Abstract
Objective
Sodium nitroprusside-enhanced CPR (SNPeCPR) consists of active compression decompression (ACD), impedance threshold device (ITD), abdominal binding (AB), and large intravenous doses of sodium nitroprusside (SNP). We hypothesize SNPeCPR will significantly increase carotid blood flow and return of spontaneous circulation compared to standard CPR after prolonged ventricular fibrillation (VF) and pulseless electrical activity (PEA) cardiac arrest.
Design
Prospective randomized animal study.
Setting
Hennepin County Medical Center Animal Laboratory.
Subjects
40 Yorkshire female farm-bred pigs weighing 32 ± 2kg.
Interventions
In protocol A, 24 isoflurane anesthetized pigs underwent 15 minutes of untreated VF and were subsequently randomized to receive S-CPR (n=6), ACD CPR +ITD (n=6), or SNPeCPR (n=12) for up to 15 minutes. First defibrillation was attempted at minute 6 of CPR. In protocol B, a separate group of 16 pigs underwent 10 minutes of untreated VF followed by 3 minutes of chest compression only CPR followed by counter-shock induced PEA after which animals were randomized to S-CPR (n=8) or SNPeCPR (n=8).
Measurements and Main Results
The primary endpoint was carotid blood flow during CPR and return of spontaneous circulation (ROSC). Secondary endpoints included end-tidal CO2 (ETCO2) as well as coronary (CPP) and cerebral perfusion pressure (CerPP). After prolonged untreated VF, SNPeCPR demonstrated superior rates of ROSC when compared to S-CPR and ACD CPR+ITD (12/12, 0/6, 0/6 respectively, p<0.01). In animals with PEA, SNPeCPR increased ROSC rates when compared to S-CPR. In both groups, carotid blood flow, CPP, CerPP, and ETCO2 were increased with SNPeCPR.
Conclusions
In pigs, SNPeCPR significantly increased ROSC rates as well as carotid blood flow and ETCO2, when compared to S-CPR or ACD CPR+ITD.
Keywords: Vasodilators, CPR, neurological function, resuscitation rates, carotid blood flow
Introduction
Sodium nitroprusside (SNP) enhanced cardiopulmonary resuscitation, or SNPeCPR, is a new method of CPR. It consists of three components:
-
1)
Active compression-decompression CPR with an impedance threshold device (ACD CPR+ITD) which enhances active venous return, improves hemodynamics, and provides a superior method of thoracic compressions compared to standard CPR as previously demonstrated in animal and randomized clinical trials[1–3].
-
2)
Large doses of intravenous sodium nitroprusside to decrease peripheral vascular resistance, and
-
3)
Lower abdominal binding that mechanically increases lower body resistance to descending aortic blood flow and effectively redistributes blood flow to the thorax and brain.
We have previously shown that when SNPeCPR is applied after eight minutes of untreated ventricular fibrillation (a common clinical scenario), vital organ viability can be maintained for up to 25 minutes of continuous CPR. In the same setting, SNPeCPR significantly improves all hemodynamic parameters including carotid blood flow, end-tidal CO2 (ETCO2), rates of return to spontaneous circulation (ROSC), and 24-hour survival with good neurological outcomes compared to standard CPR (S-CPR). [4]
When VF is left untreated for more than 10 minutes, the probability of successful ROSC decreases drastically. [5] Most reported animal experiments demonstrate minimal success in resuscitation rates after 10–12 minutes of untreated VF. [6–8] Furthermore, it has been observed in many clinical trials that when CPR and resuscitation efforts are delayed more than 10 minutes, short and long-term resuscitation rates are extremely poor. [1, 9]
The effects of SNPeCPR on resuscitation rates after prolonged periods of untreated cardiac arrest have not been investigated. We hypothesized that 1) SNPeCPR can significantly improve ROSC rates after 15 minutes of untreated VF compared to S-CPR and ACD CPR+ITD and 2) SNPeCPR will improve ROSC rates following a simulated period of bystander CPR in a pulseless electrical activity model of cardiac arrest.
Materials and Methods
This study was approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation of Hennepin County Medical Center. All animal care was compliant with the National Research Council's 1996 Guidelines for the Care and Use of Laboratory Animals. All studies were performed by a qualified, experienced research team in Yorkshire female farm-bred pigs weighing 32 ± 2kg. A certified and licensed veterinarian assured the protocols were performed in accordance with the National Research Council's Guidelines.
