Abstract
Introduction
Sudden cardiac arrest (CA) is a leading cause of death worldwide. Breathing nitric oxide (NO) reduces ischemia-reperfusion (IR) injury in animal models and in patients. The objective of this study was to learn whether inhaled NO improves outcomes after CA and cardiopulmonary resuscitation (CPR).
Methods and Results
Adult male mice were subjected to potassium-induced CA for 7.5 min whereupon CPR was performed with chest compression and mechanical ventilation. One hour after CPR, mice were extubated and breathed air alone or air supplemented with 40 parts per million (ppm) NO for 23h. Mice that were subjected to CA/CPR and breathed air exhibited a poor 10-day survival rate (4/13), depressed neurological and left ventricular (LV) function, and increased caspase-3 activation and inflammatory cytokine induction in the brain. Magnetic resonance imaging revealed brain regions with marked water diffusion abnormality 24h after CA/CPR in mice that breathed air. Breathing air supplemented with NO for 23h starting 1h after CPR attenuated neurological and LV dysfunction 4 days after CA/CPR and markedly improved 10-day survival rate (11/13, P=0.003 vs Air). The protective effects of inhaled NO on the outcome after CA/CPR were associated with reduced water diffusion abnormality, caspase-3 activation, and cytokine induction in the brain and increased serum NOx levels. Deficiency of the α1 subunit of soluble guanylate cyclase (sGC), a primary target of NO, abrogated the ability of inhaled NO to improve outcomes after CA/CPR.
Conclusions
These results suggest that NO inhalation after CA and successful CPR improves outcome via sGC-dependent mechanisms.
Keywords: cardiopulmonary resuscitation, heart arrest, neurological function, magnetic resonance imaging, nitric oxide synthase, physiology
INTRODUCTION
Sudden cardiac arrest (CA) is a leading cause of death worldwide.1 Despite advances in cardiopulmonary resuscitation (CPR) methods, including the introduction of the automatic electrical defibrillator (AED) and therapeutic hypothermia (TH),2,3 fewer than 8% of adult out-of-hospital CA victims survive to hospital discharge,4 and up to 60% of survivors have moderate to severe cognitive deficits 3 months after resuscitation.5 The poor outcome after CA is at least partly due to the post-CA syndrome that includes neurological and myocardial dysfunction and systemic inflammation. While TH has proven effective in clinical studies,2,3 no pharmacological agent is available to improve outcomes of post-CA syndrome.
Nitric oxide (NO) is produced from NO synthases (NOS1, NOS2, and NOS3). One of the primary targets of NO is soluble guanylate cyclase (sGC) that generates the second messenger cGMP upon activation. sGC is a heme-containing heterodimeric enzyme composed of one α and one β subunit. In most tissues, including heart, lung, and vascular smooth muscle cells, the sGCα1β1 heterodimer is the predominant isoform. NO exerts a number of effects that would be expected to prevent IR injury including inhibition of reactive oxygen species (ROS)-producing enzymes and direct scavenging of ROS. Nonetheless, the impact of endogenous and exogenous NO in the setting of CA/CPR, a whole body IR injury complicated by systemic inflammation, is incompletely understood. In a previous study, we observed that deficiency of NOS3 or sGCα1 worsened outcomes of CA/CPR, whereas cardiomyocyte-specific overexpression of NOS3 rescued NOS3-deficient mice from myocardial and neurological dysfunction and death after CA.6 Along these lines, Dezfulian and colleagues recently reported that administration of nitrite at the initiation of CPR improved outcomes in a murine CA model, presumably by releasing NO.7 The protective effects of nitrite were associated with increased cardiac S-nitrosothiol levels and reversible inhibition of respiratory chain complex I in mitochondria. While these results suggest that NO-dependent mechanisms have protective effects in CA/CPR, systemic administration of NO-donor compounds may induce systemic vasodilation and hypotension, frequently precluding its use in patients after CA in whom blood pressure may be low and unstable.
Although originally developed as a selective pulmonary vasodilator, inhaled NO has been shown to elicit systemic effects in a variety of pre-clinical and clinical studies, without causing systemic vasodilation. For example, breathing NO attenuates myocardial IR injury in mice8 and swine9 and hepatic IR injury in patients undergoing liver transplantation.10 Based on these observations, we hypothesized that NO inhalation could improve outcomes after CA/CPR. Here, we provide evidence that breathing NO starting 1h after CPR markedly improves neurological and myocardial function and 10-day survival rate in mice after CA.
MATERIALS AND METHODS
Mice
After approval by the Massachusetts General Hospital Subcommittee on Research Animal Care, we studied 2- to 3-month-old age- and weight-matched male C57BL/6J wild-type (WT, n=105), sGCα1-deficient (sGCα1−/−, n=49)11, and NOS3-deficient mice (NOS3−/−, B6.129P2-Nos3tm1Unc/J, n=33)12 on a C57BL/6J background.
Murine CPR model
Cardiac arrest and CPR in mice was performed as previously described.6,13 Briefly, after instrumentation under anesthesia, CA was induced by administration of potassium chloride (0.08 mg/g body weight) through the femoral venous catheter. In WT and sGCα1−/− mice, after 7.5 min of CA, chest compressions were delivered using a finger at a rate of 340~360 beats per minute with resumption of mechanical ventilation (FiO2=1.0). Epinephrine was infused at 0.3 µg/min starting 30 seconds before CPR, and the infusion was continued until the heart rate (HR) became higher than 300 bpm. Return of spontaneous circulation (ROSC) was defined as the return of sinus rhythm with a mean arterial pressure (MAP) >40 mm Hg lasting at least 1 minute. Mice were weaned from mechanical ventilation and extubated at 1h after CPR. Mice were then randomized to breath air alone or air supplemented with 40 ppm NO for 23h in custom-made chambers. Core body temperature was maintained at 37°C by a warming lamp for the first hour after CPR. Thereafter, body temperature was allowed to equilibrate in an ambient temperature of 27°C in the chambers for the subsequent 23h, after which mice returned to the regular cages in room air (ambient temperature ~25°C) for the remainder of the study period. Mice subjected to sham surgery that were not subjected to CA/CPR were used as controls.
