Abstract
Background and purpose
Delayed paraplegia remains a devastating complication after ischemic spinal cord injury associated with aortic surgery and trauma. While apoptosis has been implicated in the pathogenesis of delayed neurodegeneration, mechanisms responsible for the delayed paraplegia remain incompletely understood. The aim of this study was to elucidate the role of apoptosis in delayed motor neuron degeneration after spinal cord ischemia.
Methods
Mice were subjected to spinal cord ischemia induced by occlusion of the aortic arch and left subclavian artery for 5 or 9 min. Motor function in the hind limb was evaluated up to 72h after spinal cord ischemia. Histological studies were performed to detect caspase-3 activation, glial activation, and motor neuron survival in the serial spinal cord sections. To investigate the impact of caspase-3 activation on spinal cord ischemia, outcome of the spinal cord ischemia was examined in mice deficient for caspase-3.
Results
In wild-type mice, 9 min of spinal cord ischemia caused immediate paraplegia, whereas 5 min of ischemia caused delayed paraplegia. Delayed paraplegia after 5 min of spinal cord ischemia was associated with histological evidence of caspase-3 activation, reactive astrogliosis, microglial activation, and motor neuron loss starting around 24–48h after spinal cord ischemia. Caspase-3 deficiency prevented delayed paraplegia and motor neuron loss after 5 min of spinal cord ischemia, but not immediate paraplegia after 9 min of ischemia.
Conclusion
The present results suggest that caspase-3 activation is required for delayed paraplegia and motor neuron degeneration after spinal cord ischemia.
Keywords: Spinal cord ischemia, Delayed paraplegia, Delayed neuronal death, Cleaved caspase-3, Apoptosis
Introduction
Delayed paraplegia is a devastating complication of spinal cord ischemia (SCI) which can occur after thoracic and abdominal aortic surgery for a variety of aortic pathologies including aneurysm and trauma.1 Rates of immediate and delayed onset neurologic deficits after major thoracic aortic repairs range between 4–11 %.1 Of all incidence of paraplegia (immediate and delayed combined) associated with aortic surgery, the reported incidence of delayed paraplegia varied from 12–73 %.1 Although introduction of several adjunct procedures including cerebrospinal fluid drainage reduced the incident of immediate paraplegia after aortic surgery, the incidence of delayed paraplegia has not changed.2
While immediate paraplegia is thought to be caused by an irreversible ischemic neuronal injury in the spinal cord, the mechanism responsible for delayed paraplegia is incompletely understood.3 Several potential mechanisms responsible for the development of delayed paraplegia have been proposed including delayed apoptotic neuronal death executed by caspase-3 activation.4–6 Nonetheless, role of motor neuron apoptosis and caspase-3 activation in the pathogenesis of delayed paraplegia remains controversial; some studies show presence of apoptosis in the spinal cord of animals exhibiting delayed paraplegia whereas others do not.7,8 Since majority of these studies examined the role of apoptosis using immunohistochemical detection of caspase-3 and or DNA fragmentation, no causal relationship between caspase-3 activation and delayed motor neuron death has been established to date. Furthermore, elucidation of the role of apoptosis in delayed paraplegia has been hindered by the lack of reproducible animal models in which genetic modification can be exploited to determine the molecular mechanisms.
To define the molecular mechanisms responsible for the delayed paraplegia after SCI, we have recently developed a mouse model of delayed paraplegia by modifying previously-reported mouse model of SCI.9 This model is unique in which mice that are subjected to SCI initially recover from surgery and anesthesia and exhibit ability to walk for about 24h. Subsequently, however, all mice develop delayed paraplegia starting around 30–36h after surgery. Using this robust model, we sought to determine the role of apoptotic neuronal death in the immediate and delayed paraplegia after SCI. Here, we report that caspase-3 activation is required for delayed paraplegia but not for immediate paraplegia in mice.
Materials and Methods
Mouse model of spinal cord ischemia
After approval by the Massachusetts General Hospital Subcommittee on Research Animal Care, male wild-type mice (WT, C57BL/6J, 8–10 week old, Jackson Laboratory, Bar Harbor, ME) and male mice deficient for caspase-3 backcrossed onto C57BL/6 background more than 10 generations (caspase-3−/−, 8–10 weeks old)10 were anesthetized with isoflurane and subjected to SCI according to the method described by Lang-Lazdunski and colleagues with modifications.9 Please see http://stroke.ahajournals.org for the details of surgical procedures, measurements of physiological parameters, quantal bioassay, and histological studies.
Assessment of motor neuron function
Motor function was quantified serially at pre-SCI, 8, 24, 48 and 72h after SCI by Basso Mouse Scale (BMS).11 The maximum deficit is indicated by a score of 0. While BMS score less than 6 (0 – 5) indicates paraplegia, BMS score greater than 6 (6 – 9) indicates ability to walk.
Statistics
Parametric data were presented as mean±SD. ANOVA followed by Bonferroni and Tukey-Kramer tests or Student’s t-test was used to compare parametric data. The quantal bioassay was based on logistic analysis.12 The difference between the probability of producing delayed and immediate paraplegia in 50 % of mice (P50d and P50i, respectively) was graphically demonstrated by computer construction of an ischemic duration-probability of paraplegia curve for each group.13 Changes in BMS were analyzed by two-way repeated measures ANOVA followed by Turkey test. The number of viable neurons between experimental groups at individual time points was compared with Mann-Whitney test. P values less than 0.05 were considered significant.
