Abstract
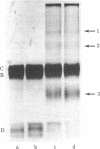
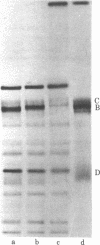
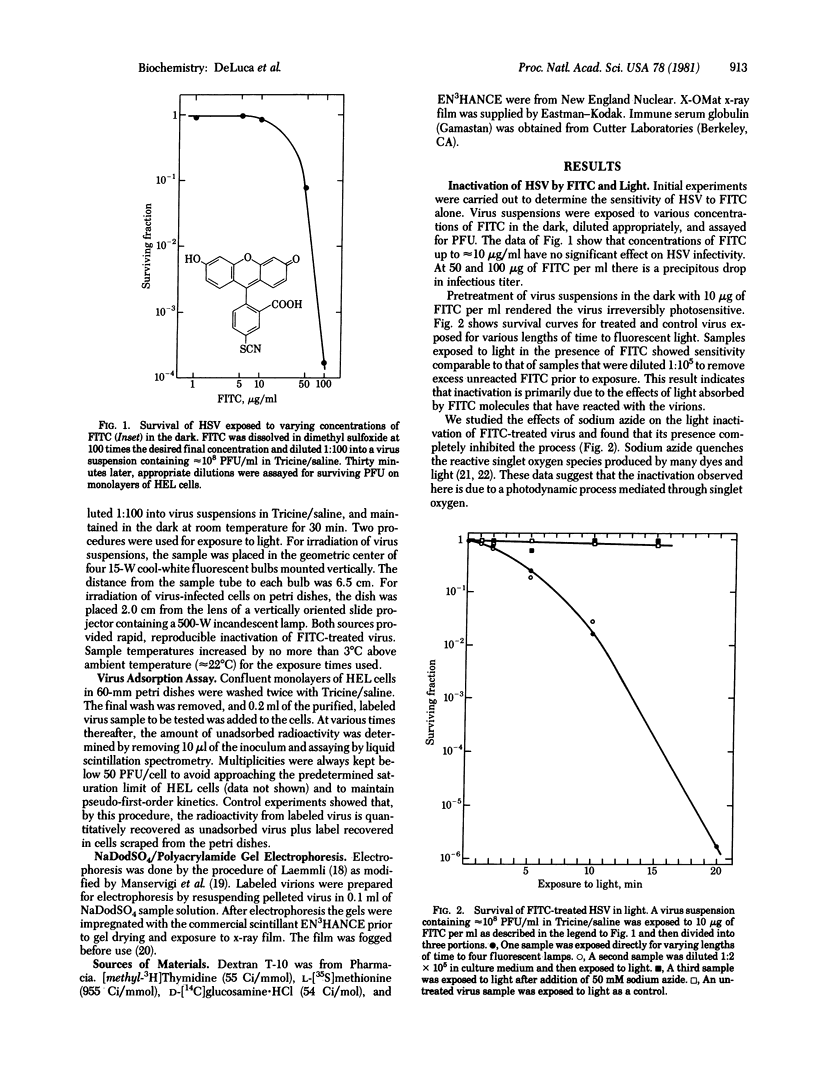
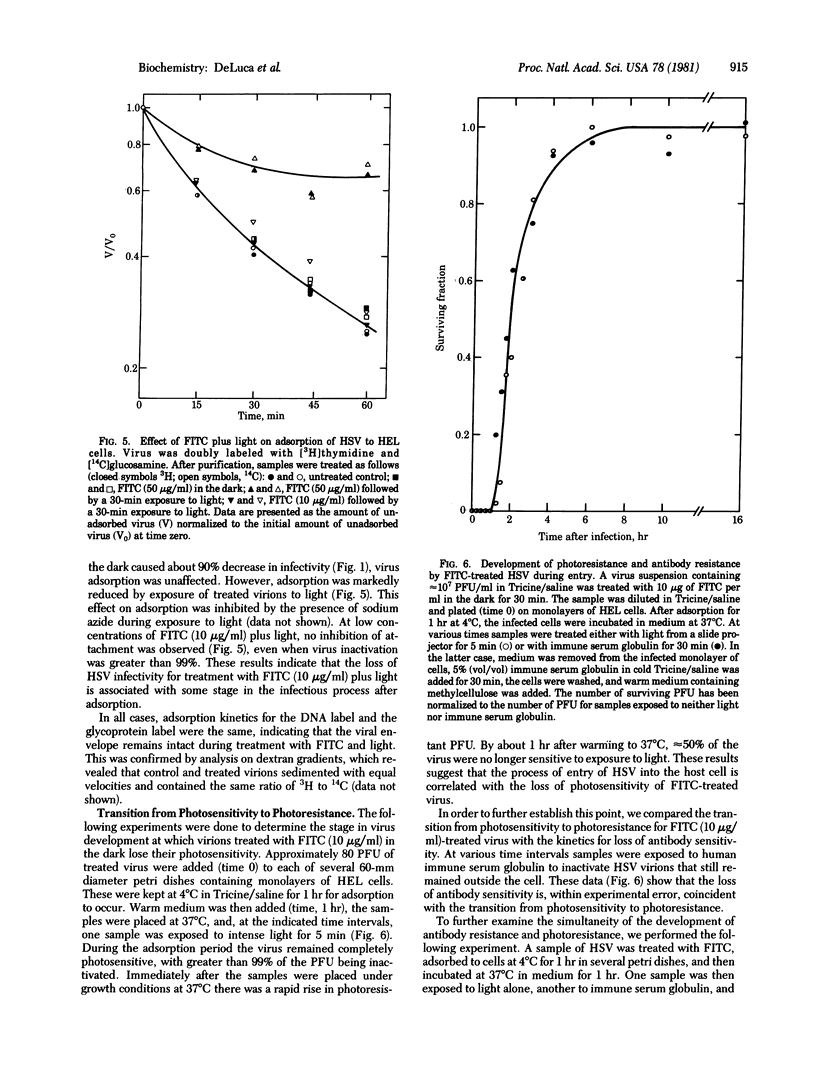
Herpes simplex virus type 1 is photosensitized by treatment with fluorescein isothiocyante (FITC). The inactivation of FITC-treated virions upon subsequent exposure to light is inhibited by the presence of sodium azide, suggesting the involvement of singlet oxygen in the process. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis revealed that treatment with FITC plus light induces crosslinks in viral envelope glycoproteins. Treatment of virions with high concentrations of FITC (50 micrograms/ml) plus light causes a reduction in the adsorption of the virus to monolayers of human embryonic lung cells. For lower concentrations of FITC (10 micrograms/ml) plus light, treated virions adsorb to the host cells, but remain sensitive to light until entry occurs. The loss of light sensitivity coincides with the development of resistance to antibodies. These results are most consistent with a mechanism of entry for herpes simplex virus involving fusion of the viral membrane with the plasma membrane of the host cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang T. W., Weinstein L. Eczema herpeticum. Treatment with methylene blue and light. Arch Dermatol. 1975 Sep;111(9):1174–1175. doi: 10.1001/archderm.111.9.1174. [DOI] [PubMed] [Google Scholar]
- Friedrich E. G., Jr Relief for Herpes vulvitis. Obstet Gynecol. 1973 Jan;41(1):74–77. [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. H., Gardner H. L., Brown D., Wallis C., Rawls W. E., Melnick J. L. Herpes genitalis treated by photodynamic inactivation of virus. Am J Obstet Gynecol. 1973 Dec 15;117(8):1144–1146. doi: 10.1016/0002-9378(73)90768-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Thompson J. E., Kruuv J. Photoinduced crosslinking of membrane proteins by fluorescein isothiocyanate. Biochem Biophys Res Commun. 1978 Nov 14;85(1):344–350. doi: 10.1016/s0006-291x(78)80048-5. [DOI] [PubMed] [Google Scholar]