Because of their sensitivity to prolonged CA,6,14 NOS3−/− mice were subjected to CA for only 6.5 min. Subsequent procedures, including CPR, in NOS3−/− mice were conducted as described above.
Assessment of neurological function
Neurological function was assessed at 24 and 96h after CA/CPR or sham surgery using a previously-reported neurological function scoring system.6,13,15 Briefly, five parameters were assessed and scored: level of consciousness (no reaction to pinching of tail = 0, poor response to tail pinch = 1, normal response to tail pinch = 2), corneal reflex (no blinking = 0, sluggish blinking = 1, normal blinking = 2), respirations (irregular breathing pattern = 0, decreased breathing frequency with normal pattern = 1, normal breathing frequency and pattern = 2), coordination (no movement = 0, moderate ataxia = 1, normal coordination = 2), and movement/activity (no spontaneous movement = 0, sluggish movement = 1, normal movement = 2). Total score was reported as the neurological function score (total possible score = 10).
Assessment of right ventricular systolic pressure
In a group of WT mice, right ventricular (RV) systolic pressure was measured 1h after CPR (before initiation of NO inhalation) or sham surgery using a conductance pressure-volume catheter (SPR-839, Millar Instruments Inc., Houston, TX) inserted into the RV via right jugular vein.
Effects of NO inhalation on myocardial function
Left ventricular (LV) function was examined 4 days after CPR in WT mice that were subjected to CA/CPR and breathed air or air supplemented with NO or sham surgery. Mice were anesthetized with fentanyl 250 µg/kg and ketamine 100 mg/kg IP and LV function was measured with a conductance pressure-volume catheter, as previously described.16 Hemodynamic data were analyzed using a computer program (PVAN version 3.6, Millar Instruments).
Acquisition and analysis of MRI
To investigate the degree of ischemic brain injury after CA/CPR, diffusion-weighted imaging (DWI) was performed 24h after CA/CPR in mice that breathed air (n=6) or air supplemented with NO (n=7) using standard MRI acquisition and analysis methods as described previously (see Online Supplement for details).17 Apparent diffusion coefficient (ADC), calculated at each imaging voxel (3-dimensional volume element) from whole-brain images with two different diffusion weightings, reflects a single best measurement of the rate of water diffusion at that location. For quantitative analysis of the brain regions with abnormal water diffusion, average ADC values were calculated in anatomically distinct brain regions of interest (ROI) determined based on the Allen Mouse Brain Atlas,18 including ventral lateral hippocampus (Hipp), caudoputamen (CPu), lateral cortex (Cortex), and whole brain (Total). Average ADC values (µm2/ms) were computed in each mouse across each ROI, and group average ADC values of mice that breathed air or NO were reported for each ROI.
Measurement of serum nitrate/nitrite levels
Concentrations of nitrite and nitrate were measured in serum samples obtained at 24h after CA/CPR or sham surgery with a Nitrate/Nitrite Fluorometric Assay Kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions.
Detailed description of reagents and protocol for quantitative RT-PCR and histological studies are provided in Supplemental Methods.
Statistical Analysis
All data are expressed as mean±SEM. Continuous data were analyzed using unpaired t-test, two-way repeated measures ANOVA, or one-way ANOVA with a Holm-Sidak or Bonferroni post hoc test. Differences in survival rates were analyzed by Log-rank test. Sigmastat 3.01a (Systat Software Inc., San Jose, CA) and GraphPad Prism 5.0 (GraphPad Software Inc. La Jolla, CA) were used for statistical analyses. We did not correct overall for multiple testing because the biological consistency of the set of results indicated a strong underlying consistent effect of breathing NO.
RESULTS
Inhaled NO improves survival rate at 10 days after cardiac arrest and CPR
ROSC was achieved in all 105 WT mice. Three WT mice died soon after extubation and were, therefore, excluded from further analysis. There was no difference between treatment groups in the CPR time to ROSC, the total epinephrine dose, blood pressure, and heart rate 1h after CPR (Supplemental Table 1). The partial pressure of oxygen (PaO2) and oxygen saturation (SaO2) of arterial blood samples obtained at 2h after CPR (1h after initiation of air or NO breathing) did not differ between mice that breathed air or air supplemented with NO (data not shown). Body temperature was maintained at 37±0.5°C for the first hour after CPR. After mice were placed in the chambers at an ambient temperature of 27°C, body temperature fell to ~30°C within 3h but returned to baseline within 24h. There was no difference in core body temperature between mice that breathed air or air supplemented with NO for the first 24h after CA/CPR (data not shown). While only 4 out of 13 mice that breathed air survived 10 days after CPR, 11 out of 13 mice that breathed NO for 23 hours starting 1h after CPR survived for 10 days (P=0.003, Figure 1).
Figure 1.
Survival rate of wild-type mice during the first 10 days after cardiac arrest and CPR. Air, mice subjected to CA/CPR and breathed air for 23h starting 1h after CPR. iNO, mice subjected to CA/CPR and breathed air supplemented with NO for 23h starting 1h after CPR. *P=0.003 vs Air by Log-rank test.
