Abstract
Continuous monitoring of one’s performance is invaluable for guiding behavior towards successful goal attainment by identifying deficits and strategically adjusting responses when performance is inadequate. In the present study, we exploited the advantages of event-related functional magnetic resonance imaging (fMRI) to examine brain activity associated with error-related processing after severe traumatic brain injury (sTBI). fMRI and behavioral data were acquired while 10 sTBI participants and 12 neurologically-healthy controls performed a task-switching cued-Stroop task. fMRI data were analyzed using a random-effects whole-brain voxel-wise general linear model and planned linear contrasts. Behaviorally, sTBI patients showed greater error-rate interference than neurologically-normal controls. fMRI data revealed that, compared to controls, sTBI patients showed greater magnitude error-related activation in the anterior cingulate cortex (ACC) and an increase in the overall spatial extent of error-related activation across cortical and subcortical regions. Implications for future research and potential limitations in conducting fMRI research in neurologically-impaired populations are discussed, as well as some potential benefits of employing multimodal imaging (e.g., fMRI and event-related potentials) of cognitive control processes in TBI.
Keywords: traumatic brain injury, TBI, cognitive control, performance monitoring, anterior cingulate cortex, ACC
1. Introduction
Physical and neurobehavioral impairments are common sequelae of traumatic brain injury (TBI; Horn & Sherer, 1999), however, even in patients with good neurological recovery, persistent cognitive deficits are among the most pronounced and frequent complaints of TBI survivors (Cicerone et al., 2005; Lovell & Franzen, 1994). Severity-related impairments in “cognitive control,” a set of higher-order executive processes supported by the prefrontal cortex and critical to executive function (Lorist, Boksem, & Ridderinkhof, 2005; Miller, 2000; Miller & Cohen, 2001), are thought to underlie some aspects of enduring cognitive dysfunction after brain injury (Larson, Farrer, & Clayson, in press; Larson, Perlstein, Demery, & Stigge-Kaufman, 2006; Larson, Stigge-Kaufman, Schmulfass, & Perlstein, 2007a; Perlstein, Cole, Dixit, & Demery, 2004; Perlstein, Larson, Dotson, & Kelly, 2006; Scheibel et al., 2007; Seignourel et al., 2005; Soeda et al., 2005), and current theories of neurobehavioral dysfunction in TBI have been based on observed impairments in cognitive control component processes (Anderson, Levin, & Jacobs, 2002; Burgess & Robertson, 2002; Larson et al., 2006; Larson et al., 2007a; Levine, Katz, Dade, & Black, 2002; Perlstein et al., 2006).
Numerous functional magnetic resonance imaging (fMRI) studies (i.e., Carter, Botvinick, & Cohen et al., 1999; MacDonald, Cohen, Stenger, & Carter, 2000) suggest that cognitive control comprises two broad component processes implemented in a closely interactive, yet dissociable frontal neural network: a regulative/strategic component supporting the maintenance of task goals, allocation of limited attentional resources, and the implementation of top-down control (MacDonald et al., 2000), and an anterior cingulate cortex (ACC)-mediated evaluative component that supports conflict processing and performance monitoring (i.e., Carter & van Veen, 2007; Kerns et al., 2004; van Veen & Carter, 2002; van Veen & Carter, 2006). These evaluative monitoring processes serve to adjust behavioral performance toward goal attainment based on the detection of performance errors (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004). Continuous performance monitoring is important for guiding behavior towards successful goal-attainment by detecting deficiencies and strategically adjusting responses when current performance is inadequate. An understanding of the neural basis underlying error-related processing is critical not only to identifying the mechanisms through which cognitive control is executed, but also because impairments in self-awareness in TBI patients may partially arise from impaired performance-monitoring abilities (Larson & Perlstein, 2009; O’Keeffe, Dockree, & Robertson, 2004).
Whereas numerous studies of impaired executive function following brain injury have focused their attention on examining the impairment of top-down regulative processes of cognitive control that rely heavily on the dlPFC (i.e., Christodoulou et al., 2001; Larson et al., 2006; McAllister et al., 2001; Perlstein, Cole, Dixit, & Demery, 2004, Perlstein et al., 2006; Seignourel et al., 2005), research examining the impairment of performance monitoring functions of cognitive control, or the potential role that alterations in ACC function contribute to cognitive dysfunction after brain injury is limited (i.e., Larson et al., 2007a; Scheibel et al., 2003; Soeda et al., 2005). Importantly, available neuroimaging and electrophysiological findings from studies conducted both in- and-outside of our laboratory have provided evidence demonstrating alterations in ACC-mediated evaluative activity in TBI patients. Specifically, electrophysiological studies have demonstrated that TBI patients display attenuated scalp-recorded event-related potential (ERP) components thought to reflect ACC-mediated evaluative monitoring aspects of control including the conflict-related N450 (Perlstein et al., 2006), error-related negativity (ERN; Larson et al., 2007a; Stemmer, Segalowitz, Witzke, & Schonle, 2004), and feedback-related negativity (FRN; Larson, Kelly, Stigge-Kaufman, Schmalfuss, & Perlstein, 2007b). Similarly, alterations in ACC-mediated evaluative activation have also been observed using fMRI, however, results have been contradictory. For example, Soeda and colleagues (2005) observed reduced ACC activation in TBI patients during completion of a modified Stroop task that elicited a high degree of response conflict, Scheibel and colleagues (2007) observed greater ACC activation in TBI patients during completion of a stimulus-response compatibility cognitive control task. Findings from both studies (Scheibel et al., 2007; Soeda et al. 2005) suggest that neural networks mediating cognitive control and evaluative processes of control are disrupted after brain injury; however, these findings do not account for error-related activity, and methodological limitations (i.e., use of blocked fMRI designs) in the studies described above also preclude full interpretation of results.
In the present study, we build upon available research findings and address the methodological limitations described above by exploiting the advantages of event-related fMRI which enables us to separately evaluate correct- and incorrect-trial response activity and, therefore, to examine potential alterations of activity reflecting error-related processing after TBI. Specifically, we tested the hypothesis that in comparison to healthy controls, patients with severe TBI (sTBI) would show smaller magnitude error-related activation of the ACC during completion of a cued-Stroop task, a cognitive task that has been found to reliably elicit a high degree of cognitive control and response conflict (Kerns et al., 2004; West, 2003).
2. Materials and methods
2.1 Participants
Ten individuals with sTBI were recruited from two Northern Florida trauma and rehabilitation hospitals and the local community, including meetings of the Florida Brain Injury Association, the Brain and Spinal Cord Injury Program of Florida, and local Brain Injury Association support groups. Twelve demographically-similar control participants were recruited by advertisement from the local community. All individuals provided written informed consent in accordance with procedures established by the University of Florida Health Science Center Institutional Review Board and received financial compensation for participation in the study. All ten participants with sTBI and eleven of the control participants also participated in our electrophysiological studies of cognitive control and error processing (Larson et al., 2007a; Larson et al., 2009; Larson & Perlstein, 2009).
Severity of TBI was determined by medical record review of lowest post-resuscitation Glasgow Coma Scale (GCS) score (Teasdale & Jennett, 1974); sTBI was defined as a GCS score <9. Neuroradiological findings taken from acute computerized tomography (CT) scans and neuroradiologist interpretation of the current structural MRI scans. Duration of loss of consciousness (LOC) and duration of post-traumatic amnesia (PTA) were acquired from medical record review or, when LOC and PTA information were not available in medical records, from structured participant and significant other interview (King, et al., 1997; McMillan, Jongen, & Greenwood, 1996). Data for LOC and PTA indicated all TBI participants met criteria for sTBI as traditionally defined by LOC > 6 hours and/or PTA > 7 days (Bigler, 1990; Bond, 1986). Only patients who did not exhibit current PTA were included.
