Abstract
BACKGROUND
Preclinical findings suggest that the inhibition of NMDA glutamatergic neurotransmission may have beneficial effects in the treatment of opioid dependence. AIMS: We hypothesized that memantine, a low-potency, uncompetitive NMDA receptor antagonist, would be safe and effective when used as an adjunct to oral naltrexone in the treatment of opioid dependence, particularly in preventing relapse to opiate use in detoxified individuals.
METHODS
Opioid-dependent participants (N =112) were enrolled. Following detoxification all participants were inducted onto oral naltrexone and were randomized to receive memantine 15 mg bid (N=27), memantine 30 mg bid (N=27) or placebo (N=27) for 12-weeks in combination with naltrexone 50 mg/d and individual relapse-prevention therapy. The primary outcome was the retention in treatment since treatment dropout is most commonly associated with relapse to opiate use.
RESULTS
Twenty-six percent of participants withdrew from treatment prior to starting naltrexone. Of those that were randomized 35% completed 4 weeks only, and 24% completed all 12 weeks of treatment. There was no significant difference in treatment retention or heroin use, opiate withdrawal symptoms and craving between the groups treated with memantine versus placebo.
CONCULSION
Thus, the efficacy of memantine 30 or 60 mg/d as an adjunct to oral naltrexone for the treatment of opiate dependence was not supported.
Keywords: Opiate dependence, Pharmacotherapy trials, Naltrexone, NMDA receptors
1. Introduction
Opiate use, particularly use of prescription analgesics, remains a serious public health problem affecting a growing number of individuals in the US (SAMHSA, 2010). While agonist maintenance with methadone or buprenorphine remains the treatment of choice for individuals with a chronic and relapsing course of opioid dependence (Mattick et al., 2009; Mattick et al., 2008), use of agonists remains controversial for the treatment of youth, newly diagnosed patients, or prescription opioid abusers. Further, agonist maintenance is not available or acceptable to many patients, nor is it universally effective. Naltrexone, a mu-opioid antagonist, acts by a different mechanism and offers an alternative approach to treatment (Johansson et al., 2006). Naltrexone blocks the effects of opioids, while producing no agonist effects itself, and thus may be helpful to patients who are not suitable for agonist maintenance or have failed prior trials of agonist treatment.
Naltrexone initiation is preferably implemented directly following detoxification and as such it often coincides with the symptomatic treatment of the residual opiate withdrawal with hypnotics, anxiolytics, and non-opiate analgesics. However, the effectiveness of this approach has been limited as a significant proportion of patients continue to report physical discomfort and, attributing these withdrawal symptoms to naltrexone, make the decision to discontinue naltrexone during the initiation phase and relapse to opioid use.
One of the approaches to improve the tolerability of naltrexone induction would involve targeting pathways that mediate residual opiate withdrawal and those responsible for relapse during stabilization on naltrexone. Glutamatergic neurotransmission, an important component of the endogenous reward system, is a viable target. Glutamate receptor antagonists attenuate drug-conditioned response, reduce the development and expression of physical dependence, and attenuate the reinforcing effects of opioids in preclinical models (Bisaga and Popik, 2000; Gass and Olive, 2008).
Memantine is an NMDA glutamatergic receptor antagonist that has been in clinical use for close to 20 years for the treatment of Parkinson’s Disease, spasticity, and dementia (Parsons et al., 1999). As an uncompetitive NMDA receptor antagonist, memantine blocks transmission when this receptor is in the active (open) state, such as in conditions of excess glutamate concentration (Parsons et al., 2008). Compared to other NMDA receptor antagonists such as ketamine or phencyclidine, memantine is a low affinity antagonist with good tolerability at clinically relevant doses (20 to 40 mg/day). Memantine is well tolerated in humans, with mild positive subjective effects and possible thymoleptic and anxiolytic effects (Skolnick and Krystal, 2002). Memantine has produced encouraging results in animal laboratory models of opioid dependence, where it decreased aversive and physical signs of morphine withdrawal (Harris et al., 2008; Maldonado et al., 2003; Medvedev et al., 1998; Popik and Danysz, 1997; Popik and Skolnick, 1996), attenuated rewarding and reinforcing effects of morphine (Aguilar et al., 2009; Popik et al., 2003; Ribeiro Do Couto et al., 2004; Semenova et al., 1999), and blocked morphine-induced reinstatement of place preference, an animal model of relapse (Popik et al., 2006; Ribeiro Do Couto et al., 2005). In human laboratory studies memantine, given in a single dose of 60 mg, attenuated the severity of opioid withdrawal (Bisaga et al., 2001) and memantine given repeatedly at the doses of 30 and 60 mg/d reduced heroin craving and other positive subjective effects of heroin though it did not affect heroin self-administration (Comer and Sullivan, 2007). A small controlled trial of medication given to detoxified opiate dependent patients in a residential program showed that memantine 30 mg/d was superior to placebo in reducing protracted opiate withdrawal symptoms (anhedonia, depression, anxiety), heroin craving, and in decreasing treatment dropout (Krupitsky et al., 2002).