Inhaled NO prevents water diffusion abnormality in the brain 24h after cardiac arrest and CPR
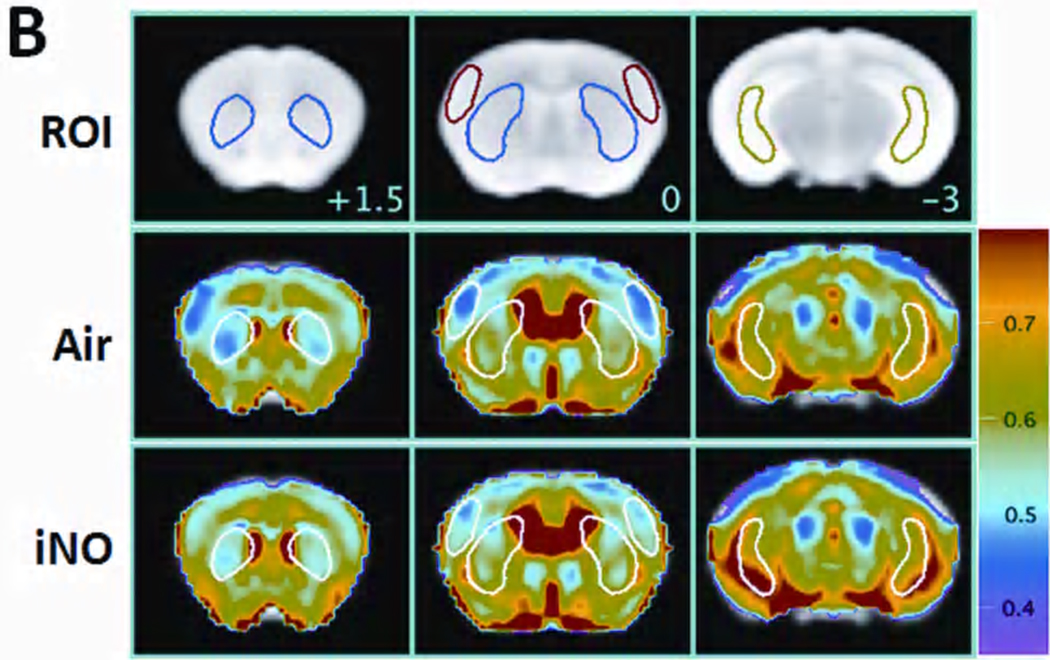
MRI acquired 24h after CA/CPR in mice that breathed air showed areas of hyperintense DWI in the brain (Figure 2A). Hyperintense DWI signal (or reduced ADC signal) is a measure of brain edema presumably due to disruption of ion pump homeostasis and membrane failure.19,20 Breathing NO for 23h starting 1h after CPR largely prevented the development of hyperintense DWI. The degree of abnormal water diffusion was quantitated by calculating the average ADC in several ROIs including the ventral-lateral hippocampus, caudoputamen, and lateral-frontal cortex (Figure 2B). Breathing NO prevented the reduction of ADC values in each ROI and across the whole brain (Figure 2C). These results suggest that NO breathing reduced the development of the ischemia-induced edema in the brain 24h after CA/CPR.
Figure 2.
Panel A. Representative diffusion-weighted image (DWI) of mice 24h after CA/CPR that breathed air (Air) or air supplemented with NO (iNO). White arrows indicate areas of hyperintense DWI. Panel B. Representative MR images showing three brain slices containing regions of interest [ROI]. Slice positions are identified in millimeters (+1.5, 0, or −3 mm) with respect to bregma in the coordinate space of the Allen Mouse Brain Atlas. Colored outlines indicate portions of ROI (blue = caudoputamen, red = lateral cortex; green = ventral lateral hippocampus) that intersect with these slice planes (see Supplemental Methods for further information). Average ADC values of the slice plane for mice (n=6) that breathed Air after CA/CPR [Air]. Average ADC values of the slice plane for mice (n=7) that breathed NO after CA/CPR [iNO]. Color bar on the right side indicates color-code for ADC values (µm2/ms). Panel C. Average ADC values of each 3 dimensional ROI (Hipp = ventral lateral hippocampus, CPu = caudoputamen, Cortex = lateral cortex, total = total brain) across all planes in mice that breathed air (Air, n=6) or NO (iNO, n=7) after CA/CPR. *P<0.05 vs Air.
Inhaled NO prevents neurological dysfunction 4 days after cardiac arrest and CPR
While neurological function did not differ between surviving mice that breathed air or NO at 1 day after CA/CPR, the neurological function score at 4 days after CA/CPR was better in surviving mice that breathed air supplemented with NO than in mice that breathed air alone (P<0.01, Figure 3A). These results suggest that breathing NO prevented the development of neurological dysfunction 4 days after CA/CPR in mice.
Figure 3.
Neuroprotective effects of inhaled NO. Panel A. Neurological function score in surviving mice at 24 and 96h after CA/CPR. Dead mice (indicated by score = 0) were excluded from the statistical analysis. *P<0.01 vs Air by unpaired t-test. Panel B. Representative photomicrographs of brain sections of mice that breathed air or air supplemented with NO after CA/CPR showing cleaved caspase 3-immunoreactive neurons (brown-colored cells) at 4 days after CPR. Size bar = 250 µm. Panel C. Number of neurons per mm2 containing cleaved caspase 3 in the CA-1 region of the hippocampus. *P<0.05 vs Air. N=4 for each group.
Inhaled NO prevents neuronal apoptosis after cardiac arrest and CPR
Histological studies revealed that the number of neurons containing activated caspase 3 in the CA1 region of the hippocampus was markedly increased at 4 days after CA/CPR in mice that breathed air (Figure 3 B and C). Breathing NO starting 1h after CPR prevented caspase 3 activation in the hippocampal neurons. These results suggest that NO inhalation starting 1h after CPR prevents neuronal apoptosis in the brain.
Inhaled NO prevents myocardial dysfunction after cardiac arrest and CPR
There was no difference in HR and MAP among mice at ROSC or 1h after CPR that were subsequently randomized to breath air or air supplemented with NO (Supplemental Table 1). Furthermore, there was no difference in right ventricular systolic pressure at 1h after CPR between mice subjected to CA and mice subjected to sham operation, suggesting the absence of pulmonary hypertension after CA (data not shown).
Four days after CA/CPR, indices of LV systolic and diastolic function, LV end-systolic pressure (LVESP), LV end-diastolic pressure (LVEDP), maximum rate of developed LV pressure (dP/dtmax), minimum rate of developed LV pressure (dP/dtmin), cardiac output (CO), arterial elastance (Ea), end-systolic elastance (Ees), Ees/Ea, preload-recruitable stroke work (PRSW), and the time constant of isovolumic relaxation (τ), were markedly impaired in mice that breathed air compared to sham-operated mice (Table 1). Inhaled NO attenuated the impairment of HR, dP/dtmax, dP/dtmin, CO, Ees, Ees/Ea, and τ at 4 days after CA/CPR. These results show that inhalation of NO for 23h starting 1h after CPR ameliorates post-CA myocardial dysfunction at 4 days after CA/CPR in mice.
Table 1.