Animals in both protocols had the same preparation.
Preparatory Phase
The surgical preparation, anesthesia, data monitoring, and recording procedures used in this study have been previously described. [10] Under aseptic surgical conditions, we used initial sedation with intramuscular ketamine (7 mL of 100 mg/mL, Ketaset, Fort Dodge Animal Health, Fort Dodge, Iowa) followed by inhaled isoflurane. A catheter (Mikro-Tip Transducer, Millar Instruments Houston, Texas) was placed through a burr hole to enable real-time recording of intracranial pressure. Central aortic blood pressure was recorded continuously with a catheter (Mikro-Tip Transducer, Millar Instruments, Houston, Texas) placed in the descending thoracic aorta. A second Millar catheter was inserted in the right atrium via the right external jugular vein. An ultrasound flow probe (Transonic 420 series multichannel, Transonic Systems, Ithaca, New York) in the left common carotid artery was placed to quantify carotid blood flow (ml/min). All data were recorded with a digital recording system (Superscope II version 1.295, GW Instruments, Somerville, Massachusetts). Partial pressure of end-tidal CO2 (ETCO2), tidal volume, minute ventilation, and blood oxygen saturation were continuously measured with a respiratory monitor (CO2SMO Plus, Novametrix Medical Systems, Wallingford, Connecticut).
Measurements and Recording
Aortic pressure, right atrial pressure, intracranial pressure, ETCO2, and carotid blood flow were continuously recorded. Coronary perfusion pressure (CPP) was calculated from the difference between right atrial pressure and aortic pressure during the decompression phase of CPR. Cerebral perfusion pressure (CerPP) was calculated from the difference between the mean values of aortic pressure and intracranial pressure based on the recorded waveforms. Ultrasound derived carotid blood flow was reported in ml/sec. ROSC was defined using the Utstein guidelines for uniform reporting in animal research. [11] Arterial blood gases were obtained at baseline, at 5 min CPR, and 30 minutes following ROSC.
Experimental Protocol
Upon completion of the surgical preparation, VF was induced by delivering direct current via a temporary pacing wire (Daig Division, St Jude Medical, Minnetonka, Minnesota) positioned in the right ventricle. The ventilator was disconnected from the endotracheal tube. In each protocol, treatment assignment was randomized during the untreated VF period based on a computer generated randomized list.
After 15 min of untreated VF (protocol A) and 10 minutes of VF (protocol B), closed-chest standard CPR (standard compression mode) was performed with a pneumatically driven automatic piston device (Pneumatic Compression Controller, Ambu International, Glostrup, Denmark) as previously described. [12] Uninterrupted chest compressions (100 compressions/min) with a 50% duty cycle and a compression depth of 25% of the anterior-posterior chest diameter were provided. During CPR, asynchronous positive-pressure ventilations were delivered to simulate Advanced Life Support with a manual resuscitator bag (Smart Bag, O2 Systems, Toronto, Ontario, Canada). The fraction of inspired oxygen was 1.0, the tidal volume was maintained at ~10ml/kg and the respiratory rate was 10 breaths/min.
Manual abdominal binding was added to provide approximately 50 lbs of force (upper body weight of one investigator leaning forward at a 45 degree angle as measured on a scale) to the lower half of the abdomen.
The investigators were blinded to hemodynamics during CPR. Blinding of ACD CPR and abdominal binding was not possible.
Protocol A
A total of 24 animals had untreated VF for 15 minutes and were subsequently randomized to: 1) SNPeCPR (12 animals) or 2) S-CPR (6 animals)[13], or 3) ACD CPR +ITD (6 animals)[1].
SNPeCPR group
This group immediately received ACD CPR +ITD+AB as previously described. [4] In addition, animals received 2 mg of intravenous sodium nitroprusside at minute 1 of CPR and 1 mg at minute 3 of CPR. The first defibrillation shock (150 J biphasic) was attempted at minute 6 of CPR. If after 3 shocks animals did not have successful ROSC, they received 0.015mg/kg of epinephrine intravenously. If ROSC was not achieved within the next two minutes, another dose of 1 mg of SNP was administered and the above cycle was repeated (only two animals required epinephrine). CPR was continued for up to 15 minutes at which point if there was no ROSC, the attempted resuscitation was terminated.