Potential participants were excluded from the study for the following reasons: history of schizophrenia or bipolar disorder, substance abuse disorder, attention-deficit hyperactivity disorder, learning disability, inpatient psychiatric treatment predating brain injury, clinically-significant depression or anxiety predating brain injury by no more than two years, or substance use within two weeks of testing or of sustained abuse over the past year. In addition, any individual with any type of prior TBI, penetrating head injury, or neurological disorder (i.e., stroke, seizure disorder) not directly related to the TBI was excluded from participation. Non-native English speakers, individuals below 18 or above 55 years of age, patients with language comprehension deficits, dominant hand or finger mobility impairments, uncorrected visual impairment, current anti-epileptic medication use, color-blindness, or patients involved in current litigation were also excluded from participation.
Demographic characteristics for all participants are presented in Table 1; injury characteristics (i.e., duration of LOC) and neuroradiological findings for individuals with sTBI are presented in Table 2. Participants with TBI were at least 3 months post-injury, with the exception of one TBI survivor (2 months post-injury) whose functional abilities were sufficient to return to work. Gender distribution was not significantly different between groups, χ2(1) = 0.22, p = .69 (TBI: 6 male/4 female; Control: 6 male/6 female), and groups did not significantly differ for age, education, and parental education (all ps > .05; see Table 1).
Table 1.
Demographic and Negative Affect Data for Severe TBI and Control participants.
| Severe TBI (N = 10) | Control (N = 12) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age (yrs) | 25.1 | 7.3 | 20–40 | 22.9 | 6.4 | 19–42 |
| Educational Level (yrs) | 13.9 | 1.7 | 12–17 | 14.7 | 1.2 | 13–17 |
| Parental Educational Level (yrs) | 14.0 | 2.4 | 12–19 | 14.3 | 3.1 | 8–17 |
| Time Since Injury (months) | 8.2 | 3.7 | 2–13 | -- | -- | -- |
| Initial Glasgow Coma Scale Score | 3.6 | 1.3 | 3–6 | -- | -- | -- |
| Loss of Consciousness (days) | 22.9 | 18.1 | 1–60 | -- | -- | -- |
| Post-traumatic Amnesia (days) | 28.3 | 14.9 | 13–60 | -- | -- | -- |
| NAART Errors* | 32.6 | 10.2 | 14–43 | 23.8 | 5.5 | 15–36 |
| NAART IQ Estimate | 102.4 | 7.9 | 94–117 | 109.3 | 4.3 | 99–116 |
| BDI-II Score* | 13.1 | 8.5 | 1–31 | 5.6 | 5.4 | 0–18 |
| STAI-State | 32.2 | 7.7 | 20–45 | 29.5 | 7.0 | 20–42 |
| STAI-Trait | 33.5 | 10.1 | 21–48 | 31.8 | 8.0 | 23–50 |
Note: BDI-II = Beck Depression Inventory—2nd Edition; NAART = North American Adult Reading Test; STAI=State-Trait Anxiety Inventory.
Mean difference significant at p<.05 by independent-samples t-test.
Table 2.
Injury Characteristics and Neuroradiological Information for all Participants with TBI (N=10).
| Age (yrs) | Sex | Etiology | GCS | LOC (days) | PTA (days) | Time Post Injury (months) | Neuro-Radiological Findings |
|---|---|---|---|---|---|---|---|
| 21 | M | MVA | 3 | 42 | 21 | 10 | Skull fracture and right subdural hematoma |
| 22 | F | MVA | 3 | 7 | 21 | 10 | Bilateral frontal contusions but no other physical injuries |
| 20 | F | MVA | 3 | 14 | 14 | 12 | 1.5 cm high right frontal shear injury in the white matter; some subarachnoid blood in the quadrigeminal cistern |
| 25 | F | MVA | 3 | 28 | 28 | 11 | Left occipital condyle fracture |
| 21 | M | MVA | 3 | 28 | 42 | 6 | Unavailable |
| 21 | M | Motorcycle Accident | 3 | 12 | 33 | 8 | Right temporal intracranial hemorrhage |
| 37 | M | Motorcycle Accident | 3 | 28 | 36 | 7 | Small bilateral intraventricular hemorrhages with no shift |
| 40 | F | MVA | 6 | 1 | 15 | 13 | Left temporal occipital subarachnoid hemorrhage; multiple skull fractures |
| 24 | M | Boating Accident | 3 | 9 | 13 | 2 | Right temporal lobe epidural and subdural hematomas; right anterior middle cranial fossa hematoma |
| 20 | M | MVA | 6 | 60 | 60 | 3 | Left frontal subdural hematoma, multiple frontoparietal contusions and an orbital fracture |
2.2. Assessment of Functioning
All participants underwent a comprehensive screening of medical, psychiatric, and psychosocial history, including assessment of pre- and post-morbid functioning and self-reported symptomatology. Estimation of premorbid intellectual functioning was determined using the North American Adult Reading Test (NAART; Blair & Spreen, 1989; Spreen & Strauss, 1991). As shown in Table 1, relative to controls, participants with TBI committed significantly greater NAART errors, resulting in a significantly lower estimate of premorbid intellectual functioning in TBI participants. However, mean NAART-estimated premorbid WAIS-R FSIQ scores (Spreen & Strauss, 1991), while different between groups, both fell within the average range of intellectual functioning (Wechsler, 1981).
Participants also completed the Beck Depression Inventory – Second Edition, (BDI-II; Beck, Steer, & Brown, 1996) and State-Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, & Lushene, 1970), to assess current levels of depressive and anxiety symptomatology, respectively. As shown in Table 1, groups did not differ in the extent to which they endorsed symptoms of either state or trait anxiety; however, TBI participants endorsed significantly greater depressive symptoms than the control group. Though mean BDI-II scores were greater for TBI than control participants, means for both group were below clinical cut-off levels for depression (i.e., 13 for mild depression; Beck et al., 1996). Additionally, although one control participant and four TBI patients had BDI-II scores reflective of mild depression (i.e., 14; Beck et al., 1996), the overall pattern of functional imaging results did not significantly differ when high-negative affect individuals (i.e., BDI ≥ 14) were excluded from subsequent follow-up analyses, and as such, all participants were included in the analyses reported below2.
2.3. Cognitive Activation Task
Participants were scanned while they performed a version of the task-switching cued-Stroop task (Cohen, Barch, Carter, & Servan-Schreiber, 1999) which was also used in our previous behavioral (Seignourel et al., 2005) and ERP (Perlstein et al., 2006) studies. The task is schematicized in Figure 1. At the beginning of each trial, participants were presented visually with an instructional cue (the word “color” or “word”) followed after a delay by the probe (i.e., Stroop) stimulus. Participants were instructed to respond manually to the stimulus as designated by the instructional cue, as quickly and accurately as possible. Participants performed two tasks as specified by the instructional cue: word reading and color naming. In the word-reading task, participants silently read the probe word; in the color-naming task, they silently named the printed color of the probe word. Three font colors and color words were used (red, green, blue) and presented in each of two congruency conditions (congruent, incongruent). Congruent stimuli consisted of one of the three color names presented in its own color (i.e., “RED” printed in red); incongruent stimuli consisted of a color name presented in one of the two remaining colors (i.e., “RED” printed in blue). Participants were instructed to respond manually, as quickly and accurately as possible, by pressing one of three color-coded response keys using the index, middle, and ring fingers on their right hand.
Figure 1.
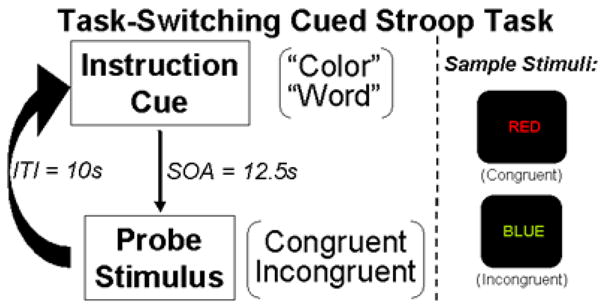
Schematic of the task-switching cued Stroop task. As shown, task trials comprised an instructional cue followed after a delay by a stimulus probe to which the participant responded with a button press.