Left ventricular function 4 days after cardiac arrest and CPR
| Sham (n=4) |
Air (n=5) |
iNO (n=5) |
|
|---|---|---|---|
| HR, bpm | 673±7 | 482±26* | 591±28*# |
| LVESP, mmHg | 100±6 | 58±4* | 73±5* |
| LVEDP, mmHg | 2±1 | 4±1* | 2±0# |
| dP/dtmax, mmHg/s | 18153±1862 | 6159±1007* | 10461±803*# |
| dP/dtmin, mmHg/s | −10599±1189 | −4235±432* | −7248±640*# |
| CO, ml/min | 12.4±0.5 | 7.6±1.0* | 11.4±0.6# |
| dP/dtmax/IP, s−1 | 231±9 | 198±27 | 206±18 |
| Ea, mmHg/µL | 5±0 | 4±1* | 4±0* |
| Ees, mmHg/µL | 26±5 | 6±1* | 15±1*# |
| Ees/Ea | 4.7±0.8 | 1.6±0.3* | 3.8±0.1# |
| PRSW, mmHg | 141±25 | 74±9* | 102±11 |
| τ, milliseconds | 4.9±0.3 | 8.2±0.6* | 5.6±0.4# |
Values are mean±SEM. Sham, sham-operated mice; Air, mice breathed air after CA/CPR; iNO, mice breathed air supplemented with NO starting 1h after CA/CPR; HR, heart rate; LVESP, left ventricular end-systolic pressure; LVEDP, left ventricular end-diastolic pressure; dP/dtmax, maximum rate of developed left ventricular pressure; dP/dtmin, minimum rate of developed left ventricular pressure; CO, cardiac output; dP/dtmax/IP, dP/dtmax divided by instantaneous pressure; Ea, arterial elastance; Ees, left ventricular end-systolic ventricular elastance; PRSW, preload-recruitable stroke work; τ, time constant of isovolumic relaxation.
P<0.05 vs sham-operated mice,
P<0.05 vs Air (by one-way ANOVA with a Bonferroni post hoc test)
Inhaled NO increased serum levels of nitrite and nitrate 24h after CA/CPR
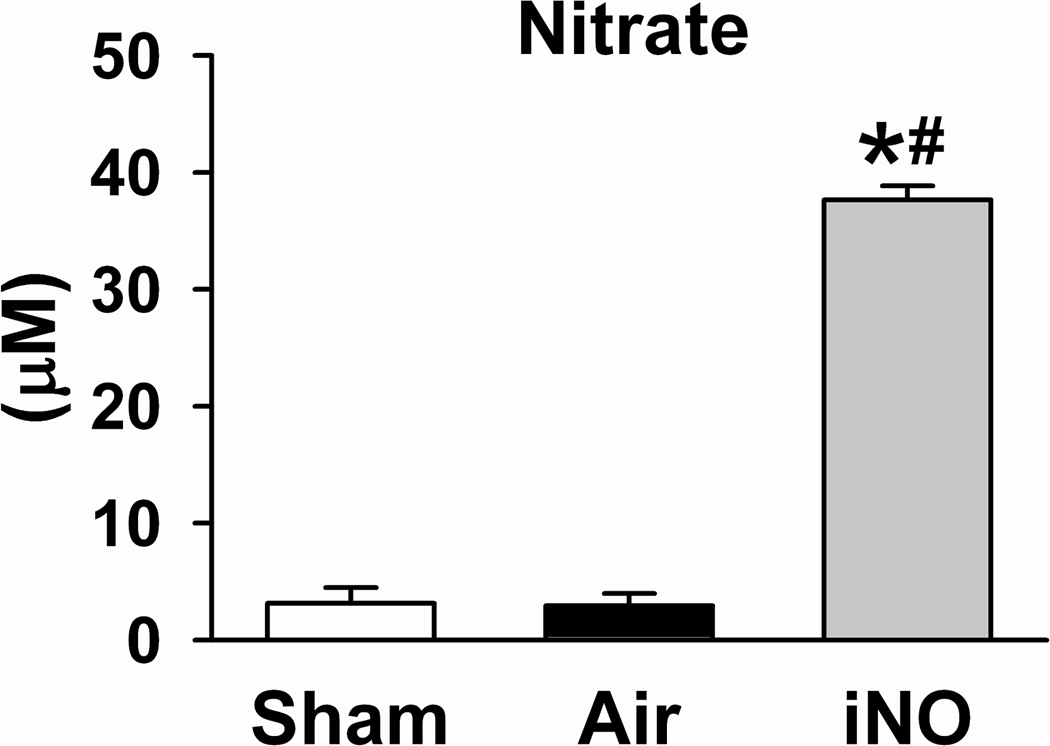
Cardiac arrest and CPR did not affect serum levels of nitrite and nitrate in mice that breathed air alone 24h after CA/CPR. Breathing air supplemented with NO for 23h markedly increased serum nitrite and nitrate levels compared to sham-operated mice (P<0.05 for nitrite and P<0.0001 for nitrate vs Sham) and mice that breathed air alone after CPR (P<0.01 for nitrite and P<0.0001 for nitrate vs Air, Figure 4).
Figure 4.
Serum nitrite and nitrate concentrations in mice 24h after sham surgery (Sham), after CA/CPR and breathing air (Air), or after CA/CPR and breathing air supplemented with NO (iNO) for 23h starting 1h after CPR. N=6–9. *P<0.05 vs Sham. #P<0.05 vs Air.
Deficiency of sGCα1, but not NOS3, abolishes the salutary effects of inhaled NO on survival rate at 10 days after CA/CPR
To elucidate the mechanisms responsible for the beneficial effects of NO inhalation on survival after CA/CPR, we examined whether or not inhaled NO improves outcomes of CA/CPR in sGCα1−/− mice.
While ROSC was achieved in all 49 sGCα1−/− mice, 10 mice died soon after extubation and, therefore, were excluded from further analysis. The early mortality rate (in the first 2h after CPR) was higher in sGCα1−/− than in WT mice (3/105 WT mice died; P=0.0007 vs WT). In mice that survived long enough to be randomized to breath air alone or air supplemented with NO for survival study (n=8 in each group), NO inhalation did not prevent neurological dysfunction on day 3 after CPR in sGCα1−/− mice (neurological function score = 6±1 in mice that breathed air and 5±1 in mice that breathed NO, P=NS). Three out of 8 sGCα1−/− mice that breathed air survived 10 days after CA/CPR. Inhalation of NO for 23 h starting 1h after CPR did not improve the survival rate in sGCα1−/− mice (4 out of 8 survived, Figure 5).