S-CPR group
This group received S-CPR as recommended but the AHA Guidelines 2010 with an advanced airway.[14, 15] Chest wall decompression was passive.
ACD CPR+ITD group
The third group received ACD CPR with an inspiratory impedance threshold device (ITD) (ResQPOD® Advanced Circulatory Systems, Roseville, Minnesota) as previously described.[16]
The first dose of epinephrine (0.015mg/kg) was given in the S-CPR and ACD CPR +ITD at minute 5 of CPR and every 5 minutes thereafter if ROSC was not achieved after the first 3 DC shocks.
All survivors were followed for 1 hour. A transthoracic echocardiogram was performed at that time to evaluate left ventricular ejection fraction from the right parasternal window (providing a similar view as the long and short parasternal windows for humans) (16). Ejection fraction was assessed with trace planimetry and reported by a blinded echocardiographer unrelated to the study.
Intracardiac echocardiographic evaluation of left ventricular dimensions during CPR
12 animals (6 randomized to SNPeCPR and 6 to SCPR) had an intracardiac ultrasound probe (Acuson Acunav™ diagnostic ultrasound catheter, Siemens, Washington DC, USA) placed in the RV and the left ventricular short axis view centered at the mid papillary level obtained during the untreated VF period. After initiation of CPR and at minute 5, before the first DC shock, video images were collected and the diameters of the LV cavity and septal wall were measured at the end of the decompression phase just before the initiation of the next compression cycle for 3 cycles. Measurements of the 3 cycles were averaged and were reported as the individual measurements for each animal. The end-diastolic (end of decompression) LV cavity and septal wall dimensions were reported for each of the two primary interventions (SNPeCPR and S-CPR) as mean ± SD.
Protocol B
16 animals had 10 minutes of untreated VF followed by 3 minutes of chest compression only CPR (simulating bystander CPR). This was followed by a single 150J DC shock at minute 3 of CPR to induce PEA based on the model described in the study by Halperin et al.[17] All animals developed PEA as documented by a 10 second pause at minute 3 of CPR when central aortic pressure and ECG were reviewed. Subsequently, animals were randomized in two groups: 1) S-CPR with 0.015mg/kg epinephrine (8 controls) at minute 5 and then every 5 minutes and 2) SNPeCPR (8 animals) performed as in Protocol A (simulating an ACLS intervention). CPR efforts were continued for up to 15 minutes and if ROSC was not achieved the study was terminated. Survivors were followed for one hour. If animals developed VF at any time during the CPR efforts, defibrillation was attempted. Animals with successful ROSC were followed for one hour.
Statistical analysis
Values are expressed as mean ± standard deviation. Baseline data between groups were compared using the t-test. Hemodynamics and blood gases during CPR were analyzed with single factor ANOVA for Protocol A and with non-paired t-test for protocol B. A 2-tailed Fischer exact test was used to compare ROSC rates. A p-value of <0.05 was considered statistically significant. LV dimensions were compared between the groups using a non-paired t-test.
Results
There were no significant differences between baseline hemodynamic and blood gas characteristics in the animals that received SNPeCPR and the controls in either protocol.
Protocol A (15 minutes of untreated VF)
After 15 minutes of untreated VF, SNPeCPR achieved ROSC in 12/12 animals with an average of 2±1 shocks. S-CPR and ACD CPR+ITD with epinephrine was uniformly unsuccessful in achieving ROSC (0/6 and 0/6 respectively).
SNPeCPR significantly improved coronary perfusion pressure and carotid blood flow compared with both control groups. The carotid blood flow during CPR was similar to baseline carotid blood flow pre-arrest. (Table 1)
Table 1. Hemodynamics and Resuscitation Rates for Protocol A.