As the profile of memantine based on pre-clinical and early clinical work strongly suggested that given at 30 and 60 mg/d dose memantine might be effective in the treatment of heroin dependence we have decided to launch a clinical trial. We hypothesized that decreasing glutamatergic neurotransmission using memantine could be effective in modulating glutamate-dependent mechanisms that contribute to relapse and discontinuation of treatment by individuals receiving naltrexone. To test this, we conducted a placebo-controlled trial of memantine in detoxified opioid dependent individuals that started receiving naltrexone and were being discharged to outpatient care. We hypothesized that memantine would reduce protracted symptoms of opiate withdrawal and heroin craving, resulting in a decrease of opioid use and prevention of relapse in detoxified individuals treated with oral naltrexone.
2. Methods
2.1. Participants
Individuals who applied for treatment at Columbia University’s Substance Treatment and Research Service (STARS) outpatient clinic in New York City, USA, were recruited for this study. Clinical screening included the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (SCID Axis I/P version; (First et al., 1995) and a clinical interview assessing substance abuse severity. Medical assessment included history, laboratory tests, electrocardiogram (ECG), a physical examination, and a psychiatric evaluation. Included were men and women 18–60 years old, who met DSM-IV criteria for current opioid dependence and used opioids daily. Individuals with major affective or psychotic disorder were excluded. Other exclusion criteria included: 1) regular use of methadone; 2) history of accidental opioid overdose, 3) ongoing treatment with prescription opioids or psychotropic agents; 4) physiological dependence on alcohol or sedative-hypnotics, 5) unstable physical disorders which might make participation hazardous.
2.2. Study Procedures
Following study consent, participants were admitted to an inpatient unit at the New York State Psychiatric Institute for the purpose of detoxification and naltrexone induction. We have used a modification of a buprenorphine-assisted, rapid opioid detoxification and naltrexone induction procedure (Collins et al., 2005). Briefly, participants were stabilized on buprenorphine for 1 day, followed by a washout period of 1–2 days, then given an increasing daily dose of naltrexone (12.5 mg, 25 mg, 50 mg, 100 mg) while precipitated withdrawal symptoms were treated with clonidine, clonazepam, and other adjuvant medications. On the second day of naltrexone induction, participants were randomized by a research pharmacy to receive one of the two doses of memantine (30 or 60 mg/day) or placebo. Participants were discharged with small supplies and week-long tapering schedules for adjuvant medications that they have been receiving in the hospital (clonidine, trazodone, and zolpidem).
Following discharge, participants continued treatment with memantine or placebo for 12 weeks. Both participants and study personnel were blind to medication assignment. Memantine tablets were encapsulated with 25 mg of riboflavin, added by the research pharmacy of the New York State Psychiatric Institute, as a urine marker to assess compliance. A matching capsule containing folic acid tablets and riboflavin was used as a placebo. Throughout the 12 weeks of the study, participants received 3 medication capsules daily (1 in the morning and 2 in the evening), each containing memantine 10 mg, memantine 20mg, or placebo, each encapsulated with 25 mg of riboflavin. The memantine dose was gradually increased to a target dose of 30 or 60 mg/d over the 1- or 2-week period respectively. We selected the maximum target dose of memantine 60 mg/day to ensure that brain levels would be sufficient to achieve antagonist effects at the NMDA receptor (Danysz et al., 1997). This dose has been safely administered in human laboratory studies (Bisaga et al., 2001; Collins et al., 2007); however, it is higher than doses used in patients with Alzheimer’s disease (up to 20 mg/d).
All participants received naltrexone during the 12-week study. Naltrexone was administered under supervision in the clinic in doses of 100 mg on Mondays and Wednesdays and 150 mg on Fridays. Participants were given an emergency supply of naltrexone to take at home in case of a missed visit (Carroll et al., 2001). Participants were required to attend the clinic three times per week. During each visit, participants gave an observed urine specimen and completed self-report measures of drug use, craving, and mood. A nurse or a physician also obtained vital signs and administered naltrexone under supervision. Participants met with a research psychiatrist once per week to monitor their progress in treatment, and review medication safety and adherence. All urine specimens were tested on-site for opiates and two samples per week were sent to the laboratory for a full toxicology panel. Each urine sample submitted during the trial was observed under UV light for riboflavin fluorescence indicating adherence with study medication. Participants who did not come for a visit to submit a urine sample for 14 consecutive days during the 12-week medication trial were classified as study drop-outs. Participants were reimbursed $10 per week during the treatment period and $25 for follow-up visits, for their time taken to complete the research assessments.
All participants received a psychosocial intervention that covered the motivational and cognitive-behavioral techniques incorporated in Behavioral Naltrexone Therapy (BNT) (Rothenberg et al., 2002). Therapy was provided by clinical psychologists trained in BNT and relapse prevention. All treatment sessions were provided within an individual therapy framework. Therapy sessions were audiotaped for supervisory and adherence purposes. Therapists participated in weekly supervision sessions to assure adherence to the intervention procedures and to prevent therapeutic drift.