Figure 5.
Survival rate of sGCα1−/− mice during the first 10 days after cardiac arrest and CPR. Air sGCα1−/−, sGCα1−/− mice subjected to CA/CPR and breathed air. iNO sGCα1−/−, sGCα1−/− mice subjected to CA/CPR and breathed air supplemented with NO for 23h starting 1h after CPR. N=8 in each group. There was no difference in survival rates between the two groups.
We considered the possibility that the failure of inhaled NO to improve the outcome in sGCα1−/− mice was that the injury induced by CA/CPR was too severe to be rescued by breathing NO. To test this hypothesis, we examined whether inhaled NO could improve outcomes in a strain of mice, NOS3−/− mice, that also manifest increased sensitivity to CA/CPR.6,13 All NOS3−/− mice that were subjected to 7.5 or 7 min CA died within 24h after CPR, confirming that NOS3−/− mice were more sensitive to CA/CPR than WT and sGCα1−/− mice. In NOS3−/− mice subjected to CA for 6.5 min, mean survival time was greater in those mice that breathed air supplemented with NO than in those that breathed air alone (3±1 vs 1±0 days, respectively; P=0.0064 by Log-rank test). These observations demonstrate that mice that are more sensitive to prolonged CA than sGCα1−/− mice can be rescued by NO inhalation after CA/CPR. Taken together, these results suggest that the protective effects of inhaled NO on neurological function and survival after CA/CPR are at least in part mediated via sGC-dependent mechanisms.
Inhaled NO prevents the induction of inflammatory cytokines in WT but not in sGCα1−/− mice
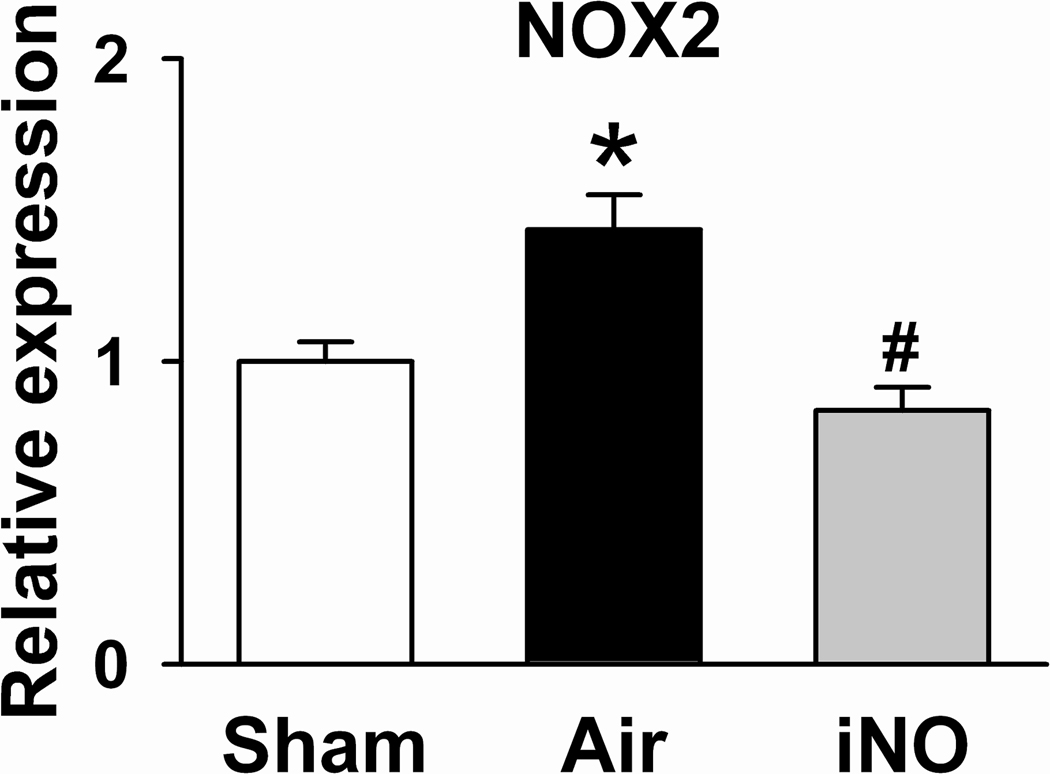
Expression of genes encoding tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and gp91phox (NOX2, a subunit of NADPH oxidase) were markedly greater in the brain cortex of WT mice that were subjected to CA/CPR and breathed air 24h after CA/CPR than in those of sham-operated mice (Figure 6). Breathing NO prevented the induction of TNF-α, IL-6, and NOX2 in the brain of WT mice subjected to CA/CPR.
Figure 6.
Expression of genes encoding tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β, and gp91phox (NOX2, a subunit of NADPH oxidase) in the brain cortex of WT mice 24h after sham surgery (Sham), after CA/CPR and breathing air (Air), or after CA/CPR and breathing air supplemented with NO (iNO) for 23h starting 1h after CPR. N=4–8. *P<0.05 vs Sham. #P<0.05 vs Air.
While CA/CPR induced TNF-α, IL-1β, and NOX2 gene expression in the brains of sGCα1−/− mice, the ability of NO inhalation to prevent the induction of these genes was abolished by sGCα1 deficiency (Supplemental Figure 2). Taken together, these observations suggest that NO breathing exerts anti-inflammatory and anti-oxidant effects in the brain after CA/CPR via sGC-dependent mechanisms.
DISCUSSION
The current study demonstrates that NO inhalation at 40 ppm for 23h starting at 1h after successful CPR markedly improves myocardial and neurological function and survival rate at 10 days after CA/CPR in mice. The neuroprotective effects of inhaled NO were associated with attenuation of the CA/CPR-induced abnormality in water diffusion detected using brain MRI at 1 day after CA and with prevention of caspase 3 activation in the hippocampal neurons at 4 days after CA/CPR. The salutary impact of inhaled NO on the outcome of CA/CPR was also associated with the inhibition of inflammatory cytokine induction in the brain and increased serum levels of nitrite and nitrate. Finally, deficiency of sGCα1, but not NOS3, abrogated the protective effects of inhaled NO on the 10-day survival rate, neurological function, and inflammatory cytokine induction after CA/CPR. Taken together, these observations suggest that breathing NO after successful CPR confers organ protection and improves survival, at least in part, via sGC-dependent mechanisms.