Timing parameters for trial events were: cue-probe delay = 12.5s, probe-cue delay = 10s, for a total trial duration of 22.5s. Instructional cues and probe stimuli were presented for 1.5s each. Participants performed 16 blocks of 12 trials each for a total of 192 trials distributed equally across task conditions (i.e., color naming, word reading, congruent, and incongruent). Trial conditions were presented pseudo-randomly with the constraint that an equal number of conditions occurred within each block.
All participants were trained in color-button response mapping to at least 80% prior to entering the scanner. Though participants received feedback regarding their performance on the color-button response mapping training, they did not receive feedback on their in-scanner performance on the cued-Stroop task. E-Prime software 1.1 (Psychology Software Tools, Pittsburgh, PA) was used to generate stimuli and record behavioral response accuracy and RTs and an Integrated Functional Imaging System (IFIS; Psychology Software Tools, Pittsburgh, PA) 5-button MR compatible response box was used to acquire participant’s behavioral responses.
2.4. Functional Image Data Acquisition and Reduction
MRI scanning was conducted using a research-dedicated Siemens Allegra 3-Tesla MRI head scanner equipped with a standard head radio frequency coil. Task stimuli were presented using an LCD screen mounted above the participant’s head with IFIS hardware. Functional images were acquired in 35 axial slices rotated approximately 30° above the anterior commissure-posterior commissure (AC-PC) line using a T2*-weighted EPI pulse sequence (repetition time, TR=2500ms; echo time, TE=30ms; flip angle, FA=90°; field of view, FOV=24cm; 64 × 64 voxels at 3.75mm3 with .4mm slice gap). The 30° AC-PC line offset was used to decrease signal loss from the orbitofrontal cortex due to susceptibility artifact (McClure, Laibson, Loewenstein, & Cohen, 2004). Prior to functional scanning, a T1-weighted MP-RAGE high-resolution 3D anatomical image was acquired (160 1-mm thick slices; TR = 2000ms; TE = 4.13ms; FA = 8°; matrix = 512 × 512 voxels; FOV = 24cm) for evaluation of structural abnormalities, and to enable transformation of functional data into standard reporting space (Talairach & Tournoux, 1988). Each of 16 12-trial blocks was 4 min and 35s in duration, and total functional scanning time was approximately 80 min. Scan acquisition was time-locked to each trial-event onset (i.e., cue and probe) and lasted the entire duration of each 22.5s trial, allowing for acquisition of 108 total volumes per functional run (i.e, 9 images per trial).
Imaging data were processed using BrainVoyager (BVQX 2.10s; Brain Innovation, Maastricht, the Netherlands). Image preprocessing consisted of rigid-body 3-dimensional motion correction using trilinear interpolation, slice-scan time correction using sinc interpolation to account for potential timing differences across individual-slice acquisition, spatial smoothing with a 3D 8-mm full-width at half maximum (FWHM) Gaussian kernel to accommodate between-subject differences in brain anatomy, voxel-wise linear detrending, and high-pass filtering of frequencies below 3 cycles per time course to remove low-frequency nonlinear drifts. Initial co-registration of functional images to their respective high resolution three-dimensional anatomical volumes was completed using standard BVQX co-registration procedures; subsequent manual alignment was conducted as needed based on visual inspection of alignment adequacy. To enable groupwise analyses of functional imaging data, all images were spatially normalized into standard stereotactic Talairach space (Talairach & Tournoux, 1988) using the standard 9-parameter landmark Talairach method defined on each individual’s anatomical volume.
2.5. Data Analyses
2.5.1. Behavioral Performance Data
Stroop response time (RT) and error rate data were analyzed separately using JMP 6.0.3 software (SAS Institute Inc., Cary, NC, USA). For each participant and condition, median correct-trial RTs and mean error rates were calculated. RTs and error rates were analyzed separately using 2-Group × 2-Task (word reading, color naming) × 2-Condition (congruent, incongruent) mixed-model restricted maximum likelihood analyses of variance (REML-ANOVAs). Interaction effects were decomposed using least-square means contrasts. Where relevant, Cohen’s d with pooled standard deviation (Cohen, 1988) for between-group comparisons is reported as a measure of effect size.
2.5.2. fMRI Data
Imaging data were analyzed using two complementary approaches—between-group and individual-group—based on whole-brain voxel-wise statistical tests and follow-up contrasts on signal intensity in identified regions. In both cases, the percent transformed functional time courses were analyzed using a two-step general linear modeling (GLM) approach (Friston et al., 1995) using BrainVoyager QX v1.10.4. First, a separate fixed-effects GLM was specified for each participant, with separate predictors created for accuracy (correct, incorrect) and each trial event (cue, probe), collapsed across task and congruency conditions and resulting in a total of 4 predictors. The hemodynamic response for each event was estimated by convolving each regressor with a standard two-gamma function (Boynton, Engel, Glover & Heeger, 1996; onset = 0, response undershoot ratio = 6, time to response peak = 5s, time to undershoot peak = 15s, response dispersion = 1, undershoot dispersion = 1) spanning two functional volumes associated with the onset of cue events (to capture the sustained component of activation), and one functional volume associated with the onset of probe events (to capture the transient component of activation). For each voxel and trial event/accuracy condition a parameter estimate (β) was generated that indicated the strength of covariance between the data and the hemodynamic response function (HRF). Second, probe-related imaging data were analyzed with voxel-wise, mixed-model, 2-Group (controls, TBIs) × 2-Accuracy (correct, incorrect) analyses of variance (ANOVAs; subject = random factor) wherein linear contrasts on parameter estimates comparing probe-related correct and incorrect trials were calculated for each participant, and the results were submitted to group analyses that treated inter-subject variability as a random effect. Planned statistical contrasts examined error-related activity (error vs. correct trials) within each group for the individual-group analyses and, for the between-group analyses, a between-group ANOVA examined the Group x Accuracy interaction. A significance level of p<.01 and a 12-voxel three-dimensional contiguity (Forman et al., 1995; as estimated using the BrainVoyager plugin written by Fabrizio Esposito to extend from 2D to 3D statistical maps; Goebel, Esposito & Formisano, 2006) were used as thresholds for statistical maps; these criteria ensure that the probability of Type I error is less than 5% (Forman et al., 1995). Anatomical localization of suprathreshold activity was determined using the “nearest gray matter search” option of Talairach Daemon software (http://ric.uthsca.edu/project/talairachdaemon.html; Lancaster et al., 2000) by overlaying statistical maps onto the reference structural image transformed into standard reporting coordinates (Talairach & Tournoux, 1988).
3. Results
3.1. Task Performance
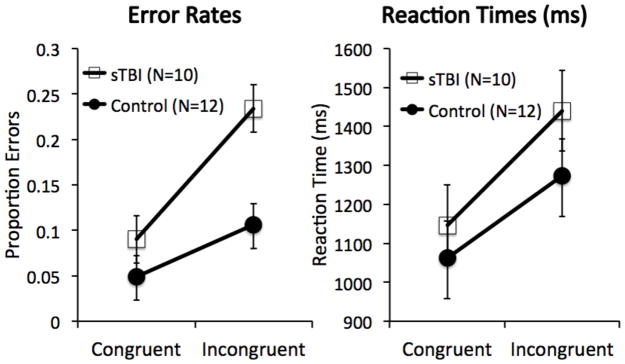
RTs and error rates for the cued-Stroop task (Figure 2) were positively and not significantly correlated for control, r(11) = .26, p > .41, or TBI participants, r(9) = .54, p > .10, suggesting that speed-accuracy trade-off was not a significant factor in task performance for either group.
Figure 2.
Behavioral performance on the cued-Stroop task as a function of Group and Congruency. (Left) Error rates; (Right) Reaction times. Error bars reflect standard errors.
