Abstract
Besides their slow genomic actions, estrogens also induce rapid physiological responses. To be functionally relevant, these effects must be associated with rapid changes in local concentrations of estrogens. Rapid changes in aromatase activity (AA) controlled by calcium-dependent phosphorylations of the enzyme can alter in a rapid manner local estrogen concentrations, but so far this mechanism was identified only in the avian (quail) brain. We show here that AA is also rapidly down-regulated by phosphorylating conditions in quail ovary homogenates and in various cell lines transfected with human aromatase (HEK 293, Neuro2A, and C6). Enzymatic activity was also rapidly inhibited after depolarization of aromatase-expressing HEK 293 cells with 100 mm KCl, and activity was fully restored when cells returned to control conditions. Western blot analysis demonstrated that the reduction of enzymatic activity is not due to protein degradation. We next investigated by site-directed mutagenesis the potential implication in the control of AA of specific aromatase residues identified by bioinformatic analysis. Mutation of the amino acids S118, S247, S267, T462, T493, or S497 to alanine, alone or in combination, did not block the rapid inhibition of enzymatic activity induced by phosphorylating conditions, but basal AA was markedly decreased in the S118A mutant. Altogether, these results demonstrate that the rapid inhibition of AA is a widespread and fully reversible process and that phosphorylation of specific residues modulate AA. These processes provide a new general mechanism by which local estrogen concentration can be rapidly altered in the brain and other tissues.
Estrogens have been the focus of intense research for decades, yet numerous questions remain concerning the dynamic of estrogens synthesis and action. The transcriptional activity of estrogens plays major roles in the regulation of physiology and behavior (1–4) but also multiple pathologies (2, 5, 6). It has recently become clear that estrogens also exert nongenomic actions affecting a variety of cellular signaling pathways (3, 8, 9) that have profound consequences for numerous physiological and behavioral processes (10, 11). However, the critical question of the mechanisms able to acutely control estrogen synthesis has received little attention.
17β-Estradiol (E2) is synthesized in various tissues, including the ovaries and brain, by the conversion of testosterone catalyzed by the enzyme aromatase (12–14). Changes in aromatase activity (AA) often reflect changes in concentration of the enzymatic protein that are mediated in a relatively slow fashion (hours to days) by changes in the production of the enzyme at the transcriptional level (15–17). In addition to this slow genomic regulation, studies of quail aromatase performed in our laboratory indicated that AA can also be modulated within minutes by mechanisms that cannot possibly involve changes in the concentration of the enzymatic protein. Indeed, AA in the preoptic area/hypothalamic (POA/HYP) region is rapidly and significantly reduced in male Japanese quail after 5 min of interaction with a female (18). Additionally, we showed that a potassium-induced depolarization or the activation of glutamatergic receptors rapidly (within 5 min) and reversibly down-regulate AA in quail POA/HYP blocks maintained in vitro (19, 20). Similarly, retrodialysis of glutamate in the aromatase-rich caudomedial nidopallium of the zebra finch (Taeniopygia guttata) telencephalon significantly reduces within minutes the local concentration of E2 (21), supporting the idea that the glutamatergic inhibition of AA observed in quail POA/HYP explants also takes place in vivo.
Pharmacological experiments indicate that these rapid changes in quail AA might be mediated by phosphorylation processes directly affecting the enzyme. Indeed, preincubation for 15 min of POA/HYP homogenates in phosphorylating conditions (increased but physiological concentrations of ATP, Mg2+, and Ca2+) significantly inhibits the enzymatic activity (22). This inhibitory effect of ATP/Mg2+/Ca2+ is largely abolished by coincubation with inhibitors of several protein kinases, including protein kinase C (PKC) and protein kinase A (PKA) (23) or with acid phosphatase (24), further indicating that AA inhibition is mediated by phosphorylation processes. Moreover, an increased density of phosphorylated serine, threonine, and tyrosine residues was observed in immunoprecipitated aromatase when aromatase was subjected to phosphorylating conditions, supporting the idea that aromatase itself is phosphorylated (23).
With the exception of the in vivo dialysis study on zebra finches (21), this suite of experiments was, however, carried out exclusively on quail brain tissue so that the relevance of the above-mentioned conclusions for other tissue or species could not be evaluated. The present studies were initiated with three objectives in mind: 1) define whether the rapid inhibition of AA by phosphorylation processes is specific to the neuronal environment or whether it is observed in other aromatase-rich tissues such as the ovary and its follicles; 2) test whether the fast reduction of enzymatic activity is unique to quail aromatase or whether human aromatase, stably expressed in the human embryonic kidney (HEK) 293, mouse Neuro2A, or rat C6 cell lines is also modulated via phosphorylations, and lastly; 3) investigate by site-directed mutagenesis the potential implication of specific aromatase amino acids in the control of rapid changes in AA.
Materials and Methods
Animals and tissue homogenization
A first set of experiments were carried out on adult (>8 wk old), sexually mature, egg-laying female Japanese quail (Coturnix japonica) obtained from our breeding colony. Aromatase activity was measured in the fourth and fifth follicles in the egg-laying sequence (F4 and F5) and the rest of the ovary (see Supplemental Materials and Methods, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). These follicles were selected for separate analyses because earlier studies had demonstrated that they represent the stage of follicle maturation in which aromatase is the most active (25). Animal protocols were approved by the Ethics Committee for the Use of Animals at the University of Liège (Liège, Belgium).
Phosphorylation sites
Consensus phosphorylation and kinase-binding sites in the human aromatase sequence were analyzed using public domain softwares (NETPHOS 2.0 PREDICTION server at http://www.cbs.dtu.dk/services/NetPhos/ and http://www.cbs.dtu.dk/services/NetPhosK/).
Plasmid and aromatase mutants
We used a plasmid containing the human aromatase under the control of a cytomegalovirus promoter (pNH-CMV/AROM-BSD or pRc/CMV/AROMc-myc, expressing a c-myc tag in C terminal of the human aromatase) to express wild-type human aromatase in different eukaryotes cell lines (see below). Aromatase mutant expression plasmids were constructed by a primer-directed plasmid amplification using pNH-CMV/AROM-BSD as a template using primers described in Supplemental Table 1 (See Supplemental Materials and Methods).
Cell culture and transfection
HEK293, Neuro2A (mouse neuroblastoma), and C6 (rat glioma) cells were grown in DMEM medium (4.5 g/liter glucose + pyruvate + glutamine; Invitrogen, Merelbeke, Belgium) supplemented with 10% fetal bovine serum (Invitrogen) at 37 C in an atmosphere of 5% CO2 and 95% air. Cells were grown to 50% confluence on six-well culture plates 24 h before transfection using TransIT-LT1 transfection reagent following the protocol recommended by the provider (Mirus, Madison, WI; see Supplemental Materials and Methods). Transfected cells went through at least 10 passages before being assayed for AA. Cells were rapidly rinsed with DMEM, resuspended in KTH (150 mm KCl, 10 mm Tris-base, HEPES, ph 7.2) buffer and stored at −80 C until used. Before aromatase assay, samples went through two additional freeze/thaw cycles to lyse the cells.