Results
Spinal cord ischemia causes immediate or delayed paraplegia
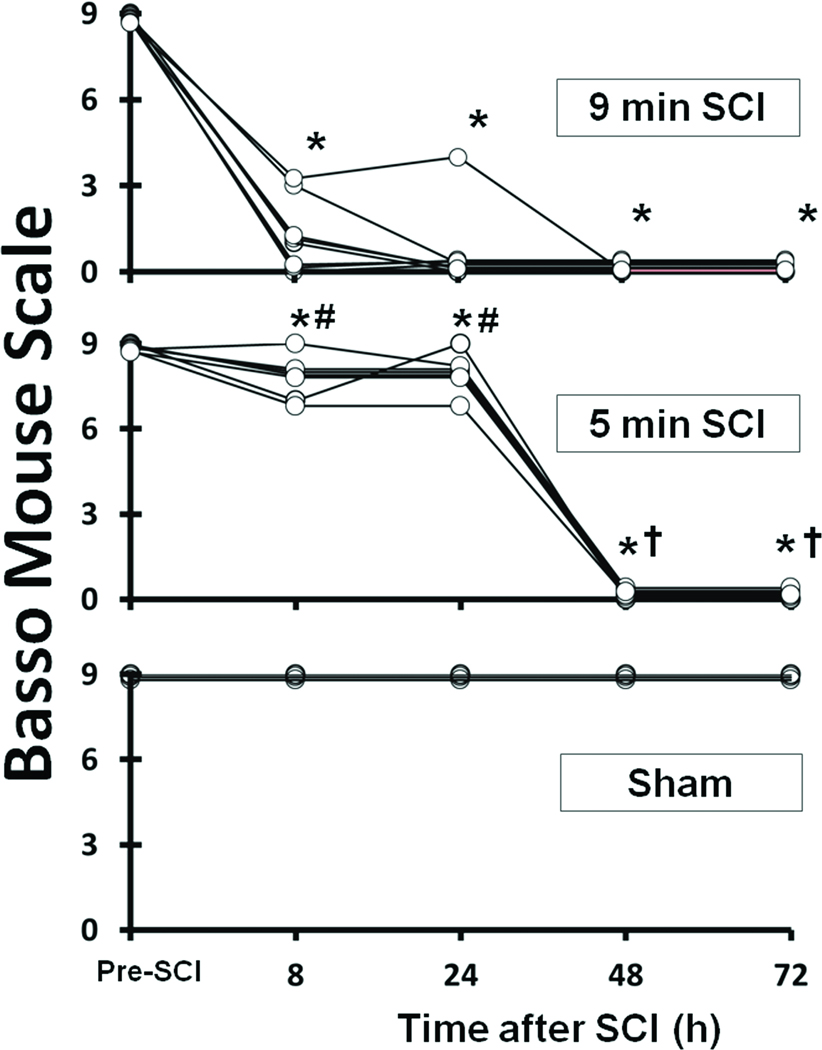
All mice subjected to 9 min SCI showed immediate paraplegia that persisted up to 72h after SCI (P<0.01 vs sham and pre-SCI, Figure 1). All mice subjected to 5 min SCI showed modest and transient motor weakness (BMS = 6 – 9) at 30 min after reperfusion followed by gradual recovery over the next 24–30h (Figure 1). Up to approximately 8h after 5 min SCI, motor deficit manifested as ataxia and partial weakness in place-stepping reflex with preserved ability to walk (P<0.05 vs 9 min SCI at 8 and 24h after SCI, Figure 1). The motor function of lower extremities of mice subjected to 5 min SCI gradually worsened starting around 30h after reperfusion and all mice exhibited complete paraplegia by 48h after reperfusion (Figure 1). Long-term outcomes after SCI (6 weeks) were examined in subgroups of mice that were subjected to 9 or 5 min SCI. While all mice that were subjected to 9 min SCI died within 7 days, 20% of mice survived up to 6 weeks after 5 min of SCI (Figure S1). Once became paraplegic, no mice showed any recovery of hind limb motor function until death or up to 6 weeks after SCI of either duration. Sham-operated mice did not show any neurological deficits throughout the experiments (Figure 1).
Figure 1.
Time course of Basso Mouse Scale (BMS) in mice subjected to 9 min SCI (n = 6), 5 min SCI (n = 8), or sham operation (n = 3). *P<0.05 vs sham and pre-ischemia (pre-SCI). #P<0.05 vs 9 min SCI at corresponding time point. †P<0.05 vs 8h after 5 min SCI.
Quantal bioassay for the relationship between the duration of SCI and neurological function
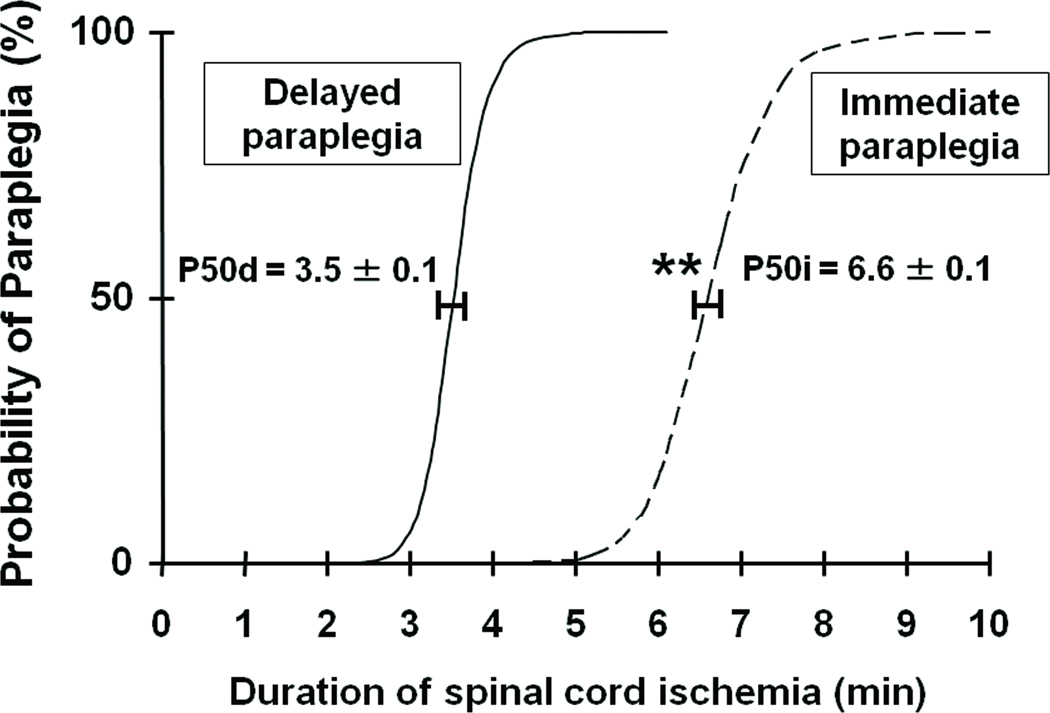
Incidence of paraplegia (BMS = 0 – 5) increased as the duration of SCI increased (Table S1 and Figure 2). In the current model, shorter than 3 min of SCI appeared to be well-tolerated that did not cause motor deficit. While many mice exhibited ability to walk (BMS = 6 – 9) up to 24h after SCI after shorter durations of aortic clamping (from 3.5 to 7.5 min), majority of the mice developed complete paraplegia by 48h of reperfusion (delayed paraplegia). All mice subjected to more than 8 min of SCI showed motor deficit in the hind limb immediately after recovering from anesthesia without any recovery up to 72h after SCI (immediate paraplegia). Quantal bioassay analysis revealed that the P50i and P50d were 6.6±0.1 min and 3.5±0.1 min, respectively (Figure 2, P<0.01).
