Abstract
Estrogen receptor (ER) is a key regulator of mammary gland development and is also implicated in breast tumorigenesis. Because ER-mediated activities depend critically on coregulator partner proteins, we have investigated the consequences of reduction or loss of function of the coregulator repressor of ER activity (REA) by conditionally deleting one allele or both alleles of the REA gene at different stages of mammary gland development. Notably, we find that heterozygosity and nullizygosity for REA result in very different mammary phenotypes and that REA has essential roles in the distinct morphogenesis and functions of the mammary gland at different stages of development, pregnancy, and lactation. During puberty, mice homozygous null for REA in the mammary gland (REAf/f PRcre/+) showed severely impaired mammary ductal elongation and morphogenesis, whereas mice heterozygous for REA (REAf/+ PRcre/+) displayed accelerated mammary ductal elongation, increased numbers of terminal end buds, and up-regulation of amphiregulin, the major paracrine mediator of estrogen-induced ductal morphogenesis. During pregnancy and lactation, mice with homozygous REA gene deletion in mammary epithelium (REAf/f whey acidic protein-Cre) showed a loss of lobuloalveolar structures and increased apoptosis of mammary alveolar epithelium, leading to impaired milk production and significant reduction in growth of their offspring, whereas body weights of the offspring nursed by females heterozygous for REA were slightly greater than those of control mice. Our findings reveal that REA is essential for mammary gland development and has a gene dosage-dependent role in the regulation of stage-specific physiological functions of the mammary gland.
The majority of mammary gland development takes place in distinct stages of postnatal development and physiological changes in the mammary gland during puberty, pregnancy, lactation, and involution are tightly controlled by the orchestrated actions of ovarian steroid hormones and growth factors (1–4). Throughout recurrent estrous or menstrual cycles, the epithelial ducts and branches increase, whereas in pregnancy and lactation, alveolar units proliferate and differentiate into milk-secretory cells (1, 4, 5). The ovarian steroid hormones, estrogens and progestins, are key mediators of ductal morphogenesis and are mitogenic for mammary epithelial cells (4, 6). These physiological effects of hormones are mediated through the estrogen receptor (ER) and progesterone receptor (PR), both members of the nuclear receptor superfamily of ligand-activated transcription factors (7).
ERα has been shown to play a pivotal role in mammary gland development. Mammary glands of mice with knockout of ERα showed only a rudimentary ductal structure that failed to invade the mammary fat pad, demonstrating that ERα is required for normal ductal elongation and outgrowth during puberty (8–11). However, exploring the role of ERα in the mammary gland beyond puberty was not possible, due to the infertility of knockout of ERα mice, until Khan and co-workers (12) developed whey acidic protein (WAP)-driven Cre-mediated conditional ERα knockout mice. Their studies demonstrated that the ablation of ERα in mammary epithelium during late pregnancy and lactation resulted in a loss of ductal side-branching and lobuloalveolar structures and decreased the proliferation of alveolar progenitors (12). These observations in both conventional and conditional ERα knockout mouse models have demonstrated that ERα profoundly impacts multiple developmental stages of the mammary gland, puberty, pregnancy, and lactation.
It is now well established that the transcriptional activity of ERα is modulated by a delicate balance between coactivator and corepressor proteins (13, 14). Changes in the expression of receptor coactivators or corepressors can affect the transcriptional activity of the estradiol (E2)-ER complex and is shown to underlie various disorders of estrogen target tissues (15). Although many coactivators for ERα are known, few corepressors have been identified (14, 16). Repressor of ER activity (REA) was initially identified as an ER-interacting coregulator that repressed the activity of E2 (17, 18). Because in a previous work (19) we found that the conventional homozygous deletion of REA resulted in embryonic lethality, we were not able to study the role of REA in postnatal mammary gland development and function. However, conventional heterozygous (REA+/−) animals were viable and, interestingly, displayed a phenotype in which enhanced ER function was observed in the mammary gland, suggesting that REA is an important repressive modulator of mammary gland development (20). Additional lines of study suggest that REA might also act as a brake on breast carcinogenesis. REA expression levels were shown to be inversely correlated with tumor grade and positively correlated with ER in breast cancer (21). Also, the F-box protein S-phase kinase-associated protein-2B (Skp2B), which is often overexpressed in breast cancers, interacts with REA, resulting in degradation of the REA protein, and mouse mammary tumor virus-Skp2B mice overexpressing Skp2B developed mammary tumors (22), suggesting that altered levels of REA might be linked to breast tumorigenesis.
In this study, we sought to dissect the physiological roles of the coregulator REA at distinct stages of mammary gland development. To this end, we generated two types of mammary-specific conditional REA knockout animals using a cre-loxP recombination strategy. To define the role of REA during puberty or after late pregnancy, respectively, we used PR-Cre knockin mice (23) or WAP-Cre transgenic mice (24), respectively. In this first report of conditional REA knockout in the mammary gland and its physiological consequences, we have found that heterozygosity and nullizygosity for REA resulted in very different mammary gland phenotypes. Although mice with mammary-specific conditional knockout of only one REA allele displayed an enhancement of mammary ductal outgrowth and enhanced mammary gland activities, mice homozygous null for REA showed impaired mammary gland morphogenesis and a loss of lobuloalveolar structure associated with reduced epithelial cell proliferation and increased apoptosis that greatly impacted lactational ability. Our study reveals a critical role for REA gene dosage in normal mammary gland development and function during multiple distinct physiological stages, puberty, pregnancy, lactation, and involution.
Materials and Methods
Generation of transgenic REA knockout mice
All animals were maintained in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals, and all procedures were approved by the University of Illinois and Baylor College of Medicine Institutional Animal Care and Use Committees. Adult female C57BL mice were purchased from Harlan Co. (Indianapolis, IN).
The mouse BAC library clone 284H12 (Invitrogen, Carlsbad, CA) was used as the template for mouse REA genomic DNA to generate a targeting vector. The targeting vector contained two loxP sequences flanking the REA gene exons 2 through 6, which are required for REA repressive activity and for interaction with ER. Flippase recognition target (Frt)-floxed neomycin-resistance gene (NEO) was inserted between intron 6 and the 5′-loxP site. The targeting vector contained flanking 5.2-kb (5′-targeting arm) and 3.0-kb (3′-targeting arm) mouse REA and neighboring sequences for homologous recombination. In addition, the herpes simplex virus thymidine kinase gene was located outside of the REA sequence and served as a negative drug-selection marker. The sequence of the targeting vector was verified using the BigDye DNA Sequencing kit (PE Applied Biosystems, Foster City, CA). Embryonic stem cell work was carried out as previously described (19).