Aromatase activity assay
AA was quantified by measuring the tritiated water production from its specific substrate [1β-3H] androstenedione (26, 27) with methods previously validated in our laboratory for avian and mammalian species (28, 29). Aliquots (50 μl) of tissue homogenate or cell lysate were added to 50 μl of KTH (control) or 50 μl of KTH containing ATP (0–8 mm), Mg2+ (10 mm), and Ca2+ (0–2 mm), conditions known to induce phosphorylations (PO4). Samples were preincubated for 15 min at 37 C. They were then transferred to an ice/water bath. Twenty microliters of 10 mm EGTA in KTH were quickly added to each sample to chelate divalent ions. Forty microliters of 125 nm [1β-3H] androstenedione in KTH [final concentration 25 nm, shown previously to be a saturating concentration for measuring AA (30)] were dispensed to all tubes. Forty microliters of reduced nicotinamide adenine dinucleotide phosphate (NADPH) in KTH were then added to reach a final concentration of 1.2 mm. All tubes were quickly capped and incubated in a shaking bath for 10 min at 37 C.
To assess AA in intact transfected cells and to define the effect of their depolarization, 24-well plates were coated with PolyOrnithine (1 mg/ml; Sigma Aldrich, Bornem, Belgium) and cells grown to reach 80–90% confluence in DMEM and 10% fetal bovine serum. One hour before the enzymatic assay, cells were rinsed twice with PBS and incubated for 1 h in DMEM serum. Medium was then discarded and cells were preincubated in DMEM (control) or DMEM containing KCl 100 mm (KCl) to induce cell depolarization. Kinase inhibitors (stauroporine 1 μm or genistein 50 μm) were added to some of the wells to test whether the effect of depolarization on AA requires phosphorylation events. Preincubation medium was then rapidly discarded and replaced by DMEM containing 25 nm [1β-3H] androstenedione to quantify AA during a 10-min incubation at 37 C. The incubation volume was in all cases 250 μl.
In all conditions (ovarian or cell homogenates and cell cultures), the enzymatic reaction was stopped by cooling the samples on ice and adding 400 μl of ice-cold 10% trichloroacetic acid containing 2% activated charcoal and, for cell cultures only, 50 μl of 10 mg/ml BSA. The tritiated water was then purified and quantified as previously described (30) and results expressed in picomoles of tritiated water produced per hour after correction of the counts for quenching, recovery (95%), blank values [obtained by addition of the aromatase inhibitor R83842 in excess (active enantiomer of R76713) (31)] and percentage (91%) of tritium in β-position in the substrate (30).
Western blots and ATP incorporation
We performed Western blot analysis on total proteins or proteins purified on c-myc agarose (see Supplemental Materials and Methods). Aromatase was visualized using rabbit antihuman aromatase antibody [1:1000 (32, 33)], phosphoserines were identified with rabbit antiserine antibody (1:1000; StressMark, Victoria, BC, Canada) and aromatase expression was normalized with β-actin (1:10,000, Clone AC-74; Sigma). The OD of the bands was measured by image analysis (ImageJ, Wayne Rasband; National Institutes of Health, Bethesda, MD) on images obtained from an ImageQuant LAS4000 analyzer (GE Healthcare Life Sciences, Indianapolis, IN). In parallel, cell lysate from HEK293 cells containing the pRC/CMV-AROM/c-myc or pRC/CMV alone was incubated in control condition (KTH) or in phosphorylating conditions for 15 min at 37 C in the presence of [γ-32P]-ATP (3000 Ci/mmol, 10 mCi/ml; PerkinElmer, Courteboeuf, France; see Supplemental Materials and Methods).
Statistics
All aromatase assays were performed in triplicate (assay variability) on a variable number of independent replicates (biological variability). Means of triplicates were always calculated first and considered as the raw data in all statistical analyses. The variance indicated in the paper refers only to differences between independent replicates (indicated in the figure legends). Data were analyzed by Student t tests or one- and two-way ANOVA including repeated designs when different treatments had been tested on the same samples (SPSS version 17.0; SPSS, Chicago, IL). When appropriate, ANOVA were followed by post hoc tests (Tukey's highest significant difference test adapted for repeated measures or Fisher's least significant difference test). Data from the gonads were log transformed to normalize the distribution. Differences were considered significant for P < 0.05. All data are presented by their means and sem.
Results
Gonadal aromatase
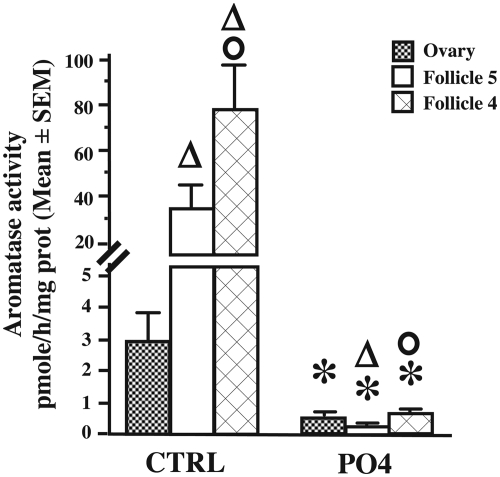
Detectable levels of AA were observed in the ovary and in follicles F5 and F4, but the enzymatic activity was higher in the isolated follicles. A mixed-design, two-way ANOVA identified a significant difference between tissues (F2,16 = 14.570, P < 0.001; Fig. 1). A 15-min preincubation in phosphorylating conditions (8 mm ATP, 2 mm Ca2+, and 10 mm Mg2+) resulted in a drastic and significant reduction of AA in all tissues (F1,16 = 221.250, P < 0.0001). There was a significant interaction between tissues and phosphorylating conditions (F2,18 = 24.235, P < 0.0001). Student t tests adapted for post hoc comparisons indicated that AA in F4 and F5 was higher than in the ovary in control but not in phosphorylating conditions. AA was also higher in F4 than in F5 in both conditions (see Fig. 1 for details).
Fig. 1.
Aromatase activity expressed in picomoles per hour per milligram protein in the ovary and follicles F4 and F5 of Japanese quail. Homogenates of these tissues were preincubated for 15 min in control (CTRL) or phosphorylating (PO4) conditions before enzymatic assay. Data (seven independent replicates) were analyzed by a mixed-design, two-way ANOVA followed by post hoc tests whose results are presented at the top of bars as follows: *, P < 0.05 vs. control conditions in the same tissue; Δ, P < 0.05 vs. ovary, same conditions; o, P < 0.05 vs. F5 same conditions. Values are presented as mean ± sem.
Human aromatase expression
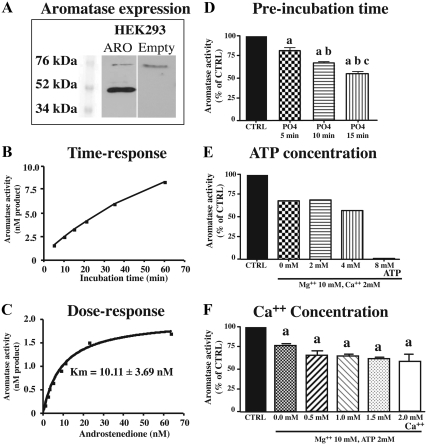
Human aromatase was stably expressed in the HEK293 cell line. Western blot analysis of proteins with an antibody targeting human aromatase showed a specific band at the expected size (52 kDa, Fig. 2A) plus another nonspecific band at 76 kDa that was also present in nontransfected HEK293 cells. Enzyme activity assays confirmed the presence of active aromatase in transfected HEK293 cells. Accumulation of the enzymatic product increased in a linear fashion over time for at least 30 min (Fig. 2B) and the apparent affinity of the transfected enzyme [Michaelis-Menten constant (Km)] estimated by saturation analysis was similar (10.11 ± 3.69 nm) to the Km of native human aromatase [Fig. 2C (34, 35)]. In the presence of a saturating concentration of substrate (25 nm), AA was 240 ± 22.27 pmol · h−1 · mg protein−1.