Figure 2.
Percentage of paraplegic mice as a function of the duration of SCI in minutes. The curves labeled “delayed” (solid line) and “immediate” (dashed line) show the probabilities of developing delayed and immediate paraplegia, respectively, calculated by quantal bioasay. The horizontal bars represent standard deviation at the probability of producing delayed and immediate paraplegia in 50 % of mice (P50d and P50i, respectively). **P<0.01 vs P50d.
Time course of neurodegeneration after SCI
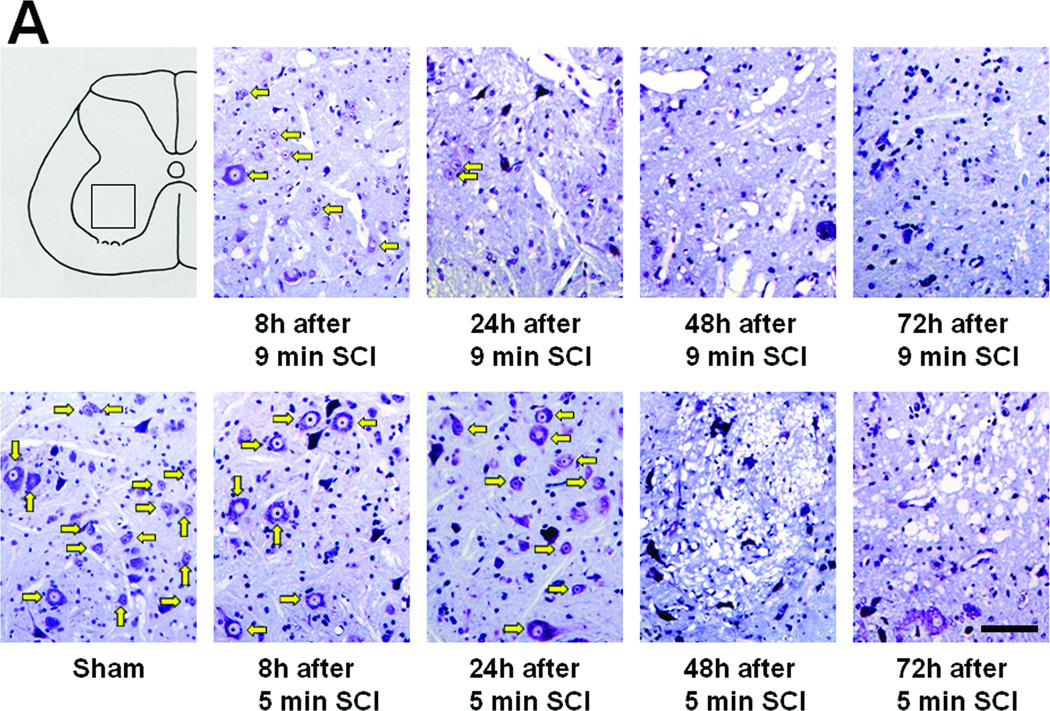
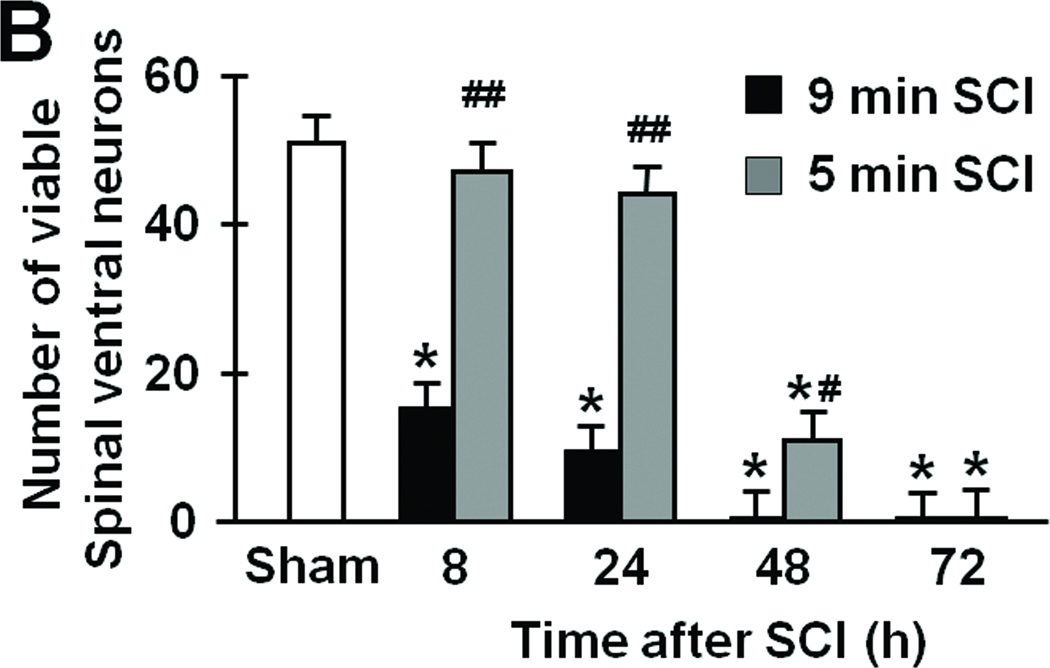
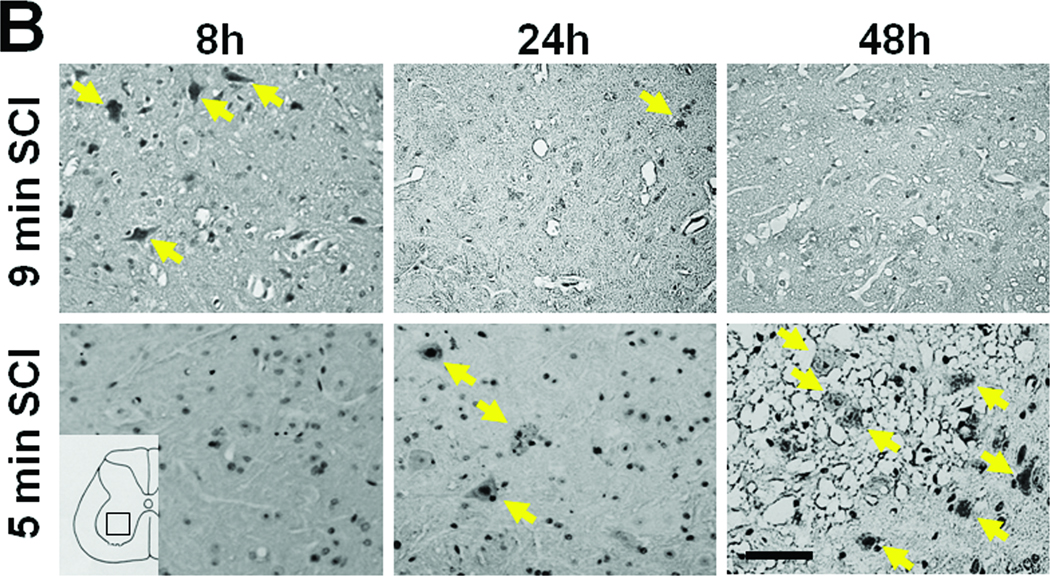
In the spinal cord of mice subjected to 9 min SCI, neurons in the ventral horn started to degenerate at 8h after SCI. Extensive neuronal loss appeared to have completed by 24h of reperfusion without further changes up to 72h after 9 min SCI (Figure 3 A and B). In contrast, 5 min SCI did not affect the number and appearance of neurons in the ventral horn at 8 and 24h after reperfusion (Figure 3 A and B). However, the ventral horns of mice subjected to 5 min SCI exhibited extensive cellular loss with marked cavitation at 48 and 72h after SCI. Although the structure of the ventral horn appeared to be better preserved at 72h after 9 min of SCI than after 5 min of SCI, extensive neuronal loss after SCI of both durations was confirmed by the absence of NeuN-positive neurons at 72h after 9 or 5 min of SCI (Figure 3C).
Figure 3.