It is increasingly recognized that post–CA care after ROSC can improve the likelihood of patient survival with good neurological function. Clinical trials showed that TH conferred neuroprotective effects when it was applied for 12–24h starting minutes to hours after successful CPR from CA due to ventricular fibrillation.2,3 The apparent presence of a temporal therapeutic window after successful CPR is consistent with the observations that many of the mechanisms responsible for the post-CA brain injury are executed over hours to days following ROSC.21–24 These post-CA pathogenetic pathways include excitotoxicity, neuroinflammation, disrupted ion channel homeostasis, and membrane failure, as well as pathological activation of proteases and cell death signalling.21,22 The protective effects of breathing NO for 23h beginning 1h after successful CPR, observed in the current study, further support the notion that outcomes of sudden CA can be improved by implementing innovative therapies in the “post-CA golden hours” after successful CPR.
Conventional histopathological assessment of brain injury requires brain sections from individual animals sacrificed at separate time points after injury. These methods not only diminish the statistical power but may also introduce artifacts due to the post-mortem tissue preparation. In the current study, mice that were successfully resuscitated from 7.5 min of CA and breathed air exhibited a marked abnormality in water diffusion in the hippocampus, caudoputamen, and cortex 24h after CPR. The presence of abnormal DWI signals in the vulnerable regions of the brain 1 day after CA/CPR correlated with worse neurological function and increased apoptosis of hippocampal neurons 4 days after CPR, as well as decreased rate of survival at 10 days. In contrast, NO breathing markedly attenuated the development of abnormality in water diffusion in the brain and improved neurological outcomes and survival rate. These observations are consistent with a recent clinical study that showed that diffuse cortical abnormalities in DWI are associated with poor outcomes in patients resuscitated from CA.25 Hyperintense DWI signals indicate the presence of brain edema presumably due to disruption of ion pump function and membrane failure. The current observations, therefore, suggest that NO inhalation after successful CPR can preserve ion pump homeostasis and membrane integrity early after CA/CPR.
Although the greatest proportion of the post-CA mortality and morbidity is caused by global ischemic brain damage, the severity of myocardial dysfunction correlates with poor neurological outcome.26 We found that the degree of LV dysfunction 4 days after CPR was markedly attenuated in mice that breathed NO. These observations support the correlation between myocardial dysfunction and poor neurological outcomes and survival after CA/CPR.
RV dysfunction may also contribute to the circulatory failure after CA/CPR.27 Given the ability of inhaled NO to selectively reduce pulmonary artery pressure, it is conceivable that breathing NO improved outcomes of CA/CPR by reducing RV afterload. However, we did not find the evidence of pulmonary hypertension in WT mice 1h after CA/CPR (before initiation of NO inhalation). Because inhaled NO reduces pulmonary artery pressure only in the presence of pulmonary hypertension, it is unlikely that inhaled NO improved outcomes after CA/CPR by reducing RV afterload in our model.
Neuroinflammation triggered by the whole-body IR injury associated with CA/CPR hinder the neurological recovery from prolonged CA. We observed that CA/CPR markedly upregulated the expression of genes encoding inflammatory cytokines and NADPH oxidase in the brain of WT mice that breathed air, but not in WT mice that breathed air supplemented with NO. These observations suggest that NO inhalation prevents neuroinflammation after CA/CPR. Furthermore, these results demonstrate a correlation between neuroinflammation, neurological dysfunction, and mortality after CA/CPR.
NO elicits biological effects via sGC-dependent and/or -independent mechanisms. To determine the role of sGC in the protective effects of inhaled NO on the outcome of CA/CPR, we studied sGCα1−/− mice. We observed that sGCα1-deficiency increased the early mortality rate (in the first 2h after CPR) when compared to WT mice after CA/CPR, consistent with our previous report.6 While the cause of these early deaths is unknown, we previously reported that sGCα1 deficiency markedly exacerbated LV dysfunction early after CA/CPR.6 After excluding the mice that died early after CPR, sGCα1−/− mice that breathed air had 10-day survival rate comparable to that in WT mice that breathed air after CA/CPR. These observations suggest that sGC activity is critically important for initial recovery after CA/CPR but may not be necessary for long-term survival after CA/CPR. In contrast, sGCα1-deficiency abolished the ability of NO inhalation to inhibit the induction of inflammatory cytokines in the brain and to improve neurological function and 10-day survival rate after CA. These observations suggest that protective effects of inhaled NO on the outcome of CA/CPR are largely mediated via sGC-dependent mechanisms.
Inhaled NO may exert systemic effects via interaction with circulating bone marrow (BM)-derived cells (e.g. leukocytes) as they transit lungs. Alternatively, some NO, once inhaled, may escape scavenging by hemoglobin and be converted to relatively stable NO-metabolites (e.g., nitrite, S-nitrosothiols) that can regenerate NO in the periphery and directly protect neurons.28,29 In fact, in the present study, we found that breathing NO increased levels of nitrite and nitrate 24h after CA/CPR. We previously reported that neutrophils are required for inhaled NO to reduce MI size in WT mice subjected to transient left coronary artery occlusion.8 Along these lines, we recently observed that NO breathing markedly decreased MI size in WT but not in sGCα1−/− mice.30 Furthermore, breathing NO decreased MI size in chimeric sGCα1−/− mice carrying WT BM generated by BM transplantation. These results raise the possibility that the neuroprotective effects of inhaled NO after CA/CPR maybe mediated by BM-derived cells in a sGC-dependent manner.
Our data does not exclude the possibility that sGC-independent mechanisms could contribute to the protective effects of inhaled NO on peripheral organs after CA/CPR. It is conceivable that NO modifies functions of enzymes and ion channels in a sGC-independent manner.7,31 For example, ischemic preconditioning has been shown to protect cardiomyocytes from subsequent IR injury by preventing Ca2+ overload via S-nitrosylation-mediated inhibition of L-type Ca2+ channel α1 subunit.32 Further studies are warranted to elucidate the mechanisms responsible for the protective effects of inhaled NO on the outcome after CA/CPR.