Female REAflox/flox mice (25) were mated with male PR-Cre knockin mice (23) to generate the REAflox/flox PRcre/+ mice. Briefly, female homozygous REA-floxed mice (REAflox/flox) were mated with male homozygous PR-Cre mice (PRcre/cre) to produce offspring (REAflox/+ PRcre/+) that are heterozygous for both mutations. These heterozygous mice (REAflox/+ PRcre/+) were then bred with each other to produce conditional REA homozygous mutant mice that carried two REA floxed alleles and the Cre gene. In these mice, Cre-mediated excision of floxed REA led to a null mutation of this gene in PR-expressing cell lineages. To generate the REAflox/+ WAP-Cre bigenic mouse model, female REAflox/flox mice were mated with male WAP-Cre transgenic mice (24, 26).
Whole-mount staining of mouse mammary glands
The fourth inguinal mammary glands were excised, spread onto a glass microscope slide, and fixed in 4% paraformaldehyde for 2 h at 4 C. The samples were washed with PBS and stained in carmine alum solution overnight at room temperature. The samples were then dehydrated using stepwise ethanol concentrations and defatted in xylene overnight.
Histology and immunostaining and 5-bromo-2-deoxyuridine (BrdU) incorporation assay
Hematoxylin and eosin staining or immunohistochemistry was performed as previously described (27). In brief, the tissues were fixed in 10% buffered formalin phosphate for 24 h at room temperature, transferred to 70% ethanol, and then embedded in paraffin; 4-μm sections were subjected to hematoxylin and eosin staining or immunostaining using antibodies to REA (07-234; Millipore Co., Billerica, MA), PR (A0098; Dako Co., Glostrup, Denmark), ERα (6F11; Novocastra, Newcastle upon Tyne, UK), or caspase-3 (AF835; R&D Systems, Minneapolis, MN).
For BrdU incorporation assay, mice were injected ip with 30 μg BrdU/g body weight (BD Biosciences, San Jose, CA) 2 h before killing. Mammary glands were fixed, embedded in paraffin, and tissue sections were stained with BrdU antibody (3D4; BD Biosciences).
Western blot analysis
Immunoblotting was performed as previously described (19). The fourth inguinal mammary gland was removed at the indicated developmental stage and snap frozen in liquid nitrogen. Protein lysates were prepared using ice-cold lysis buffer [25 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 1% sodium dodecyl sulfate] supplemented with protease inhibitors (complete EDTA free; Roche, Indianapolis, IN) and phosphatase inhibitors (Phospho-stop; Roche). Protein concentrations were determined by the BCA Protein Assay System (Pierce, Rockford, IL). Proteins (20–50 μg) were separated on SDS-PAGE gels, transferred onto nitrocellulose membranes, and subjected to immunoblotting with anti-REA (07-234; Millipore Co.), anti-signal transducer and activator of transcription 5 (STAT5) (9363; Cell Signaling Technology, Beverly, MA), antiphospho-STAT5 (C11C5; Cell Signaling Technology), and anti-ERK2 (D2; Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
RNA isolation and real-time PCR
Total RNA was isolated from mammary glands at the indicated developmental stage using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. One microgram of total RNA was reverse transcribed and analyzed by real-time PCR as described previously (19).
Terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling (TUNEL) staining
Apoptosis was detected by using the In Situ Cell Death Detection kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's protocol. Briefly, deparaffinized and rehydrated sections were incubated at 37 C for 15 min with 20 μg/ml of proteinase K in 10 mm Tris-HCl (pH 7.6). Sections were washed twice with PBS and incubated for 1 h at 37 C with TUNEL reaction mixture in a humidified chamber. After the incubation, sections were rinsed three times with PBS and counterstained with 4′,6-diamidino-2-phenylindole.
Statistical analysis
Statistical comparisons between two experimental groups were evaluated using the Student's t test (two-sample assuming unequal variance). Results are expressed as mean ± sd, and P < 0.05 was assigned as significant. Pup's body weight data were analyzed using the generalized linear model (GLM) with repeated measures ANOVA (28) for mean weight over a period of 20 d to test the dam's genotype effect on pups' body weight (ANOVA) (SAS 9.2; SAS Institute, Inc., Cary, NC), and P < 0.05 was considered statistically significant.
Results
Generation of REA conditional knockout mice and REA expression in the developing mammary gland
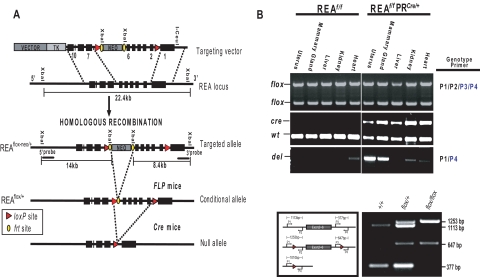
To investigate the biological role of REA in mammary gland development and morphogenesis, we generated REA conditional knockout mice by Cre-loxP recombination in which REA function was abrogated in PR-expressing mammary cells. As shown in Fig. 1A, Cre-mediated recombination will eliminate REA exon 2 through 6, which are required for interaction with ER and repressive activity of ER-target genes (17, 18). This deletion might generate aberrantly spliced mRNA transcripts connecting exon 1 to exon 7. However, mRNA transcribed from the knockout allele creates a frameshift, resulting in a premature stop codon at the end of exon 7, and it therefore will be degraded by the nonsense-mediated mRNA decay pathway. Genotyping confirmed the ablation of REA alleles in mammary glands of virgin mice (Fig. 1B).
Fig. 1.
Design of the conditional targeting vector and strategy for disruption of the REA gene using PR-Cre. A, The targeting vector, described in more detail in the text, contains positive (NEO) and negative [thymidine kinase (TK)] selection markers, two frt sites that flank a neomycin resistant gene cassette (indicated as ovals), and two loxP sequences (triangles). The REAflox-frt-neo allele was created by homologous recombination in embryonic stem cells, and the REAflox/+ allele was derived from a REAflox-frt-neo allele through in vivo Flippase recombination enzyme (FLP)-mediated recombination. Finally, the PR-Cre mice were used to generate the conditional deletion of the REA gene by Cre-mediated excision in PR expressing cell lineages. B, Genomic DNA isolated from the indicated tissues was genotyped by PCR.