(A) Representative photomicrographs of the lumber spinal cord sections of mice subjected to 9 or 5 min SCI showing cresyl violet (Nissl staining)-positive neurons. Yellow arrows indicate viable spinal ventral neurons. (B) Time-dependent changes in the number of viable spinal ventral neurons after 9 or 5 min SCI. N=4–7. * P<0.01 vs sham-operated mice. ## P<0.01 vs 9 min SCI. # P<0.05 vs 9 min SCI. (C) Representative photomicrographs of lumber spinal cord sections of mice showing NeuN-positive neurons (green) 72h after 9 or 5 min SCI. Size bars = 100 µm.
Spinal cord ischemia activates astrocytes and microglia
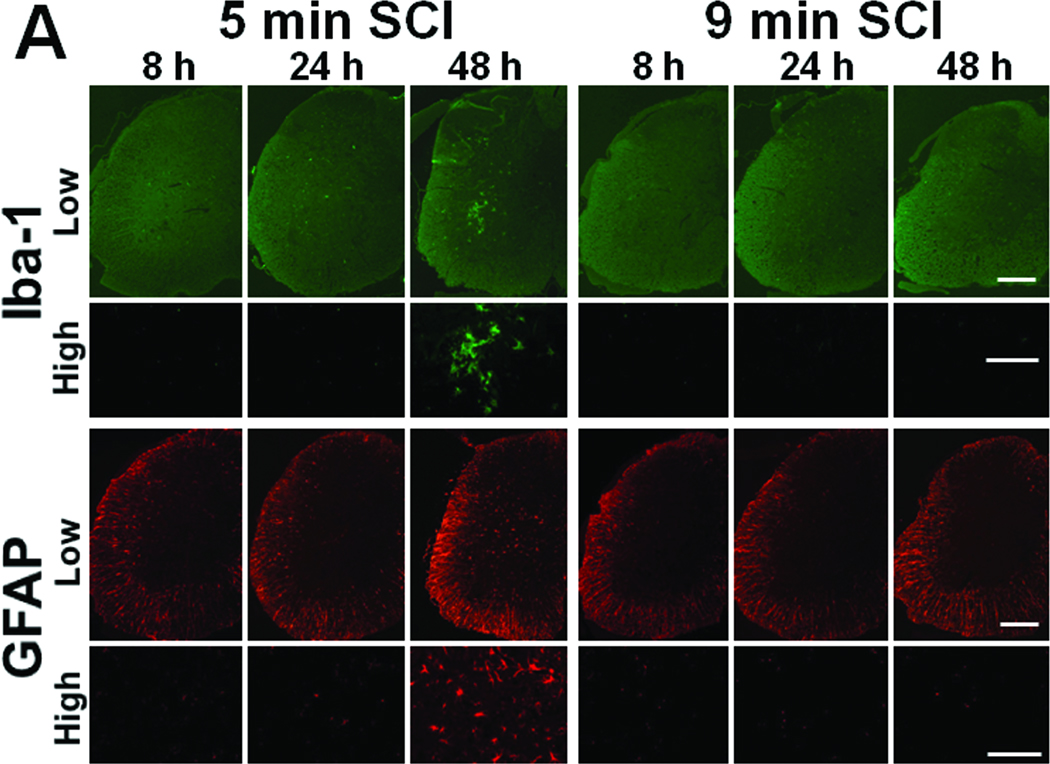
To examine the mechanisms responsible for the delayed extensive cavitation observed in the spinal cord of mice subjected to 5 min SCI, activation of astrocytes and microglia was assessed by immunohistochemical staining of glial fibrillary acidic protein (GFAP) and ionized calcium binding adaptor molecule 1(Iba-1), respectively. While only few reactive astrocytes and activated microglia were observed at 8 and 24h after 5 min SCI (Figure 4A), number of reactive astrocyte and activated microglia markedly increased at 48 h after 5 min SCI (Figure 4A). In contrast, in the spinal cord of mice subjected to 9 min SCI, no reactive astrocyte or activated microglia were observed up to 48h after reperfusion (Figure 4A). These results suggest that delayed inflammatory reaction associated with glial activation may have contributed to the extensive spinal cord damage and delayed paraplegia after 5 min SCI.