2.3. Outcome Measures and Data Analyses
The primary aim of the study was to compare the retention rates (time to drop out) of participants across the three treatment arms in the 12-week trial. Secondary outcomes were: weekly proportion of participants who used opiates (dichotomous), weekly average Clinical Global Impression (CGI) severity score (continuous), weekly average CGI improvement score (continuous), weekly craving (defined as any craving score >0 during the week; categorical), and weekly average ratings of opiate withdrawal symptoms (SOWS: Subjective Opiate Withdrawal Scale: continuous). Retention rates were compared using Kaplan-Meier curves and log-rank statistics. Longitudinal secondary outcomes were analyzed using mixed effect models with appropriate link functions. The two-way interaction between treatment and time (i.e., week) was assessed and was retained in the final models if found significant. All interaction terms were evaluated at a significance level of 15%. PROC GLIMMIX in SAS was used to conduct these analyses. All analyses were conducted based on the intent-to-treat principle. All statistical tests were two-tailed and employed an alpha significance level of .05, unless otherwise stated.
Safety of the treatment was evaluated based on reports of adverse events (AEs). The incidence of treatment-emergent AEs was defined as AEs that occurred after the first administration of study medication. The overall incidence of treatment-emergent AEs described as mild, moderate or severe was compared across treatment arms using chi-square tests or Fisher’s exact tests. A Data and Safety Monitoring Board met at the middle point of the study to review enrollment, medication tolerability, and to conduct a preliminary data analysis. Results of a preliminary data analysis are reported here. Because there was no evidence of any efficacy, the DSMB recommended stopping the study.
3. Results
3.1. Sample description
We screened in person 476 individuals for eligibility (See Figure 1 for the CONSORT Flow Diagram). Of those who were screened, 193 individuals declined to participate (159 failed to complete the evaluation and were lost to follow-up, 22 wanted immediate detoxification, 12 were not interested in inpatient detox or treatment medications) and 78 individuals were not eligible to participate (27 had significant medical problems, 12 had significant psychiatric co-morbidities, 4 were taking other psychotropic medications, 2 were using methadone regularly, and 33 were not eligible for other reasons). In addition, 94 participants entered other treatment studies conducted concurrently at our clinic.
Figure 1.
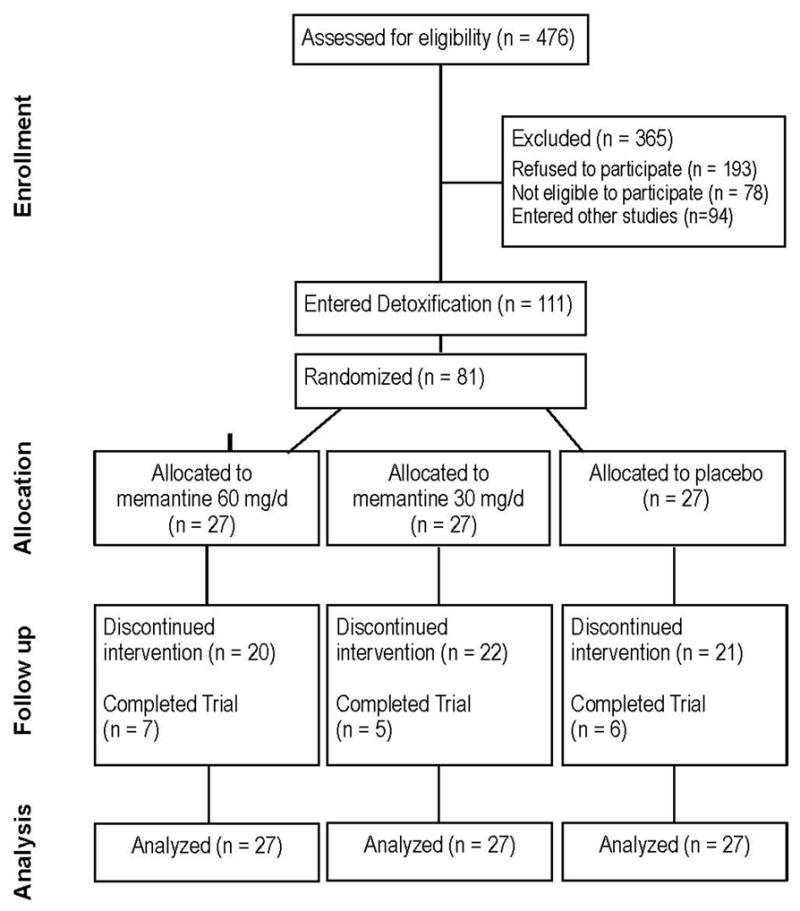
Consort diagram summarizing participant flow
A total of 111 individuals consented to the study and entered the inpatient detoxification protocol. Participants were on average 41 years of age (SD=10.1), mostly male (81%), either White (48%) or Hispanic (30%). The majority of participants were daily intranasal heroin users, using on average 7.5 bags heroin/day (range 2–30). Twenty-nine of those participants (26%) decided to withdraw from study participation during the first 4–5 days of detox. After receiving the first dose of naltrexone participants were stratified based on their age; 36 years and younger vs. 37 years or older and the baseline level of heroin use; 5 or less bags/d vs. 6 or more bags/d (29 high age/high use, 33 high age/low use, 15 low age/high use, and 5 low age/low use). After stratification, participants were randomized equally to three study arms; memantine 30mg (n=27), memantine 60mg (n=27), and placebo (n=27). Demographic characteristics of the 81 randomized participants are presented in Table 1. None was found to be significantly different across the three groups.
