Abstract
We analyzed the relationships among clinical variables, histology, 1p/19q status, and outcome in 95 patients with oligodendroglial tumors.
The study enrolled adult patients who underwent first-time surgery for a supratentorial oligodendroglial tumor at Oslo University Hospital, Rikshospitalet. Tumors were: 27 oligodendrogliomas, WHO grade II; 32 oligoastrocytomas, WHO grade II; 16 anaplastic oligodendrogliomas, WHO grade III; 14 anaplastic oligoastrocytomas, WHO grade III; and 6 glioblastomas with a major oligodendroglial component, WHO grade IV. The clinical files were reviewed. Three neuropathologists evaluated the histological slides independently. Loss-of-heterozygosity analysis for 1p and 19q was performed by PCR.
Favorable prognostic factors from univariate analyses included seizures as presenting symptom, female sex, location in the frontal lobe, low WHO grade, classic histology, absence of gemistocytic cells, and combined 1p/19q loss. Solitary 19q loss was a negative prognostic marker. 1p/19q status was of prognostic significance in both tumors with classic and nonclassic oligodendroglial histology. In the multivariate analysis, WHO grade II (P< .001), frontal tumor location (P= .002), and combined 1p/19q loss (P< .001) remained favorable prognostic variables.
Our results suggest that tumor location, WHO grade, and 1p/19q status are important independent variables associated with survival in oligodendroglial tumors. The study suggests that solitary 19q loss is a negative prognostic variable and that 1p/19q loss is associated with prolonged survival also in oligodendroglial tumors without classic histology.
Keywords: oligodendroglioma, 1p, 19q, prognostic
Introduction
Gliomas are classified into groups according to their hypothetical line of differentiation. The cellular origin of these tumors is unknown, but it has been postulated that they derive from stem cells or from differentiated glial cells that suffer dedifferentiation and later acquire features resembling those of astrocytes, oligodendrocytes, or ependymal cells.1
Oligodendrogliomas (ODs) are well-differentiated diffuse gliomas composed of cells morphologically resembling oligodendroglia, occurring predominantly in adulthood and typically located in the cerebral hemispheres. Oligoastrocytomas (OAs) are composed of 2 distinct neoplastic cell types morphologically resembling the tumor cells in ODs and diffuse astrocytomas.1
Oligodendroglial tumors used to be relatively rare compared with astrocytomas in earlier reports, comprising approximately 4% of primary brain tumors.2 However, the entity remains a challenge to neuropathologists, as neither clear diagnostic criteria nor specific immunohistochemical markers exist.3,4 The incidence of ODs has increased since the recognition of their chemosensitivity, and this entity has come to exceed the frequency of astrocytomas in many institutions.5,6 An important step forward has been the detection of combined 1p/19q loss as the molecular genetic signature of oligodendroglial tumors. Combined 1p/19q loss is associated with longer progression-free survival and better overall survival (OS), as well as favorable response to both chemo- and radiotherapy in patients with WHO grade III oligodendroglial tumors.7,8 The prognostic relevance of this genotype in low-grade tumors is less clear, but it has been identified as a strong, non–treatment-related prognostic factor for OS in patients with diffusely infiltrating WHO grade II gliomas.9 Some have reported 1p/19q status as a prognostic factor in only tumors with classic oligodendroglial histology, suggesting that the relevance of molecular genetic testing may depend on the presence or absence of classic histological features.10 Although combined loss of 1p/19q is the predominant genotype in oligodendroglial tumors, loss of 19q and retention of 1p (solitary 19q loss) is sometimes encountered. The prognostic impact of solitary 19q loss is less studied, and the published results are conflicting.10–13
The main objective of this study was to analyze the relationships between clinical variables, histology, and genetic aberrations in a large, single-institution series of oligodendroglial tumors. In particular, we wanted to study whether the prognostic relevance of 1p/19q molecular genetic status was limited to tumors with classic oligodendroglial histology or may be of importance also in oligodendroglial tumors without classic histological features. In addition, we wanted to investigate the prognostic impact of solitary 19q loss.
