Abstract
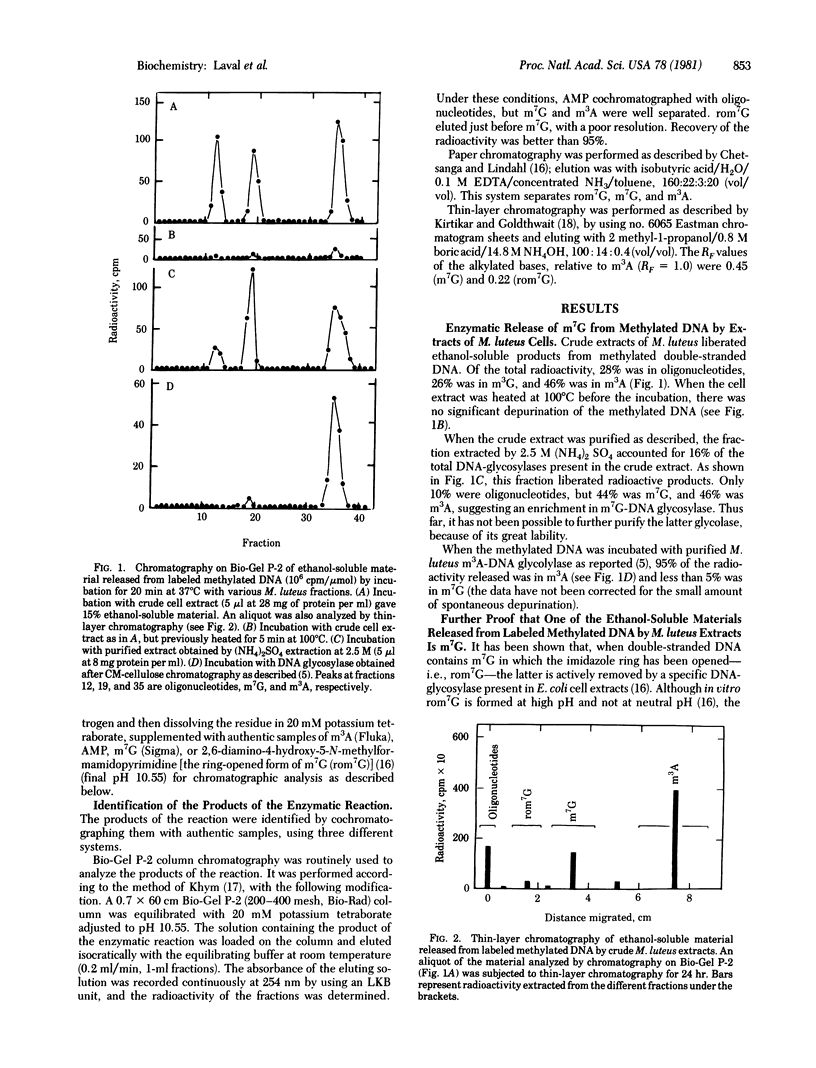
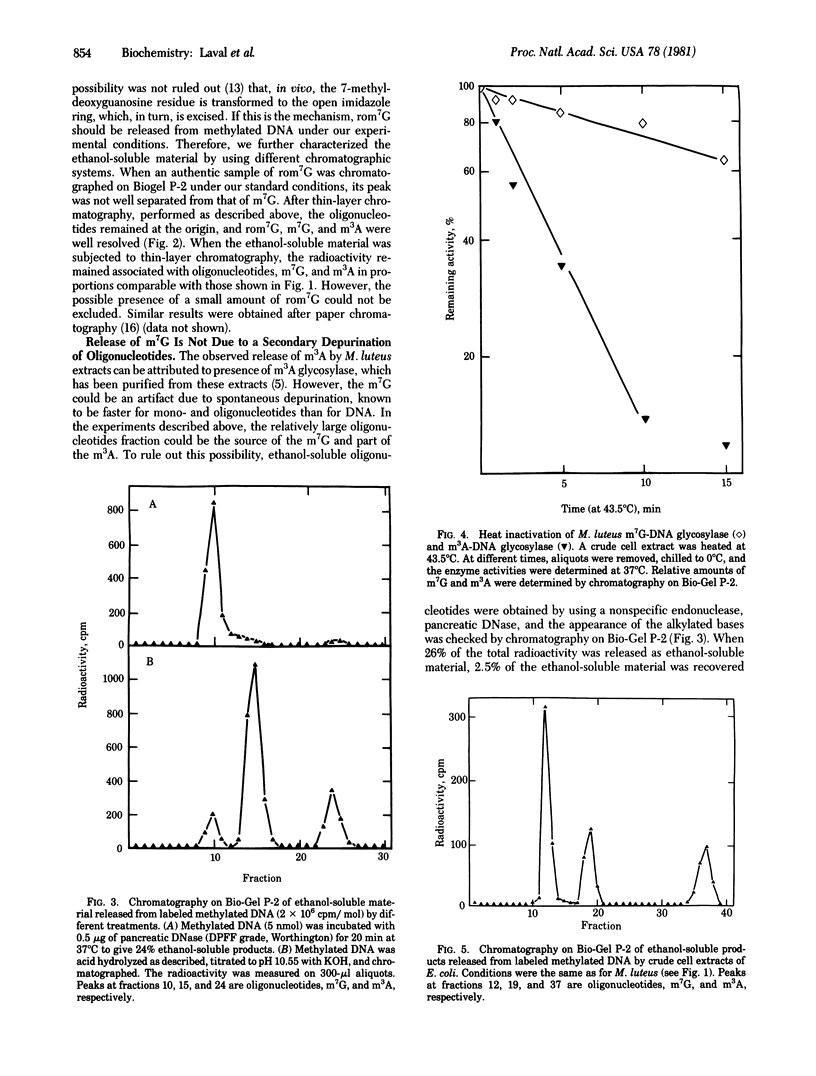
Cell extracts from Micrococcus luteus release both free 3-methyladenine and free 7-methylguanine from alkylated DNA. The glycosylase activity responsible for the liberation of 7-methylguanine is not 3-methyladenine-DNA glycosylase, which, when purified, does not liberate it. Furthermore, the heat inactivation rates of the two enzymatic activities are different. The release of 7-methylguanine by chemical depurination of ethanol-soluble oligonucleotides has been ruled out. A similar activity releasing 7-methylguanine is also found in Escherichia coli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodell W. J., Singer B., Thomas G. H., Cleaver J. E. Evidence for removal at different rates of O-ethyl pyrimidines and ethylphosphotriesters in two human fibroblast cell lines. Nucleic Acids Res. 1979 Jun 25;6(8):2819–2829. doi: 10.1093/nar/6.8.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent T. P. Partial purification and characterization of a human 3-methyladenine-DNA glycosylase. Biochemistry. 1979 Mar 6;18(5):911–916. doi: 10.1021/bi00572a028. [DOI] [PubMed] [Google Scholar]
- Chetsanga C. J., Lindahl T. Release of 7-methylguanine residues whose imidazole rings have been opened from damaged DNA by a DNA glycosylase from Escherichia coli. Nucleic Acids Res. 1979 Aug 10;6(11):3673–3684. doi: 10.1093/nar/6.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei J. V., Swenson D. H., Warren W., Lawley P. D. Alkylation of deoxyribonucleic acid in vivo in various organs of C57BL mice by the carcinogens N-methyl-N-nitrosourea, N-ethyl-N-nitrosourea and ethyl methanesulphonate in relation to induction of thymic lymphoma. Some applications of high-pressure liquid chromatography. Biochem J. 1978 Sep 15;174(3):1031–1044. doi: 10.1042/bj1741031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran P., Lindahl T. Enzymatic excision of free hypoxanthine from polydeoxynucleotides and DNA containing deoxyinosine monophosphate residues. J Biol Chem. 1978 Sep 10;253(17):5877–5879. [PubMed] [Google Scholar]