Values are shown ± SD. CPR with SNPeCPR, ACD CPR+ITD and S-CPR.
| Base line | 2 min CPR | 6 min CPR | # shocks to 1st sucesful defibrilation | ||
|---|---|---|---|---|---|
| SNPeCPR | SBP | 18±14 | 67±9 | 95±13 | 2±1 |
| DBP | 81±15 | 29±4 | 48±9 | ||
| RA | 2±1 | 6±2 | 7±2 | ||
| CPP | 79±8 | 23±2 | 41±6 | ||
| CBF | 403±70 | 427±92 | 489±122 | ||
| S-CPR | SBP | 107±20 | 55±6* | 68±8* | N/A |
| DBP | 78±12 | 12±3* | 28±5* | ||
| RA | 2±2 | 2±2* | 3±2* | ||
| CPP | 76±11 | 10±3* | 25±3* | ||
| CBF | 389±82 | 99±45 * | 56±32 * | ||
| ACD+ITD CPR | SBP | 109±13 | 62±7* | 71±12* | N/A |
| DBP | 76±9 | 16±3* | 32±9* | ||
| RA | 2±1 | 0±1* | 0±2* | ||
| CPP | 73±8 | 16±2* | 32±6* | ||
| CBF | 393±78 | 188±55 * | 166±81 * | ||
All pressures in mm Hg, all flows in ml/min. SBP= systolic blood pressure, DBP= diastolic blood pressure, RA= right atrial pressure, CPP= coronary perfusion pressure, CBF= carotid blood flow.
means statistically significant difference with p<0.05 compared to SNPeCPR.
Blood gases demonstrated less metabolic acidosis in the SNPeCPR groups 5 minutes into CPR and a normalization of pH and acidosis within 30 minutes of ROSC. Additionally, ETCO2 was significantly higher during CPR in the SNPeCPR group. (Table 2)
Table 2. Arterial Blood Gasses during CPR and after ROSC.
Mean ± SD. Arterial blood gas measurements at baseline, during CPR, and after ROSC.
| Baseline | 5 min CPR | 30 min ROSC | ||
|---|---|---|---|---|
| SNPeCPR | pH | 7.35±0.2 | 7.2±0.1 | 7.38±0.06 |
| pCO2 | 43±3 | 46±6 | 36±46 | |
| pO2 | 95±13 | 182±63 | 102±13 | |
| HCO3 | 23±2 | 15±3 | 21±2 | |
| SaO2 | 98±2 | 100 | 100 | |
| ETCO2 | 38±4 | 32±4 | 37±4 | |
| S-CPR | pH | 7.41±0.2 | 7.11±0.1* | N/A |
| pCO2 | 42±4 | 46±6 | N/A | |
| pO2 | 93±8 | 195±65 | N/A | |
| HCO3 | 22±2 | 10±4* | N/A | |
| SaO2 | 96±3 | 100 | N/A | |
| ETCO2 | 38±4 | 8±3* | N/A | |
| ACD CPR+ITD | pH | 7.37±0.1 | 7.13±0.1* | N/A |
| pCO2 | 39±3 | 43±7 | N/A | |
| pO2 | 91±7 | 172±57 | N/A | |
| HCO3 | 24±3 | 11±3* | N/A | |
| SaO2 | 97±5 | 100 | N/A | |
| ETCO2 | 38±4 | 15±4* | N/A | |
Partial pressures in torr, SaO2= percent oxygen saturation, HCO3= bicarbonate, ETCO2= end-tidal CO2..
means statistically significant difference with p<0.05 compared to SNPeCPR.
Intra-CPR left ventricular dimensions
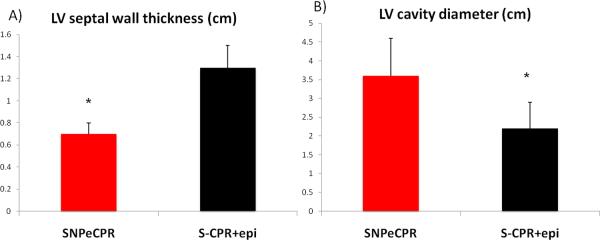
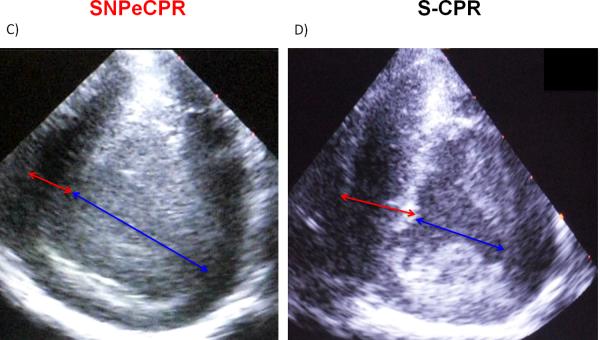
During SNPeCPR the left ventricular end-of-decompression mid cavity diameter was significantly longer and the septal wall was significantly thinner than the S-CPR with epinephrine. (Figure 1)
Figure 1. Intra-CPR Left Ventricular Dimensions in S-CPR and SNPeCPR.
all measurements are recorded at end-diastole (end of thoracic decompression). The blue arrow represents the LV cavity dimensions and the red arrow represents the interventricular septal thickness. * means statistically significant difference with p<0.05 compared to SNPeCPR.