Table 1.
Demographic characteristics of the participants randomized to placebo, memantine 30mg, and memantine 60mg (N=81)*
| Characteristic | Placebo (n=27) | Memantine 30mg (n=27) | Memantine 60mg (n=27) | p-value |
|---|---|---|---|---|
| mean (SD) or n (%) | ||||
| Age (years) | 40.5 (9.6) | 41.5 (9.4) | 42.0 (10.3) | 0.86 |
| Male (n) | 20 (74.1) | 24 (88.9) | 22 (81.5) | 0.37 |
| Race/Ethnicity | ||||
| Hispanic | 6 (22.2) | 10 (37.0) | 8 (29.6) | 0.41 |
| Black | 5 (18.5) | 4 (14.8) | 10 (37.0) | |
| White | 14 (51.9) | 11 (40.7) | 8 (29.6) | |
| Other | 2 (7.4) | 2 (7.4) | 1 (3.7) | |
| Education (n) | ||||
| Less than or equal to high school | 12 (44.4) | 10 (40.0) | 14 (63.6) | 0.53 |
| Some college | 9 (33.3) | 8 (32.0) | 5 (22.7) | |
| College or more | 6 (22.2) | 7 (28.0) | 3 (13.6) | |
| Employment status | ||||
| Full-time | 8 (29.6) | 9 (33.3) | 5 (19.2) | 0.15 |
| Part-time | 0 (0) | 2 (7.4) | 5 (19.2) | |
| Unemployed/others | 19 (70.4) | 16 (59.3) | 16 (61.5) | |
| Currently married | 4 (14.8) | 2 (8.0) | 4 (18.2) | 0.63 |
| Pattern of Opiate Use at baseline | ||||
| Duration of use (yrs) | 14.7 (10.5) | 14.5 (11.2) | 14.9 (11.5) | 0.99 |
| Amount of daily use (bags)** | 8.3 (6.4) | 6.4 (3.5) | 7.2 (3.9) | 0.46 |
| Intravenous route | 5 (18.5) | 8 (29.6) | 8 (29.6) | 0.63 |
| Prescription opiate users | 2 (7.4) | 4 (14.8) | 2 (7.4) | 0.57 |
| Baseline Drug and Alcohol Use | ||||
| Alcohol | ||||
| Days used in last 30 days | 1.0 (0–3.0) | 1.0 (0–12.0) | 1.0 (0–7.0) | 0.59 |
| Amount spent per day in last 30 days ($) | 2.5 (1.8–3.5) | 2.0 (1.0–3.3) | 3.0 (1.8–4.0) | 0.75 |
| Marijuana | ||||
| Days used in last 30 days | 0.0 (0–1.25) | 0.0 (0–0) | 0.0 (0–2.0) | 0.28 |
| Amount/day in last 30 days (#joints) | 1.0 (1.0–1.8) | 1.0 (0) | 1.0 (1.0–3.0) | 0.43 |
| Cocaine | ||||
| Days used in last 30 days | 1.0 (0–10.0) | 0.0 (0–2.0) | 0.0 (0–1.0) | 0.24 |
| Amount/day in last 30 days ($) | 20 (10–21.3) | 45 (18.8–50) | 20 (10–43.8) | 0.06 |
Frequencies may not sum to N=81 due to missing values. Percentages may not add up to 100 due to rounding.
One bag of heroin contains 50–100 mg of powder, which contains 50–70% of pure heroin.
3.2. Primary Outcome: retention in treatment
Of the 81 randomized participants, 35% (n=28) completed at least 4 weeks of treatment, and 24% (n=20) completed all 12 weeks of the trial. The survival curves in Figure 2 describe retention in treatment for the placebo, memantine 30mg, and memantine 60mg groups. Retention to week 12 was slightly higher in PBO (26%), compared to memantine 30mg (19%) and memantine 60mg (22%) groups. However, the difference in retention rates across the three groups was not statistically significant (P=0.32).
Figure 2.