Figure 4.
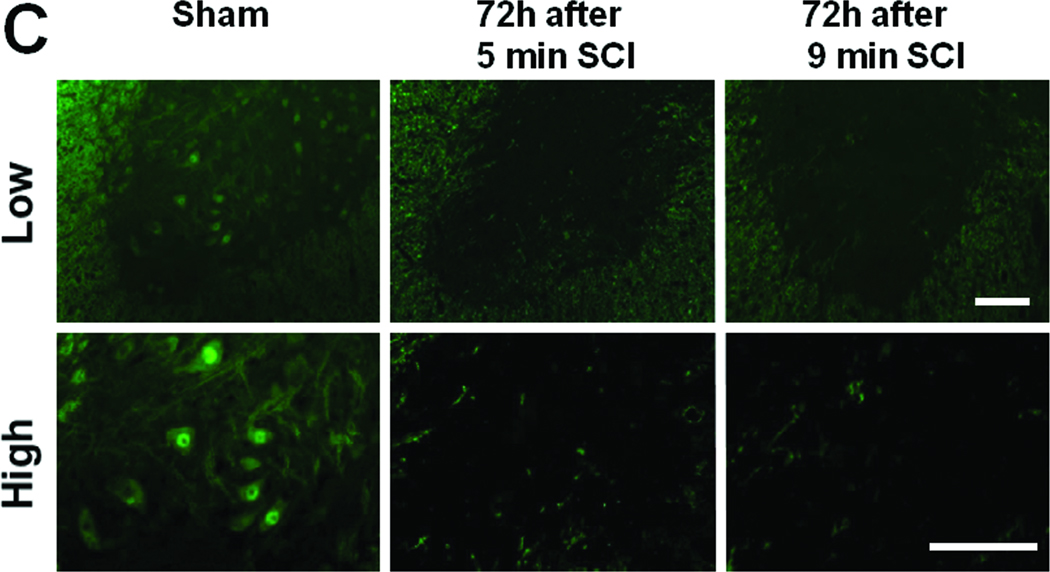
(A) Representative photomicrographs of the lumber spinal cord sections of mice subjected to 9 or 5 min SCI showing Iba-1 or GFAP-immunoreactive cells at 8, 24, or 48h after reperfusion. Size bar = 100 µm for low and 200 µm for high magnification pictures. (B) Representative photomicrographs of the ventral horn of the lumber spinal cord sections of mice subjected to 9 or 5 min SCI showing cleaved caspase-3-immunoreactive neurons. Yellow arrows indicate cleaved caspase3-immunoreactive neurons. Size bar = 100 µm.
Caspase-3 activation precedes the onset of delayed paraplegia
A small number of cleaved caspase-3-positive neurons were observed in the ventral horn of the spinal cord of mice at 8h after 9 min SCI (Figure 4B). However, only a few cleaved caspase-3-positive neurons were found at 24 and 48h after 9 min SCI, presumably because of the extensive necrosis and disappearance of the spinal ventral neurons. In contrast, the number of ventral horn neurons with positive cleaved caspase-3 immunoreactivity markedly increased at 24 and 48h after 5 min SCI (Figure 4). These results suggest caspase-3 activation precedes the onset of extensive neuroinflammation, neurodegeneration, and delayed paraplegia after 5 min SCI.