Left Ventricular Ejection Fraction at one hour
In the SNPeCPR group, left ventricular function at 60 minutes post-ROSC was low normal with no wall motion abnormalities and an ejection fraction calculated as 52±9%.
Protocol B
(Counter-shock induced PEA after 10 minutes of untreated VF and 3 minutes of CCO-CPR).
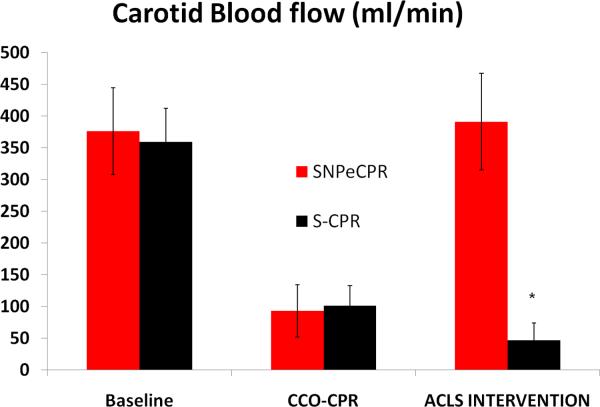
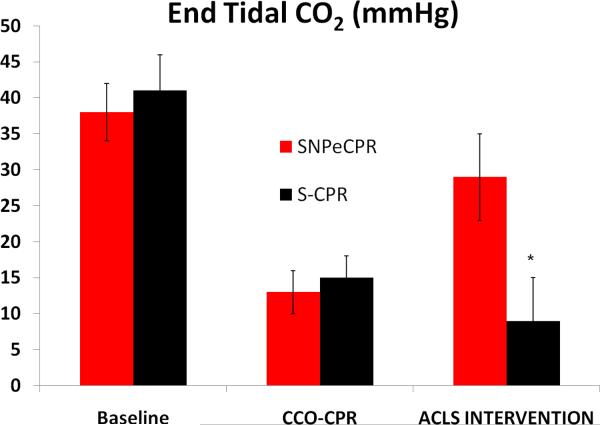
SNPeCPR resulted in significant improvement in carotid blood flow and ETCO2 compared to standard CPR with epinephrine when it was applied after chest compression only CPR. (Figure 2a, b) SNPeCPR resulted in a significant improvement in ROSC compared to S-CPR (7/8 versus 0/8, p<0.01). (Table 3)
Figure 2. Carotid Blood Flow and End-Tidal CO2.
(A) Carotid blood flow at baseline, during chest-compression only CPR, and during advanced cardiac life support in protocol B. Note the significant increase in flow during ACLS in animals receiving SNPeCPR compared to S-CPR. (B) End-tidal CO2 at baseline, during chest-compression only CPR, and during ACLS. Note the significant increase in ETCO2 in animals receiving SNPeCPR compared to S-CPR. * means statistically significant difference with p<0.05 compared to SNPeCPR.
Table 3. Hemodynamics and Resuscitation Rates for Protocol B.
Values are shown ± SD. 10 minutes of untreated VF followed by 3 minutes of CCO-CPR.
| Baseline | CCO-CPR | SNPeCPR | 1 hour Survival | ||
|---|---|---|---|---|---|
|
SNPeCPR
ACLS Group |
SBP | 111±9 | 42±10 | 85±13 | 7/8 |
| DBP | 74±10 | 18±4 | 40±5 | ||
| RA | 2±1 | 2±2 | 6±7 | ||
| CPP | 72±8 | 16±3 | 34±5 | ||
| ICP | 11±3 | 22±3 | 27±6 | ||
| CerPP | 82±8 | 8±4 | 36±5 | ||
| Baseline | CCO-CPR | S-CPR | 1 hour Survival | ||
|
S-CPR
ACLS Group |
SBP | 108±10 | 45±8 | 52±5* | 0/8 * |
| DBP | 68±7 | 18±5 | 22±4* | ||
| RA | 1 ±2 | 4±3 | 3±4* | ||
| CPP | 67±6 | 14±2 | 19±8* | ||
| ICP | 12±4 | 21±3 | 20±5* | ||
| CerPP | 76±9 | 10.5±3 | 17±3* | ||
All pressures in mm Hg, all flows in ml/min. SBP= systolic blood pressure, DBP= diastolic blood pressure, RA= right atrial pressure, CPP= coronary perfusion pressure, ICP= intracranial pressure, CerPP= cerebral perfusion pressure, CCO-CPR= chest compression only CPR.