Fig. 2.
Characterization of human aromatase expressed in HEK293 cells (A–C) and characterization of the effects of phosphorylating conditions on the inhibition of human aromatase activity expressed by transfected HEK293 cells (D–F). In all cases, aromatase activity was measured from cell lysate. A, Western blots of transfected HEK293 proteins with an antibody targeting human aromatase, showing a band at the expected size (52 kDa). Another band at is also apparent at 76 kDa but is also present in untransfected HEK293 cells. B, Accumulation of product derived from androstenedione aromatization over time (from 2 to 60 min). C, Saturation curves obtained by incubating transfected HEK293 cells for 10 min with increasing concentrations of substrate (in nanomolar concentration), providing estimates of the apparent Km of aromatase for its substrate (four independent replicates). D, Effect of preincubation duration in the presence of 2 mm ATP, 10 mm Mg2+, and 2 mm Ca2+ (PO4) compared with control conditions. Aromatase activity is expressed as percentage of controls (CTRL). a, P < 0.001 vs. CTRL; b, P < 0.001 vs. 5 min; c, P < 0.01 vs. 10 min (6 independent replicates). E, Effect of ATP concentration (from 0 to 8 mm) during a 15-min preincubation in a medium also containing 10 mm Mg2+ and 2 mm Ca2+. CTRL were preincubated in the absence of Mg2+, Ca2+ and ATP. Assays were not repeated and no statistical analysis is present. F, Effect of Ca2+ concentration (from 0 to 2 mm) in the preincubation medium also containing 10 mm Mg2+ and 2 mm ATP. CTRL were preincubated in the absence of Mg2+, Ca2+, and ATP. Preincubation was performed during 15 min. a, P < 0.05 vs. CTRL (six independent replicates). Values are presented as mean ± sem.
Phosphorylating conditions inhibit human AA
A 15-min preincubation in conditions stimulating phosphorylations [2 mm ATP, 10 mm Mg2+, 2 mm Ca2+ (19)] significantly reduced activity of human aromatase from HEK293 lysates compared with matched control samples (56.30 ± 1.69% of activity found in control, paired t test: DF = 10, t = 8.253, P < 0.001). Similar reductions were observed after exposure to phosphorylating conditions for AA expressed in Neuro2A (67.24 ± 2.70% of control, DF = 5, t = 12.08, P < 0.001) or C6 (51.5% of control, one single assay) cells.
Characterization of phosphorylating conditions for human aromatase
To define the optimal phosphorylating conditions for human aromatase expressed in HEK293 cells, we examined the effects of the preincubation time, ATP concentration, and calcium concentration on enzyme activity. Preincubation for various durations (5, 10, or 15 min) of HEK293 lysate with 2 mm ATP, 2 mm Ca2+, and 10 mm Mg2+ had a significant effect on AA compared with activity in samples preincubated for 15 min in control conditions (F3, 20 = 79.43, P < 0.001; Fig. 2D). The decrease in activity was significantly stronger after longer preincubation times (see results of post hoc tests in Fig. 2D). The concentration of ATP, in the presence of 10 mm Mg2+ and 2 mm Ca2+, also played an important role in AA reduction (Fig. 2E). Fifteen-minute preincubation with Mg2+ and Ca2+ without ATP caused a reduction of enzymatic activity, but a high concentration of ATP (8 mm) led to an almost complete suppression of activity. Lower ATP concentrations (2 or 4 mm) had no major effect compared with 0 mm ATP. In addition, the presence of 10 mm Mg2+ and 2 mm ATP during the 15-min preincubation significantly decreased AA (F5,6 = 12.63, P = 0.004), but the addition of increasing concentrations of calcium up to 2 mm had no additional effect (Fig. 2F).
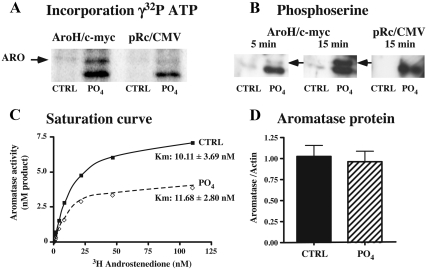
To define whether phosphorylating conditions targeted the aromatase protein itself, we engineered a modified human aromatase containing a c-myc tag to allow its immunoprecipitation. Cells transfected with this construct were then incubated with [γ-32P]-ATP in phosphorylating or nonphosphorylating (control) conditions. As depicted in Fig. 3A, a 32P-labeled protein was detected at the expected molecular mass for aromatase c-myc in phosphorylating conditions, whereas only a faint band was present in control conditions. Cells transfected with an empty vector did not show that band. In addition, the immunoprecipitated protein visualized with antiphosphoserine similarly showed an immunoreactive band at the expected molecular weight after 5 min of incubation of the cell lysate in phosphorylating conditions (8 mm ATP, 2 mm Ca2+, and 10 mm Mg2+). The intensity of this band was stronger after 15 min incubation in phosphorylating conditions (Fig. 3B). That band was present neither in control conditions nor in the cells transfected with the empty vector in control or phosphorylating conditions.
Fig. 3.
Effect of phosphorylating conditions on aromatase phosphorylation (A and B), aromatase kinetics (C), and aromatase protein concentration (D) in homogenates of HEK293 transfected with human aromatase. A, Incorporation of [γ32P]-ATP by aromatase. HEK-293 cells transfected with an empty pRc/CMV control vector (lanes 3 and 4) or a vector encoding c-myc-tagged human aromatase (lanes 1 and 2) were incubated in control conditions (CTRL, lanes 1–3) or in phosphorylating conditions (10 mm Mg2+, 2 mm Ca2+ PO4, lanes 2–4) for 15 min. B, Western blot using an antiphosphoserine antibody. HEK-293 cells transfected with a vector encoding c-myc-tagged human aromatase (lanes 1–4) were incubated in control conditions (CTRL, lanes 1 and 3) or in phosphorylating conditions (PO4, lanes 2 and 4) for 5 or 15 min. Empty pRc/CMV plasmid was used as a negative control (lanes 5 and 6, 15 min incubation). It can be noted that a band at a lower molecular mass (∼50 kDa) was observed in phosphorylating conditions from cells transfected with the empty vector or with the vector containing aromatase, in both [γ32P]-ATP incorporation and Western blot using phosphoserine antibody. C, Saturation curve analysis showing that the maximum velocity but not the apparent Km of aromatase is affected by preincubation for 10 min in phosphorylating conditions [three independent replicates, each incubated in CTRL or PO4 conditions (PO4: 10 mm Mg2+, 2 mm Ca2+, and 2 mm ATP), with increasing concentration of substrate]. D, Bar graph showing that the preincubation in phosphorylating conditions for 15 min did not affect aromatase protein concentration determined by Western blot compared with control conditions (CTRL). The protein concentration is expressed as the ratio of the mean OD of the aromatase to the mean OD of actin (values are presented as mean ± sem, seven independent replicates).
We also tested whether the reduction of enzymatic activity under phosphorylating conditions was due to a change in the affinity of the enzyme for its substrate. The preincubation of HEK293 lysate with 2 mm ATP, 2 mm Ca2+, and 10 mm Mg2+ for 15 min in the presence of increasing concentrations of substrate did not change the apparent Km compared with control conditions (Fig. 3C; paired t test: df = 3, t = 1.171, P = 0.33) The same conclusion was apparently true for aromatase expressed in Neuro2A cells (control: 14.10 ± 2.84 nm, n = 2; PO4: 10.99 ± 1.89 nm, n = 2; not shown). In addition, Western blot analysis of aromatase expression in HEK293 cells exposed or not to these phosphorylating conditions indicated that the fast reduction of AA is not associated with a change in concentration of the enzymatic protein (paired t test: df = 6, t = 0.961, P = 0.37; Fig. 3D).
