Abstract
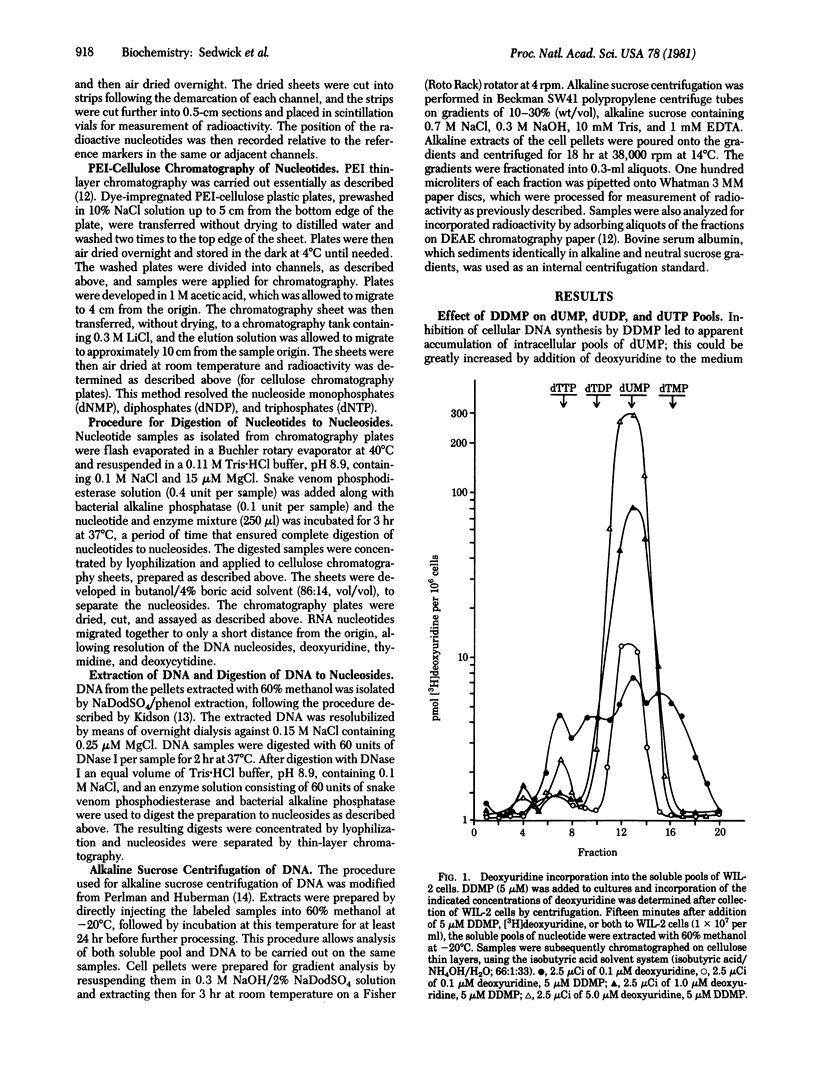
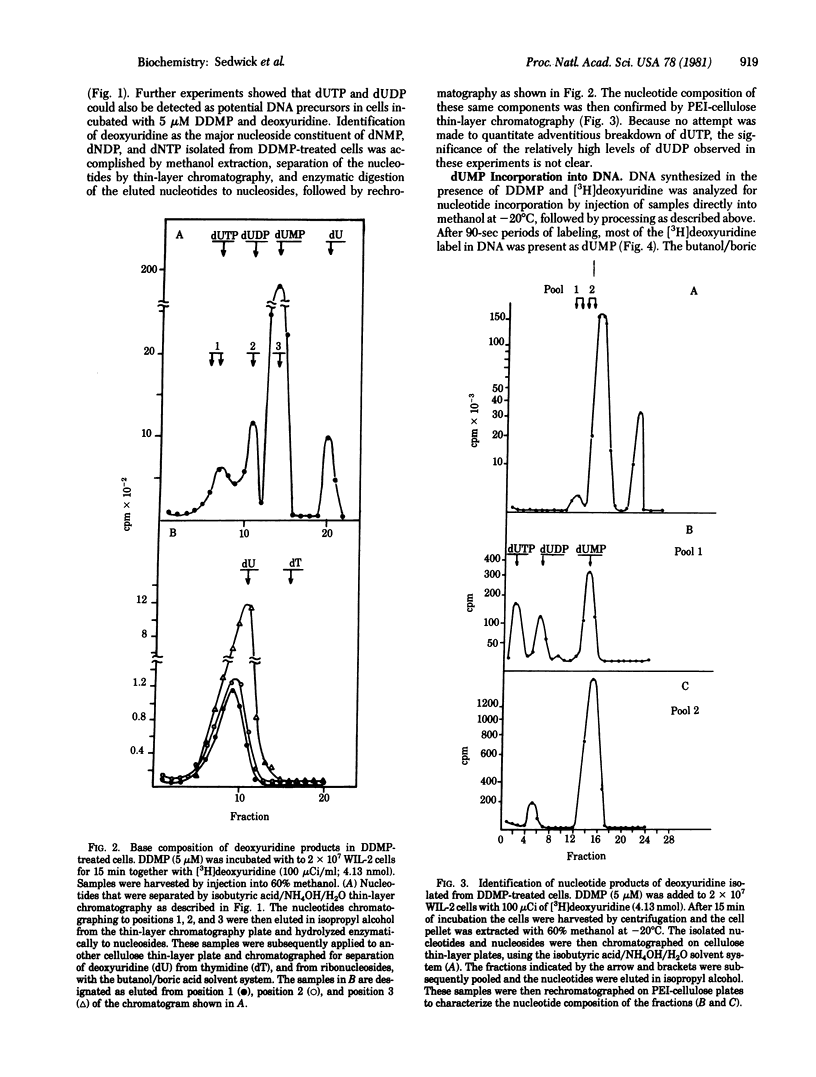
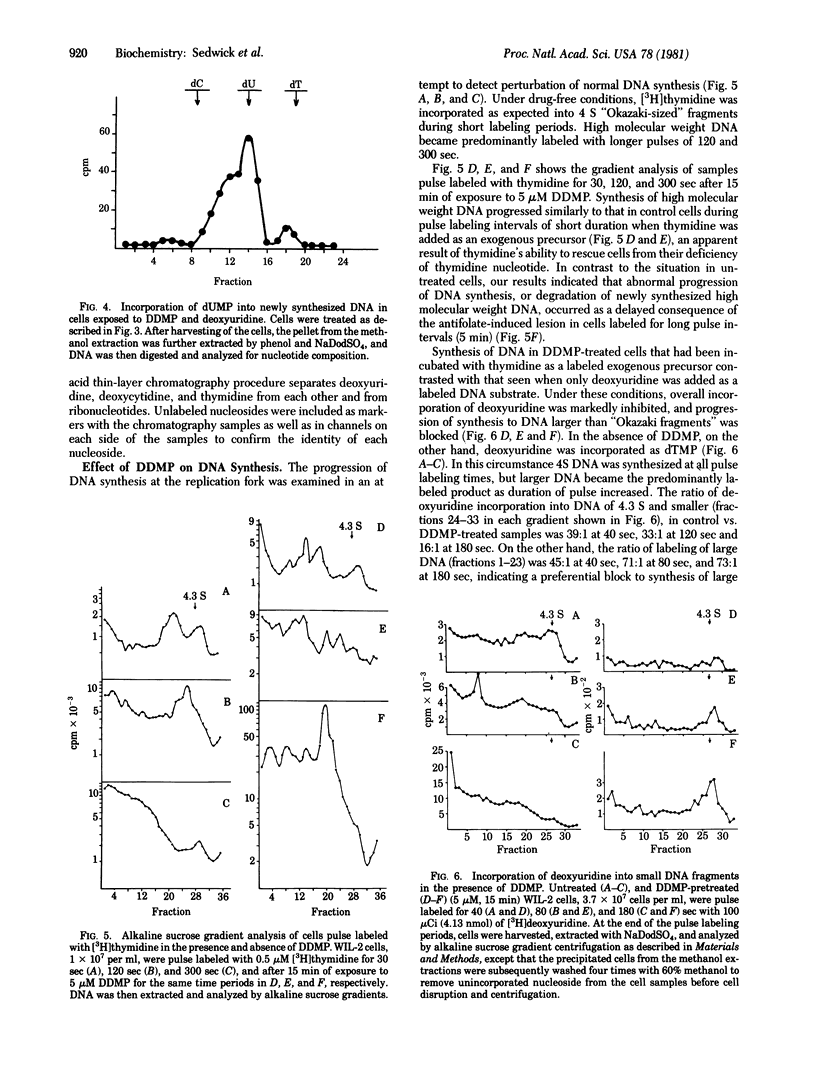
In vitro exposure of a human lymphoblastoid cell line (WIL-2) to the antifolate metoprine (DDMP), when followed by the addition of exogenous deoxyuridine, led to intracellular accumulation of deoxyuridine triphosphate (dUTP) and incorporation of deoxyuridine monophosphate (dUMP) into DNA. When newly synthesized DNA was extracted from DDMP-treated cells that had been labeled with deoxyuridine for up to 3 min, most of the DNA synthesized was no larger than 4 S on alkaline sucrose gradients. In contrast, the predominant form of newly synthesized alkali-stable DNA in cells not treated with drug was larger than 4 S. Abnormal progression of DNA synthesis, degradation of newly synthesized DNA, or both occurred as a delayed consequence of DDMP treatment in the absence of exogenous deoxyuridine when thymidine was used to label DNA of DDMP-treated stability of antifolate-induced misincorporation of dUMP into DNA was not elucidated, it was clear that antifolates can directly perturb the quality as well as the quantity of DNA synthesized by drug-treated cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima T., Akiyoshi H., Fujii S. A new deoxyuridine-5'-triphosphatase in Yoshida sarcoma cells involved in deoxyuridine 5'-triphosphate metabolism. Cancer Res. 1977 Jun;37(6):1598–1601. [PubMed] [Google Scholar]
- Friedberg E. C., Ganesan A. K., Minton K. N-Glycosidase activity in extracts of Bacillus subtilis and its inhibition after infection with bacteriophage PBS2. J Virol. 1975 Aug;16(2):315–321. doi: 10.1128/jvi.16.2.315-321.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M., Bleile B., Tseng B. Y. Methotrexate-induced misincorporation of uracil into DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1956–1960. doi: 10.1073/pnas.77.4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstrom R. H., Tseng B. Y., Goulian M. The incorporation of uracil into animal cell DNA in vitro. Cell. 1978 Sep;15(1):131–140. doi: 10.1016/0092-8674(78)90089-2. [DOI] [PubMed] [Google Scholar]