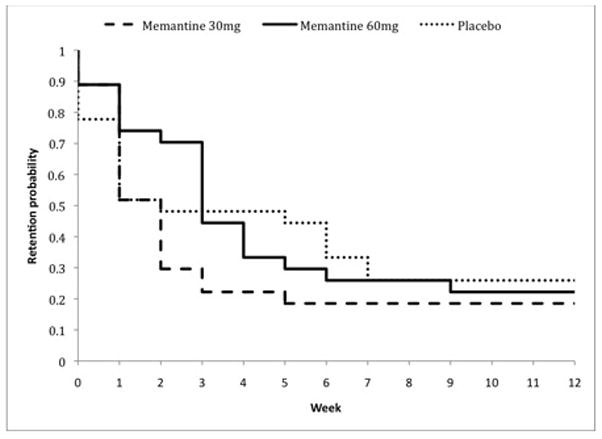
Kaplan-Meier curve of patient retention throughout the 12-week medication trial, by treatment condition
Among the 61 participants who dropped out of the trial, 10 were removed prior to completing detoxification (8 were no longer interested in naltrexone and 2 were removed by investigators, one for medical and one for psychiatric deterioration), 32 stopped attending the clinic appointments and most likely resumed opiate use, 6 continued heroin use over prolonged period of time and were referred out, 5 stopped taking naltrexone and attending citing medical reasons, 4 moved out of state, 3 stopped attending due to work requirement and one was removed by investigators after accidental overdose. There were no differences between those who completed the trial and those who did not in demographic or clinical characteristics.
3.3. Secondary outcomes
Opiate Use
The proportions of participants who used opiates during the trials were not significantly different across the three treatment groups (P=.97). Nonetheless, a significant time effect (P=.008) suggests that the proportion of participants who used opiates decreased in all treatment arms over the twelve-week trial. This decrease was comparable across treatments, as no significant interaction between treatment and time was found. This finding is most likely due to the fact that active users left treatment while abstinent participants remained.
Clinical Global Impression (CGI)
The overall CGI severity and improvement scores of participants remaining in treatment were low during the trial suggesting that patients remaining in treatment had low severity of disease and were significantly improved from baseline. CGI scores did not differ across the three treatment groups (P=.56 and P=.16 respectively). There was a statistically significant time effect on both measures (P<.001), suggesting that CGI severity scores and the CGI improvement scores decreased over time over time in all three groups. No significant treatment by time interaction was found.
Withdrawal Symptoms and Craving
No significant difference in SOWS scores was observed across the three treatment arms (P=.24), although a significant time effect (P<.001) suggests that SOWS scores decreased considerably for all treatment groups throughout the course of the trial. No significant time-by-treatment interaction was found. Intensity of craving did not differ across treatments and remained low and relatively constant over time. There was no significant effect of either treatment (P=.49), or time (P=0.1), or the interaction of treatment by time.
3.4. Medication Adherence
The number of memantine capsules taken was assessed at each visit using a structured calendar-based interview and confirmed using a visual inspection of the sample for riboflavin fluorescence. Medication adherence was quantified as the proportion of days with compliance of 80% or more capsules taken during the 12 weeks of the treatment trial. The median rate (and interquartile range) of medication adherence was 90% (67%–100%) for the memantine 30mg group, 79% (50%–95%) for the memantine 60mg group, and 87% (58%–97%) for the placebo group (P=.65).
Furthermore, blood samples for memantine levels were collected at weeks 4, 8 and 12. Blood levels were measured in 83% of participants that remained in treatment at week 4. No memantine was detected in participants randomized to receive placebo. Across all three measurements mean memantine blood level for the 30 mg group was 61.5 ng/ml (SD 37.4; range 0 to 102) and for 60 mg group was 97.7 ng/ml (SD 91.8; range 0 to 266), confirming that participants in the 60-mg group were taking a higher mean dose of memantine.
3.5. Medication Tolerability, and Adverse Effects
The mean maximum tolerated dose of memantine (of the maximum 3 capsules per day where each capsule contained 20 mg for high dose group, 10 mg in low-dose group, and 0 mg in placebo group) was 2.0 ± 1.3 capsules/day in the placebo group, 1.6 ± 1.4 capsules/day in the memantine 30mg arm, and 2.4 ± 1.1 capsules/day in the memantine 60mg arm (P=.57). Thirty-three participants required dose reductions, with 12 from the placebo group, nine from the memantine 30mg group and 12 from the memantine 60mg group (P=.63). The most frequent reasons given for dose reductions were insomnia, gastrointestinal distress, headaches and dizziness.
Fifty-nine percent of the placebo group, 37% of the memantine 30mg group and 63% of the memantine 60mg group reported experiencing some adverse effects (Table 2). There were no significant differences across treatment groups (P=.12). While some side effects had been attributed to the study medications, such claims were not significantly different across the three treatment groups (P=.81). None of the side effects was sustained and there were no participants who reported discontinuing the study because of AEs.
Table 2.