Material and Methods
The study included adult patients who underwent first-time surgery for a supratentorial oligodendroglial tumor at Oslo University Hospital, Rikshospitalet. Only cases with known 1p/19q status were included. At our hospital, oligodendroglial tumors have been tested for 1p/19q loss since 2001. The study included consecutive cases with a histological diagnosis of OD or OA operated on between 2001 and 2007. Also included were selected cases operated on between 1998 and 2000, in which 1p/19q analysis had been performed retrospectively. The study included 1 patient operated on in 1998, 1 in 1999, 3 in 2000, 6 in 2001, 17 in 2002, 11 in 2003, 19 in 2004, 11 in 2005, 15 in 2006, and 11 in 2007. Informed consent was obtained from all patients, and the study was approved by the Regional Committee for Medical Ethics of Southern Norway.
Ninety-five patients (58 men and 37 women) with a median age of 43 years (range 19–72 y) at surgery were included. The tumors were: 27 ODs, WHO grade II; 32 OAs, WHO grade II; 16 anaplastic ODs, WHO grade III; 14 anaplastic OAs, WHO grade III; and 6 glioblastomas with a major oligodendroglial component, WHO grade IV (GBO).
The clinical files were reviewed and the following variables registered: main presenting symptom, duration of symptoms before surgery, tumor location (right or left hemisphere, lobe, involvement of several lobes), tumor resection (gross total resection [GTR], partial resection, biopsy), number of resections, postoperative treatment (radio- or chemotherapy), and date of death. Tumor resection was evaluated by a neurosurgeon from postoperative MR or CT images. GTR was defined as more than 95% reduction of tumor volume. Postoperative MR images were available in 76 cases. In 18 (mostly biopsy) cases, only CT images were available. In 1 case of subtotal resection, there were no postoperative images. All histological slides were reviewed individually by 3 neuropathologists. The following histological variables were evaluated: “classic” oligodendroglial histology, gemistocytic cells, and calcifications. As previously described,14 the histology was considered “classic” if the proportion of tumor cells with uniform, round nuclei and perinuclear halos was considered larger than 50% by at least 2 observers. Likewise, gemistocytic cells were considered present if reported by at least 2 observers. The estimated proportion of gemistocytic cells had to constitute more than 5% of the tumor cells in order to be reported. Calcifications were considered present if reported by at least 1 neuropathologist.
PCR to detect loss of heterozygosity at 1p and 19q was performed as previously described.14,15 Combined 1p and 19q loss was defined as loss of all informative microsatellite markers. Five cases were not included due to either insufficient amount or reduced quality of DNA.
Statistics
Continuous variables were described with median and range; categorical variables with proportions and percentages. Crude associations were analyzed with a chi-squared test or Fisher's exact test. Follow-up was defined as the time from date of surgery until date of death or November 2009, whichever occurred first. The shortest interval between date of surgery and November 2009 was 28 months. The outcome was defined as alive versus deceased.
Twelve clinical, histological, and molecular genetic variables were evaluated using survival analysis methods. The variables included age, gender, main presenting symptom, duration of symptoms before surgery, tumor location, WHO grade, classic oligodendroglial histology, calcifications, gemistocytic cells, combined 1p/19q loss, solitary 19q loss, and GTR. The crude survival data were analyzed using the Kaplan–Meier method. Univariate and multivariate analyses were performed using Cox regression. The effect of significant variables was quantified using hazard ratios and 95% confidence intervals (CIs). All reported P-values were two-sided, and P < .05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows, version 16.
Results
Patient, tumor, and treatment characteristics are summarized in Table 1. Univariate and multivariate analyses of prognostic factors for overall survival are presented in Table 2 and in a supplementary table.
Table 1.