Recovery of aromatase activity after depolarization
We previously showed that a KCl-induced depolarization triggers, presumably through the phosphorylation of aromatase mediated by the resulting Ca2+ influx, a pronounced but transient and reversible inhibition of AA in quail POA/HYP explants (19). We investigated here whether similar K+-dependent changes would also affect human aromatase expressed in intact (nonhomogenized) HEK293 cells.
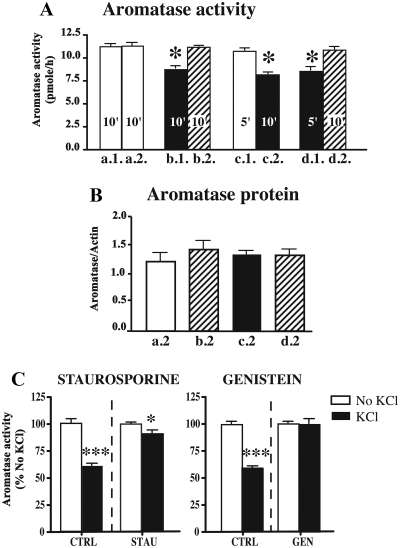
In a first step, cells were preincubated on polyornithine-coated plates in DMEM or DMEM + 100 mm KCl for 10 min (Fig. 4A, respectively, a.1 and b.1 in) or for 5 min (Fig. 4A, c.1 and d.1) before incubation with the aromatase substrate for 10 min. The supernatants were then collected for analysis, cells were washed twice with PBS, and, in a second step, cells were preincubated again for 10 min with DMEM (Fig. 4A, a.2, b.2, d.2) or DMEM + KCl (Fig. 4A, c.2) before being assayed once more for AA. Two independent one-way ANOVA comparing separately AA after the first and the second preincubation showed a significant effect of the treatment (respectively F3,16 = 16.13 and F3,16 = 28.96, P < 0.0001). AA measured after the first preincubation was significantly reduced after 5 or 10 min preincubation with KCl (Fig. 4A, respectively, b.1 and d.1) compared with DMEM alone (Fig. 4A, a.1 and c.1). There was no difference between 5 or 10 min preincubation in control DMEM alone (Fig. 4A, respectively, a.1 and c.1). After the second preincubation, AA was similarly decreased after exposure to KCl 100 mm (Fig. 4A, c.2) compared with control conditions (Fig. 4A, a.2, b.2, d.2), and there was no significant AA difference between samples preincubated in DMEM alone (Fig. 4A a.2, b.2, and d.2).
Fig. 4.
Bar graphs representing the effects of a KCl-induced depolarization on the human aromatase activity expressed by HEK293 intact cells (A), aromatase protein concentration as determined by Western blot on these cells (B), and blocking of KCl-induced reduction of aromatase activity by kinase inhibitors (C). A, Cells were submitted to two successive preincubation treatments (1 and 2) and aromatase was assayed at the end of each preincubation. The duration of preincubations varied from experiment to experiment and is indicated in the corresponding bars. Four groups of experiments were performed in which cells were incubated in sequence in DMEM followed by DMEM (a1–a2), DMEM + 100 mm KCl followed by DMEM (b1–b2 and d1–d2), or DMEM followed by DMEM + 100 mm KCl (c1–c2). *, P < 0.05 vs. matched control conditions. B, Bar graph representing the aromatase protein concentration expressed as the ratio of the mean OD of the aromatase to the mean OD of actin. Proteins were collected after the last assay in each of the four experiments described in A (a2, b2, c2, and d2). The shading of the bars corresponds to the shading in part A of the figure (DMEM, white; DMEM + KCl, black; DMEM after DMEM + KCl, hatched; values are presented as mean ± sem, five independent replicates). C, Bar graph representing the inhibition of aromatase activity by KCl, whereas the addition of 1 μm staurosporine or 50 μm genistein blocked the effect of KCl-induced depolarizations on AA. *, P < 0.05 vs. no KCl condition, same treatment; ***, P < 0.001 vs. no KCl, same treatment.
Paired t test comparing AA from the same cells after the first and second preincubations confirmed that a 5- or 10-min preincubation with KCl significantly reduced AA compared with the control counterpart (b: t = 6.761, df = 4, P = 0.0025; c: t = 4.495, df = 4, P = 0.0109; d: t = 7.944, df = 4, P = 0.0014, see Fig. 4B). These differences remain significant (P ≤ 0.05), even after a Bonferroni correction to adjust the alpha critical level for multiple t tests. In contrast, maintaining the cells in preincubating control conditions had no effect on AA (a: df = 4, t = 0.245, P = 0.812).
At the end of the second experimental step, cells were collected and expression of the aromatase protein was quantified by Western blot analysis, with actin used as an internal standard (Fig. 4B). A one-way ANOVA comparing these aromatase to actin ratios showed that aromatase protein concentration was present and similar in all conditions, therefore ruling out the degradation of the protein as a potential mechanism for the reduction of AA (F3,16 = 0.4285 P = 0.73).
Moreover, the addition of 1 μm staurosporine (serine/threonine kinase inhibitor) or 50 μm genistein (tyrosine kinase inhibitor) blocked the effect of KCl-induced depolarizations on AA (Fig. 4C). Although KCl reduced the activity down to 60% of control activity, treatment with kinase inhibitors maintained the activity to 89 (staurosporine) or 95% (genistein) of control values (see Fig. 4C). Altogether these data show that a KCl-induced depolarization rapidly reduces AA via phosphorylation mechanisms and that this activity is fully restored when cells are replaced in control conditions.
Aromatase site-directed mutagenesis
The NETPHOS 2.0 program identified 19 putative phosphorylation sites with a consensus score higher than the critical value of 0.5; they concerned 10 of the 27 serine, five of the 24 threonine, and four of the 17 tyrosine residues (Table 1). The parallel search for consensus sequences corresponding to protein kinases that were shown to affect quail AA during the pharmacological experiments [i.e. PKC and PKA (23)], on the other hand, pointed to six different residues, five serines (S118, S167, S247, S267, S497) and one threonine (T143). Additional kinases that were not investigated during pharmacological experiments in quail also displayed consensus sequences in the human aromatase sequence, and a few of them had a consensus score higher than 0.5 (data not shown).
Table 1.