Summary of Adverse Events reported in greater than 5% of randomized participants.
| Number (%) of participants
|
|||
|---|---|---|---|
| Placebo (N=27) | Memantine 30 mg/d (N=27) | Memantine 60 mg/d (N=27) | |
| Number of Participants who were removed from trial because of SAEs* | 0 | 1 | 0 |
| Number of Participants with at least 1 TEAE* | 16 | 10 | 17 |
| Number of Participants requiring dose reduction | 12 | 9 | 12 |
| TEAEs* | |||
| Headache | 5 (18.5) | 2 (7.41) | 3 (11.1) |
| Insomnia | 7 (25.9) | 8 (29.6) | 9 (33.3) |
| Body Aches | 2 (7.41) | 1 (3.7) | 2 (7.41) |
| Dizziness | 3 (11.1) | 2 (7.41) | 2 (7.41) |
| Nausea/Vomiting | 1 (3.7) | 1 (3.7) | 5 (18.5) |
| Weakness | 1 (3.7) | 3 (11.1) | 1 (3.7) |
| Diarrhea | 4 (14.8) | 2 (7.41) | 4 (14.8) |
| GI Distress | 7 (25.9) | 0 (0.00) | 7 (25.9) |
AE, adverse event, SAE, serious adverse event; TEAE, treatment-emergent adverse event. No significant differences were detected between treatment conditions.
There were four Serious Adverse Events (SAEs) that occurred in this study. Three SAEs occurred during detoxification prior to participants’ receiving memantine or placebo and included hypotensive crisis, stabilized with IV infusions in two participants and one episode of fainting with head injury. One SAE occurred following discharge in a patient treated with memantine 30 mg who resumed heroin use and had an episode of overdose requiring hospitalization, with full recovery. Review of laboratory and ECG parameters revealed no clinically remarkable changes from baseline. There were no differences between treatment groups at the end of treatment in terms of weight, blood pressure (SBP and DBP), heart rate, respiration, and oral temperature.
4. Discussion
The results of this randomized, placebo-controlled trial suggest that memantine (30 or 60 mg/d), is not effective as an adjunct to oral naltrexone combined with weekly individual therapy for the treatment of opioid dependence in recently detoxified individuals. There were no significant differences between the memantine and placebo treatment groups in the retention in treatment, likelihood of heroin use, CGI ratings, opiate withdrawal symptoms or the level of heroin craving during the 12 weeks of the trial. Overall, memantine appeared to be well tolerated in this population; frequency of adverse effects and of requests to lower medication dose in memantine groups were comparable to those of placebo. The majority of participants complained of adverse effects during the first 1–3 weeks following discharge from the inpatient unit, and although many participants believed that these might have been related to the study medication, requests for dose reduction of medications occurred with equal frequency in the medication and the placebo groups. This finding suggests that adverse effects were more likely the result of protracted withdrawal, rather than medication side-effects.
Our findings do not support results of preclinical and human laboratory studies predicting effectiveness of memantine in opiod dependence (Aguilar et al., 2009; Bisaga et al., 2001; Comer and Sullivan, 2007; Harris et al., 2008; Maldonado et al., 2003; Popik and Danysz, 1997; Popik and Skolnick, 1996; Popik et al., 2003, 2006; Ribeiro Do Couto et al., 2004, 2005; Semenova et al., 1999). In addition results of the present trial differ from an earlier trial showing effectiveness of memantine in detoxified opioid dependent individuals residing for a month on the inpatient unit (Krupitsky et al., 2002). There are several possible reasons for the observed lack of memantine’s efficacy in the present study. We hypothesized that memantine would decrease the aversive effects of residual and naltrexone precipitated withdrawal, attenuate exaggerated reactivity to conditioned cues, and decrease heroin craving. In turn, these effects would assure continued compliance with naltrexone and sustained abstinence.
Almost all participants experienced distressing symptoms of moderate severity in the first 1-2 weeks following discharge. Memantine did not appear to reduce withdrawal symptoms during this vulnerable period, as no measurable difference on SOWS was observed, and ratings of craving remained low in both groups. It is possible that concomitant naltrexone might have exacerbated protracted withdrawal, thus masking the potential ameliorative effects of memantine that have been observed by Krupitsky and colleagues (2002) in inpatients undergoing detoxification. While a replication of the study testing memantine only is warranted, this approach is suboptimal for outpatients since discharging participants following detoxification without the protective effects of a prescribed agonist or antagonist results in unacceptably high risks of relapse and overdose. Perhaps a study that initiates treatment with memantine in the inpatient setting during the first month of treatment and continues it in the outpatient setting will minimize the high risk of relapse that usually occurs in the first month.
Second, the most significant dropout occurred during the first week after discharge. However, at this time patients had had only a brief exposure to memantine, often at lower doses used early in the titration schedule. Such modest exposure may have been insufficient for the desired effects of memantine to emerge. Therefore, the adopted design might have been insufficient to adequately test the effectiveness of memantine. A trial that includes treatment with memantine from the first day of detoxification, perhaps with more aggressive dose titration might have been more appropriate. Moreover, oral naltrexone has weak effectiveness to retain patients in treatment, particularly in the absence of intensive behavioral therapy, and this could have obscured any beneficial effect of memantine. Hence, future trials should be considered combining memantine with long-acting preparations of naltrexone, which have better retention over the first 1–2 months and provide a more robust platform to determine effectiveness of adjunctive medications (Brooks et al., 2010).