Patient characteristics. When not otherwise specified, data are given as number (%) of patients
| Variable | No. | (%) |
|---|---|---|
| Sex | ||
| Male | 58 | (61) |
| Female | 37 | (39) |
| Age in years | ||
| Mean (95% CI) | 45 (42–47) | |
| Median | 43 | |
| Range | 19–72 | |
| Main presenting symptom | ||
| Seizures | 57 | (61) |
| Headache/signs of raised intracranial pressure | 16 | (17) |
| Other | 20 | (22) |
| Data not available | 2 | |
| Duration of symptoms (months) | ||
| Mean (95% CI) | 17 (10–25) | |
| Median | 3 | |
| Range | 0–192 | |
| Data not available | 2 | |
| Localization | ||
| Frontal lobe | 60 | (63) |
| Temporal lobe | 17 | (18) |
| Other | 18 | (19) |
| 1p/19q loss | ||
| Present | 52 | (55) |
| Absent | 43 | (45) |
| Solitary 19q loss | ||
| Present | 9 | (10) |
| Absent | 85 | (90) |
| Inconclusive result | 1 | |
| WHO grade | ||
| II | 59 | (64) |
| III | 30 | (32) |
| IV | 6 | (6) |
| Classic histology | ||
| Present | 48 | (51) |
| Absent | 47 | (49) |
| Calcifications | ||
| Present | 41 | (43) |
| Absent | 54 | (57) |
| Gemistocytic cells | ||
| Present | 25 | (27) |
| Absent | 70 | (74) |
| Initial treatment | ||
| Surgery | ||
| Gross total resection | 42 | (44) |
| Subtotal resection | 41 | (43) |
| Biopsy | 12 | (13) |
| Adjuvant therapy | ||
| Chemotherapy | 43 | (45) |
| Radiotherapy | 25 | (26) |
| Treatment at progression | ||
| Repeated surgery | 23 | (24) |
| Chemotherapy | 41 | (43) |
| Radiotherapy | 29 | (31) |
| Follow-up time in months | ||
| Median | 50 | |
| Mean | 54 (49–60) | |
| Range | 4–132 | |
| Status | ||
| Deceased | 28 | (29) |
| Alive | 67 | (71) |
Table 2.
Univariate and multivariate analysis of prognostic factors for overall survival
| Variable | All Cases (n = 95) |
||
|---|---|---|---|
| E/N | Univariate Analysis, Hazard Ratio (95% CI) | Multivariate Analysis, Hazard Ratio (95% CI) | |
| Age | |||
| <40 years | 9/36 | 1.0 | |
| ≥40 years | 19/59 | 1.6 (0.7–3.5) | |
| Gender | |||
| Female | 5/37 | 1.0 | |
| Male | 23/58 | 3.5* (1.3–9.1) | |
| Seizures as presenting symptom | |||
| Yes | 11/57 | 1.0 | |
| No | 17/36 | 3.2** (1.5–6.9) | |
| Duration of symptoms | |||
| <24 months | 26/75 | 3.8 (0.9–16.1) | |
| ≥24 months | 2/18 | 1.0 | |
| Location in frontal lobe | |||
| Yes | 9/60 | 1.0 | 1.0 |
| No | 19/35 | 4.8*** (2.2–10.6) | 3.8** (1.7–8.6) |
| Location in temporal lobe | |||
| Yes | 9/17 | 3.4** (1.5–7.7) | |
| No | 19/78 | 1.0 | |
| WHO grade | |||
| II | 9/59 | 1.0 | 1.0 |
| III and IV | 19/36 | 4.9*** (2.2–10.8) | 4.9*** (2.2–11.0) |
| Classic histology | |||
| Yes | 8/48 | 1.0 | |
| No | 20/47 | 3.5** (1.6–8.1) | |
| Calcifications | |||
| Yes | 11/41 | 1.0 | |
| No | 17/54 | 1.5 (0.7–3.2) | |
| Gemistocytic cells | |||
| Yes | 13/25 | 3.0** (1.4–6.3) | |
| No | 15/70 | 1.0 | |
| Combined 1p/19q loss | |||
| Yes | 4/52 | 1.0 | 1.0 |
| No | 24/43 | 12.2*** (4.2–35.5) | 8.8*** (3.0–26.3) |
| Solitary 19q loss | |||
| Yes | 8/9 | 9.1*** (3.9–21.6) | |
| No | 19/85 | 1.0 | |
| GTR | |||
| Yes | 9/42 | 1.0 | |
| No | 19/53 | 2.0 (0.9–4.5) | |
Abbreviations: E/N, number of events (deaths) among the cases; CI, confidence interval. *P < .05; **P < .01; ***P < .001.