Analysis by the NETPHOS 2.0 program of all serine, threonine, and tyrosine residues of the human aromatase-deduced amino acid sequence for the presence of phosphorylation consensus sites
| Pos | Context | Score | Pred |
|---|---|---|---|
| Serine predictions (n = 10) | |||
| 15 | YNITSIVPE | 0.005 | . |
| 45 | YEGTSSIPG | 0.636 | S |
| 46 | EGTSSIPGP | 0.065 | . |
| 61 | GPLISHGRF | 0.191 | . |
| 72 | MGIGSACNY | 0.004 | . |
| 90 | RVWISGEET | 0.992 | S |
| 98 | TLIISKSSS | 0.096 | . |
| 100 | IISKSSSMF | 0.072 | . |
| 101 | ISKSSSMFH | 0.843 | S |
| 102 | SKSSSMFHI | 0.765 | S |
| 113 | HNHYSSRFG | 0.260 | . |
| 114 | NHYSSRFGS | 0.435 | . |
| 118a | SRFGSKLGL | 0.291 | . PKA (0.72) |
| 153 | MKALSGPGL | 0.633 | S |
| 167 | VCAESLKTH | 0.976 | S PKC (0.64) |
| 182 | VTNESGYVD | 0.470 | . |
| 199 | MLDTSNTLF | 0.006 | . |
| 211 | PLDESAIVV | 0.067 | . |
| 238 | FFKISWLYK | 0.022 | . |
| 247a | KYEKSVKDL | 0.997 | S PKC (0.79) |
| 267a | RRRISTEEK | 0.998 | S PKA (0.87) |
| 312 | PDTMSVSLF | 0.059 | . |
| 314 | TMSVSLFFM | 0.014 | . |
| 363 | FIYESMRYQ | 0.194 | . |
| 470 | QCVESIQKI | 0.036 | . |
| 478 | IHDLSLHPD | 0.824 | S |
| 497a | TPRNSDRCL | 0.995 | S PKA (0.61) |
| Threonine predictions (n = 5) | |||
| 14 | HYNITSIVP | 0.045 | . |
| 25 | MPAATMPVL | 0.105 | . |
| 32 | VLLLTGLFL | 0.005 | . |
| 44 | NYEGTSSIP | 0.442 | . |
| 94 | SGEETLIIS | 0.205 | . |
| 143 | ELWKTTRPF | 0.859 | T PKC (0.51) |
| 144 | LWKTTRPFF | 0.032 | . |
| 162 | VRMVTVCAE | 0.800 | T |
| 170 | ESLKTHLDR | 0.093 | . |
| 179 | LEEVTNESG | 0.032 | . |
| 189 | VDVLTLLRR | 0.053 | . |
| 198 | VMLDTSNTL | 0.122 | . |
| 201 | DTSNTLFLR | 0.225 | . |
| 268 | RRISTEEKL | 0.941 | T |
| 280 | MDFATELIL | 0.011 | . |
| 292 | RGDLTRENV | 0.451 | . |
| 310 | AAPDTMSVS | 0.309 | . |
| 338 | KEIQTVIGE | 0.632 | T |
| 392 | VKKGTNIIL | 0.012 | . |
| 414 | PNEFTLENF | 0.206 | . |
| 453 | AILVTLLRR | 0.008 | . |
| 462a | FHVKTLQGQ | 0.025 | . |
| 484 | HPDETKNML | 0.433 | . |
| 493a | EMIFTPRNS | 0.973 | T p38MAPK (0.51) |
| Tyrosine predictions (n = 4) | |||
| 11 | NPIHYNITS | 0.077 | . |
| 41 | LVWNYEGTS | 0.014 | . |
| 52 | PGPGYCMGI | 0.278 | . |
| 76 | SACNYYNRV | 0.106 | . |
| 77 | ACNYYNRVY | 0.515 | Y |
| 81 | YNRVYGEFM | 0.075 | . |
| 112 | KHNHYSSRF | 0.069 | . |
| 184 | NESGYVDVL | 0.992 | Y |
| 220 | KIQGYFDAW | 0.092 | . |
| 241 | ISWLYKKYE | 0.046 | . |
| 244 | LYKKYEKSV | 0.030 | . |
| 361 | ENFIYESMR | 0.976 | Y |
| 366 | ESMRYQPVV | 0.077 | . |
| 386 | VIDGYPVKK | 0.118 | . |
| 424 | KNVPYRYFQ | 0.158 | . |
| 426 | VPYRYFQPF | 0.013 | . |
| 441 | CAGKYIAMV | 0.770 | Y |
For each residue, the table lists the position in the sequence (Pos), the context in which the potential phosphoacceptor amino acid is found (Context), the consensus phosphorylation score (from 0.0 to 1.0; Score), and a mark for all predicted phosphorylation sites with a score higher than 0.5 (Pred). The last column indicates the specific kinases that match the consensus sequences and their score (in parentheses). S, Serine; T, threonine; Y, tyrosine.
The six sequences that were modified through site-directed mutagenesis.
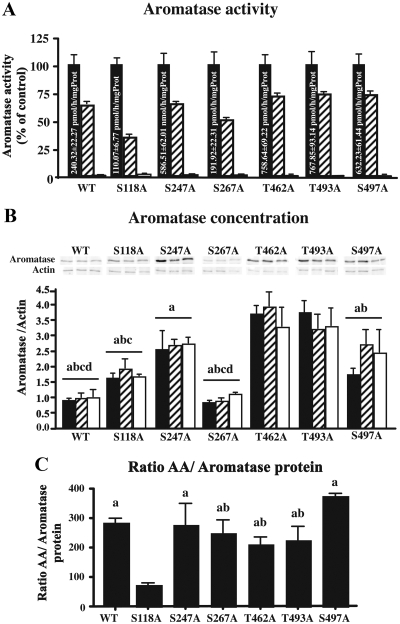
As a result of these analyses, we focused our attention on six different residues: S247, S267, and S497 that had high scores in both the predictive phosphorylation sites and PKA or PKC recognition consensus sequence; T462 and T493 that correspond to positions S455 and S486 in quail aromatase, two residues that were predicted to be involved in the phosphorylation of aromatase quail (23); and finally S118, based on previous data suggesting its role during phosphorylation-dependent control of human aromatase (36). Six different mutants S/T to A were produced to determine the potential importance of these residues in the rapid modulation of AA by phosphorylating conditions (preincubation with 2 or 8 mm ATP, 2 mm Ca2+, and 10 mm Mg2+ for 15 min). All mutants still expressed AA, and phosphorylating conditions markedly reduced this enzymatic activity in the six different mutants (S118A, S247A, S267A, T462A, T493A, S497A) to approximately the same extent as in wild-type enzyme (Fig. 5A). In all cases inhibition was more pronounced after exposure to the highest concentration of ATP. Data are presented after transformation in percentage of the corresponding controls, but raw data were analyzed by a mixed-design, two-way ANOVA that confirmed the enzymatic inhibition by phosphorylating conditions (F2,42 = 460.12, P < 0.0001). In addition, there was a significant difference in general activity between the different clones (F6,42 = 27.10, P < 0.0001, see values in control conditions in Fig. 5A) and a significant interaction between the conditions and the clones (F12,42 = 25.10, P < 0.0001).
Fig. 5.
Aromatase activity and expression in WT enzyme and enzyme modified by site-directed mutagenesis in control conditions (black bars) and after preincubation with 2 mm Ca2+, 10 mm Mg2+, and 2 mm (hatched bars) or 8 mm (white bars) ATP. All assays were performed in four independent replicates. A, Mean aromatase activity for each clone in the control and phosphorylating conditions. Aromatase activity in phosphorylating conditions is expressed as percentage of the mean activity compared in control conditions. The absolute enzymatic activities in control conditions are printed in the corresponding bar. B, Representative Western blots and bar graphs summarizing aromatase concentration in the different clones and experimental conditions. Aromatase expression in the bar graphs is normalized for each clone with actin expression. a, P < 0.05 vs. T462A; b, P < 0.05 vs. T493A; c, P < 0.05 vs. S247A; d, P < 0.05 vs. S497A. C, Bar graph representing the ratio of aromatase activity over the aromatase protein concentration for the WT and mutant enzyme preincubated in control conditions. a, P < 0.05 vs. S118A; b, P < 0.05 vs. S497A. Values are presented as mean ± sem. All assays were performed in four independent replicates.