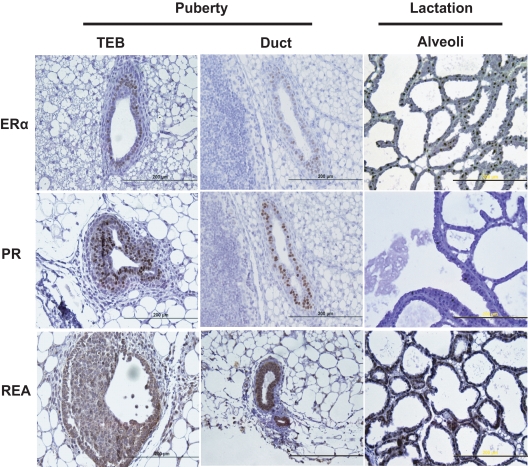
Before studying the mammary glands of the REA conditional knockout mice, we first examined the expression pattern of REA during normal mammary gland development, and as shown in Fig. 2, almost all mammary cells were positive for REA. However, PR was only expressed in body cells and a subgroup of luminal epithelial cells during puberty, thus restricting loss of REA expression to the body cells within the terminal end buds (TEB) and to a subgroup of luminal epithelial cells in the pubertal gland (Fig. 2). Because we found that REAf/f PRCre/+ mice were infertile (25), PR-Cre-mediated REA knockout animals were only used in our studies to examine the role of REA in mammary gland development before pregnancy. Furthermore, the number of mammary cells expressing PR was previously shown to be significantly reduced during pregnancy, lactation, and involution (29), and we also confirmed very low PR expression at lactation day (L)14 by immunohistochemistry (Fig. 2).
Fig. 2.
ERα, PR, and REA expression in the developing mammary gland and at L14. Immunohistochemical detection of ERα, PR, and REA in TEB and ducts of the mammary glands of virgin female mice at 6 wk of age and in alveoli of the mammary glands of L14 mice. Scale bars, 200 μm.
Therefore, for later mammary gland studies exploring the role of REA during pregnancy and lactational stages, we used a different REA knockout strategy involving WAP-Cre mice (24, 26). Additionally, we assessed the pattern of ERα expression, because we were particularly interested in how REA might impact ERα signaling during mammary gland development. In the pubertal mammary gland, as shown in Fig. 2, the expression pattern for ERα was similar to that observed for PR, but ERα was quite high during the late lactation period (L14).
Mammary ductal outgrowth is impaired in mammary glands lacking both alleles of REA but is enhanced in mammary glands lacking only one allele of REA
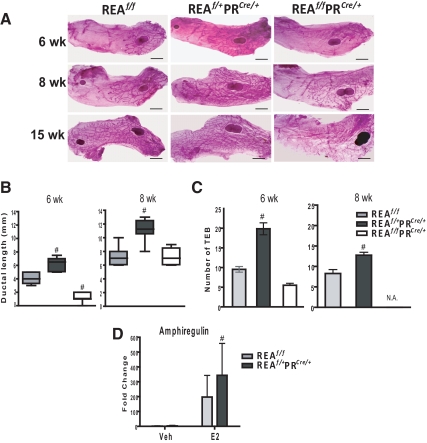
ERα signaling is known to direct ductal elongation and branching, which occurs through the proliferation of TEB (30). To determine the contribution of REA in pubertal mammary gland development, we analyzed the status of ductal elongation and branching in the virgin mammary glands of REAf/f, REAf/+PRCre/+ (heterozygous knockout), and REAf/f PRCre/+ (homozygous knockout) mice. Because normal ductal elongation begins at about 4 wk of age and ends by 10 wk (31), mammary glands were isolated and whole mounted over this time period, and the extent of ductal outgrowth (distance from the lymph node to the end of the longest extended duct) was assessed. Differences in mammary ductal outgrowth of pubertal period mice were apparent in REAf/f, REAf/+PRCre/+, and REAf/fPRCre/+ mice (Fig. 3, A and B). Mammary ductal morphogenesis was severely impaired in glands from mice homozygous null for REA. Very interestingly, in comparison with glands from REAf/f mice, ductal morphogenesis was accelerated in the mammary glands of REAf/+PRCre/+ mice, as evidenced by longer ductal length (Fig. 3, A, center panels, and B). The number of TEB was also significantly higher in glands from REAf/+PRCre/+ mice vs. REAf/f mice (Fig. 3C). TEB were very small and not developed in REAf/fPRCre/+mammary glands and are therefore not shown (designated as not available) in Fig. 3C.
Fig. 3.
Mammary ductal outgrowth is accelerated in mice heterozygous for REA but severely impaired in mice homozygous null for REA. Mice heterozygous for REA show hyperstimulation of amphiregulin upon E2 treatment. A, Whole-mount staining of the number four inguinal mammary glands from REAf/f, REAf/+ PRCre/+, and REAf/f PRCre/+ virgin mice at 6, 8, and 15 wk of age. Scale bars, 2 mm. B, Ductal length of the mammary glands at 6 wk of age. Distance from the lymph node to the end of the longest extended duct was measured. The horizontal line in box plots represents the median length from eight mammary glands per group. C, The number of TEB per mammary gland was counted in whole mounts of number four inguinal glands (n = 8 mammary glands per group) at 6 wk of age. D, REAf/f and REAf/+ PRCre/+ mice at 6 wk of age were ovariectomized, allowed to rest for 2 wk, and were then injected with oil vehicle (Veh) or E2 (0.05 μg/g of body weight/d) for 5 d. Amphiregulin mRNA was measured by qRT-PCR. n = 7 mice per group, mean ± sd; #, P < 0.05 vs. wild type. N.A., Not available.
At 15 wk of age, mice homozygous null for REA displayed even more severe defects in mammary morphogenesis (Fig. 3A), and even at 24 wk of age, the mammary ductal network of REAf/f PRCre/+ mice had barely invaded the fat pad, and side branching was almost completely absent, whereas control REAf/f females exhibited extensive branching and normal fat pad invasion (data not shown). These observations that mammary ductal outgrowth was accelerated and enhanced by loss of one allele of REA, but reduced by loss of both alleles, indicate that mammary gland development is sensitive to the gene dosage of REA.
Because ovarian function is very important in the control of mammary morphogenesis, we examined and found that the conditional REA knockout animals retained normal ovarian function, including follicle maturation, ovulation, and corpora lutea formation similar in numbers to that of wild-type (flox/flox) mice (25). Examination of vaginal epithelial cytology and cyclicity also demonstrated that the conditional REA knockout animals had normal estrous cycles (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Reduction of REA in the mammary gland of REAf/+PRCre/+ mice enhances E2-stimulated amphiregulin expression
Because we observed accelerated mammary ductal outgrowth in the absence of one allele of REA and because E2 is known to be critical for this process, we hypothesized that REA might repress estrogen-mediated mammary ductal outgrowth. In an attempt to identify factors that might be responsible for the accelerated mammary ductal outgrowth in the REAf/+PRCre/+ mice, we examined the expression of amphiregulin, because amphiregulin is the major paracrine mediator of ductal outgrowth and is directly regulated by E2 (3, 32). Mice were ovariectomized at 6 wk of age, and after 2 wk, they were treated with E2 or control vehicle for 5 d. As shown in Fig. 3D, up-regulation of amphiregulin in response to E2 was significantly greater in the mammary gland of REAf/+PRCre/+ mice, suggesting that the accelerated mammary outgrowth in these animals is associated with hyperstimulation of amphiregulin by E2. However, we do not know if this represents a true transcriptional effect of REA or a difference in the abundance of responsive cells expressing amphiregulin.