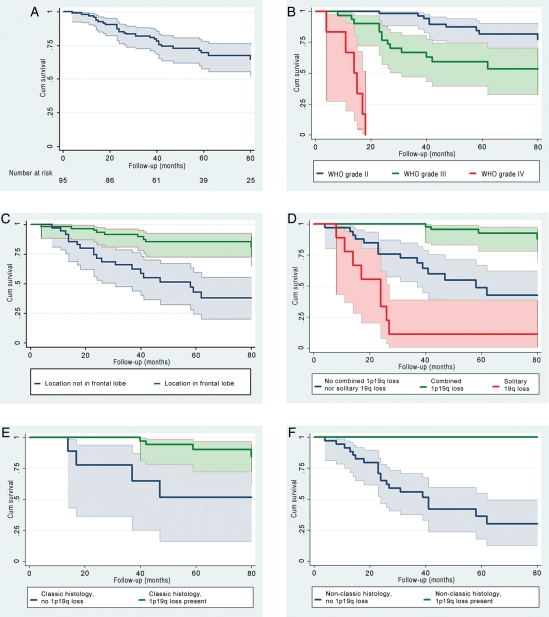
The male:female ratio was 1.6:1. Median age in men was 45 years (range: 19–72 y) and in women 39 years (range: 28–66 y). The median follow-up period was 50 months (range: 4–132 mo). Twenty-eight patients (29%) died during the observation period: all 6 patients with GBO, 13 of 30 patients (43%) with WHO grade III tumors, and 9 of 59 (15%) with WHO grade II tumors. The 1-, 2-, and 5-year survival rates were 96% (CI = 89%–98%), 85% (CI = 76%–91%), and 69% (CI = 58%–78%), respectively, for the whole group (Fig. 1A); 100%, 98% (CI = 86%–100%), and 82% (CI = 66%–90%) for patients with WHO grade II tumors (Fig. 1B); and 93% (CI = 76%–98%), 78% (CI = 57%–78%), and 53% (CI = 39%–74%) for the WHO grade III cases (Fig. 1B). Favorable prognostic factors from univariate analyses included seizures as presenting symptom, female gender, location in the frontal lobe (Fig. 1C), low WHO grade, classic histology, absence of gemistocytic cells, and combined 1p/19q loss. Solitary 19q loss was a negative prognostic factor. Eight of the 9 patients with tumors harboring solitary 19q loss died in the study period. The median survival for this group was 24 months (range: 8–94 months). Kaplan–Meier analyses revealed 3 prognostic groups based on the molecular genetic results (Fig. 1D). Patients with tumors demonstrating combined 1p/19q loss had the longest survival times, and cases with solitary 19q loss had the shortest, whereas patients with tumors harboring neither combined 1p/19q loss nor solitary 19q loss had an intermediate prognosis (P< .001). Loss of 1p/19q was significantly associated with prolonged survival in patient groups that both received and did not receive adjuvant chemo- and/or radiotherapy (P< .001 and P= .01, respectively).
Fig. 1.
Overall survival curves for the total group of 95 patients (A), according to WHO grade (B), tumor location (C), and 1p/19q status (D). Overall survival curves for cases demonstrating classic oligodendroglial histology with and without 1p/19q loss (E) and tumors demonstrating nonclassic histology with and without 1p/19q loss (F). Shaded areas illustrate 95% confidence intervals. Abbreviations: Cum Survival, cumulative survival.
Kaplan–Meier analyses demonstrated that 1p/19q loss was a significant positive prognostic variable in tumors with classic as well as nonclassic histology (P= .002 and P= .001, respectively; Fig. 1E and F). In the classic histology group, 4 of 39 cases with 1p/19q loss died in the study period, as opposed to 4 of 9 cases without combined 1p/19q loss. There was no death among the 13 patients with nonclassic histology and combined 1p/19q loss, whereas 20 of 34 patients with nonclassic histology and absence of 1p/19q loss died in the study period.
Due to a limited sample size, we could not include all variables found to be of prognostic significance in the univariate analyses in a multivariate Cox model. The variables selected for multivariate analysis were 1p/19q status, WHO grade, and classic histology. Of these, 1p/19q status (P= .001) and WHO grade (P< .001) remained statistically significant, but classic histology did not (P= .3). In addition, a second model including the variables 1p/19q status, WHO grade, and tumor location (frontal lobe vs others) was tested. All variables in this second model were significantly associated with survival; the results are presented in Table 2.