- Kidson C. Deoxyribonucleic acid secondary structure in the region of the replication point. J Mol Biol. 1966 May;17(1):1–9. doi: 10.1016/s0022-2836(66)80089-x. [DOI] [PubMed] [Google Scholar]
- Kuhnlein U., Lee B., Linn S. Human uracil DNA N-glycosidase: studies in normal and repair defective cultured fibroblasts. Nucleic Acids Res. 1978 Jan;5(1):117–125. doi: 10.1093/nar/5.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Moran R. G., Mulkins M., Heidelberger C. Role of thymidylate synthetase activity in development of methotrexate cytotoxicity. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5924–5928. doi: 10.1073/pnas.76.11.5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M. DNA intermediates at the Escherichia coli replication fork: effect of dUTP. Proc Natl Acad Sci U S A. 1978 Jan;75(1):238–242. doi: 10.1073/pnas.75.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Huberman J. A. Asymmetric Okazaki piece synthesis during replication of simian virus 40 DNA in vivo. Cell. 1977 Dec;12(4):1029–1043. doi: 10.1016/0092-8674(77)90167-2. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Kutler M., Frazer T., Brown O. E., Laszlo J. New dose-time relationships of folate antagonists to sustain inhibition of human lymphoblasts and leukemic cells in vitro. Cancer Res. 1979 Sep;39(9):3612–3618. [PubMed] [Google Scholar]
- Sekiguchi M., Hayakawa H., Makino F., Tanaka K., Okada Y. A human enzyme that liberates uracil from DNA. Biochem Biophys Res Commun. 1976 Nov 22;73(2):293–299. doi: 10.1016/0006-291x(76)90706-3. [DOI] [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. Deoxyuridine triphosphatase of Escherichia coli. Purification, properties, and use as a reagent to reduce uracil incorporation into DNA. J Biol Chem. 1978 May 10;253(9):3305–3312. [PubMed] [Google Scholar]