means statistically significant difference with p<0.05 compared to SNPeCPR.
Five animals that received SNPeCPR developed spontaneous electromechanical coupling that resulted in ROSC. The other two animals that had successful ROSC, converted into course VF after 5 and 6 minutes respectively. VF was successfully shocked into sinus tachycardia In S-CPR animals, PEA degenerated into fine VF and asystole that was not amenable to DC shocks. Only two animals in the S-CPR had DC shocks attempted, both were unsuccessful.
Arterial blood gases showed significantly greater metabolic acidosis in the S-CPR group.
Discussion
Our study, for the first time, has demonstrated that sodium nitroprusside enhanced CPR (SNPeCPR) has the ability to significantly improve resuscitation rates in two porcine models of prolonged untreated cardiac arrest due to ventricular fibrillation and counter-shock-induced PEA. S-CPR in the same settings was uniformly unsuccessful in restoring ROSC.
SNPeCPR provided superior hemodynamics and optimized forward blood flow (as evidenced by significantly increased carotid blood flow and ETCO2) while also delivering higher vital organ perfusion pressures. Carotid blood flow was 8 to 10-fold higher in the SNPeCPR group compared S-CPR with epinephrine in conjunction with higher coronary and cerebral perfusion pressures. The addition of a vasodilator in the setting of a very effective mechanical CPR platform led to >3-fold increase of ETCO2. This supports our initial contention that flow can be nearly normalized during CPR and can lead to significant improvement in resuscitation rates even after prolonged untreated cardiac arrest. We are unaware of any other clinically practical, non-invasive CPR method that could achieve consistent ROSC rates after 15 minutes of untreated VF.
SNPeCPR led to a significant improvement in hemodynamics when it was used as a simulated ACLS intervention after chest compression only CPR and counter-shock induced PEA. We hypothesize that large doses of SNP given during simulated ACLS combined with mechanical adjuncts optimize vital organ perfusion pressures and redirect blood flow to the brain and thorax, significantly improving myocardial perfusion, and facilitating spontaneous electro-mechanical coupling and ROSC. In 2 animals, PEA converted into very coarse VF that was successfully shocked into sinus tachycardia. Conversely, S-CPR animals that received epinephrine all degenerated after 5–7 minutes into fine VF or asystole that was not amenable to successful defibrillation.
Contrary to the previous description by Kern et al., we did not observe significant left ventricular dysfunction in the animals that received SNPeCPR in both protocols despite similar or longer untreated cardiac arrest times.[18] However, we did not have the ability to resuscitate any of the control animals. Exogenous nitric oxide donation has been shown to be protective from ischemiareperfusion injury after prolonged arrest in transplanted hearts in a scenario similar to cardiac arrest, although in the present study nitric oxide was not directly examined.[19]
We did identify a significantly larger left ventricular cavity and thinner walls when SNPeCPR was performed compared to S-CPR with epinephrine. This could be a direct effect of SNP or the effect of higher coronary perfusion. Regardless of the etiology of this observation, it is clear that much higher blood flow can be achieved through a larger, flaccid left ventricle compared to a smaller cavity with thicker, partially contracted walls. Ischemic contracture has been previously described during CPR and indicates a poor prognosis.[20, 21] Other investigators' efforts have focused on reducing ischemic contracture as a target to improve blood flow and outcomes during CPR.[22, 23] SNPeCPR appears to achieve improved left ventricular mechanics, however the mechanism remains elusive.