Combined 1p/19q loss and location in the frontal lobe were the only variables of prognostic significance in WHO grade II tumors (Supplementary Table). In the WHO grade III group, the factors of prognostic significance from univariate analyses included GTR, tumor location, classic histology, combined 1p/19q loss, and solitary 19q loss. Due to a small number of patients and few events, multivariate analysis on data grouped according to WHO grade was not performed.
Seizures were the most frequent presenting symptom, reported in 57 of the cases (61%). This was more common in grade II than in grade III or IV tumors (P= .008) and in patients younger than 43 years (P= .003). Symptoms of increased intracranial pressure were more common in grades III and IV tumors (P= .001) and in patients older than 49 years (P= .013). Symptoms of increased intracranial pressure were associated with solitary 19q loss (P= .024).
Combined 1p/19q loss was associated with classic histology (P< .001), calcifications (P< .001), absence of gemistocytic cells (P< .001), WHO grade II status (P= .046), and location in the frontal lobe (P= .002). Among WHO grade III cases, there was a higher proportion of GTR for tumors located in the frontal lobe (P= .035) and that had 1p/19q loss (P= .009).
Solitary 19q loss was only detected in anaplastic tumors and was associated with the absence of classic histology (P= .011), presence of gemistocytic cells (P= .004), and location in the temporal lobe (P= .002).
WHO grade III and IV tumors (P= .030), location in the temporal lobe (P= .047), and solitary 19q loss (P= .013) were more common in males than in females.
Discussion
In this study we analyzed the relationship between several clinical variables, histological findings, genetic aberrations, and outcome in 95 patients with oligodendroglial tumors. We found that low WHO grade, frontal tumor location, and combined 1p/19q loss were favorable and independent prognostic variables. The study also suggests that solitary 19q loss is a negative prognostic variable and that 1p/19q loss is associated with prolonged survival also in oligodendroglial tumors without classic histology.
WHO Grade
ODs and OAs are graded as II (low-grade) or III (high-grade or anaplastic) according to the WHO classification.1 The presence of necrosis is compatible with a grade III OD, but OAs with necrosis should be regarded as glioblastomas (with OD component; GBOs), WHO grade IV, as large studies suggest that necrosis is associated with significantly worse prognosis in mixed gliomas.8,16 In our study, approximately 15% and 43% of the patients with WHO grades II and III tumors, respectively, had died in the observation period. Our results are in line with those reported by others,1 although median survival times could not be calculated due to a relatively short observation period.
The 1-, 2-, and 5-year survival rates for WHO grade II cases were 100%, 98%, and 82%, respectively. WHO grade II ODs are typically slowly growing tumors with median survival times from 10 to 17 years and reported 5- and 10-year survival rates of 71% and 54%, respectively.1 A median survival time of 6.3 years and 5- and 10-year survival rates of 58% and 32%, respectively, have been reported in WHO grade II OAs.17
In our study, the 1-, 2-, and 5-year survival rates for patients with WHO grade III tumors were 93%, 78%, and 53%, respectively. Reported median survival time for WHO grade III ODs is between 4 and 5 years,7,8 and the 5-year survival rate is approximately 40%. The prognosis for patients with WHO grade III OAs is worse than for pure ODs of similar grade but better than for patients with glioblastomas. A median survival time of 2.8 years and 5- and 10-year survival rates of 36% and 9%, respectively have been reported.17
In our study, all of the 6 patients with GBO died during the observation period. The median survival of 14 months (data not shown) is almost similar to that observed in ordinary glioblastomas,18,19 although no conclusion can be drawn from such a small number of cases. Whether or not there is any clinical significance to identifying an oligodendroglial component in glioblastomas is unclear. Most studies have shown a molecular pattern comparable to that of ordinary glioblastomas and that GBOs rarely display 1p/19q loss, although a slightly longer median survival in patients with GBO compared with patients with ordinary glioblastomas have been reported.20–22
Tumor Location
Our study revealed that patients with tumors located in the frontal lobes lived significantly longer than patients with tumors in other lobes. Conflicting results have been reported on this matter. Some studies have shown that patients with frontal lobe ODs live significantly longer than patients with tumors located elsewhere,23–25 while others have failed to demonstrate any prognostic relevance of tumor location.2 One might suggest that an association between tumor location and overall survival in OD patients could at least in part be explained by genetic findings, since an association between 1p/19q loss and tumor location has been reported.26 However, in our study the prolonged survival of patients with frontal tumors cannot be entirely explained by the genetic changes, since both tumor location and 1p/19q status remained independent prognostic factors in the multivariate analysis. Neither can the prolonged survival seen in patients with frontally located tumors be explained by a higher frequency of GTRs when all cases were analyzed, but there was a significant association between GTR and frontal location among WHO grade III tumors.