Caspase-3 deficiency prevents delayed paraplegia in mice
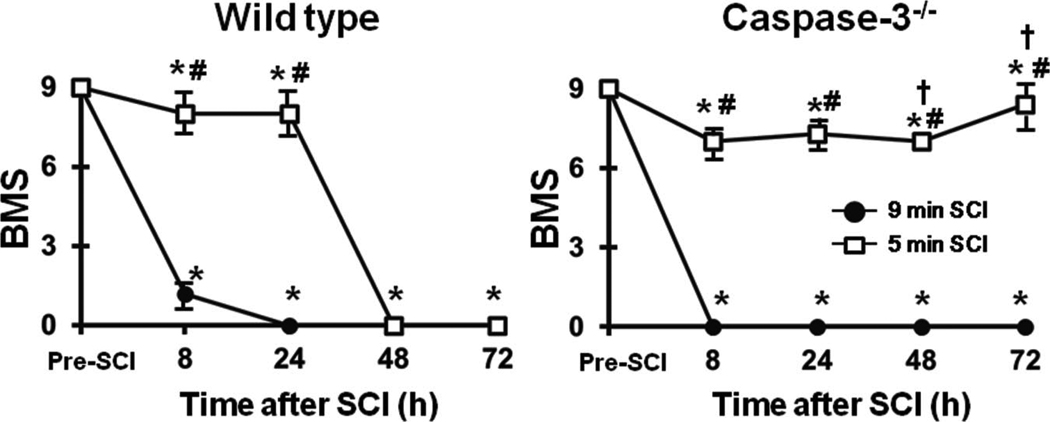
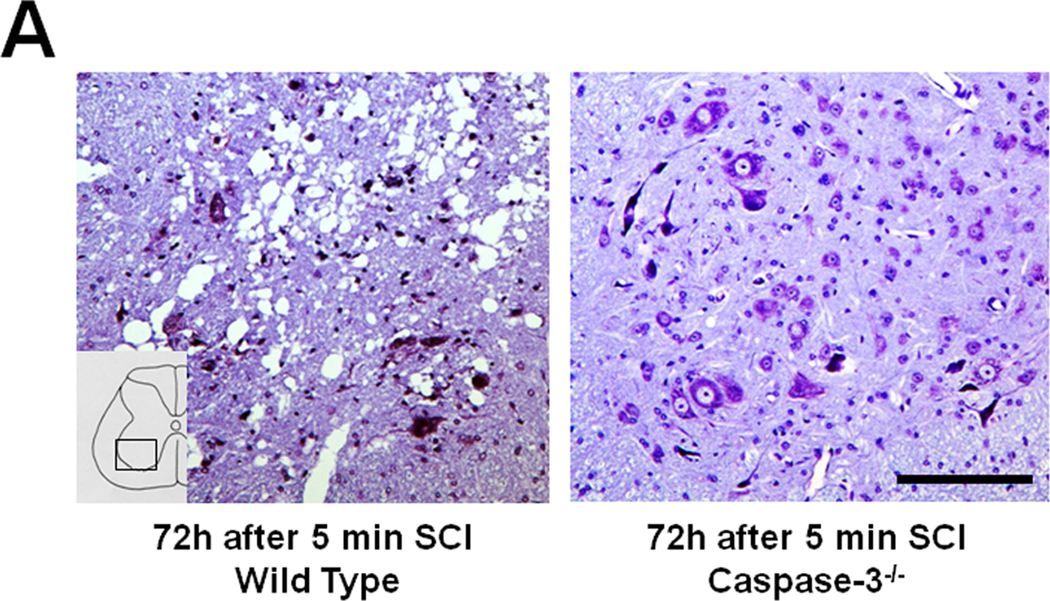
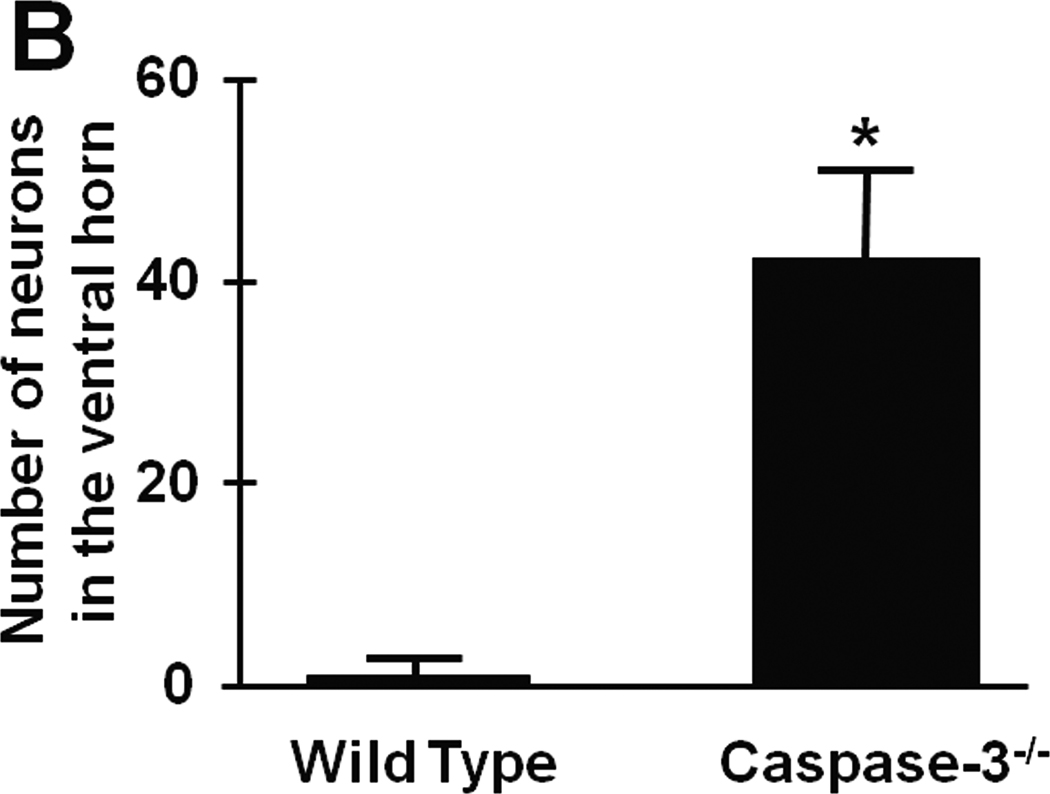
To determine the role of caspase-3 on the immediate and delayed onset paraplegia after SCI, we examined outcomes of SCI in caspase-3−/− mice. There was no difference in physiological parameters including regional spinal cord blood flow during SCI between WT and caspase-3−/− mice (Tables S2-S4). Similar to WT mice, caspase-3−/− mice that were subjected to 9 min SCI exhibit flaccid paraplegia immediately after recovery from anesthesia (Figure 5). Histopathological analysis revealed extensive neuronal loss in the spinal cord of caspase-3−/− mice harvested at 72h after 9 min SCI (data not shown). In contrast to WT mice that developed delayed paraplegia after 5 min SCI, caspase-3−/− mice that were subjected to 5 min SCI exhibited near normal motor function of lower extremity up to 72h after reperfusion (P<0.01 vs WT 48 and 72h after 5 min SCI, Figure 5). Histopathological analysis of the spinal cord of caspase-3−/− mice subjected to 5 min SCI revealed normal appearances of the spinal ventral gray matter at 72h after reperfusion (Figure 6A). There was statistically significant difference (P<0.01) in the number of Nissl-positive spinal cord neurons at 72h after reperfusion between WT and caspase-3−/− mice subjected to 5 min SCI (Figure 6B).