3.1.1. Reaction Time
A mixed-model 2-Group × 2-Task × 2-Congruency REML ANOVA revealed only a significant main effect of congruency, F(1,148) = 118.88, p < .0001, reflecting the expected Stroop RT interference effect wherein RTs were longer during the incongruent than congruent condition. There was also a trend-level Group x Congruency interaction, F(1, 148) = 3.20, p < .08, indicating that sTBI participants showed marginally greater RT interference (293.9 ms ± 190.6) than controls (211.0 ms ± 116.6).
3.1.2. Error Rates
A 2-Group × 2-Task × 2-Congruency REML-ANOVA revealed significant main effects of group, F(1,20) = 6.97, p < .02, and congruency, F(1,148) = 54.38, p < .0001. TBI participants committed significantly more errors than controls, and error rates were higher to incongruent than congruent stimuli. A significant Group x Congruency interaction, F(1,148) = 10.11, p < .002, reflected greater error-rate interference in patients than controls; least-squares means contrasts revealed that the two groups did not differ in error rates on the congruent condition, t(20) = 1.18, p > .24, d = .51, but did differ on the incongruent condition, t(20) = 3.68, p < .001, d = 1.54, while both groups showed significant error-rate interference, ts ≥ 3.11, ps < .0025, ds ≥ 2.44.
3.2. Imaging Data1
3.2.1. Between-Groups Analysis
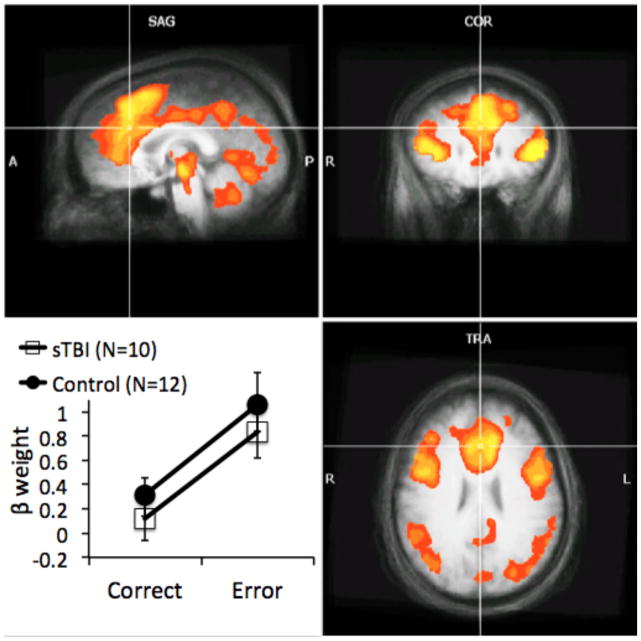
The between-groups comparison used voxel-wise Group x Accuracy ANOVAs to provide a direct quantitative comparison of signal intensities across the two groups. Significant main effects of accuracy, reflecting greater activity following incorrect than correct responses, were obtained in a number of regions previously shown to be engaged during error processing, including the ACC extending into the supplementary motor area, bilateral insula, inferior frontal gyrus, bilateral inferior parietal lobule and precuneus. Table 3 provides locations at peak t-values for separable clusters of error-related activation; Figure 3 illustrates regions of error-related activation. The main effect of group revealed that activity was greater in TBI patients than controls in 14 regions, whereas activity in 13 regions was greater in controls than patients (Table 4).
Table 3.
Brain regions in 10 patients with TBI and 12 healthy comparison participants that showed significant clusters of activity in voxel-wise ANOVAs of Group (TBI vs. Control) and Performance Accuracy (correct vs. incorrect) showing main effects of accuracy (incorrect > correct).
| Region of Change | Brodmann Area | # voxels | Coordinatesa |
tb | pb | Z-valueb | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| L Red Nucleus | -- | 25 | −3 | −16 | −8 | 5.87 | <0.000001 | 4.88 |
| R PHG | 28 | 14 | 24 | −22 | −5 | 5.26 | 0.000001 | 4.88 |
| R Claustrum | -- | 410 | 30 | 17 | 7 | 7.64 | <0.000001 | 4.88 |
| L IFG | 45 | 336 | −45 | 23 | 7 | 8.71 | <0.000001 | 4.88 |
| L ACG | 32 | 327 | −12 | 29 | 31 | 6.42 | <0.000001 | 4.88 |
From the atlas of Talairach and Tournoux (1988).
Determined from the voxel showing the maximal t value in each cluster.
Note: IFG = inferior frontal gyrus; PHG = parahippocampal gyrus; ACC = anterior cingulate gyrus; R = right; L = left.
Figure 3.
Brain regions illustrating error-related activity (incorrect > correct). Results from the between-group analyses showing a significant main effect of accuracy. Statistical maps are superimposed on the three-dimensional structural MRI averaged over all participants. Graphic insert at lower left illustrates beta weights showing the main effect of accuracy in a region of the midline anterior cingulate, F(1,20) = 20.65, p < .0002 which exhibited neither a main effect of group, F(1,20) < 1, p > .40, nor a Group x Accuracy interaction, F(1,20) < 1, p > .94, Error bars reflect standard errors. (L = left; R = right; Talairach coordinates of images shown = -1 26 27).
Table 4.
Brain regions in 10 patients with TBI and 12 healthy comparison participants that showed significant clusters of activity in voxel-wise ANOVAs of Group (TBI vs. Control) and Performance Accuracy (correct vs. incorrect) showing main effects of group.
| Region of Change | Brodmann Area | # voxels | Coordinatesa |
tb | pb | Z-valueb | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Greater Activity in Control than in TBI Participants
| ||||||||
| R Cerebellum | -- | 783 | 3 | −37 | −35 | 6.44 | <0.000001 | 4.88 |
| L Fusiform Gyrus | 37 | 44 | −36 | −52 | −11 | 4.38 | 0.000034 | 4.14 |
| L STG | 38 | 17 | −45 | 5 | −11 | 3.26 | 0.0016 | 3.16 |
| R LN | -- | 25 | 24 | −19 | −2 | 3.80 | 0.00028 | 3.63 |
| R Thalamus | -- | 24 | 9 | −10 | 16 | 4.61 | 0.000014 | 4.34 |
| L Caudate | -- | 120 | −12 | −7 | 19 | 4.04 | 0.00012 | 3.85 |
| R SFG | 10 | 25 | 24 | 50 | 25 | 3.27 | 0.0015 | 3.17 |
| L Precuneus | 7 | 57 | −21 | −64 | 31 | 6.04 | <0.000001 | 4.88 |
| R Precuneus | 7 | 23 | 21 | −70 | 34 | 3.78 | 0.00029 | 3.62 |
| R ACG | 24 | 69 | 3 | −22 | 34 | 4.35 | 0.000038 | 4.12 |
| L MFG | 9 | 17 | −30 | 35 | 37 | 3.62 | 0.0005 | 3.48 |
| L Precentral Gyrus | 4 | 33 | −42 | −10 | 53 | 3.72 | 0.00036 | 3.57 |
| L Frontal Sub-Gyral | 6 | 17 | −18 | 5 | 58 | 3.43 | 0.00094 | 3.31 |
|
| ||||||||
| Greater Activity in TBI than in Control Participants
| ||||||||
| R Lingual Gyrus | 18 | 28 | 9 | −79 | −5 | −3.67 | 0.00043 | 3.52 |
| L Lingual Gyrus | -- | 243 | −18 | −76 | 1 | −5.05 | 0.000003 | 4.66 |
| R STG | 22 | 92 | 57 | −10 | 7 | −4.07 | 0.0001 | 3.89 |
| R Posterior Cingulate | 23 | 21 | 6 | −55 | 13 | −3.47 | 0.00082 | 3.35 |
| L Insula | 13 | 61 | −42 | −13 | 22 | −3.95 | 0.00016 | 3.78 |
| L SOG | 19 | 62 | −36 | −76 | 25 | −4.62 | 0.000014 | 4.34 |
| R ACG | 32 | 113 | 15 | 17 | 31 | −4.60 | 0.000015 | 4.33 |
| R Cingulum | -- | 34 | 15 | −19 | 31 | −3.84 | 0.00024 | 3.67 |
| L MFG | 8 | 357 | −45 | 11 | 37 | −6.02 | <0.000001 | 4.88 |
| R IPL | 40 | 323 | 39 | −31 | 40 | −5.37 | 0.000001 | 4.88 |
| R MFG | 6 | 175 | 42 | 5 | 40 | −4.58 | 0.000016 | 4.31 |
| L Precentral Gyrus | 4 | 370 | −27 | −22 | 46 | −5.50 | <0.000001 | 4.88 |
| L SPL | 7 | 13 | −33 | −50 | 58 | −3.30 | 0.0014 | 3.19 |
| R Precentral Gyrus | 4 | 18 | 15 | −25 | 70 | −3.74 | 0.00033 | 3.59 |
From the atlas of Talairach and Tournoux (1988).