Our study has several limitations. First, we did not assess neurological function. A study on the effects of SNPeCPR on neurologic function has been completed and will be reported shortly. Second, although the rationale for implementing several interventions at one time is outlined in the introduction, this combination of interventions makes it difficult to assess each individual impact on the observed result. In our recent study we have demonstrated the need for SNP in addition to the mechanical platform in order to optimize neurological function at 24 hours.[4] Third, we cannot be certain that the results from our porcine model can be translated to humans. Finally, our models used open coronary arteries without stenosis, and therefore the applicability of SNPeCPR in an ischemic model of cardiac arrest needs to be further investigated.
Conclusions
SNPeCPR significantly increased ROSC rates, carotid blood flow, ETCO2, coronary and cerebral perfusion pressures after prolonged VF arrest and counter shock induced PEA when compared to S-CPR.
Acknowledgments
Financial support: The study was funded by an Institutional, Division of Cardiology grant at the University of Minnesota and R01 HL108926-01 NIH grant to Dr. Yannopoulos.
Footnotes
Conflict of interest: The authors have not disclosed any potential conflicts of interest.
Disclosure: Preliminary data from this manuscript were reported in the Resuscitation Science Symposium in Chicago 2010
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Aufderheide TP, Frascone RJ, Wayne MA, Mahoney BD, Swor RA, Domeier RM, Olinger M, Holcomb RG, Tupper DE, Yannopoulos D, et al. Comparative Effectiveness of an Impedance Threshold Device and Active Compression Decompression CPR versus Standard CPR for Treatment of Out-of Hospital Cardiac Arrest. The Lancet. 2011;377:301–311. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plaisance P, Lurie KG, Payen D. Inspiratory impedance during active compression-decompression cardiopulmonary resuscitation: a randomized evaluation in patients in cardiac arrest. Circulation. 2000;101(9):989–994. doi: 10.1161/01.cir.101.9.989. [DOI] [PubMed] [Google Scholar]
- 3.Wolcke BB, Mauer DK, Schoefmann MF, Teichmann H, Provo TA, Lindner KH, Dick WF, Aeppli D, Lurie KG. Comparison of standard cardiopulmonary resuscitation versus the combination of active compression-decompression cardiopulmonary resuscitation and an inspiratory impedance threshold device for out-of-hospital cardiac arrest. Circulation. 2003;108(18):2201–2205. doi: 10.1161/01.CIR.0000095787.99180.B5. [DOI] [PubMed] [Google Scholar]
- 4.Yannopoulos D, Matsuura T, Schultz J, Rudser K, Halperin H, Lurie KG. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves survival with good neurological function in a porcine model of prolonged cardiac arrest. Crit Care Med. 2011 doi: 10.1097/CCM.0b013e31820ed8a6. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummins RO, Thies W. Encouraging early defibrillation: the American Heart Association and automated external defibrillators. Ann Emerg Med. 1990;19(11):1245–1248. doi: 10.1016/s0196-0644(05)82282-6. [DOI] [PubMed] [Google Scholar]
- 6.Högler S, Sterz F, Sipos W, Schratter A, Weihs W, Holzer M, Janata A, Losert U, Behringer W, Tichy A, et al. Distribution of neuropathological lesions in pig brains after different durations of cardiac arrest. Resuscitation. 2010;81:1577–1583. doi: 10.1016/j.resuscitation.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Berg R, Hilwig R, Berg M, Berg D, Samson R, Indik J, Kern K. Immediate post-shock chest compressions improve outcome from prolonged ventricular fibrillation. Resuscitation. 2008;78(1):71–76. doi: 10.1016/j.resuscitation.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staffey KS, Dendi R, Brooks LA, Pretorius AM, Ackermann LW, Zamba KD, Dickson E, Kerber RE. Liquid ventilation with perfluorocarbons facilitates resumption of spontaneous circulation in a swine cardiac arrest model. Resuscitation. 2008;78(1):77–84. doi: 10.1016/j.resuscitation.