Figure 5.
Time-dependent changes of motor function indicated by Basso Mouse Scale (BMS) after 9 or 5 min SCI in WT and caspase-3−/− mice. *P<0.05 vs pre-SCI. #P<0.05 vs 9 min SCI at corresponding time point. †P<0.05 vs WT at 48 and 72h after 5 min SCI.
Figure 6.
(A) Spinal cord histopathology at the lumbar spinal cord at 72h after 5 min SCI in WT and caspase-3−/− mice. Size bar = 100 µm. (B) The number of viable spinal ventral neurons at 72h after 5 min SCI in WT and caspase-3−/− mice (n=3 each). *P<0.01 vs WT mice.
Discussion
In the present study, we found that 9 and 5 min of SCI produced immediate- and delayed-onset paraplegia, respectively, in mice. Once became paraplegic, no mice showed any recovery of motor function in this model up to 6 weeks after SCI of either duration. Although degeneration of the spinal cord neurons and motor deficit became apparent 8h after 9 min SCI, neurodegeneration and paralysis did not manifest until 36–48h after 5 min SCI in WT mice. The delayed neurodegeneration and paraplegia after 5 min SCI were associated with reactive astrogliosis and microglial activation, as well as marked cavitation, in the ventral horn at 48h after reperfusion. Caspase-3 activation after 5 min SCI precedes the onset of glial activation and extensive neuronal loss. Of note, while 9 min SCI produced immediate paraplegia in caspase-3−/− mice, caspase-3 deficiency prevented delayed motor neuron loss and paraplegia after 5 min SCI. These observations suggest that caspase-3 activation is required for delayed neurodegeration and paraplegia after spinal cord ischemic injury.
A variety of animal models including dog14, rabbit4, baboon15, pig16, and rat17 have been used to study spinal cord ischemia. The rabbit model of SCI has been particularly popular since it can produce delayed and immediate paraplegia by temporarily occluding the infrarenal aorta. However, the arterial blood supply of the spinal cord in rabbit is almost purely segmental and different from that in human.18,19 In rats and mice,9 one anterior and two posterior spinal arteries supply the spinal cord, which is similar to human. Moreover, availability of the variety of genetically-altered mouse strains is a clear advantage of mouse models of human diseases compared to rats and rabbit.
We modified a previously-reported mouse model of SCI by Lang-Lazdunski and colleagues9 by improving the stability of the model using endotracheal intubation and mechanical ventilation during anesthesia and surgery. The robustness of our model enabled us to determine the relationship between the duration of SCI and neurological outcomes (Figure 2). The duration-dependent acceleration of the onset of paraplegia after SCI observed in this study is consistent with what was reported in rabbits. 20 Whether or not duration of ischemia affects outcomes of SCI in human is incompletely understood. Individual difference in extent of collateral blood supply and or surgical techniques is likely to have significant impact on the outcome of SCI. Nonetheless, our results suggest that severity of ischemia determines the timing of onset of neurodegeneration and paraplegia after SCI in mice. Furthermore, the high long-term mortality rate of paraplegic mice observed in the current study is consistent with a clinical report that showed decreased survival rate in patients who developed delayed paraplegia after thoracoabdominal aortic operations.21
Delayed paraplegia and neurodegeneration after 5 min SCI were associated with marked cavitation in the ventral horn at 48 and 72h after reperfusion. The extensive cellular loss was associated with reactive astrogliosis and microglial activation at 48h after 5 min SCI. While the role of activated astrocytes and microglia after SCI is incompletely understood, it is possible that necrosis and/or apoptosis activates proinflammatory and phagocytotic activity of glial cells promoting tissue damage.22 The current observation that caspase-3 activation preceded the glial activation supports this hypothesis. In contrast to 5 min SCI, no cavitation or glial activation were observed after 9 min SCI. Although the reason for the differing impact of 5 and 9 min SCI may be multi-factorial, it is conceivable that 9 min of ischemic insult is too severe for any cells to survive in the ischemic spinal cord regions.
Role of caspase-3 activation and apoptosis in the pathogenesis of delayed motor neuron death has been implicated primarily based on immunohistochemical detection of cleaved caspase-3 in the spinal cord of rabbits that exhibited delayed paraplegia after SCI.4–6 However, the role of caspase-3 in delayed motor degeneration has been questioned because of the failure of a conventional caspase inhibitor to prevent delayed paraplegia7 and lack of caspase-3 activation and apoptosis in the spinal cord of rabbits.8 These conflicting results are at least in part due to the limited potency and/or toxicity of chemical caspase inhibitors and lack of reproducible and standardizable animal models of SCI. In the current study, using caspase-3−/− mice, we demonstrated that caspase-3 is required for the SCI-induced development of delayed paraplegia, but not immediate paraplegia, in mice. Time-dependent changes of the SCI-induced caspase-3 activation in the spinal cord support the critical role of caspase-3 in the pathogenesis of delayed paraplegia (see Figure 4). Although the precise mechanism thereby caspase-3-deficiency prevents SCI-induced delayed paraplegia remains to be determined, our results suggest that inhibition of apoptotic cell death and glial activation in caspase-3−/− mice contributed to the preserved spinal cord neurons after 5 min of SCI. To elucidate the role of caspases in the pathogenesis of delayed paraplegia, further studies using genetically-altered mouse models and new generation caspase inhibitors are warranted.
Summary
In summary, the current study demonstrates that SCI in mice can cause immediate or delayed-onset motor neuron degeneration and paraplegia depending on the duration of ischemia. Our results also provide definitive evidence that caspase-3 activation is required for the development of delayed paraplegia after 5 min of SCI in mice. These observations have important clinical implications suggesting the preventive effects of caspase-3 inhibition in spinal cord ischemia induced by surgery or trauma. Further, the unique mouse model of delayed paraplegia described here provides important experimental platform to further examine the molecular mechanisms of delayed motor neuron degeneration.