Determined from the voxel showing the maximal t value in each cluster.
Note: IPL = inferior parietal lobule; LN = lentiform nucleus; MFG = middle frontal gyrus; SFG = superior frontal gyrus; SOG = superior occipital gyrus; SPL = superior parietal lobule; STG = superior temporal gyrus; ACG = anterior cingulate gyrus; R = right; L = left.
More central to the aims of the current research was the predicted Group x Accuracy interaction (Table 5) in the ACC, as well as a region of the cerebellum. In contrast to our predictions, follow-up examination of beta weights for activity in the right ACC cluster that emerged in the Group x Accuracy interaction revealed greater activation in sTBI patients, but not controls, to incorrect trials only (Figure 4); TBI patients showed greater error-related ACC activity than controls, t(20) = 2.62, p < .01), while the two groups did not differ in ACC activity to correct responses, t(20) < 1.0, p > .15), as determined by least-squares means contrasts. Unexpectedly, paired t-tests showed that while TBI patients showed significant error-related increases in activity within this region of the ACC, t(9) = 5.13, p < .0003, controls did not, t(11) < 1.0, p > .5.
Table 5.
Brain regions in 10 patients with TBI and 12 healthy comparison participants that showed significant clusters of activity in voxel-wise ANOVAs of Group (TBI vs. Control) and Performance Accuracy (correct vs. incorrect) showing significant Group x Accuracy interactions.
| Region of Change | Brodmann Area | # voxels | Coordinatesa |
tb | pb | Z-valueb | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| L Cerebellum# | -- | 24 | −12 | −49 | −35 | 3.64 | 0.0005 | 3.48 |
| R ACG* | 32 | 13 | 18 | 14 | 31 | −3.17 | 0.002 | 3.09 |
From the atlas of Talairach and Tournoux (1988).
Determined from the voxel showing the maximal t value in each cluster.
activation greater in TBI patients;
activation greater in controls.
Note: ACG = anterior cingulate gyrus; R = right; L = left.
Figure 4.
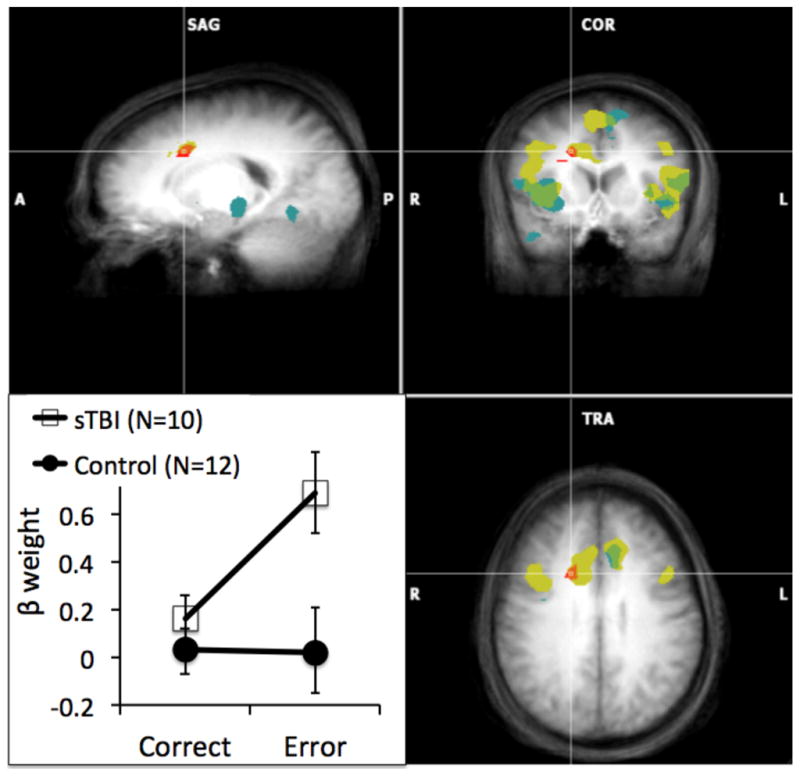
Brain regions of patients with TBI and healthy comparison participants illustrating significant error-related activity (incorrect > correct). Results from the individual-group analyses: Yellow illustrates significant error-related activity in TBI patients, blue illustrates error-related activity in healthy controls, and green illustrates error-related activity in in which the TBI patients and healthy controls overlap. Results from the between-group analysis: Red illustrates a region of the anterior cingulate cortex (ACC) in which there is a Group x Accuracy interaction; graph insert in lower left illustrates beta weights showing the interaction effect. Error bars reflect standard errors. Statistical maps are superimposed on the three-dimensional structural MRI averaged over all participants. (L = left; R = right; Talairach coordinates of images shown = 18 11 34).
3.2.2. Individual-Group Analysis
These analyses employed voxel-wise linear contrasts comparing effect of accuracy (i.e., error vs. correct probe response) to evaluate error-related activity within the two groups separately. Results for both groups showed several cortical regions wherein error-related activity exceeded correct trial activity, and were largely similar with respect to the number of regions showing suprathreshold activation (see Table 6).
Table 6.
Brain regions showing significantly greater error-related activity following incorrect than correct probe response revealed in the individual-group analyses of 12 healthy controls and 10 TBI participants.
| Region of Change | Brodmann Area | # Voxels | Coordinatesa |
tb | pb | Z-valueb | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Control participants
| ||||||||
| L Cerebellum | -- | 60 | −15 | −46 | −32 | 5.02 | 0.000003 | 4.66 |
| L STN | -- | 128 | −9 | −10 | −8 | 5.20 | 0.000001 | 4.88 |
| R Insula | 13 | 146 | 30 | 14 | −5 | 5.44 | 0.000001 | 4.88 |
| L IFG | 45 | 189 | −45 | 23 | 7 | 5.93 | <0.000001 | 4.88 |
| R MTG | 39 | 18 | 46 | −67 | 19 | 5.00 | 0.000003 | 4.66 |
| L Cingulate Gyrus | 32 | 18 | −12 | 26 | 31 | 4.50 | 0.000022 | 4.24 |
| L Precuneus | 9 | 29 | −6 | −49 | 43 | 4.65 | 0.000012 | 4.37 |
| L MFG | 6 | 36 | −12 | 8 | 55 | 5.37 | 0.000001 | 4.88 |
|
| ||||||||
| TBI participants
| ||||||||
| R Claustrum | -- | 362 | 30 | 17 | 7 | 5.26 | <0.000001 | 4.88 |
| L IFG | 45 | 298 | −48 | 20 | 10 | 6.51 | <0.000001 | 4.88 |
| R Cingulate Gyrus | 24 | 238 | 12 | 14 | 31 | 5.30 | 0.000001 | 4.88 |
| L MFG | 6 | 50 | −12 | 29 | 34 | 4.82 | 0.000006 | 4.53 |
From the atlas of Talairach and Tournoux (1988).