Impact of the conditional deletion of the REA gene in the mammary gland epithelium during pregnancy and lactation
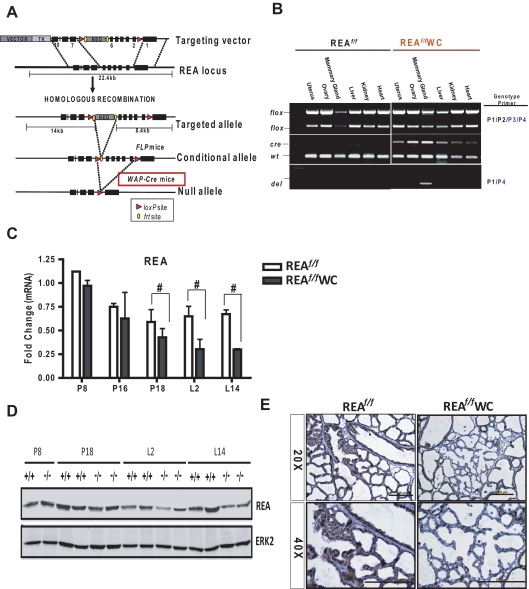
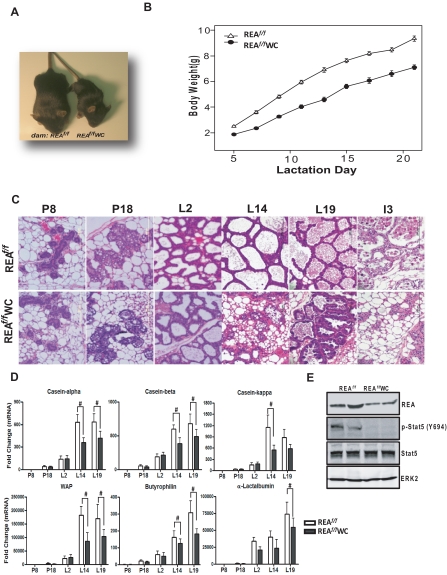
Because we found that REAf/f PRCre/+ females were infertile, we were unable to explore the physiological roles of REA during pregnancy and lactation with REAf/f PRCre/+ mice. Therefore, we generated REA conditional knockout mice from mice that carry a WAP-Cre transgene (24) and REA floxed alleles (REAf/f WAP-Cre) (Fig. 4A). The WAP-Cre transgene was shown to be expressed exclusively in mammary epithelium during late pregnancy and through lactation and to be activated by prolactin and Stat5 signaling (33–35). Because WAP is expressed in differentiating mammary epithelial cells, use of WAP-Cre transgenic mice allowed us to delete the REA gene only in differentiated cells and to examine whether REA is needed for the maintenance of differentiated mammary epithelium after it has undergone pregnancy-induced proliferation and differentiation (34, 36). As shown in Fig. 4B, ablation of the REA alleles was mammary gland-specific among the various tested organs, as examined by genotyping. Using quantitative RT-PCR (qRT-PCR) and immunoblotting, we also confirmed the reduction of REA in REAf/f WAP-Cre mammary tissues starting at late pregnancy [pregnancy day (P)18] through lactation (L2 and L14) (Fig. 4, C and D). As shown in Fig. 4E, uniform REA protein expression was observed in REAf/f epithelium at L14, whereas about half of the epithelial cells were devoid of REA in REAf/f WAP-Cre mice.
Fig. 4.
Conditional knockout of the REA gene in the mammary epithelium during pregnancy and lactation using WAP-Cre. A, Schematic diagram of the REA gene-targeting strategy. B, Genomic DNA isolated from the indicated tissues was genotyped by PCR. REA (C) mRNA and protein (D) were analyzed by qRT-PCR or immunoblotting, respectively, in the mammary gland at different stages of pregnancy and lactation. The data are mean ± sd, and the REA mRNA expression is presented as relative expression normalized to wild-type P8. E, Immunohistochemical detection of REA in REAf/f and REAf/f WAP-Cre mammary glands at L14. WC, WAP-Cre. Scale bars, 200 μm; #, P < 0.05 vs. wild type.
Pups nursed by REAf/f WAP-Cre mothers show greatly reduced weight gain
Although the REAf/f WAP-Cre dams could support their litters during lactation, REA deficiency in the epithelium of the mammary glands profoundly impaired growth of the offspring. All litters born to REAf/f WAP-Cre dams survived until the time of weaning (postnatal d 21), but these pups exhibited low body weights (Fig. 5, A and B). To carefully examine the time line of lactation impairment of the REAf/f WAP-Cre mice, pup body weights were measured throughout postnatal development from d 2 to 20 (PND 2–20). On PND2, the litter sizes were normalized to six pups. As shown in Fig. 5B, the average body weights of pups nursed by REAf/f WAP-Cre mothers were consistently less than that of pups from REAf/f females (34% less at L7), with the growth retardation of the pups being even more severe thereafter (∼50% lower body weight at d 20). Statistical analysis of this data using the GLM with repeated measures ANOVA (28) showed that the body weights of pups nursed by REAf/f WAP-Cre mothers were significantly different from those nursed by the REAf/f mothers (GLM, F1,358 = 2280; P < 0.0001).
Fig. 5.
REA is required for the maintenance of differentiated lobuloalveolar structure. A, Representative photograph of pups nursed for 20 d by REAf/f or REAf/f WAP-Cre (WC) mothers. B, The body weights of pups nursed by REAf/f or REAf/f WAP-Cre mice during the first 20 d of postnatal development. The dam's genotype has a significant effect on the body weights of pups, with reduced weight gain of the pups nursed by REAf/f WAP-Cre dams (GLM, F1,358 = 2280; P < 0.0001). GLM with repeated measures ANOVA. The data are mean ± sem (n = 20 per group). C, Representative hematoxylin- and eosin-stained mammary gland sections from REAf/f and REAf/f WAP-Cre at different stages of pregnancy, lactation, and involution. Magnification, ×40. D, qRT-PCR analysis of genes involved in milk protein expression (casein-α, casein-β, casein-κ, and WAP), lactose synthesis (α-lactalbumin), and milk lipid secretion (butyrophilin) at different stages of pregnancy (d 8 and 18) and lactation (d 2, 14, and 19). The data are mean ± sd (n = 10 per group), and mRNA levels are illustrated as relative expression normalized to 36B4 by wild-type P8. E, Mammary gland lysates were analyzed by immunoblotting for REA and phospho-Stat5. ERK2 served as a loading control. #, P < 0.05 vs. wild type.
