [DOI] [PubMed] [Google Scholar]
- 24. Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. 2006. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis 44:419–424 [DOI] [PubMed] [Google Scholar]
- 25. Pangas SA, Jorgez CJ, Tran M, Agno J, Li X, Brown CW, Kumar TR, Matzuk MM. 2007. Intraovarian activins are required for female fertility. Mol Endocrinol 21:2458–2471 [DOI] [PubMed] [Google Scholar]
- 26. Rabinovici J, Spencer SJ, Jaffe RB. 1990. Recombinant human activin-A promotes proliferation of human luteinized preovulatory granulosa cells in vitro. J Clin Endocrinol Metab 71:1396–1398 [DOI] [PubMed] [Google Scholar]
- 27. Hillier SG, Yong EL, Illingworth PJ, Baird DT, Schwall RH, Mason AJ. 1991. Effect of recombinant activin on androgen synthesis in cultured human thecal cells. J Clin Endocrinol Metab 72:1206–1211 [DOI] [PubMed] [Google Scholar]
- 28. Bingham EL, Cheng SP, Woods Ignatoski KM, Doherty GM. 2009. Differentiation of human embryonic stem cells to a parathyroid-like phenotype. Stem Cells Dev 18:1071–1080 [DOI] [PubMed] [Google Scholar]
- 29. Joyce KL, Porcelli J, Cooke PS. 1993. Neonatal goitrogen treatment increases adult testis size and sperm production in the mouse. J Androl 14:448–455 [PubMed] [Google Scholar]
- 30. Anderson RA, Cambray N, Hartley PS, McNeilly AS. 2002. Expression and localization of inhibin α, inhibin/activin βA and βB and the activin type II and inhibin β-glycan receptors in the developing human testis. Reproduction 123:779–788 [DOI] [PubMed] [Google Scholar]
- 31. Tomaszewski J, Joseph A, Archambeault D, Yao HHC. 2007. Essential roles of inhibin βA in mouse epididymal coiling. Proc Natl Acad Sci USA 104:11322–11327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Russel LD, Ettlin RA, Sinha Hikim AP, Clegg ED. 1990. Histopathology of the testis: quantitative evaluation of testis histopathology. In: Russel LD, Ettlin RA, Hikim S, Clegg ED. eds. Histological and histopathological evaluation of the testis. Clearwater, FL: Cache River Press; 254–264 [Google Scholar]
- 33. Franchimont P, Millet D, Vendrely E, Letawe J, Legros JJ, Netter A. 1972. Relationship between spermatogenesis and serum gonadotrophin levels in azoospermia and oligospermia. J Clin Endocrinol Metab 34:1003–1008 [DOI] [PubMed] [Google Scholar]
- 34. Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O'Shaughnessy PJ. 2004. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology 145:318–329 [DOI] [PubMed] [Google Scholar]
- 35. Mancini RE, Seiguer AC, Lloret AP. 1969. Effect of gonadotrophins on the recovery of spermatogenesis in hypophysectomized patients. J Clin Endocrinol Metab 29:467–478 [DOI] [PubMed] [Google Scholar]
- 36. Walker WH. 2009. Molecular mechanisms of testosterone action in spermatogenesis. Steroids 74:602–607 [DOI] [PubMed] [Google Scholar]
- 37. Roberts V, Meunier H, Vaughan J, Rivier J, Rivier C, Vale W, Sawchenko P. 1989. Production and regulation of inhibin subunits in pituitary gonadotropes. Endocrinology 124:552–554 [DOI] [PubMed] [Google Scholar]
- 38. Buzzard JJ, Loveland KL, O'Bryan MK, O'Connor AE, Bakker M, Hayashi T, Wreford NG, Morrison JR, de Kretser DM. 2004. Changes in circulating and testicular levels of inhibin A and B and activin A during postnatal development in the rat. Endocrinology 145:3532–3541 [DOI] [PubMed] [Google Scholar]
- 39. Barakat B, O'Connor AE, Gold E, de Kretser DM, Loveland KL. 2008. Inhibin, activin, follistatin and FSH serum levels and testicular production are highly modulated during the first spermatogenic wave in mice. Reproduction 136:345–359 [DOI] [PubMed] [Google Scholar]
- 40. Mithraprabhu S, Mendis S, Meachem SJ, Tubino L, Matzuk MM, Brown CW, Loveland KL. 2010. Activin bioactivity affects germ cell differentiation in the postnatal mouse testis in vivo. Biol Reprod 82:980–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boitani C, Stefanini M, Fragale A, Morena AR. 1995. Activin stimulates Sertoli cell proliferation in a defined period of rat testis development. Endocrinology 136:5438–5444 [DOI] [PubMed] [Google Scholar]
- 42. Buzzard JJ, Farnworth PG, De Kretser DM, O'Connor AE, Wreford NG, Morrison JR. 2003. Proliferative phase Sertoli cells display a developmentally regulated response to activin in vitro. Endocrinology 144:474–483 [DOI] [PubMed] [Google Scholar]
- 43. Toebosch AM, Robertson DM, Trapman J, Klaassen P, de Paus RA, de Jong FH, Grootegoed JA. 1988. Effects of FSH and IGF1 on immature rat Sertoli cells: inhibin alpha and beta-subunit mRNA levels and inhibin secretion. Mol Cell Endocrinol 55:101–105 [DOI] [PubMed] [Google Scholar]