From the viewpoint of translating the current results into clinical benefit, it is of particular importance that NO inhalation started 1h after successful CPR and continued for 23h markedly improves neurological and myocardial function and survival rate 10 days after CA/CPR. For example, NO inhalation can be started after patients are transferred to hospital and informed consent obtained. To date, TH is the only therapeutic approach that is proven to improve outcomes after CA/CPR when applied hours after successful CPR.2,3 Since body temperature of mice were allowed to decrease to ~30°C during NO inhalation in the first 24h after CA/CPR in the current study, our data suggests that NO breathing may confer protection in the setting of mild hypothermia. Nonetheless, effects of combination of inhaled NO with TH, compared to either alone, on outcomes after CA/CPR remains to be formally determined in future studies.
There are several limitations in the current study. The induction of CA by bolus administration of potassium chloride may have limited clinical relevance. However, we believe this model provides a valuable platform for elucidating the molecular mechanisms of organ dysfunction associate with CA/CPR and the impact of inhaled NO on the post-CA syndrome. All mice were anesthetized when subjected to CA/CPR. It is possible that drugs used to induce anesthesia may impact outcomes of CA/CPR.
In summary, the current study revealed robust protective effects of NO inhalation on the outcome of CA/CPR in mice. Breathing NO at 40 ppm for 23h starting 1h after successful CPR markedly improved myocardial and neurological function and survival rate 10 days after CA/CPR, at least in part, via sGC-dependent mechanisms. The ability of “delayed” NO breathing to prevent the post-CA brain injury and promote survival in mice, if extrapolated to human beings, is highly clinically relevant and may serve as the experimental basis for future clinical trials in which effects of inhaled NO on the outcome after CA/CPR are examined. We anticipate that the established safety profile of NO inhalation33 will enable the rapid translation of findings in animal models to patients suffering from the post-CA syndrome.
Clinical Perspective.
Sudden cardiac arrest is one of the leading causes of death worldwide. Despite advances in resuscitation techniques, fewer than 8% of the 300,000 adults who experience cardiac arrest in the US each year survive to hospital discharge, and up to 60% of survivors have long lasting neurological deficits. While therapeutic hypothermia has proven effective in clinical studies, no pharmacological agent is available to improve outcome from cardiac arrest. Although originally developed as a selective pulmonary vasodilator, inhaled NO has been shown to have systemic effects in a variety of pre-clinical and clinical studies without causing systemic vasodilation. In the current study, we found that breathing a low concentration of NO starting 1h after successful CPR for 23h markedly improves long-term neurological and cardiac outcomes and survival in mice subjected to cardiac arrest and CPR. The ability of NO breathing to improve outcomes after cardiac arrest when begun after CPR, if extrapolated to human beings, makes inhaled NO a practical therapeutic approach, which can be initiated after patients are transferred to hospital. Furthermore, because inhaled NO does not cause systemic hypotension, in contrast to systemic NO-donors, it is uniquely suited for the treatment of post-cardiac arrest patients in whom blood pressure is often unstable. We anticipate that the established safety profile of NO inhalation will enable the rapid translation of findings in animal models to patients suffering from the post-cardiac arrest syndrome.
Supplementary Material
ACKNOWLEDGEMENTS
Authors wish to thank Dr. Warren M. Zapol for valuable comments.
FUNDING SOURCES:
This work was supported by funding from the Jikei University School of Medicine (KK); a Resuscitation Fellowship Award from the American Heart Association (AHA) and Philips 09POST2220133 (PYS); an AHA Scientist Development Grant 10SDG2610313 (ESB); an AHA Grant-in-Aid GIA0855887D (PKL); NIH R01 grants DA26108 (PKL), GM79360 (FI), and HL101930 (FI); and a sponsored research agreement between MGH and IKARIA Inc (KDB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURES:
Drs. Bloch and Ichinose have obtained patents relating to the use of inhaled NO. These patents are assigned to Massachusetts General Hospital, which has licensed them to IKARIA and Linde Gas Therapeutics, Lidingo, Sweden.
Reference List
- 1.Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, Samson RA, Kattwinkel J, Berg RA, Bhanji F, Cave DM, Jauch EC, Kudenchuk PJ, Neumar RW, Peberdy MA, Perlman JM, Sinz E, Travers AH, Berg MD, Billi JE, Eigel B, Hickey RW, Kleinman ME, Link MS, Morrison LJ, O'Connor RE, Shuster M, Callaway CW, Cucchiara B, Ferguson JD, Rea TD, Vanden Hoek TL. Part 1: Executive Summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S640–S656. doi: 10.1161/CIRCULATIONAHA.110.970889. [DOI] [PubMed] [Google Scholar]
- 2.The Hypothermia after Cardiac Arrest Study Group. Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of Comatose Survivors of Out-of-Hospital Cardiac Arrest with Induced Hypothermia. New England Journal of Medicine. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.WRITING GROUP; Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics--2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 5.Roine RO, Kajaste S, Kaste M. Neuropsychological sequelae of cardiac arrest. JAMA. 1993;269:237–242. [PubMed] [Google Scholar]