- Li J. L., Jerkofsky M. A., Rapp F. Demonstration of oncogenic potential of mammalian cells transformed by DNA-containing viruses following photodynamic inactivation. Int J Cancer. 1975 Feb 15;15(2):190–202. doi: 10.1002/ijc.2910150204. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C., Wallis C., Melnick J. L., Kuns M. D. Photodynamic treatment of herpes keratitis. Infect Immun. 1972 Feb;5(2):169–171. doi: 10.1128/iai.5.2.169-171.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M., Mednis B. Electron microscopy of herpes simplex virus. I. Entry. J Virol. 1968 May;2(5):507–516. doi: 10.1128/jvi.2.5.507-516.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. G., Oxman M. N., Clark J. E., Arndt K. A. Failure of neutral-red photodynamic inactivation in recurrent herpes simplex virus infections. N Engl J Med. 1975 Nov 6;293(19):945–949. doi: 10.1056/NEJM197511062931901. [DOI] [PubMed] [Google Scholar]
- Person S., Knowles R. W., Read G. S., Warner S. C., Bond V. C. Kinetics of cell fusion induced by a syncytia-producing mutant of herpes simplex virus type I. J Virol. 1975 Jan;17(1):183–190. doi: 10.1128/jvi.17.1.183-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Li J. L., Jerkofsky M. Transformation of mammalian cells by DNA-containing viruses following photodynamic inactivation. Virology. 1973 Oct;55(2):339–346. doi: 10.1016/0042-6822(73)90173-6. [DOI] [PubMed] [Google Scholar]
- Roome A. P., Tinkler A. E., Hilton A. L., Montefiore D. G., Waller D. Neutral red with photoinactivation in the treatment of herpes genitalis. Br J Vener Dis. 1975 Apr;51(2):130–133. doi: 10.1136/sti.51.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes W., Keller G., Woog J., Vickroy T., Deering R., Keith A. Inactivation of lipid-containing viruses by hydrophobic photosensitizers and near-ultraviolet radiation. Photochem Photobiol. 1979 Apr;29(4):785–790. doi: 10.1111/j.1751-1097.1979.tb07767.x. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]