To determine whether the inhibition of AA was due to a reduction in aromatase protein content, Western blots were performed on the different clones in the three different conditions. Phosphorylating conditions did not affect aromatase concentration (F2,42 = 0.98, P = 0.4), but this concentration was significantly different in the different clones (F6,42 = 12.51, P < 0.0001, see Fig. 5B). However, there was no interaction between clones and conditions (F12,42 = 1.08, P = 0.38). We finally calculated the ratio of AA over normalized aromatase concentration obtained by Western blot to obtain an index of the intrinsic AA. This analysis identified a significant difference between mutants (one way ANOVA, F6,27 = 5.36, P = 0.002). Several clones had a ratio of activity over concentration similar to what is observed in the native wild-type enzyme, but this ratio was slightly (although not significantly) higher in the S497A mutant and significantly reduced in the S118A mutant (Fig. 5C).
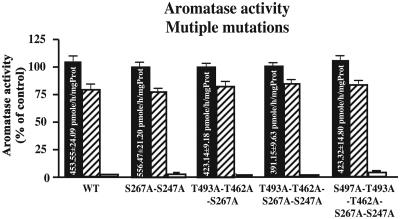
We also analyzed the enzymatic activity of the human aromatase containing various combinations of the S/T to A mutations studied above (Fig. 6). All mutants still expressed AA, and phosphorylating conditions markedly reduced this enzymatic activity in four different combinations of mutants [including the combined five mutations (S267A-S247A, T493A-T462A-S267A, T493A-T462A-S267A-S247A, S497A-T493A-T462A-S267A-S247A)]. As observed with single mutations, inhibition was more pronounced after exposure to the highest concentration of ATP. A mutated human aromatase containing the six mutations investigated above was not generated because S118A by itself caused a marked reduction of aromatase activity, independently of phosphorylating conditions. Data are presented after transformation in percentage of the corresponding controls, but raw data were analyzed by a mixed-design, two-way ANOVA that confirmed the enzymatic inhibition by phosphorylating conditions (F2,68 = 2046.00, P < 0.0001). In addition, there was a significant difference in general activity between the different clones (F4,42 = 31.68, P < 0.0001, see values in control conditions in Fig. 6) and a significant interaction between the conditions and the clones (F8.68 = 5.748, P < 0.0001).
Fig. 6.
Aromatase activity and expression in WT enzyme and enzyme modified by site-directed mutagenesis (multiple mutations) in control conditions (black bars) and after preincubation with 2 mm Ca2+, 10 mm Mg2+, and 2 mm (hatched bars) or 8 mm (white bars) ATP. Mean aromatase activity for each clone in the control and phosphorylating conditions is shown. Aromatase activity in phosphorylating conditions is expressed as percentage of the mean activity compared in control conditions. The absolute enzymatic activities in control conditions are printed in the corresponding bar. Values are presented as mean ± sem. All assays were performed in six independent replicates.
Discussion
We show here that AA is rapidly (within minutes) inhibited by conditions promoting protein phosphorylation in the quail ovary and in various cell lines expressing human aromatase. We also demonstrate that AA inhibition by phosphorylating conditions is reversible and that the basal activity of the human enzyme is markedly affected if serine 118 is mutated to an alanine (S118A). Mutation of this and five other residues, alone or in combination, did not, however, affect the rapid inhibition of enzymatic activity by phosphorylating conditions.
Phosphorylation of specific amino acid residues is generally a reversible process catalyzed by the competing activities of protein kinases and phosphatases (37). This mechanism offers a tight control of protein activity, either through changes in receptor affinity or changes in enzyme kinetics. Our laboratory originally demonstrated that AA is rapidly modulated in Japanese quail hypothalamic explants by phosphorylating conditions (19, 23, 24). More recently AA was shown to vary rapidly in the Japanese quail hypothalamus after sexual interactions with a female (18, 38) and in the telencephalon of zebra finch after social interactions (39).
The present work extends this mechanism of rapid control of AA to the human enzyme and to other cellular environments, including ovarian cells (quail) and various mammalian cell lines. High but physiological concentrations of ATP-Mg-Ca, known to enhance protein phosphorylation (19, 40, 41), inhibited within 5–15 min AA in quail ovary and in rat glial, mouse neural, and human kidney cell lines expressing human aromatase. Therefore, the rapid inhibition of AA is a general control mechanism of estrogen production that is not limited to the neuronal cell environment. In addition, we confirm previous observations (40) that aromatase itself is phosphorylated by phosphorylating conditions, suggesting that the rapid changes in enzymatic activity result from a direct action on the enzymatic protein itself rather than another regulatory protein that would secondarily regulate AA.
Importantly, we also demonstrate that the rapid mechanism of inhibition of human AA does not involve aromatase degradation because enzyme concentration is not affected. Furthermore, the enzymatic activity can be fully restored within minutes after removal of the inhibitory conditions. Although our previous pharmacological studies defined the potential importance of several protein kinases, including members of the AGC family, the exact involvement of specific phosphatases was not clear (27). The reversibility of AA inhibitions demonstrated here for human aromatase expressed in HEK293 cells and the absence of protein degradation support the idea that the interplay between phosphorylation and dephosphorylation rapidly modulate the activity of the enzyme.
We also showed that phosphorylating conditions do not affect the apparent enzyme affinity for its substrate but change the maximum velocity of reaction. Among the 14 amino acid residues involved in the tethering of androstenedione in the binding pocket of aromatase, only one residue could potentially be affected by phosphorylation [T310 (42)], reinforcing the observation that phosphorylation does not affect the affinity for the substrate. The phosphorylation of aromatase is therefore likely to concern other residues in the catalytic site of the enzyme to significantly slow down the aromatization process. Aromatization is a complex mechanism involving numerous reaction steps, including 19-hydroxylation and 10-methylation (43, 44). These different steps are currently not fully understood, and discussing the implication of specific residues for each step would therefore be purely speculative.
The role of specific phosphorylated residues in the rapid inhibition of AA
Because numerous phosphorylation consensus sites are present on the aromatase amino acid sequence in various species, we previously proposed that phosphorylations directly affect the aromatase protein rather than another protein that would secondarily regulate AA (19). This notion is supported by Western blot experiments demonstrating an increased phosphorylation of S, T, and Y residues at the level of the aromatase band purified by immunoprecipitation under phosphorylating conditions that inhibit AA (23). Previous pharmacological experiments on quail hypothalamus homogenates also indicated that the inhibition of AA by phosphorylation was catalyzed by the activity of two S/T kinases, PKA and PKC (23). Based on this knowledge and on the prediction scores for residue phosphorylation derived from bioinformatics analysis, six amino acids, including two threonines and four serines, were mutated to alanine in the human aromatase sequence to determine their potential role in the control of AA by phosphorylations.
The six mutated proteins as well as the combination of the different mutations still expressed a significant level of AA, although absolute enzymatic activity was significantly affected in several mutants, even when activity was corrected for the amount of enzymatic protein present in the assays. Surprisingly, however, none of these mutations blocked the rapid effect of phosphorylating conditions on aromatase inhibition. These two aspects of the results must be considered independently.