Determined from the voxel showing the maximal t value in each cluster.
Note: IFG = inferior frontal gyrus; MFG = middle frontal gyrus; MTG = middle temporal gyrus; STN = subthalamic nuclei; R = right; L = left.
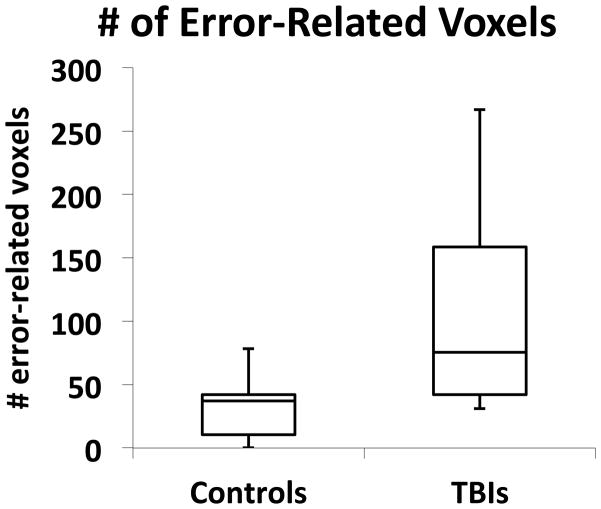
3.2.4. Spatial Extent of Error-Related Activation
We also examined the overall spatial extent of error-related activation within each group. Based on previous findings of more widespread activation in TBI survivors than neurologically-normal controls, we predicted that the current sample of TBI patients would similarly show greater spatial extent of error-related activation. Toward this end, we calculated the median number of error-related suprathreshold voxels (p < .01, 12 voxel contiguity) for each subject and, since the number of clusters of activation differed across participants, calculated the median number of suprathreshold voxels across clusters for each participant. As the variances across groups were unequal (as revealed by Levine’s test, F(1, 19) = 12.97, p < .002), we employed Welch ANOVA testing for equal means allowing unequal variances across group. In accord with predictions, TBI patients showed a larger median number of voxels activated during error-related activity (Mean = 103.5 ± SD = 81.7) than controls (Mean = 32.1 ± SD = 25.6), F(1, 10.6) = 7.03, p < .025, Cohen’s d =1.11.3
4. Discussion
We examined error-related brain activity in survivors of sTBI using event-related fMRI acquired in the context of performing a Stroop task. Our primary aim was to determine if, relative to demographically-similar healthy controls, sTBI participants exhibit altered error-related activity reflective of impaired performance monitoring. Our initial prediction was that sTBI patients would exhibit reduced error-related activity in the ACC. However, contrary to this prediction, sTBI patients showed greater magnitude of error-related activation within the ACC and more extensive cortical and subcortical activation. This combination of findings suggests that while TBI patients demonstrated greater errors than controls during task-performance, the postulated neural underpinnings of error-related processing within our sample of TBI patients was largely intact, though more widespread. Our findings of greater ACC activation and increased spatial extent in sTBI participants than controls suggests altered neural substrates for error-related processing in sTBI patients.
Consistency of our findings is mixed when compared with previous fMRI studies that report alteration of activation within the ACC following TBI (i.e., Easdon, Levine, O’Connor, Tisserand, & Hevenor, 2004; Scheibel et al., 2007; Soeda et al., 2005). For example, reduction in ACC activation has been reported in patients with TBI during completion of a “go-stop” task (Easdon et al., 2004) and a Stroop task (Soeda et al., 2005), findings that may reflect injury-related disruption of neural networks. In contrast, Scheibel et al. (2007) observed greater magnitude of activation within the ACC in TBI patients compared to orthopedic-injury controls during completion of a stimulus-response incompatibility task. These findings may suggest compensation for inefficient cognitive processes in the neural networks mediating cognitive control.
One possible explanation for the contradictory findings across studies may be due to methodological differences. For example, both Soeda and colleagues (2005) and Easdon and colleagues (2004) employed fixed-effects analyses of their imaging data, which may bias there results due to the influence of single observations, while use of block design fMRI by Scheibel et al. (2007) potentially confounds examination of several task-relevant effects by including both correct and incorrect trials in their analyses. Methodological advantages in the present study such as use of random-effects analyses and event-related fMRI acquisition may help shed light on these important issues. For example, rather than collapse activation for incorrect and correct trials, we directly compared activation for correct and incorrect trials, an important distinction given that findings of intact ACC activation by Scheibel et al. (2007) were collapsed across successful and incorrect trials.
Irrespective of methodological differences, our findings suggest that sTBI patients demonstrated greater magnitude and extent of activation of brain regions within and outside our primary region of interest (i.e., ACC) than controls during error-related processing. However, this increased activation did not improve task-performance, as TBI patients committed significantly greater errors across conditions than controls. That is, individuals with TBI had more diffuse and stronger activity than controls. Parsimoniously, this would suggest that damage to the brain affects normal error processing, and that post-injury, alternate and additional forms of processing that have yet to be understood occur that may or may not improve task performance. Whereas some researchers may suggest such diffuse activation represents the inefficient use of neural resources, the apparently greater magnitude and extent of activation of brain regions outside our primary region of interest (i.e. ACC) in TBI patients may reflect compensatory plasticity following injury, as implementation of error-related processes may be partially dependent on all active regions during task performance. For example, activation of these dispersed regions might serve to properly allocate neural resources during task performance. In addition, activated regions may serve to inhibit other brain regions that might interfere and would otherwise prove detrimental to task performance. One way to possible explore this issue may be through the examination of the inverse (Negative) Bold signal (for review of this method the reader is instructed to the following articles: Frostig, Lieke, Ts’o, & Grinvald, 1990; Shmuel et al., 2002); however, examination of working memory functions following TBI lend partial support to this hypothesis (i.e., Kim et al., 2009; McAllister et al., 2001; Newsome et al., 2007; Turner & Levine, 2008).
These results stand somewhat in contrast to electrophysiological studies from our lab showing decreased event-related potential (ERP) correlates of performance monitoring—namely, the error-related negativity component of the ERP—in individuals with sTBI relative to controls (i.e., Larson et al., 2007a). Notably, all of the individuals with sTBI and 11 of 12 controls in the current study also participated in the electrophysiology study. Thus, differences cannot be accounted for by differences in the sample. Methodological differences, however, may play a role in these seemingly disparate findings. Event-related potentials rely on synchronous activation of a group of similarly-oriented apical dendrites which spread to the scalp by virtue of volume conduction. Diffuse activity, therefore, is often spread across multiple electrodes and not easily detected in ERP analyses that are focused on small groups of electrodes chosen based on a priori hypotheses and voltage-related scalp maps. Furthermore, multiple simultaneously active neural generators can lead to changes in ERP morphology and amplitude depending on neuronal orientation (see Luck, 2005). Thus, it is possible that the increased diffuse activity in individuals with sTBI was seen as a reduced-amplitude ERN. This possibility is supported by a recent multimodal imaging study that examined electrophysiological (ERPs) and hemodynamic (fMRI) reflections of error-related processing in healthy participants (Doñamayor, Heilbronner & Münte (2011). This study found several cortical and subcortical areas, including the ACC, superior frontal gyrus, precentral gyrus, inferior frontal gyrus, and inferior parietal lob involved in error processing. An additional methodological difference which could potentially account for the inconsistent findings between the Larson et al. (2007a) ERN findings and current findings of increased ACC activity in TBI patients is task related. Specifically, in contrast to the current study, the Larson et al. (2007a) study used a single-trial Stroop task that did not include a stimulus-preceding cue. Error rates in that study were modestly lower than those observed in the present study, possibly suggesting that the working memory burden imposed by task-instructional cueing may also play a role in the discrepant findings across these two studies. Nevertheless, the use of multiple modalities in similar groups of patients across studies from our group represent a strength of this research. Future studies directly addressing these methodological differences are needed to clarify these seemingly disparate study findings.