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plaisance P, Lurie KG, Vicaut E, Martin D, Gueugniaud PY, Petit JL, Payen D. Evaluation of an impedance threshold device in patients receiving active compression-decompression cardiopulmonary resuscitation for out of hospital cardiac arrest. Resuscitation. 2004;61(3):265–271. doi: 10.1016/j.resuscitation.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Yannopoulos D, Matsuura T, McKnite S, Goodman N, Idris A, Tang W, Aufderheide TP, Lurie KG. No assisted ventilation cardiopulmonary resuscitation and 24-hour neurological outcomes in a porcine model of cardiac arrest. Crit Care Med. 2010;38(1):254–260. doi: 10.1097/CCM.0b013e3181b42f6c. [DOI] [PubMed] [Google Scholar]
- 11.Idris AH, Becker LB, Ornato JP, Hedges JR, Bircher NG, Chandra NC, Cummins RO, Dick W, Ebmeyer U, Halperin HR, et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a task force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Institute of Critical Care Medicine, the Safar Center for Resuscitation Research, and the Society for Academic Emergency Medicine. Writing Group. Circulation. 1996;94(9):2324–2336. doi: 10.1161/01.cir.94.9.2324. [DOI] [PubMed] [Google Scholar]
- 12.Shultz JJ, Coffeen P, Sweeney M, Detloff B, Kehler C, Pineda E, Yakshe P, Adler SW, Chang M, Lurie KG. Evaluation of standard and active compression-decompression CPR in an acute human model of ventricular fibrillation. Circulation. 1994;89(2):684–693. doi: 10.1161/01.cir.89.2.684. [DOI] [PubMed] [Google Scholar]
- 13.Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S729–767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 14.Berg RA, Hemphill R, Abella BS, Aufderheide TP, Cave DM, Hazinski MF, Lerner EB, Rea TD, Sayre MR, Swor RA. Part 5: adult basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S685–705. doi: 10.1161/CIRCULATIONAHA.110.970939. [DOI] [PubMed] [Google Scholar]
- 15.Sayre MR, Koster RW, Botha M, Cave DM, Cudnik MT, Handley AJ, Hatanaka T, Hazinski MF, Jacobs I, Monsieurs K, et al. Part 5: adult basic life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122(16 Suppl 2):S298–324. doi: 10.1161/CIRCULATIONAHA.110.970996. [DOI] [PubMed] [Google Scholar]
- 16.Lurie KG, Coffeen P, Shultz J, McKnite S, Detloff B, Mulligan K. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91(6):1629–1632. doi: 10.1161/01.cir.91.6.1629. [DOI] [PubMed] [Google Scholar]
- 17.Halperin HR, Lee K, Zviman M, Illindala U, Lardo A, Kolandaivelu A, Paradis NA. Outcomes from low versus high-flow cardiopulmonary resuscitation in a swine model of cardiac arrest. Am J Emerg Med. 2010;28(2):195–202. doi: 10.1016/j.ajem.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Kern KB, Hilwig RW, Berg RA, Rhee KH, Sanders AB, Otto CW, Ewy GA. Postresuscitation left ventricular systolic and diastolic dysfunction. Treatment with dobutamine. Circulation. 1997;95(12):2610–2613. doi: 10.1161/01.cir.95.12.2610. [DOI] [PubMed] [Google Scholar]
- 19.Ali IS, Gandhi M, Finegan BA, Koshal A, Clanachan AS. Cardioprotection by activation of NO/cGMP pathway after cardioplegic arrest and 8-hour storage. Ann Thorac Surg. 1998;65(5):1303–1309. doi: 10.1016/s0003-4975(98)00182-9. [DOI] [PubMed] [Google Scholar]
- 20.Takino M, Okada Y. Firm myocardium in cardiopulmonary resuscitation. Resuscitation. 1996;33:101–106. doi: 10.1016/s0300-9572(96)00995-1. [DOI] [PubMed] [Google Scholar]
- 21.Klouche K, Weil MH, Sun S, Tang W, Povoas HP, Kamohara T, Bisera J. Evolution of the stone heart after prolonged cardiac arrest. Chest. 2002;122(3):1006–1011. doi: 10.1378/chest.122.3.1006. [DOI] [PubMed] [Google Scholar]
- 22.Gazmuri RJ, Ayoub IM, Hoffner E, Kolarova JD. Successful ventricular defibrillation by the selective sodium-hydrogen exchanger isoform-1 inhibitor cariporide. Circulation. 2001;104(2):234–239. doi: 10.1161/01.cir.104.2.234. [DOI] [PubMed] [Google Scholar]
- 23.Ayoub IM, Kolarova J, Kantola RL, Sanders R, Gazmuri RJ. Cariporide minimizes adverse myocardial effects of epinephrine during resuscitation from ventricular fibrillation. Crit Care Med. 2005;33(11):2599–2605. doi: 10.1097/01.ccm.0000186773.88576.83. [DOI] [PubMed] [Google Scholar]