REA is required for the maintenance of differentiated mammary lobuloalveolar structure
During pregnancy and lactation, marked changes occur in the mammary gland. Pregnancy changes include an increase of ductal branching and formation of alveolar buds, and these alveolar buds progressively differentiate into individual alveoli that ultimately become milk-producing lobules during the second half of pregnancy (2). After parturition, the secretory lobuloalveolar structures become more apparent with extended luminal space (37). To determine the impact of conditional REA ablation on developmental changes in the mammary gland during pregnancy and lactation, we examined the mammary gland morphology from REAf/f and REAf/f WAP-Cre females at multiple points during pregnancy and lactation. As shown in Fig. 5C, the number of alveoli in the REAf/f mammary gland was greatly increased from P8 to P18, and proteins (purple/pink in hematoxylin- and eosin-stained sections) and lipids begin to appear in the alveolar lumen and the alveolar epithelial cells, respectively, as development proceeded. Fully differentiated alveoli were observed at L2 and were expanded further until lactation was complete (Fig. 5C). Morphological changes in the REAf/f WAP-Cre mammary gland were similar to control mice until L2 (Fig. 5C). However, at L14, the alveoli of the REAf/f WAP-Cre mammary gland began to collapse and adipocytes began to reappear in the mammary gland (Fig. 5C), and the epithelium also appeared disorganized.
After weaning of the pups, the mammary gland goes through a process of involution, involving both apoptosis and remodeling (2, 38). Interestingly, the histology of the mammary glands of REAf/f and REAf/f WAP-Cre mice during involution was very different. As shown in Fig. 5C, involution was dramatically accelerated in REAf/f WAP-Cre mice. After 3 d of weaning (involution d 3), the REAf/f mammary glands still maintained the secretory alveolar structures with apoptotic bodies visible in luminal spaces, whereas most of the alveoli in REAf/f WAP-Cre mice were already collapsed. These observations indicate that REA is required for normal lobuloalveolar development and maintenance of differentiated lobuloalveolar structure during pregnancy, lactation, and involution.
REA is required for Stat5-mediated gene expression, lactose synthesis, and lipid secretion
The observed growth retardation of offspring nursed by REAf/f WAP-Cre mice and the abnormal lobuloalveolar structures in the mammary gland of these mice led us to suspect that the content of milk produced by REAf/f WAP-Cre mice might be inadequate. Mouse milk contains about 12% protein (several casein proteins, α-lactalbumin, WAP, lactoferrin, secretory immunoglobulin A, and others), 30% lipid, and 5% lactose (37). We therefore examined the production of milk proteins in the REAf/f WAP-Cre mice at different stages of pregnancy and lactation. As shown in Fig. 5D, mRNA expression for major milk proteins, the caseins and WAP, was dramatically up-regulated as lactation progressed in the control (REAf/f) mice. However, expression of these genes was significantly dampened in the mammary gland of REAf/f WAP-Cre mice. α-Lactalbumin, an essential and limiting cofactor for lactose synthase (2), and butyrophilin, known to be involved in milk lipid secretion (2, 39), were also greatly reduced in the mammary gland of REAf/f WAP-Cre mice (Fig. 5D). Of note, there was significant reduction of the expression of these milk-associated genes at L14, which is approximately the same time we observed collapse of alveoli in the REAf/f WAP-Cre mammary gland.
Because these milk protein genes are known to be regulated by prolactin via phosphorylation of Stat5 (26, 40), we examined whether a diminished activation of Stat5 in the mammary gland of REAf/f WAP-Cre mice might be responsible for the compromised milk protein gene expression. As shown in Fig. 5E, phosphorylated Stat5 was dramatically reduced in the mammary gland of REAf/f WAP-Cre mice at L14 compared with REAf/f mice, although there was no change in total Stat5 protein. These data imply that deficiency of REA in the mammary gland epithelium contributes to an altered mammary differentiation and signaling, leading to compromised milk production.
Pups nursed by REAf/+WAP-Cre females displayed increased weight gain
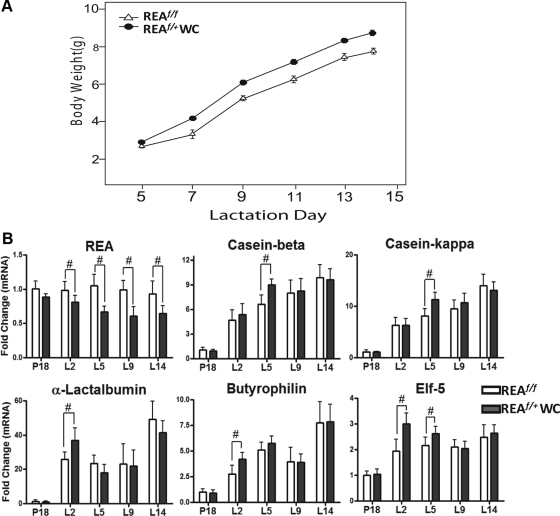
Because we observed accelerated mammary ductal outgrowth in the REAf/+PRCre/+ mammary gland during the pubertal period, we wished to examine the impact of loss of only one allele of REA on mammary gland development during pregnancy and lactation. To this end, the body weight of pups nursed by REAf/+WAP-Cre females was measured, and the expression of genes involved in milk production was examined. As shown in Fig. 6A, the average body weights of pups nursed by REAf/+WAP-Cre dams were significantly greater than those of pups nursed by REAf/f females (GLM, F1,472 = 156; P < 0.0001). Consistent with these pup weight findings, there was significantly greater expression of genes involved in milk production (caseins, α-lactalbumin, butyrophilin, and ets domain transcription factor (Elf-5)) in the mammary gland of REAf/+WAP-Cre females (Fig. 6B) early in lactation (L2 and L5).
Fig. 6.
Increased body weight gain of pups nursed by REAf/+WAP-Cre (WC) females and its correlation with increased milk-related gene expression. A, The body weights of pups nursed by REAf/f or REAf/+WAP-Cre mice during postnatal development. The dam's genotype has a significant effect on the body weights of pups, with increased weight gain of the pups nursed by REAf/+ WAP-Cre dams (GLM, F1,472 = 156; P < 0.0001). GLM with repeated measures ANOVA. The data are mean ± sem (n = 39 or 40 per group). B, mRNA levels of REA, casein-β, casein-κ, α-lactalbumin, butyrophilin, and Elf-5 were examined by qRT-PCR at different stages of pregnancy (d 18) and lactation (d 2, 5, 9, and 14). The data are mean ± sd (n = 8 per group), and the expression was illustrated as relative expression normalized to 36B4 by wild-type P18. #, P < 0.05 vs. wild type.