- Khym J. X. Group separation of ribonucleotides, ribonucleosides, and purine and pyrimidine bases on polyacrylamide gel columns. Anal Biochem. 1974 Apr;58(2):638–641. doi: 10.1016/0003-2697(74)90234-6. [DOI] [PubMed] [Google Scholar]
- Kirtikar D. M., Goldthwait D. A. The enzymatic release of O6-methylguanine and 3-methyladenine from DNA reacted with the carcinogen N-methyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1974 May;71(5):2022–2026. doi: 10.1073/pnas.71.5.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval J. Recent progress in excision repair of DNA. Biochimie. 1978;60(10):1123–1134. doi: 10.1016/s0300-9084(79)80346-6. [DOI] [PubMed] [Google Scholar]
- Laval J. Two enzymes are required from strand incision in repair of alkylated DNA. Nature. 1977 Oct 27;269(5631):829–832. doi: 10.1038/269829a0. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J. Specific excision of methylation products from DNA of Escherichia coli treated with N-methyl-N'-nitro-N-nitrosoguanidine. Chem Biol Interact. 1970 Aug;2(2):154–157. doi: 10.1016/0009-2797(70)90047-5. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Margison J. M., Montesano R. Methylated purines in the deoxyribonucleic acid of various Syrian-golden-hamster tissues after administration of a hepatocarcinogenic dose of dimethylnitrosamine. Biochem J. 1976 Sep 1;157(3):627–634. doi: 10.1042/bj1570627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margison G. P., Pegg A. E. Enzymatic release of 7-methylguanine from methylated DNA by rodent liver extracts. Proc Natl Acad Sci U S A. 1981 Feb;78(2):861–865. doi: 10.1073/pnas.78.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Anraku Y., Lehman I. R. Deoxyribonucleic acid ligase. Isolation and physical characterization of the homogeneous enzyme from Escherichia coli. J Biol Chem. 1973 Nov 10;248(21):7495–7501. [PubMed] [Google Scholar]
- Nicoll J. W., Swann P. F., Pegg A. E. Effect of dimethylnitrosamine on persistence of methylated guanines in rat liver and kidney DNA. Nature. 1975 Mar 20;254(5497):261–262. doi: 10.1038/254261a0. [DOI] [PubMed] [Google Scholar]