Supplementary Material
Acknowledgements
Funding Source: This work was supported by grant-in-aid for scientific research from the Ministry of Education of Japan (B) 22390299 to Dr. Kakinohana and National Institutes of Health grant R01HL101930 to Dr. Ichinose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures: None
References
- 1.Wong DR, Coselli JS, Amerman K, Bozinovski J, Carter SA, Vaughn WK, et al. Delayed spinal cord deficits after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2007;83:1345–1355. doi: 10.1016/j.athoracsur.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 2.Coselli JS, Lemaire SA, Koksoy C, Schmittling ZC, Curling PE. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg. 2002;35:631–639. doi: 10.1067/mva.2002.122024. [DOI] [PubMed] [Google Scholar]
- 3.Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, et al. Ischemic Delayed Neuronal Death : A Mitochondrial Hypothesis. Stroke. 1995;26:1478–1489. doi: 10.1161/01.str.26.8.1478. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi T, Sakurai M, Abe K, Sadahiro M, Tabayashi K, Itoyama Y, et al. Apoptosis of Motor Neurons With Induction of Caspases in the Spinal Cord After Ischemia · Editorial Comment. Stroke. 1998;29:1007–1013. doi: 10.1161/01.str.29.5.1007. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai M, Nagata T, Abe K, Horinouchi T, Itoyama Y, Tabayashi K. Survival and death-promoting events after transient spinal cord ischemia in rabbits: induction of Akt and caspase3 in motor neurons. J Thorac Cardiovasc Surg. 2003;125:370–377. doi: 10.1067/mtc.2003.112. [DOI] [PubMed] [Google Scholar]
- 6.Lin R, Roseborough G, Dong Y, Williams GM, Wei C. DNA damage and repair system in spinal cord ischemia. J Vasc Surg. 2003;37:847–858. doi: 10.1067/mva.2003.150. [DOI] [PubMed] [Google Scholar]
- 7.Lapchak PA, Araujo DM, Weir CJ, Wei J, Zivin JA. Effects of intrathecal administration of a cell permeant caspase inhibitor, boc-D-fluoromethylketone (BDFMK), on behavioral deficits following spinal cord ischemia: a dose-response analysis. Brain Res. 2003;959:183–190. doi: 10.1016/s0006-8993(02)03739-3. [DOI] [PubMed] [Google Scholar]
- 8.Kiyoshima T, Fukuda S, Matsumoto M, Iida Y, Oka S, Nakakimura K, et al. Lack of Evidence for Apoptosis as a Cause of Delayed Onset Paraplegia After Spinal Cord Ischemia in Rabbits. Anesthesia & Analgesia. 2003;96:839–846. doi: 10.1213/01.ANE.0000047268.41102.D4. [DOI] [PubMed] [Google Scholar]
- 9.Lang-Lazdunski L, Matsushita K, Hirt L, Waeber C, Vonsattel JP, Moskowitz MA, Dietrich WD. Spinal cord ischemia. Development of a model in the mouse. Stroke. 2000;31:208–213. doi: 10.1161/01.str.31.1.208. [DOI] [PubMed] [Google Scholar]
- 10.Woo M, Hakem R, Soengas MS, Duncan GS, Shahinian A, Kagi D, et al. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998;12:806–819. doi: 10.1101/gad.12.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 12.Zivin JA, Waud DR. Quantal bioassay and stroke. Stroke. 1992;23:767–773. doi: 10.1161/01.str.23.5.767. [DOI] [PubMed] [Google Scholar]
- 13.Zivin JA, DeGirolami U. Spinal cord infarction: a highly reproducible stroke model. Stroke. 1980;11:200–202. doi: 10.1161/01.str.11.2.200. [DOI] [PubMed] [Google Scholar]
- 14.Simpson JI, Eide TR, Schiff GA, Clagnaz JF, Hossain I, Tverskoy A, et al. Intrathecal magnesium sulfate protects the spinal cord from ischemic injury during thoracic aortic cross-clamping. Anesthesiology. 1994;81:1493–1499. [PubMed] [Google Scholar]
- 15.Svensson LG, Von Ritter CM, Groeneveld HT, Rickards ES, Hunter SJ, Robinson MF, et al. Cross-clamping of the thoracic aorta. Influence of aortic shunts, laminectomy, papaverine, calcium channel blocker, allopurinol, and superoxide dismutase on spinal cord blood flow and paraplegia in baboons. Ann Surg. 1986;204:38–47. doi: 10.1097/00000658-198607000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lips J, de Haan P, Bouma GJ, Jacobs MJ, Kalkman CJ. Delayed detection of motor pathway dysfunction after selective reduction of thoracic spinal cord blood flow in pigs. J Thorac Cardiovasc Surg. 2002;123:531–538. doi: 10.1067/mtc.2002.118048. [DOI] [PubMed] [Google Scholar]
- 17.Taira Y, Marsala M. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke. 1996;27:1850–1858. doi: 10.1161/01.str.27.10.1850. [DOI] [PubMed] [Google Scholar]
- 18.DeGirolami U, Zivin JA. Neuropathology of experimental spinal cord ischemia in the rabbit. J Neuropathol Exp Neurol. 1982;41:129–149. doi: 10.1097/00005072-198203000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Dommisse GF. THE BLOOD SUPPLY OF THE SPINAL CORD: A Critical Vascular Zone in Spinal Surgery. J Bone Joint Surg Br. 1974;56-B:225–235. [PubMed] [Google Scholar]
- 20.Zivin JA, DeGirolami U, Hurwitz EL. Spectrum of neurological deficits in experimental CNS ischemia. A quantitative study. Arch Neurol. 1982;39:408–412. doi: 10.1001/archneur.1982.00510190026008. [DOI] [PubMed] [Google Scholar]
- 21.Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357–368. [PubMed] [Google Scholar]
- 22.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.