Complete REA loss of function induces apoptosis of differentiated mammary epithelial cells
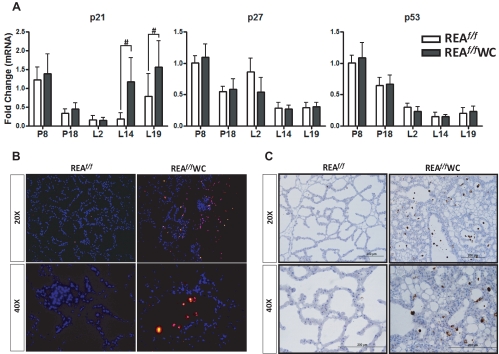
Because the REAf/f WAP-Cre mice displayed earlier alveoli collapse in the mammary gland, we investigated whether mammary epithelial cells in REAf/f WAP-Cre mice had a higher apoptotic rate, and to do so, we examined the expression of the cell cycle-regulatory proteins, p21, p27, and p53. The cyclin-dependent kinase inhibitor, p21, was significantly up-regulated in the mammary glands of REAf/f WAP-Cre compared with wild type, whereas expression of the two other genes, p27 and p53, was not affected by REA deletion (Fig. 7A). We also monitored the extent of apoptosis in the wild-type and conditional REA mutant mammary glands at L14 by TUNEL analysis, and we observed markedly increased apoptotic cell death in the REAf/f WAP-Cre mammary gland, as evidenced by increased fluorescence (Fig. 7B). Similarly, we examined activation of the apoptosis-promoting executioner, caspase-3, and as shown in Fig. 7C, cells positive for active caspase-3 were present only in the REAf/f WAP-Cre mammary glands, and apoptotic bodies were visible in luminal spaces. Collectively, these data indicate that loss of REA in the mammary epithelium during late pregnancy and lactation results in increased apoptosis and an inability to maintain the differentiated mammary alveolar structure required for optimal lactational capability.
Fig. 7.
REAf/f WAP-Cre mice display increased apoptosis of mammary epithelial cells. A, The cell cycle inhibitor p21 levels are elevated in the mammary gland of REAf/f WAP-Cre (WC) mice, as measured by qRT-PCR. The data are mean ± sd and are illustrated as relative expression normalized to 36B4 by wild-type P8. B, Representative fluorescence images of TUNEL staining in mammary gland sections from REAf/f and REAf/f WAP-Cre mammary glands of L14 mice. C, Immunohistochemical detection of active caspase-3 expression in the REAf/f and REAf/f WAP-Cre mammary glands of L14 mice. #, P < 0.05 vs. wild type.
Discussion
Studies in these mammary-specific conditional REA knockout mice have uncovered a critical relationship between gene dosage of REA and mammary gland development and functional activities at various physiological stages of the mammary gland. Genetic deletion of both REA alleles severely impeded mammary ductal morphogenesis during puberty and maintenance of differentiated lobuloalveolar structures during pregnancy and lactation, whereas a reduction of REA dosage by single allele deletion enhanced mammary ductal elongation and TEB formation during puberty and elicited hyperresponsiveness to E2. We have demonstrated that the consequences of complete REA loss in the mammary gland epithelium in REAf/f WAP-Cre mice result in caspase activation and increased apoptosis of mammary epithelial cells that markedly impaired lactational capability, whereas increased milk protein production and weight gain of pups was observed in mammary glands or REAf/+ WAP-Cre mothers with loss of a single REA allele in the mammary epithelium.
Although both E2 and progesterone are key mediators of ductal morphogenesis, E2 is generally considered to be responsible for ductal outgrowth and minor branching, whereas progesterone leads to tertiary branching and alveolar development (4, 6, 41). Our observations of accelerated ductal outgrowth in the pubertal REAf/+ PRCre/+ mammary gland suggest that REA profoundly impacts E2-mediated ductal morphogenesis. Furthermore, in an attempt to identify genes that are responsible for this accelerated ductal elongation and are also regulated by E2, we observed in ovariectomized pubertal mice that up-regulation of amphiregulin, the major paracrine mediator of estrogen-induced ductal morphogenesis, was enhanced in the mammary glands of these REA heterozygous mice compared with control mice. However, future experiments would be needed to provide support on whether the accelerated mammary outgrowth is directly associated with amphiregulin.
Our analysis of mice that are heterozygous for REA only during late pregnancy and lactation (REAf/+WAP-Cre) showed that the average body weight of the offspring nursed by REAf/+WAP-Cre mice was slightly, but statistically significantly, higher than that of pups nursed by control mice. Consistent with these body weight findings, stimulation of the genes involved in milk production (caseins, α-lactalbumin, butyrophilin, and Elf-5) was significantly greater in the mammary gland of REAf/+WAP-Cre females at early times during lactation.
It is noteworthy that REA showed a gene dosage-dependent effect on mammary gland morphogenesis and functional activities, with heterozygous REA deletion eliciting enhanced mammary gland activities and homozygous REA deletion showing impaired development and activity. REA is an evolutionarily conserved protein also known as prohibitin 2, with reported roles in transcription, mitochondrial biogenesis, and replicative senescence (18–20, 42–48). Previous reports suggest that REA is located in subcellular regions, including the nucleus and mitochondria (46), and although the molecular functions of REA are still not fully understood, REA activity has been linked to crucial nuclear transcriptional functions as well as cell proliferation and mitochondrial activities (46, 48, 49). REA can shuttle between the mitochondria and the nucleus and is a protein that has been suggested to directly couple nuclear-mitochondrial interaction (50). Thus, the phenotypic outcomes of deletion of both REA alleles in the mammary gland (cell cycle arrest and increased apoptosis of mammary cells) might be associated with defects of mitochondrial as well as nuclear activities of REA. With only partial loss of REA, where mitochondrial activities did not appear to be impaired and apoptosis of mammary cells was not observed by us, the impact of the reduced corepressor activity of REA was apparent and enhanced response to E2 and accelerated and enhanced mammary development and lactational capabilities were revealed.
Haploinsufficiency phenomena have been observed in mice previously, but only for essential coregulators with key functions as transcriptional integrators, such as thyroid receptor-associated proteincomplex 220 kDa component (TRAP220), p300, and Brg1 (51–53). Our observations therefore highlight the relevance of REA gene dosage and its importance in the regulation of stage-specific physiological functions in the mammary gland.
Because ER-mediated transcriptional activity critically depends on coregulator proteins, altered coregulator expression has also been implicated in breast cancer (54, 55). For example, one of the well-characterized coactivator proteins for ERα, (amplified in Breast Cancer 1/steroid receptor coactivator-3), is overexpressed in more than half of primary breast tumors, and there is increased development of malignant mammary tumors in transgenic mice in which AIB-1 is overexpressed in the mammary gland (54, 56, 57).
In the present study, we found that the number of TEB was significantly increased in the mammary gland of REA heterozygous mice (REAf/+ PRCre/+) compared with control mice. TEB are the structures in the mouse mammary gland that give rise to malignant mammary tumors upon exposure to a chemical carcinogen (58, 59). Similar structures in the human breast, called terminal ductal lobular unit 1, are the sites of breast cancer initiation in women (60). Thus, it is possible that a reduced level of REA in the mammary gland might enhance susceptibility to mammary tumorigenesis. Consistent with this, REA expression levels inversely correlate with breast tumor grade and are lowest in more advanced human breast tumors (55). More recently, the F-box protein Skp2B, which is often overexpressed in breast cancers, was shown to interact with REA, resulting in degradation of the REA protein (22). In the mammary gland of mouse mammary tumor virus-Skp2B mice in which Skp2B was overexpressed, REA levels were found to be low and these mice developed mammary tumors (22), suggesting that reduced levels of REA might be linked to breast tumorigenesis.