1p/19q Status
It is currently unknown how 1p/19q loss influences the pathogenesis of a relatively indolent glioma that responds to treatment. Metaphase studies have shown that the combined loss probably is mediated by an unbalanced translocation of 19p to 1q.27,28 Several gene expression studies have suggested that ODs with combined 1p and 19q deletion have a proneuronal expression profile,29 and it has been postulated that this might result in restriction to cell migration and proliferation, reflecting 1p/19q status as a prognostic biomarker.30
In our study, solitary 19q loss was a negative prognostic factor. It was detected in only anaplastic tumors, and 8 of the 9 patients with this molecular genetic signature died during the study period. In fact, Kaplan–Meier analyses revealed 3 significantly different prognostic groups based on the molecular genetic findings; cases with combined 1p/19q loss had the best prognosis, those with solitary 19q loss the worst prognosis, and the remaining cases an intermediate prognosis. The negative prognostic impact of solitary 19q loss is in agreement with a study by McDonald et al.,10 who found that anaplastic oligodendroglial tumors with 1p intact/19q deletion tended to have a poorer prognosis. Their 12 cases of anaplastic oligodendroglial tumors with solitary 19q loss had a median survival of 210 weeks (95% CI = 83–335), compared with patients with 1p/19q codeleted tumors, who had a median survival of 605 weeks (95% CI = 303–908). Our 9 cases with solitary 19q loss had an even worse prognosis, with a median survival of only 24 months (range: 8–94 months). Loss of 19q and retention of 1p is a frequent finding in grade III and IV astrocytomas31,32 and is sometimes observed in anaplastic OAs12 or anaplastic variants of morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features.11 However, conflicting results are reported regarding the prognostic value of solitary 19q loss. Fuller et al.11 found that although solitary 19q loss was associated with grade IV gliomas, this genotype was frequently coupled with prolonged patient survival (median survival, 96 months). Perry et al.12 reported the 19q-only pattern of deletion to be associated with prolonged survival in anaplastic gliomas. Similarly, Burton et al.13 found that solitary 19q deletion was statistically significantly associated with long-term survival of their glioblastoma patients.
In our study, classic oligodendroglial histology was not an independent prognostic variable on multivariate analysis. This can at least partially be explained by the close association between histological features and 1p/19q status.14 A study by McDonald et al.10 suggested that the presence or absence of classical histological features may be an important variable in oligodendroglial tumors, since 1p/19q loss was of prognostic significance in only tumors with classic histology. In our study, the prognostic significance of 1p/19q status was not limited to cases with classic oligodendroglial histology. In fact, all of the 13 patients with tumors displaying nonclassic histology and combined 1p/19q loss survived the observation period, whereas 20 of 34 patients (59%) with nonclassic histology and absence of 1p/19q loss died. The discrepancy between our results and those in the study by McDonald et al. may at least in part be explained by our inclusion of both low- and high-grade tumors, while the McDonald study included only anaplastic tumors. Most of our cases with nonclassic histology and 1p/19q loss (11 of 13 cases) were WHO grade II tumors, whereas half of the cases (17 of 34) with nonclassic histology and absence of 1p/19q loss were anaplastic tumors. Giannini et al.33 studied 247 cases of anaplastic oligodendroglial tumors and found that overall survival in cases with nonclassic histology was significantly longer in cases with 1p/19q loss compared with those without. They did not detect significant differences in overall survival between patients with nonclassic histology with combined 1p/19q loss and patients with classic histology and combined 1p/19q loss. However, they concluded that tumors with classic histology without 1p/19q loss had a more favorable prognosis than nonclassic tumors without 1p/19q loss, indicating that histology is of some prognostic importance.