- 6.Nishida T, Yu JD, Minamishima S, Sips PY, Searles RJ, Buys ES, Janssens S, Brouckaert P, Bloch KD, Ichinose F. Protective effects of nitric oxide synthase 3 and soluble guanylate cyclase on the outcome of cardiac arrest and cardiopulmonary resuscitation in mice. Crit Care Med. 2009;37:256–262. doi: 10.1097/CCM.0b013e318192face. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, Tambouret R, Jassal DS, Raher MJ, Furutani E, Ichinose F, Gladwin MT, Rosenzweig A, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. American Journal of Physiology - Heart and Circulatory Physiology. 2006;291:H379–H384. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Huang Y, Pokreisz P, Vermeersch P, Marsboom G, Swinnen M, Verbeken E, Santos J, Pellens M, Gillijns H, Van de WF, Bloch KD, Janssens S. Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischemia and reperfusion. J Am Coll Cardiol. 2007;50:808–817. doi: 10.1016/j.jacc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 10.Lang JD, Jr, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S, Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGCα1 knockout mice. Cardiovascular Research. 2008;79:179–186. doi: 10.1093/cvr/cvn068. [DOI] [PubMed] [Google Scholar]
- 12.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. PNAS. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minamishima S, Bougaki M, Sips PY, De Yu J, Minamishima YA, Elrod JW, Lefer DJ, Bloch KD, Ichinose F. Hydrogen Sulfide Improves Survival After Cardiac Arrest and Cardiopulmonary Resuscitation via a Nitric Oxide Synthase 3-Dependent Mechanism in Mice. Circulation. 2009;120:888–896. doi: 10.1161/CIRCULATIONAHA.108.833491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beiser DG, Orbelyan GA, Inouye BT, Costakis JG, Hamann KJ, McNally EM, Hoek TLV. Genetic deletion of NOS3 increases lethal cardiac dysfunction following mouse cardiac arrest. Resuscitation. 2011 Jan 1;82(1):115–121. doi: 10.1016/j.resuscitation.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–2791. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 16.Ichinose F, Buys ES, Neilan TG, Furutani EM, Morgan JG, Jassal DS, Graveline AR, Searles RJ, Lim CC, Kaneki M, Picard MH, Scherrer-Crosbie M, Janssens S, Liao R, Bloch KD. Cardiomyocyte-Specific Overexpression of Nitric Oxide Synthase 3 Prevents Myocardial Dysfunction in Murine Models of Septic Shock. Circ Res. 2007;100:130–139. doi: 10.1161/01.RES.0000253888.09574.7a. [DOI] [PubMed] [Google Scholar]
- 17.Liu CH, You Z, Liu CM, Kim YR, Whalen MJ, Rosen BR, Liu PK. Diffusion-Weighted Magnetic Resonance Imaging Reversal by Gene Knockdown of Matrix Metalloproteinase-9 Activities in Live Animal Brains. J Neurosci. 2009;29:3508–3517. doi: 10.1523/JNEUROSCI.5332-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chi Chin M, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Feng Yuan X, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 19.Harris NG, Zilkha E, Houseman J, Symms MR, Obrenovitch TP, Williams SR. The Relationship Between the Apparent Diffusion Coefficient Measured by Magnetic Resonance Imaging, Anoxic Depolarization, and Glutamate Efflux During Experimental Cerebral Ischemia. J Cereb Blood Flow Metab. 2000;20:28–36. doi: 10.1097/00004647-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kastrup A, Neumann-Haefelin T, Moseley ME, de Crespigny A. High Speed Diffusion Magnetic Resonance Imaging of Ischemia and Spontaneous Periinfarct Spreading Depression After Thromboembolic Stroke in the Rat. J Cereb Blood Flow Metab. 2000;20:1636–1647. doi: 10.1097/00004647-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Neumar RW. Molecular mechanisms of ischemic neuronal injury. Ann Emerg Med. 2000;36:483–506. doi: 10.1067/mem.2000.110995. [DOI] [PubMed] [Google Scholar]
- 22.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, Callaway C, Clark RSB, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Jr, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. Post-Cardiac Arrest Syndrome: Epidemiology, Pathophysiology, Treatment, and Prognostication A Consensus Statement From the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 23.Fujioka M, Taoka T, Matsuo Y, Mishima K, Ogoshi K, Kondo Y, Tsuda M, Fujiwara M, Asano T, Sakaki T, Miyasaki A, Park D, Siesjo BK. Magnetic resonance imaging shows delayed ischemic striatal neurodegeneration. Ann Neurol. 2003;54:732–747. doi: 10.1002/ana.10751. [DOI] [PubMed] [Google Scholar]
- 24.Sharma HS, Miclescu A, Wiklund L. Cardiac arrest-induced regional blood-brain barrier breakdown, edema formation and brain pathology: a light and electron microscopic study on a new model for neurodegeneration and neuroprotection in porcine brain. J Neural Transm. 2011;118:87–114. doi: 10.1007/s00702-010-0486-4. [DOI] [PubMed] [Google Scholar]
- 25.Wijman CA, Mlynash M, Caulfield AF, Hsia AW, Eyngorn I, Bammer R, Fischbein N, Albers GW, Moseley M. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Ann Neurol. 2009;65:394–402. doi: 10.1002/ana.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 27.Meyer RJ, Kern KB, Berg RA, Hilwig RW, Ewy GA. Post-resuscitation right ventricular dysfunction: delineation and treatment with dobutamine. Resuscitation. 2002;55:187–191. doi: 10.1016/s0300-9572(02)00204-6. [DOI] [PubMed] [Google Scholar]
- 28.Gaston B. Summary: systemic effects of inhaled nitric oxide. Proc Am Thorac Soc. 2006;3:170–172. doi: 10.1513/pats.200506-049BG. [DOI] [PubMed] [Google Scholar]
- 29.Nagasaka Y, Fernandez BO, Garcia-Saura MF, Petersen B, Ichinose F, Bloch KD, Feelisch M, Zapol WM. Brief periods of nitric oxide inhalation protect against myocardial ischemia-reperfusion injury. Anesthesiology. 2008;109:675–682. doi: 10.1097/ALN.0b013e318186316e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagasaka Y, Buys E, Spagnolli E, Steinbicker A, Hayton S, Rauwerdink K, Brouckaert P, Zapol WM, Bloch KD. Soluble guanylate cyclase alfa 1 is required for the cardioprotective effects of inhaled nitric oxide. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00948.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohr MJ, Sun J, Aponte A, Wang G, Gucek M, Murphy E, Steenbergen C. Simultaneous Measurement of Protein Oxidation and S-Nitrosylation During Preconditioning and Ischemia/Reperfusion Injury With Resin-Assisted Capture. Circ Res. 2011;108:418–426. doi: 10.1161/CIRCRESAHA.110.232173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning Results in S-Nitrosylation of Proteins Involved in Regulation of Mitochondrial Energetics and Calcium Transport. Circ Res. 2007;101:1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 33.Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004;109:3106–3111. doi: 10.1161/01.CIR.0000134595.80170.62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.