- Pierre J., Laval J. Micrococcus luteus endonucleases for apurinic/apyrimidinic sites in deoxyribonucleic acid. 1. Purification and general properties. Biochemistry. 1980 Oct 28;19(22):5018–5024. doi: 10.1021/bi00563a013. [DOI] [PubMed] [Google Scholar]
- Pierre J., Laval J. Micrococcus luteus endonucleases for apurinic/apyrimidinic sites in deoxyribonucleic acid. 2. Further studies on the substrate specificity and mechanism of action. Biochemistry. 1980 Oct 28;19(22):5024–5029. doi: 10.1021/bi00563a014. [DOI] [PubMed] [Google Scholar]
- Riazuddin S., Lindahl T. Properties of 3-methyladenine-DNA glycosylase from Escherichia coli. Biochemistry. 1978 May 30;17(11):2110–2118. doi: 10.1021/bi00604a014. [DOI] [PubMed] [Google Scholar]
- STRAUSS B. S. Differential destruction of the transforming activity of damaged deoxyribonucleic acid by a bacterial enzyme. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1670–1675. doi: 10.1073/pnas.48.9.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E. Reconstitution of an Escherichia coli repair endonuclease activity from the separated uvrA+ and uvrB+/uvrC+ gene products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2569–2573. doi: 10.1073/pnas.75.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. All oxygens in nucleic acids react with carcinogenic ethylating agents. Nature. 1976 Nov 25;264(5584):333–339. doi: 10.1038/264333a0. [DOI] [PubMed] [Google Scholar]
- Singer B., Brent T. P. Human lymphoblasts contain DNA glycosylase activity excising N-3 and N-7 methyl and ethyl purines but not O6-alkylguanines or 1-alkyladenines. Proc Natl Acad Sci U S A. 1981 Feb;78(2):856–860. doi: 10.1073/pnas.78.2.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. The chemical effects of nucleic acid alkylation and their relation to mutagenesis and carcinogenesis. Prog Nucleic Acid Res Mol Biol. 1975;15(0):219–284. [PubMed] [Google Scholar]
- Smith G. J., Kaufman D. G., Grisham J. W. Decreased excision of O6-methylguanine and N7-methylguanine during the S phase in 10T1/2 cells. Biochem Biophys Res Commun. 1980 Feb 12;92(3):787–794. doi: 10.1016/0006-291x(80)90772-x. [DOI] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]