In the present study, the inability to detect efficacy of memantine was unlikely to be related to a small sample size or missing data, as the study was powered sufficiently to detect a moderate or greater effect size, which would be clinically significant. The target dose of memantine used in the present study was sufficient for a meaningful pharmacodynamic effect at the NMDA receptor site (Parsons et al., 2007). The average serum concentrations of memantine in the present study were in the 60-100 ng/mL range which is equivalent to the concentration of 0.3-0.6 μM and with the average serum/CSF ratio of 0.5 (Kornhuber and Quack, 1995) it is equivalent to the 0.15-0.3 μM concentration in the CSF. Memantine inhibits NMDA receptors with an approximate affinity of 1μM (Rammes et al., 2008) and therefore memantine levels achieved in the present study were most likely sufficient for a meaningful pharmacodynamic effect at the NMDA receptors. More importantly, the doses that were used in the present clinical trial were comparable with doses used in the human laboratory studies and in the pilot clinical trial showing beneficial effect of memantine.
Finally, the failure to confirm positive effects of memantine might be related to the limitations of preclinical animal and human laboratory studies in predicting results of clinical studies. Such models may have excellent construct validity but may not be adequate to predict clinically effective medication, i.e., they lack broad predictive validity (Haney, 2009). For example, it could be that the laboratory models that suggested promise of memantine were sensitive to relatively subtle effects that are too small to have an impact in the clinical arena. Alternatively, the effects detected in laboratory models, such as physical withdrawal, subjective effects of heroin and craving were not the most important for the medication to be useful in the clinical setting with treatment seekers. Instead, the reinforcing effects of heroin may play a central role in relapse and indeed in the study of Comer and Sullivan memantine did not alter the reinforcing effects of heroin (Comer and Sullivan, 2007). This suggests that more work may be needed in further developing human laboratory models so that they better capture the variables that influence the efficacy of medication in the natural ecology, and are stronger predictors of clinical efficacy.
In summary, the present findings did not support the efficacy of memantine as an adjunct to oral naltrexone for the treatment for opioid dependence, despite support for beneficial effects from preclinical models of the disease. However, replication of this finding using a design with modifications based on the present findings is warranted. Additional research should be undertaken to improve the methodology of clinical medication development, at the same time that new pharmacological targets and strategies are pursued.
Acknowledgments
Role of funding source:
Funding for this study was provided by NIDA grant DA15822 (Dr. Bisaga) and K24 DA022412 (Dr. Nunes). The NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We wish to thank Nabil Khan, Julianne Kurtz, John Lazar, Daniel Brooks and the staff of STARS treatment program as well as the pharmacy staff for their contribution to this project.
Footnotes
Contributors:
Drs. Bisaga and Nunes designed the study and wrote the protocol. Ms. Cheng conducted statistical analyses and prepared the Results section of the manuscript. Drs. Bisaga, Sullivan, Carpenter, Mariani, Levin, Raby, and Nunes implemented the study protocol. Dr. Bisaga was study Principal Investigator and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest:
In the past Dr. Bisaga received an educational grant supporting his preclinical work from Merz Pharmaceuticals, an originator of memantine and its distributor in some countries. Drs. Bisaga and Nunes currently receive medication from Alkermes for an ongoing study that is sponsored by the National Institute on Drug Abuse. All other authors declare that they have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar MA, Manzanedo C, Do Couto BR, Rodriguez-Arias M, Minarro J. Memantine blocks sensitization to the rewarding effects of morphine. Brain Res. 2009;1288:95–104. doi: 10.1016/j.brainres.2009.06.100. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Comer SD, Ward AS, Popik P, Kleber HD, Fischman MW. The NMDA antagonist memantine attenuates the expression of opioid physical dependence in humans. Psychopharmacology (Berl) 2001;157:1–10. doi: 10.1007/s002130100739. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Popik P. In search of a new pharmacological treatment for drug and alcohol addiction: N-methyl-D-aspartate (NMDA) antagonists. Drug Alcohol Depend. 2000;59:1–15. doi: 10.1016/s0376-8716(99)00107-6. [DOI] [PubMed] [Google Scholar]
- Brooks AC, Comer SD, Sullivan MA, Bisaga A, Carpenter KM, Raby WM, Yu E, O’Brien CP, Nunes EV. Long-acting injectable versus oral naltrexone maintenance therapy with psychosocial intervention for heroin dependence: a quasi-experiment. J Clin Psychiatry. 2010;71:1371–1378. doi: 10.4088/JCP.09m05080ecr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O’Connor PG, Eagan DA, Frankforter TL, Triffleman EG, Shi J, Rounsaville BJ. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: efficacy of contingency management and significant other involvement. Arch Gen Psychiatry. 2001;58:755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins ED, Kleber HD, Whittington RA, Heitler NE. Anesthesia-assisted vs. buprenorphine- or clonidine-assisted heroin detoxification and naltrexone induction: a randomized trial. JAMA. 2005;294:903–913. doi: 10.1001/jama.294.8.903. [DOI] [PubMed] [Google Scholar]