It is important to consider that performance monitoring processes of control serve to not only detect errors, but also to adjust behavioral performance toward goal attainment based on the detection of performance errors which can signal when strategic shifts in performance are necessary (Kerns et al., 2004; Ridderinkhof et al., 2004). Consequently, strategic shifts, in theory, should minimize conflict on subsequent trials and reduce additional likelihood of subsequent incorrect responses (Carter & van Veen, 2007; Kerns et al., 2004; Ridderinkhof et al., 2004; van Veen & Carter, 2002; van Veen & Carter, 2006). Thus, it is possible that although TBI patients may detect errors correctly, poor task performance may arise due to impairments in signaling when strategic shifts are needed (i.e., Larson et al., 2006).
Task performance findings indicated that both sTBI and control participants showed significant Stroop RT interference, with sTBI patients showing marginally greater RT interference than controls. Additionally, sTBI participants showed significantly greater error-rate interference than healthy controls. This pattern of behavioral results is consistent with previous literature showing increased Stroop RT and error-rate interference on versions of the cued-Stroop task (i.e., Larson et al., 2007a; Perlstein et al., 2006; Seignourel et al., 2005), supporting assertions that sTBI patients show impairments in conflict processing, particularly when required to override prepotent response tendencies.
Several methodological limitations and alternative explanations of findings of the current study warrant further discussion. For example, although all TBI participants were classified as sustaining severe injuries, the heterogeneity of this population was evidenced by individual differences in injury mechanism, injury localization, time since injury, PTA, and LOC (Lezak, Howieson, & Loring, 2004). Whereas studies using TBI participants cannot control for all these variables, studies of TBI must acknowledge and appreciate heterogeneity within the TBI population. Secondly, numerous research studies (i.e., Heeger, Huk, Geisler, & Albrecht, 2000; Logothetis & Wandell, 2004) have postulated that the blood-oxygen level dependent (BOLD) signal measured in fMRI studies is a complex function reflective of changing levels of cerebral blood flow, blood volume, and oxygen metabolism that occur as a result of neural activity. However, the hemodynamic response often lags greatly behind the neuronal activity that starts the event (Boynton et al., 1996). Moreover, as the BOLD signal measured by fMRI is reflective of vascular changes correlated with neural activity, and not neural activity directly, any observed injury-related differences may have resulted from several factors outside of those hypothesized (Hillary et al., 2002). Specifically, differences in injury-related activation patterns could reflect changes in vascular processes or changes due to cell atrophy, rather than neural processes. For example, both human and animal models of TBI have demonstrated reduced baseline levels of cerebral blood flow post-injury (i.e., Bouma, Muizelaar, Choi, Newlon, & Young, 1991). Moreover, blood flow abnormalities in patients with moderate-to-severe TBI relative to comparison subjects have been observed during completion of working memory tasks, an effect particularly concerning due to abnormalities in the frontal lobes, an area extensively imaged by researchers (i.e., Christodoulou et al., 2001; Perlstein et al., 2004). Finally, though many neurocognitive studies of TBI have employed uninjured healthy comparison groups as we have, such studies may confound behavioral features that predispose people to TBI. Other functional imaging studies, in contrast, have employed participants with extracranial orthopedic injury for comparison “to control for a host of risk factors (e.g., risk-taking behavior) that [may] predispose to injury and nonspecific effects of injury such as posttraumatic stress that could affect brain activation” (e.g., Scheibel et al., 2007, p. 36). Such potential confounds are important to address, particularly in light of the ACC differentiation observed in the present study and findings that posttraumatic stress may alter ACC activity (e.g., Hayes, LaBar, Petty, McCarthy & Morey, 2009) and that error-related ACC activity is modulated by affective factors (e.g., Larson, Kaufman, Kellison, Schmalfuss & Perlstein, 2009).
4.1. Summary and conclusions
In conclusion, results from the present study extend previous findings that the neural networks mediating cognitive control, specifically, error-related processing, are disrupted after sTBI. Despite its limitations, the current study supports the continued neurophysiological examination of complex processes of cognitive control. Future studies will aim to identify outcome measures of “real-world” functioning that will further examine the impact of these deficits and inform rehabilitation efforts.
Figure 5.
Box plots illustrating the total number of suprathrehold error-related voxels as a function of group as derived from the separate-groups contrast error > correct response. Bottom and top of each box represent the lower and upper quartiles, respectively, and the band near the middle of the box reflects the 50th percentile (median); upper and lower whiskers reflect the maximum and minimum number of voxels.3
Acknowledgments
This original research manuscript, or parts of it, have not been and will not be submitted elsewhere for publication. This work was supported by NIH grants K01-MH01857 and R21-MH073076, and by grants from the Evelyn F. McKnight Brain Research Grant Program, and the Florida Brain and Spinal Cord Injury Research Trust Fund awarded to WMP. The work was submitted by the first author in partial fulfillment of requirements for the Master of Science degree. We extend our appreciation to Ashley Carroll, Neha Dixit, Cortney Mauer, Megan McIntyre, Drew Nagle, Paul Seignourel, Floris Singletary, Allen Sirizi, and Raechel Steckley for their assistance in participant recruitment and data acquisition.
Footnotes
To examine the possibility that movement artifacts might have systematically impaired detection of cortical activation in patients, we analyzed the average movement parameters for mean estimated translational and rotational inter-scan displacement. Average translational and rotational inter-scan displacement was less than 1 voxel dimension (3.8mm) and 1°, respectively. Analysis of group and condition-related effects did not reveal any significant differences (Fs ≤ 2.81, ps ≥ .10) in movement, suggesting that observed group differences in activation were not due to systematic differences in head movement.
ACC error-related activity did not significantly correlate with BDI scores, collapsed across groups or for each group separately (rs ≤ .025, ps > .27).