Several reasons could explain the lack of effects of mutations on the rapid control of AA by phosphorylations. Although we mutated amino acids with the highest phosphorylation and kinase recognition prediction scores, other consensus sites for phosphorylations and for other types of kinases are also present in the human aromatase sequence suggesting that other amino acids could be involved. It is also possible that a combination of several phosphorylated amino acids that was not tested here is required to control AA. Multiple phosphorylated residues are often observed in vivo in a large array of proteins, including cytochromes P450, the protein family that includes aromatase (45). The control of AA by multiple kinases reinforces the idea that several amino acids must be concomitantly phosphorylated to modify AA. Finally, we cannot exclude that the control of AA by phosphorylations depends on the phosphorylation of proteins interacting with aromatase in addition to the enzyme itself. Aromatase forms an electron-transfer complex with NADPH-cytochrome P450 reductase that strictly conditions its activity (26, 46, 47). The phosphorylation of the reductase could be a control mechanism affecting the electron transfer and therefore AA. The demonstration that AA inhibition by phosphorylations is a general process that is not tissue or species specific, however, limits the number of potential candidate molecules that could be involved in this regulation.
Mutations affect basal aromatase activity
We also demonstrated here that the total enzymatic activity is markedly affected by most of the mutations of serine or threonine residues to alanine. These absolute changes are, however, difficult to interpret because aromatase expression was investigated in stable transfectants. The transfection method and antibiotic selection usually result in the integration of an uncontrolled number of vector copies at random chromosomal locations that express protein at levels that generally cannot be predicted (see, for example, Ref. 48). These factors (position effect, number of copies) contribute in a major way to the differences in enzymatic activity observed between different mutants. It should, however, be mentioned that the level of AA was in all cases significantly affected by phosphorylating conditions, independently of the basal level of expression of the protein.
To compensate for this potential bias, AA was corrected by the amount of enzymatic protein as assessed by Western blots and compared with the native [wild type (WT)] enzyme. Even after this correction, the S118A aromatase still had a markedly reduced activity, significantly lower than all other forms of the enzyme including WT. Activity of the S497A mutant was also significantly higher than in S267A, T462A, and S493A and numerically but not significantly higher than in the WT enzyme. These data strongly reinforce the notion that phosphorylations of specific residues of aromatase modulate the enzymatic activity of the protein. To our knowledge, the two residues shown here to affect AA (S118 and S497) have not been directly implicated in substrate binding, reaction catalysis, or direct interaction with the NADPH reductase (46, 49–50). Their exact function in the control of basal AA and protein stability remains to be determined. A recent in vitro study of the mouse aromatase also indicated that phosphorylation of serine S118 by S/T kinases alters AA via the modulation of aromatase stability (36).
Although several studies have described a rapid inhibition of AA by phosphorylations, an independent study showed that the phosphorylation of Y361 in human aromatase increases its activity. This Y361 phosphorylation was induced by a tyrosine-kinase c-Src activated by a ligand-bound estrogen receptor-α in breast cancer cell lines (7). These data, in addition to the effects of the S118A mutation described here, confirm the importance of the specific pattern of phosphorylation of aromatase in the control of its activity. Phosphorylation of some residues increases enzymatic activity, whereas the phosphorylation of others inhibits that same activity. This presumably explains why removal of all phosphate groups from the enzyme by alkaline phosphate resulted in a somewhat unexpected decrease in enzyme activity (24). These data strongly suggest that specific phosphorylation patterns are required to modulate aromatase activity via an increase in protein stability and thus concentration on the one hand and to rapidly and reversibly affect the enzymatic activity independently from changes in protein concentration on the other hand.
In conclusion, we show here that the rapid inhibition of AA by phosphorylating conditions is a widespread mechanism present in different tissues affecting aromatase from various species including humans. The relatively recent discovery that estrogens, and E2 particularly, are involved in a large number of nongenomic signaling processes has usually ignored the fact that synthesis of these estrogens needs to be rapidly controlled as well. We demonstrate here that phosphorylation/dephosphorylation processes provide a new widespread mechanism by which estrogen concentration could be rapidly altered in the brain and other tissues. These results highlight the possibility of defining new therapeutic targets to activate or inhibit AA in localized defined regions.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant NIH/NIMH R01 MH50388 (to J.B.). C.A.C. is a Fonds de la Recherche Scientifique-Fonds National de la Recherche Scientifique Research Associate (F.R.S.-FNRS). T.D.C. was a F.R.S.-FNRS Postdoctoral researcher and is currently a ULg Research Associate.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- Aromatase activity
- E2
- 17β-estradiol
- F4
- fourth follicle
- F5
- fifth follicle
- HEK
- human embryonic kidney
- Km
- Michaelis-Menten constant
- NADPH
- reduced nicotinamide adenine dinucleotide phosphate
- PKA
- protein kinase A
- PKC
- protein kinase C
- PO4
- phosphorylating conditions
- POA/HYP
- preoptic area/hypothalamic
- WT
- wild type.
References
- 1. Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. 2007. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931 [DOI] [PubMed] [Google Scholar]
- 2. Turner RT, Riggs BL, Spelsberg TC. 1994. Skeletal effects of estrogen. Endocr Rev 15:275–300 [DOI] [PubMed] [Google Scholar]
- 3. McEwen BS, Alves SE. 1999. Estrogen actions in the central nervous system. Endocr Rev 20:279–307 [DOI] [PubMed] [Google Scholar]
- 4. Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. 2001. Mechanisms of estrogen action. Physiol Rev 81:1535–1565 [DOI] [PubMed] [Google Scholar]
- 5. Cohen D, Eisdorfer C, Gorelick P, Luchins D, Freels S, Semla T, Paveza G, Shaw H, Ashford JW. 1993. Sex differences in the psychiatric manifestations of Alzheimer's disease. J Am Geriatr Soc 41:229–232 [DOI] [PubMed] [Google Scholar]
- 6. Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. 1996. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 348:429–432 [DOI] [PubMed] [Google Scholar]
- 7. Catalano S, Barone I, Giordano C, Rizza P, Qi H, Gu G, Malivindi R, Bonofiglio D, Andò S. 2009. Rapid estradiol/ERα signaling enhances aromatase enzymatic activity in breast cancer cells. Mol Endocrinol 23:1634–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vasudevan N, Pfaff DW. 2007. Membrane-initiated actions of estrogen in neurobiology: emerging principles. Endocr Rev 28:1–19 [DOI] [PubMed] [Google Scholar]
- 9. Moss RL, Gu Q, Wong M. 1997. Estrogen: nontranscriptional signaling pathway. Recent Prog Horm Res 52:33–68; discussion 68–69 [PubMed] [Google Scholar]
- 10. Cornil CA, Charlier TD. 2010. Rapid behavioural effects of oestrogens and fast regulation of their local synthesis by brain aromatase. J Neuroendocrinol 22:664–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Woolley CS. 2007. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol 47:657–680 [DOI] [PubMed] [Google Scholar]