- 44. Kaipia A, Penttilä TL, Shimasaki S, Ling N, Parvinen M, Toppari J. 1992. Expression of inhibin βA and βB, follistatin and activin-A receptor messenger ribonucleic acids in the rat seminiferous epithelium. Endocrinology 131:2703–2710 [DOI] [PubMed] [Google Scholar]
- 45. de Winter JP, Vanderstichele HM, Timmerman MA, Blok LJ, Themmen AP, de Jong FH. 1993. Activin is produced by rat Sertoli cells in vitro and can act as an autocrine regulator of Sertoli cell function. Endocrinology 132:975–982 [DOI] [PubMed] [Google Scholar]
- 46. Vliegen MK, Schlatt S, Weinbauer GF, Bergmann M, Groome NP, Nieschlag E. 1993. Localization of inhibin/activin subunits in the testis of adult nonhuman primates and men. Cell Tissue Res 273:261–268 [DOI] [PubMed] [Google Scholar]
- 47. Wada M, Shintani Y, Kosaka M, Sano T, Hizawa K, Saito S. 1996. Immunohistochemical localization of activin A and follistatin in human tissues. Endocr J 43:375–385 [DOI] [PubMed] [Google Scholar]
- 48. Meinhardt A, McFarlane JR, Seitz J, de Kretser DM. 2000. Activin maintains the condensed type of mitochondria in germ cells. Mol Cell Endocrinol 168:111–117 [DOI] [PubMed] [Google Scholar]
- 49. Okuma Y, O'Connor AE, Hayashi T, Loveland KL, de Kretser DM, Hedger MP. 2006. Regulated production of activin A and inhibin B throughout the cycle of the seminiferous epithelium in the rat. J Endocrinol 190:331–340 [DOI] [PubMed] [Google Scholar]
- 50. Sharpe RM, Skakkebaek NE. 2008. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril 89:e33–e38 [DOI] [PubMed] [Google Scholar]
- 51. Fisher JS, Macpherson S, Marchetti N, Sharpe RM. 2003. Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod 18:1383–1394 [DOI] [PubMed] [Google Scholar]
- 52. Mylchreest E, Wallace DG, Cattley RC, Foster PM. 2000. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to di(n-butyl) phthalate during late gestation. Toxicol Sci 55:143–151 [DOI] [PubMed] [Google Scholar]
- 53. Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE., Jr 2000. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci 58:339–349 [DOI] [PubMed] [Google Scholar]
- 54. Scott HM, Hutchison GR, Mahood IK, Hallmark N, Welsh M, De Gendt K, Verhoeven G, O'Shaughnessy P, Sharpe RM. 2007. Role of androgens in fetal testis development and dysgenesis. Endocrinology 148:2027–2036 [DOI] [PubMed] [Google Scholar]
- 55. Sarraj MA, Escalona RM, Umbers A, Chua HK, Small C, Griswold M, Loveland K, Findlay JK, Stenvers KL. 2010. Fetal testis dysgenesis and compromised Leydig cell function in Tgfbr3 (betaglycan) knockout mice. Biol Reprod 82:153–162 [DOI] [PubMed] [Google Scholar]
- 56. Adamopoulos D, Lawrence DM, Vassilopoulos P, Kapolla N, Kontogeorgos L, McGarrigle HH. 1984. Hormone levels in the reproductive system of normospermic men and patients with oligospermia and varicocele. J Clin Endocrinol Metab 59:447–452 [DOI] [PubMed] [Google Scholar]
- 57. Lawrence DM, Swyer GI. 1974. Plasma testosterone and testosterone binding affinities in men with impotence, oligospermia, azoospermia, and hypogonadism. Br Med J 1:349–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Purvis K, Brenner PF, Landgren BM, Cekan Z, Dicfalusy E. 1975. Indices of gonadal function in the human male. I. Plasma levels of unconjugated steroids and gonadotrophins under normal and pathological conditions. Clin Endocrinol (Oxf) 4:237–246 [DOI] [PubMed] [Google Scholar]
- 59. Rosen SW, Weintraub BD. 1971. Monotropic increase of serum FSH correlated with low sperm count in young men with idiopathic oligospermia and aspermia. J Clin Endocrinol Metab 32:410–416 [DOI] [PubMed] [Google Scholar]
- 60. Sina D, Schuhmann R, Abraham R, Taubert HD, Dericks-Tan JS. 1975. Increased serum FSH levels correlated with low and high sperm counts in male infertile patients. Andrologia 7:31–37 [DOI] [PubMed] [Google Scholar]
- 61. Subhan F, Tahir F, Ahmad R, Khan ZD. 1995. Oligospermia and its relation with hormonal profile. J Pak Med Assoc 45:246–247 [PubMed] [Google Scholar]
- 62. Bergmann M, Behre H, Nieschlag E. 1994. Serum FSH and testicular morphology in male infertility. Clin Endocrinol 40:133–136 [DOI] [PubMed] [Google Scholar]
- 63. de Kretser DM, Burger HG, Hudson B. 1974. The relationship between germinal cells and serum FSH levels in males with infertility. J Clin Endocrinol Metab 38:787–793 [DOI] [PubMed] [Google Scholar]
- 64. Dakouane M, Bicchieray L, Bergere M, Albert M, Vialard F, Selva J. 2005. A histomorphometric and cytogenetic study of testis from men 29–102 years old. Fertil Steril 83:923–928 [DOI] [PubMed] [Google Scholar]