We performed a test for detecting outliers (using the extreme studentized deviate, ESD, method; Rosner, 1983) in the spatial-extent data due to the presence of one control participant that showed to be a potential outlier in the normal probability plot of all subjects. Results of the ESD test revealed that this participants’ voxel count exceeded 4 standard deviations of the mean for the voxel counts of the two groups combined. Thus, results of the ESD test reveal that this participants’ voxel count was a significant outlier, Z = 4.48, p < .05. Consequently, this participants’ data were excluded from analysis of the spatial-extent data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson V, Levin HS, Jacobs R. Executive functions after frontal lobe injury: A developmental perspective. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. pp. 504–527. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2. USA: The Psychological Corporation; 1996. (BDI-II) [Google Scholar]
- Bigler ED. Neuropathology of traumatic brain injury. In: Bigler ED, editor. Traumatic Brain Injury. Austin, TX: Pro-ed; 1990. [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Bond MR. Neurobehavioral sequelae of closed head injury. In: Grant I, Adams KM, editors. Neuropsychological assessment of neuropsychological disorders. New York: Oxford University Press; 1986. pp. 347–373. [Google Scholar]
- Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. Journal of Neurosurgery. 1991;75:685–693. doi: 10.3171/jns.1991.75.5.0685. [DOI] [PubMed] [Google Scholar]
- Boynton GA, Engel SA, Glover G, Heeger D. Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Robertson IH. Principles of the rehabilitation of frontal lobe function. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2002. pp. 557–572. [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The role of the anterior cingulate cortex in executive processes of cognition. Reviews in the Neurosciences. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, and Behavioral Neuroscience. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, DeLuca J, Ricker JH, Madigan NK, Bly BM, Lange G, et al. Functional magnetic resonance imaging of working memory impairment following traumatic brain injury. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71:161–168. doi: 10.1136/jnnp.71.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, et al. Evidence-based cognitive rehabilitation: Updated review of the literature from 1998 through 2002. Archives of Physical Medicine and Rehabilitation. 2005;86:1681–1692. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum Associates; 1988. [Google Scholar]
- Cohen JD, Barch DM, Carter CS, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. Journal of Abnormal Psychology. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Doñamayor N, Heilbronner U, Münte TF. Coupling electrophysiological and hemodynamic responses to errors. Human Brain Mapping. 2011 doi: 10.1002/hbm.21305. published online 26 May 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easdon C, Levine B, O’Connor C, Tisserand D, Hevenor S. Neural activity associated with response inhibition following traumatic brain injury: an event-related fMRI investigation. Brain and Cognition. 2004;54:136–138. [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional neuroimaging. Neuroimage. 1995;6:218–229. [Google Scholar]
- Frostig RD, Lieke EE, Ts’o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of function image analysis contest (FIAC) data with Brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, LaBar KS, Petty CM, McCarthy G, Morey RA. Alterations in neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Research: Neuroimaging. 2009;172:7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger DJ, Huk AC, Geisler WS, Albrecht DG. Spikes versus BOLD: What does neuroimaging tell us about neuronal activity? Nature Neuroscience. 2000;3:631–633. doi: 10.1038/76572. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Steffener J, Biswal BB, Lange G, DeLuca J, Ashburner J. Functional magnetic resonance imaging technology and traumatic brain injury rehabilitation: guidelines for methodological and conceptual pitfalls. Journal of Head Trauma Rehabilitation. 2002;17:411–430. doi: 10.1097/00001199-200210000-00004. [DOI] [PubMed] [Google Scholar]
- Horn LJ, Sherer M. Rehabilitation of traumatic brain injury. In: Grabois KM, Garrison SJ, Hart A, Lehmkuhl LD, editors. Physical medicine and rehabilitation: the complete approach. Cambridge, MA: Blackwell Science; 1999. pp. 1281–1304. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kim YH, Yoo WK, Ko MH, Park CH, Kim ST, Na DL. Plasticity of the attentional network after brain injury and cognitive rehabilitation. Neurorehabilitation & Neural Repair. 2009;23:468–477. doi: 10.1177/1545968308328728. [DOI] [PubMed] [Google Scholar]
- King NS, Crawford S, Wenden FJ, Moss NE, Wade DT, Caldwell FE. Measurement of post-traumatic amnesia: How reliable is it? Journal of Neurology, Neurosurgery, and Psychiatry. 1997;62:38–42. doi: 10.1136/jnnp.62.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MJ, Farrer TJ, Clayson PE. Cognitive control in mild traumatic brain injury: Conflict monitoring and conflict adaptation. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2011.02.018. in press. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Perlstein WM. Awareness of deficits and error processing after traumatic brain injury. Neuroreport. 2009;20:1486–1490. doi: 10.1097/WNR.0b013e32833283fe. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kaufman DAS, Kellison IL, Schmalfuss IM, Perlstein WM. Double jeopardy! The additive consequences of negative affect on performance-monitoring decrements following traumatic brain injury. Neuropsychology. 2009;23(4):433–444. doi: 10.1037/a0015723. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kelly KG, Stigge-Kaufman DA, Schmalfuss IM, Perlstein WM. Reward context sensitivity impairment following severe TBI: An event-related potential investigation. Journal of the International Neuropsychological Society. 2007b;13:615–625. doi: 10.1017/S1355617707070762. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Perlstein WM, Demery JA, Stigge-Kaufman DA. Cognitive control impairments in traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 2006;28:968–986. doi: 10.1080/13803390600646860. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Stigge-Kaufman DA, Schmulfass IM, Perlstein WM. Performance monitoring, error processing, and evaluative control following severe TBI. Journal of the International Neuropsychological Society. 2007a;13:961–971. doi: 10.1017/S1355617707071305. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annual Review of Physiology. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Boksem MAS, Ridderinkhof KR. Impaired cognitive control and reduced cingulate activity during mental fatigue. Cognitive Brain Research. 2005;24:199–205. doi: 10.1016/j.cogbrainres.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Lovell M, Franzen M. Neuropsychological assessment. In: Silver JM, Yudofsky S, Hales RE, editors. Neuropsychiatry of traumatic brain injury. Washington: American Psychiatric Press; 1994. pp. 133–160. [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuro Image. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McMillan TM, Jongen EL, Greenwood RJ. Assessment of post-traumatic amnesia after severe closed head injury: retrospective or prospective? Journal of Neurology, Neurosurgery, and Psychiatry. 1996;60:422–427. doi: 10.1136/jnnp.60.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews: Neuroscience. 2000;1:59–66. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Newsome MR, Scheibel RS, Steinberg JL, Troyanskaya M, Sharma RG, et al. Working memory brain activation following severe traumatic brain injury. Cortex. 2007;43:95–111. doi: 10.1016/s0010-9452(08)70448-9. [DOI] [PubMed] [Google Scholar]
- O’Keeffe FM, Dockree PM, Robertson IH. Poor insight in traumatic brain injury mediated by impaired error processing?: Evidence from electrodermal activity. Cognitive Brain Research. 2004;22:101–112. doi: 10.1016/j.cogbrainres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Cole MA, Dixit NK, Demery JA. Parametric manipulation of working memory load in chronic traumatic brain injury. Journal of the International Neuropsychological Society. 2004;10:724–741. doi: 10.1017/S1355617704105110. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Larson MJ, Dotson VM, Kelly KG. Temporal dissociation of components of cognitive control dysfunction in severe TBI: ERPs and the cued-Stroop task. Neuropsychologia. 2006;44:260–274. doi: 10.1016/j.neuropsychologia.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rosner B. Percentage points for a generalized EST many-outlier procedure. Technometrics. 1983;25(2):165–172. [Google Scholar]
- Scheibel RS, Newsome MR, Steinberg JL, Pearson DA, Rauch RA, Mao H, Troyanskaya M, Sharma RG, Levin HS. Altered brain activation during cognitive control in patients with moderate to severe traumatic brain injury. Neurorehabilitation and Neural Repair. 2007;21:36–45. doi: 10.1177/1545968306294730. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Pearson DA, Faria LP, Kotrla KJ, Aylward E, Bachevalier J, et al. An fMRI study of executive functioning after severe diffuse TBI. Brain Injury. 2003;17:919–930. doi: 10.1080/0269905031000110472. [DOI] [PubMed] [Google Scholar]
- Seignourel PJ, Robins DL, Larson M, Demery JA, Cole MA, Perlstein WM. Cognitive control in closed head injury: Context maintenance dysfunction or prepotent response inhibition deficit? Neuropsychology. 2005;19:578–590. doi: 10.1037/0894-4105.19.5.578. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Andriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36:1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Soeda A, Nakashima T, Okumura A, Kuwata K, Shinoda J, Iwama T. Cognitive impairment after traumatic brain injury: a functional magnetic resonance imaging study using the Stroop task. Neuroradiology. 2005;47:501–506. doi: 10.1007/s00234-005-1372-x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. New York: Oxford University Press; 1991. [Google Scholar]
- Stemmer B, Segalowitz SJ, Witzke W, Schönle PW. Error detection in patients with lesions to the medial prefrontal cortex: An ERP study. Neuropsychologia. 2004;42:118–130. doi: 10.1016/s0028-3932(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Sterotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;ii:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Turner GR, Levine B. Augmented neural activity during executive control processing following diffuse axonal injury. Neurology. 2008;71:812–818. doi: 10.1212/01.wnl.0000325640.18235.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology and Behavior. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. Conflict and cognitive control in the brain. Current Directions in Psychological Science. 2006;15:237–240. [Google Scholar]
- Wechsler D. WAIS-R Manual. New York: Psychological Corporation; 1981. [Google Scholar]
- West R. Neural correlates of cognitive control and conflict detection in the Stroop and digit-location tasks. Neuropsychologia. 2003;41:1122–1135. doi: 10.1016/s0028-3932(02)00297-x. [DOI] [PubMed] [Google Scholar]