- Collins ED, Vosburg SK, Ward AS, Haney M, Foltin RW. The effects of acute pretreatment with high-dose memantine on the cardiovascular and behavioral effects of cocaine in humans. Exp Clin Psychopharmacol. 2007;15:228–237. doi: 10.1037/1064-1297.15.3.228. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA. Memantine produces modest reductions in heroin-induced subjective responses in human research volunteers. Psychopharmacology (Berl) 2007;193:235–245. doi: 10.1007/s00213-007-0775-2. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons CG, Kornhuber J, Schmidt WJ, Quack G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents--preclinical studies. Neurosci Biobehav Rev. 1997;21:455–468. doi: 10.1016/s0149-7634(96)00037-1. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M. Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict Biol. 2009;14:9–21. doi: 10.1111/j.1369-1600.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Rothwell PE, Gewirtz JC. Effects of the NMDA receptor antagonist memantine on the expression and development of acute opiate dependence as assessed by withdrawal-potentiated startle and hyperalgesia. Psychopharmacology (Berl) 2008;196:649–660. doi: 10.1007/s00213-007-0998-2. [DOI] [PubMed] [Google Scholar]
- Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction. 2006;101:491–503. doi: 10.1111/j.1360-0443.2006.01369.x. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Quack G. Cerebrospinal fluid and serum concentrations of the N-methyl-D-aspartate (NMDA) receptor antagonist memantine in man. Neurosci Lett. 1995;195:137–139. doi: 10.1016/0304-3940(95)11785-u. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Masalov DV, Burakov AM, Didienko TY, Romanova TN, Bespalov AY, Neznanov NG. A pilot sudy of memantine effects on protracted withdrawal (syndrome of anhedonia) in heroin addicts. Addict Disord Their Treat. 2002;1:143–146. [Google Scholar]
- Maldonado C, Cauli O, Rodriguez-Arias M, Aguilar MA, Minarro J. Memantine presents different effects from MK-801 in motivational and physical signs of morphine withdrawal. Behav Brain Res. 2003;144:25–35. doi: 10.1016/s0166-4328(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009:CD002209. doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- Medvedev IO, Dravolina OA, Bespalov AY. Effects of N-methyl-D-aspartate receptor antagonists on discriminative stimulus effects of naloxone in morphine-dependent rats using the Y- maze drug discrimination paradigm. J Pharmacol Exp Ther. 1998;286:1260–1268. [PubMed] [Google Scholar]
- Parsons CG, Rammes G, Danysz W. Pharmacodynamics of memantine: an update. Curr Neuropharmacol. 2008;6:55–78. doi: 10.2174/157015908783769671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Stoffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Popik P, Danysz W. Inhibition of reinforcing effects of morphine and motivational aspects of naloxone-precipitated opioid withdrawal by N-methyl-D-aspartate receptor antagonist, memantine. J Pharmacol Exp Ther. 1997;280:854–865. [PubMed] [Google Scholar]
- Popik P, Skolnick P. The NMDA antagonist memantine blocks the expression and maintenance of morphine dependence. Pharmacol Biochem Behav. 1996;53:791–797. doi: 10.1016/0091-3057(95)02163-9. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M, Bisaga A. Reinstatement of morphine-conditioned reward is blocked by memantine. Neuropsychopharmacology. 2006;31:160–170. doi: 10.1038/sj.npp.1300760. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M, Rygula R, Bisaga A, Bespalov AY. Effects of memantine, an NMDA receptor antagonist, on place preference conditioned with drug and nondrug reinforcers in mice. Behav Pharmacol. 2003;14:237–244. doi: 10.1097/00008877-200305000-00008. [DOI] [PubMed] [Google Scholar]
- Rammes G, Danysz W, Parsons CG. Pharmacodynamics of memantine: an update. Curr Neuropharmacol. 2008;6:55–78. doi: 10.2174/157015908783769671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Minarro J. Effects of NMDA receptor antagonists (MK-801 and memantine) on the acquisition of morphine-induced conditioned place preference in mice. Prog Neuro-psychopharmacol Biol Psychiatry. 2004;28:1035–1043. doi: 10.1016/j.pnpbp.2004.05.038. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Minarro J. NMDA glutamate but not dopamine antagonists blocks drug-induced reinstatement of morphine place preference. Brain Res Bull. 2005;64:493–503. doi: 10.1016/j.brainresbull.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Rothenberg JL, Sullivan MA, Church SH, Seracini A, Collins E, Kleber HD, Nunes EV. Behavioral naltrexone therapy: an integrated treatment for opiate dependence. J Subst Abuse Treat. 2002;23:351–360. doi: 10.1016/s0740-5472(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Semenova S, Kuzmin AV, Danysz W, Bespalov AY. Low affinity NMDA receptor channel blockers inhibit initiation of intravenous morphine self-administration in naive mice. Eur J Pharmacol. 1999;378:1–8. doi: 10.1016/s0014-2999(99)00431-8. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Krystal JH. Depression. In: Lodge D, Danysz W, Parsons C, editors. Ionotropic Glutamate Receptors as Therapeutic Targets. F.P. Graham Publishing Co; Johnson City, TN: 2002. pp. 71–90. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-38A, HHS Publication No. SMA 10–4586 Findings. Office of Applied Studies; Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. [Google Scholar]