- 12. Simpson ER. 2000. Role of aromatase in sex steroid action. J Mol Endocrinol 25:149–156 [DOI] [PubMed] [Google Scholar]
- 13. Simpson ER, Davis SR. 2001. Minireview: aromatase and the regulation of estrogen biosynthesis—some new perspectives. Endocrinology 142:4589–4594 [DOI] [PubMed] [Google Scholar]
- 14. Lephart ED. 1996. A review of brain aromatase cytochrome P450. Brain Res Rev 22:1–26 [PubMed] [Google Scholar]
- 15. Balthazart J, Foidart A. 1993. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Mol Biol 44:521–540 [DOI] [PubMed] [Google Scholar]
- 16. Roselli CE, Resko JA. 1989. Testosterone regulates aromatase activity in discrete brain areas of male rhesus macaques. Biol Reprod 40:929–934 [DOI] [PubMed] [Google Scholar]
- 17. Roselli CE, Resko JA. 1997. Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J Steroid Biochem Mol Biol 61:365–374 [PubMed] [Google Scholar]
- 18. Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. 2005. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology 146:3809–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balthazart J, Baillien M, Ball GF. 2001. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol 13:63–73 [DOI] [PubMed] [Google Scholar]
- 20. Balthazart J, Baillien M, Ball GF. 2006. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology 147:359–366 [DOI] [PubMed] [Google Scholar]
- 21. Remage-Healey L, Maidment NT, Schlinger BA. 2008. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci 11:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balthazart J, Baillien M, Ball GF. 2001. Phosphorylation processes mediate rapid changes of brain aromatase activity. J Steroid Biochem Mol Biol 79:261–277 [DOI] [PubMed] [Google Scholar]
- 23. Balthazart J, Baillien M, Charlier TD, Ball GF. 2003. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci 17:1591–1606 [DOI] [PubMed] [Google Scholar]
- 24. Balthazart J, Baillien M, Ball GF. 2005. Interactions between kinases and phosphatases in the rapid control of brain aromatase. J Neuroendocrinol 17:553–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodríguez-Maldonado E, Velázquez PN, Juárez-Oropeza MA, Pedernera E. 1996. Steroid metabolism in theca external cells from preovulatory follicles of domestic hen (Gallus domesticus). Gen Comp Endocrinol 101:173–179 [DOI] [PubMed] [Google Scholar]
- 26. Thompson EA, Jr, Siiteri PK. 1974. Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione. J Biol Chem 249:5364–5372 [PubMed] [Google Scholar]
- 27. Roselli CE, Resko JA. 1991. In vitro assay of aromatase activity in the central nervous system. In: Greenstein B. ed. Neuroendocrine research methods. Chur, Switzerland: Harwood Academic Publisher; 937–951 [Google Scholar]
- 28. Silverin B, Baillien M, Foidart A, Balthazart J. 2000. Distribution of aromatase activity in the brain and peripheral tissues of passerine and nonpasserine avian species. Gen Comp Endocrinol 117:34–53 [DOI] [PubMed] [Google Scholar]
- 29. Bakker J, Baillien M, Honda S, Harada N, Balthazart J. 2004. Relationships between aromatase activity in the brain and gonads and behavioural deficits in homozygous and heterozygous aromatase knockout mice. J Neuroendocrinol 16:483–490 [DOI] [PubMed] [Google Scholar]
- 30. Baillien M, Balthazart J. 1997. A direct dopaminergic control of aromatase activity in the quail preoptic area. J Steroid Biochem Mol Biol 63:99–113 [DOI] [PubMed] [Google Scholar]
- 31. Wouters W, De Coster R, van Dun J, Krekels MD, Dillen A, Raeymaekers A, Freyne E, Van Gelder J, Sanz G, Venet M, et al. 1990. Comparative effects of the aromatase inhibitor R76713 and of its enantiomers R83839 and R83842 on steroid biosynthesis in vitro and in vivo. J Steroid Biochem Mol Biol 37:1049–1054 [DOI] [PubMed] [Google Scholar]
- 32. Harada N. 1988. Novel properties of human placental aromatase as cytochrome P-450: purification and characterization of a unique form of aromatase. J Biochem 103:106–113 [DOI] [PubMed] [Google Scholar]
- 33. Balthazart J, Foidart A, Harada N. 1990. Immunocytochemical localization of aromatase in the brain. Brain Res 514:327–333 [DOI] [PubMed] [Google Scholar]
- 34. Cleland WH, Mendelson CR, Simpson ER. 1983. Aromatase activity of membrane fractions of human adipose tissue stromal cells and adipocytes. Endocrinology 113:2155–2160 [DOI] [PubMed] [Google Scholar]
- 35. Fiorelli G, Frediani U, Martineti V, Franchi A, Gori F, Franceschelli F, Tanini A, Serio M, Brandi ML. 1998. Aromatase expression and activity in the human leukaemic cell line FLG 29.1. J Steroid Biochem Mol Biol 66:105–112 [DOI] [PubMed] [Google Scholar]
- 36. Miller TW, Shin I, Kagawa N, Evans DB, Waterman MR, Arteaga CL. 2008. Aromatase is phosphorylated in situ at serine-118. J Steroid Biochem Mol Biol 112:95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cohen P. 2002. The origins of protein phosphorylation. Nat Cell Biol 4:E127–E130 [DOI] [PubMed] [Google Scholar]
- 38. Dickens MJ, Cornil CA, Balthazart J. 30 August 2011. Acute stress differentially affects activity in specific brain nuclei of adult male and female quail. Endocrinology 10.1210/en.2011-1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Remage-Healey L, Oyama RK, Schlinger BA. 2009. Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol 21:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Albert KA, Helmer-Matyjek E, Nairn AC, Müller TH, Haycock JW, Greene LA, Goldstein M, Greengard P. 1984. Calcium/phospholipid-dependent protein kinase (protein kinase C) phosphorylates and activates tyrosine hydroxylase. Proc Natl Acad Sci USA 81:7713–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daubner SC, Lauriano C, Haycock JW, Fitzpatrick PF. 1992. Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. J Biol Chem 267:12639–12646 [PubMed] [Google Scholar]
- 42. Ghosh D, Griswold J, Erman M, Pangborn W. 2009. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 457:219–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cole PA, Robinson CH. 1990. Conversion of 19-oxo[2 β-2H]androgens into oestrogens by human placental aromatase. An unexpected stereochemical outcome. Biochem J 268:553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wright JN, Akhtar M. 1990. Studies on estrogen biosynthesis using radioactive and stable isotopes. Steroids 55:142–151 [DOI] [PubMed] [Google Scholar]
- 45. Redlich G, Zanger UM, Riedmaier S, Bache N, Giessing AB, Eisenacher M, Stephan C, Meyer HE, Jensen ON, Marcus K. 2008. Distinction between human cytochrome P450 (CYP) isoforms and identification of new phosphorylation sites by mass spectrometry. J Proteome Res 7:4678–4688 [DOI] [PubMed] [Google Scholar]
- 46. Hong Y, Li H, Yuan YC, Chen S. 2010. Sequence-function correlation of aromatase and its interaction with reductase. J Steroid Biochem Mol Biol 118:203–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miller WL. 2005. Minireview: regulation of steroidogenesis by electron transfer. Endocrinology 146:2544–2550 [DOI] [PubMed] [Google Scholar]
- 48. Feng YQ, Seibler J, Alami R, Eisen A, Westerman KA, Leboulch P, Fiering S, Bouhassira EE. 1999. Site-specific chromosomal integration in mammalian cells: highly efficient CRE recombinase-mediated cassette exchange. J Mol Biol 292:779–785 [DOI] [PubMed] [Google Scholar]
- 49. Zhou D, Cam LL, Laughon CA, Korzekwa KR, Chen S. 1994. Mutagenesis study at the postulated hydrophobic region near the active site of aromatase cytochrome P450. J Biol Chem 269:19501–19508 [PubMed] [Google Scholar]
- 50. Kao YC, Korzekwa KR, Laughton CA, Chen S. 2001. Evaluation of the mechanism of aromatase cytochrome P450: a site directed mutagenesis study. Eur J Biochem 268:243–251 [DOI] [PubMed] [Google Scholar]
- 51. Amarneh B, Corbin CJ, Peterson JA, Simpson ER, Graham-Lorence S. 1993. Functional domains of human aromatase cytochrome P450 characterized by linear alignment and site-directed mutagenesis. Mol Endocrinol 7:1617–1624 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.