In summary, our animal models with mammary-specific conditional loss of both or only one REA allele provide in vivo evidence that REA plays important gene dosage-dependent roles in mammary gland development and in physiological activities of the mammary gland during puberty, pregnancy, and lactation stages. In the REA heterozygous mammary gland, the role of REA as a brake on nuclear hormone receptor stage-specific functional activities becomes apparent.
Supplementary Material
Acknowledgments
We thank Dr. Lothar Hennighausen of National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health for providing WAP-Cre mice and advice on their use. We also thank Karen Doty of the U54 HD055787 Microscopy Core Facility for assistance in immunohistochemistry.
This work was supported by National Institutes of Health (NIH) Grants U54 HD055787 as part of the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH Centers Program in Reproduction and Infertility Research (to B.S.K. and F.D.), P50 AT006268 from Office of Dietary Supplements (ODS), National Center for Complementary and Alternative Medicine (NCCAM), and National Cancer Institute (to B.S.K.), U54 HD07495 (to B.W.O.), R01 DK058242 (J.X.), and R01 CA077530 (J.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- 5-Bromo-2-deoxyuridine
- E2
- estradiol
- Elf-5
- ets domain transcription factor
- ER
- estrogen receptor
- Frt
- flippase recognition target
- GLM
- generalized linear model
- L
- lactation day
- P
- pregnancy day
- PND
- postnatal development
- PR
- progesterone receptor
- qRT-PCR
- quantitative RT-PCR
- REA
- repressor of ER activity
- Skp2B
- S-phase kinase-associated protein-2B
- STAT5
- signal transducer and activator of transcription 5
- TEB
- terminal end bud
- TUNEL
- terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling
- WAP
- whey acidic protein.
References
- 1. Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE. 2010. Control of mammary stem cell function by steroid hormone signalling. Nature 465:798–802 [DOI] [PubMed] [Google Scholar]
- 2. Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. 2000. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia 5:227–241 [DOI] [PubMed] [Google Scholar]
- 3. LaMarca HL, Rosen JM. 2007. Estrogen regulation of mammary gland development and breast cancer: amphiregulin takes center stage. Breast Cancer Res 9:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hennighausen L, Robinson GW. 2005. Information networks in the mammary gland. Nat Rev Mol Cell Biol 6:715–725 [DOI] [PubMed] [Google Scholar]
- 5. Hennighausen L, Robinson GW. 2001. Signaling pathways in mammary gland development. Dev Cell 1:467–475 [DOI] [PubMed] [Google Scholar]
- 6. Anderson E, Clarke RB. 2004. Steroid receptors and cell cycle in normal mammary epithelium. J Mammary Gland Biol Neoplasia 9:3–13 [DOI] [PubMed] [Google Scholar]
- 7. Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA. 2000. Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res 55:163–193; discussion 194–165 [PubMed] [Google Scholar]
- 8. Mallepell S, Krust A, Chambon P, Brisken C. 2006. Paracrine signaling through the epithelial estrogen receptor α is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA 103:2196–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Couse JF, Korach KS. 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- 10. Deroo BJ, Korach KS. 2006. Estrogen receptors and human disease. J Clin Invest 116:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, Korach KS. 2000. Induction of mammary gland development in estrogen receptor-α knockout mice. Endocrinology 141:2982–2994 [DOI] [PubMed] [Google Scholar]
- 12. Feng Y, Manka D, Wagner KU, Khan SA. 2007. Estrogen receptor-α expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci USA 104:14718–14723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- 14. McKenna NJ, O'Malley BW. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474 [DOI] [PubMed] [Google Scholar]
- 15. Gao X, Loggie BW, Nawaz Z. 2002. The roles of sex steroid receptor coregulators in cancer. Mol Cancer 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Norris JD, Paige LA, Christensen DJ, Chang CY, Huacani MR, Fan D, Hamilton PT, Fowlkes DM, McDonnell DP. 1999. Peptide antagonists of the human estrogen receptor. Science 285:744–746 [DOI] [PubMed] [Google Scholar]
- 17. Montano MM, Ekena K, Delage-Mourroux R, Chang W, Martini P, Katzenellenbogen BS. 1999. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc Natl Acad Sci USA 96:6947–6952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delage-Mourroux R, Martini PG, Choi I, Kraichely DM, Hoeksema J, Katzenellenbogen BS. 2000. Analysis of estrogen receptor interaction with a repressor of estrogen receptor activity (REA) and the regulation of estrogen receptor transcriptional activity by REA. J Biol Chem 275:35848–35856 [DOI] [PubMed] [Google Scholar]
- 19. Park SE, Xu J, Frolova A, Liao L, O'Malley BW, Katzenellenbogen BS. 2005. Genetic deletion of the repressor of estrogen receptor activity (REA) enhances the response to estrogen in target tissues in vivo. Mol Cell Biol 25:1989–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mussi P, Liao L, Park SE, Ciana P, Maggi A, Katzenellenbogen BS, Xu J, O'Malley BW. 2006. Haploinsufficiency of the corepressor of estrogen receptor activity (REA) enhances estrogen receptor function in the mammary gland. Proc Natl Acad Sci USA 103:16716–16721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simon SL, Parkes A, Leygue E, Dotzlaw H, Snell L, Troup S, Adeyinka A, Watson PH, Murphy LC. 2000. Expression of a repressor of estrogen receptor activity in human breast tumors: relationship to some known prognostic markers. Cancer Res 60:2796–2799 [PubMed] [Google Scholar]
- 22. Umanskaya K, Radke S, Chander H, Monardo R, Xu X, Pan ZQ, O'Connell MJ, Germain D. 2007. Skp2B stimulates mammary gland development by inhibiting REA, the repressor of the estrogen receptor. Mol Cell Biol 27:7615–7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. 2005. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41:58–66 [DOI] [PubMed] [Google Scholar]
- 24. Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. 1997. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res 25:4323–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park SE, Yoon S, Zhao Y, Liao L, Liu Z, Xu J, Lydon JP, DeMayo FJ, O'Malley BW, Bagchi MK, Katzenellenbogen BS. Altering coregulator concentration by conditional genetic modification: gene dosage of REA is critical for fertility and uterine function. 92nd Annual Meeting Annual Meeting of The Endocrine Society, San Diego, CA, 2010. (Abstract P3-349) [Google Scholar]
- 26. Liu X, Robinson GW, Hennighausen L. 1996. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol Endocrinol 10:1496–1506 [DOI] [PubMed] [Google Scholar]