- Sirover M. A. Induction of the DNA repair enzyme uracil-DNA glycosylase in stimulated human lymphocytes. Cancer Res. 1979 Jun;39(6 Pt 1):2090–2095. [PubMed] [Google Scholar]
- Tye B. K., Chien J., Lehman I. R., Duncan B. K., Warner H. R. Uracil incorporation: a source of pulse-labeled DNA fragments in the replication of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1978 Jan;75(1):233–237. doi: 10.1073/pnas.75.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye B. K., Lehman I. R. Excision repair of uracil incorporated in DNA as a result of a defect in dUTPase. J Mol Biol. 1977 Dec 5;117(2):293–306. doi: 10.1016/0022-2836(77)90128-0. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Nyman P. O., Lehman I. R., Hochhauser S., Weiss B. Transient accumulation of Okazaki fragments as a result of uracil incorporation into nascent DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):154–157. doi: 10.1073/pnas.74.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. S., Sedwick W. D., Korn D. Nuclear deoxyribonucleic acid polymerase. Purification and properties of the homogeneous enzyme from human KB cells. J Biol Chem. 1974 Feb 10;249(3):841–850. [PubMed] [Google Scholar]
- Williams M. V., Cheng Y. Human deoxyuridine triphosphate nucleotidohydrolase. Purification and characterization of the deoxyuridine triphosphate nucleotidohydrolase from acute lymphocytic leukemia. J Biol Chem. 1979 Apr 25;254(8):2897–2901. [PubMed] [Google Scholar]
- Wist E., Unhjem O., Krokan H. Accumulation of small fragments of DNA in isolated HeLa cell nuclei due to transient incorporation of dUMP. Biochim Biophys Acta. 1978 Sep 27;520(2):253–270. doi: 10.1016/0005-2787(78)90225-3. [DOI] [PubMed] [Google Scholar]