- 27. Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC, Bagchi MK. 2008. Peroxisome proliferator-activated receptor γ is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol 28:1770–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diggle PJ. 1988. An approach to the analysis of repeated measurements. Biometrics 44:959–971 [PubMed] [Google Scholar]
- 29. Ismail PM, Li J, DeMayo FJ, O'Malley BW, Lydon JP. 2002. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol 16:2475–2489 [DOI] [PubMed] [Google Scholar]
- 30. Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM, Lubahn DB, Schomberg DW, Smith EP. 1996. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res 51:159–186; discussion 186–158 [PubMed] [Google Scholar]
- 31. Hennighausen L, Robinson GW. 1998. Think globally, act locally: the making of a mouse mammary gland. Genes Dev 12:449–455 [DOI] [PubMed] [Google Scholar]
- 32. Ciarloni L, Mallepell S, Brisken C. 2007. Amphiregulin is an essential mediator of estrogen receptor α function in mammary gland development. Proc Natl Acad Sci USA 104:5455–5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burdon T, Sankaran L, Wall RJ, Spencer M, Hennighausen L. 1991. Expression of a whey acidic protein transgene during mammary development. Evidence for different mechanisms of regulation during pregnancy and lactation. J Biol Chem 266:6909–6914 [PubMed] [Google Scholar]
- 34. Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. 2004. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol 24:8037–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li S, Rosen JM. 1994. Distal regulatory elements required for rat whey acidic protein gene expression in transgenic mice. J Biol Chem 269:14235–14243 [PubMed] [Google Scholar]
- 36. Robinson GW, McKnight RA, Smith GH, Hennighausen L. 1995. Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development 121:2079–2090 [DOI] [PubMed] [Google Scholar]
- 37. Anderson SM, Rudolph MC, McManaman JL, Neville MC. 2007. Key stages in mammary gland development. Secretory activation in the mammary gland: it's not just about milk protein synthesis! Breast Cancer Res 9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quarrie LH, Addey CV, Wilde CJ. 1996. Programmed cell death during mammary tissue involution induced by weaning, litter removal, and milk stasis. J Cell Physiol 168:559–569 [DOI] [PubMed] [Google Scholar]
- 39. Robenek H, Hofnagel O, Buers I, Lorkowski S, Schnoor M, Robenek MJ, Heid H, Troyer D, Severs NJ. 2006. Butyrophilin controls milk fat globule secretion. Proc Natl Acad Sci USA 103:10385–10390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neville MC, McFadden TB, Forsyth I. 2002. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia 7:49–66 [DOI] [PubMed] [Google Scholar]
- 41. Deroo BJ, Hewitt SC, Collins JB, Grissom SF, Hamilton KJ, Korach KS. 2009. Profile of estrogen-responsive genes in an estrogen-specific mammary gland outgrowth model. Mol Reprod Dev 76:733–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nijtmans LG, de Jong L, Artal Sanz M, Coates PJ, Berden JA, Back JW, Muijsers AO, van der Spek H, Grivell LA. 2000. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J 19:2444–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coates PJ, Nenutil R, McGregor A, Picksley SM, Crouch DH, Hall PA, Wright EG. 2001. Mammalian prohibitin proteins respond to mitochondrial stress and decrease during cellular senescence. Exp Cell Res 265:262–273 [DOI] [PubMed] [Google Scholar]
- 44. Artal-Sanz M, Tsang WY, Willems EM, Grivell LA, Lemire BD, van der Spek H, Nijtmans LG. 2003. The mitochondrial prohibitin complex is essential for embryonic viability and germline function in Caenorhabditis elegans. J Biol Chem 278:32091–32099 [DOI] [PubMed] [Google Scholar]
- 45. Kurtev V, Margueron R, Kroboth K, Ogris E, Cavailles V, Seiser C. 2004. Transcriptional regulation by the repressor of estrogen receptor activity via recruitment of histone deacetylases. J Biol Chem 279:24834–24843 [DOI] [PubMed] [Google Scholar]
- 46. Mishra S, Murphy LC, Murphy LJ. 2006. The prohibitins: emerging roles in diverse functions. J Cell Mol Med 10:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He B, Feng Q, Mukherjee A, Lonard DM, DeMayo FJ, Katzenellenbogen BS, Lydon JP, O'Malley BW. 2008. A repressive role for prohibitin in estrogen signaling. Mol Endocrinol 22:344–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Löwer B, Wunderlich FT, von Kleist-Retzow JC, Waisman A, Westermann B, Langer T. 2008. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev 22:476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Merkwirth C, Langer T. 2009. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta 1793:27–32 [DOI] [PubMed] [Google Scholar]
- 50. Kasashima K, Ohta E, Kagawa Y, Endo H. 2006. Mitochondrial functions and estrogen receptor-dependent nuclear translocation of pleiotropic human prohibitin 2. J Biol Chem 281:36401–36410 [DOI] [PubMed] [Google Scholar]
- 51. Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T. 2000. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell 6:1287–1295 [DOI] [PubMed] [Google Scholar]
- 52. Ito M, Yuan CX, Okano HJ, Darnell RB, Roeder RG. 2000. Involvement of the TRAP220 component of the TRAP/SMCC coactivator complex in embryonic development and thyroid hormone action. Mol Cell 5:683–693 [DOI] [PubMed] [Google Scholar]
- 53. Yao TP, Oh SP, Fuchs M, Zhou ND, Ch'ng LE, Newsome D, Bronson RT, Li E, Livingston DM, Eckner R. 1998. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93:361–372 [DOI] [PubMed] [Google Scholar]
- 54. Hall JM, McDonnell DP. 2005. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol Interv 5:343–357 [DOI] [PubMed] [Google Scholar]
- 55. Murphy LC, Simon SL, Parkes A, Leygue E, Dotzlaw H, Snell L, Troup S, Adeyinka A, Watson PH. 2000. Altered expression of estrogen receptor coregulators during human breast tumorigenesis. Cancer Res 60:6266–6271 [PubMed] [Google Scholar]
- 56. Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968 [DOI] [PubMed] [Google Scholar]
- 57. Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M, Sellers WR, Brown M. 2004. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 6:263–274 [DOI] [PubMed] [Google Scholar]
- 58. Russo J, Gusterson BA, Rogers AE, Russo IH, Wellings SR, van Zwieten MJ. 1990. Comparative study of human and rat mammary tumorigenesis. Lab Invest 62:244–278 [PubMed] [Google Scholar]
- 59. Hilakivi-Clarke L. 2007. Nutritional modulation of terminal end buds: its relevance to breast cancer prevention. Curr Cancer Drug Targets 7:465–474 [DOI] [PubMed] [Google Scholar]
- 60. Russo J, Hu YF, Yang X, Russo IH. 2000. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr 17–37 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.