Other Prognostic Factors
In our study we also identified sex, seizures as main presenting symptom, and the presence of gemistocytic cells as factors of prognostic importance in the univariate analyses. A male:female ratio ranging from 1:1 to 2:0 is a common finding in OD studies.2,23 Sex is rarely reported as a prognostic variable in gliomas, although some studies have indicated that female patients with glioblastomas have a better prognosis than their male counterparts and that hormones or tumor suppressor genes on the X chromosome may be associated with longer survival.34 The association between sex and survival in our study might be caused by confounding variables, since the male patients were older and the tumors were more often of higher grade than those in females.
In most studies of oligodendroglial tumors, seizures are the most common presenting symptom, ranging in prevalence from 35% to 85% of patients.23 Seizure was also the most common presenting symptom in our study (61% of patients). Seizures as presenting symptom was more common in low- than in high-grade tumors, whereas headache or signs of increased intracranial pressure were significantly associated with anaplastic histology.
The presence of gemistocytic cells in the tumor tissue was a negative prognostic factor in our study. This was not unexpected, since gemistocytic cells were a more frequent finding in anaplastic tumors and were associated with solitary 19q loss. This is in agreement with Kros et al.,35 who reported that patients with oligodendroglial tumors containing gemistocytic cells survived approximately half as long as patients who did not have these cells in their tumors.
In our study, univariate analyses demonstrated that GTR was associated with prolonged survival in WHO grade III tumors. The extent of surgical resection has been reported to be associated with survival in patients with low- and high-grade gliomas.36–38
We found no association between patient age at the time of diagnosis and survival. Although younger age is associated with prolonged survival in patients with low-grade gliomas,38 the role of age in clinical outcome in OD patients has been a matter of controversy. Some studies have demonstrated that patients younger than 40 or 50 years survive longer than older patients,39 while this has not been confirmed by others.2
The strength of the present study is that it includes a large number of oligodendroglial tumors from a single institution with almost complete follow-up data. Also, the histological examinations have been performed by 3 experienced neuropathologists, reducing the effect of subjective interpretation. Limitations to our study include the inherent bias associated with retrospective analyses.
In conclusion, our results suggest that tumor location, WHO grade, and 1p/19q status are important independent variables associated with survival in oligodendroglial tumors. The study also suggests that solitary 19q loss is associated with anaplastic histology and reduced survival, but this must be clarified by future studies, as conflicting results have been reported. Our data indicate that 1p/19q status is of prognostic importance also in oligodendroglial tumors without classic histology, but studies including a large number of patients with both low- and high-grade tumors and long-time follow-up are needed.
Supplementary Material
Conflict of interest statement: Dr Lote has received lecture fees and reimbursement of expenses from Schering Plough after giving a lecture about gliomas for neurologists at a hospital in Norway.
Funding
This study has been funded by Oslo University Hospital and The Norwegian Cancer Society.
Supplementary Material
Acknowledgments
The authors thank Astrid Bustetun, Anne Signe Bø, and Ingun Benestad for providing excellent technical assistance. We thank Dr. Søren Bakke for his support of this project.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. WHO classification of tumors of the central nervous system. Lyon: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mork SJ, Lindegaard KF, Halvorsen TB, et al. Oligodendroglioma: incidence and biological behavior in a defined population. J Neurosurg. 1985;63:881–889. doi: 10.3171/jns.1985.63.6.0881. doi:10.3171/jns.1985.63.6.0881. [DOI] [PubMed] [Google Scholar]
- 3.Burger PC, Minn AY, Smith JS, et al. Losses of chromosomal arms 1p and 19q in the diagnosis of oligodendroglioma. A study of paraffin-embedded sections. Mod Pathol. 2001;14:842–853. doi: 10.1038/modpathol.3880400. doi:10.1038/modpathol.3880400. [DOI] [PubMed] [Google Scholar]
- 4.Giannini C, Scheithauer BW, Weaver AL, et al. Oligodendrogliomas: reproducibility and prognostic value of histologic diagnosis and grading. J Neuropathol Exp Neurol. 2001;60:248–262. doi: 10.1093/jnen/60.3.248. [DOI] [PubMed] [Google Scholar]
- 5.Burger PC. What is an oligodendroglioma? Brain Pathol. 2002;12:257–259. doi: 10.1111/j.1750-3639.2002.tb00440.x. doi:10.1111/j.1750-3639.2002.tb00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger PC, Scheithauer BW. Tumors of neuroglia and choroid plexus. In: Silverberg Steven G, editor. AFIP Atlas of Tumor Pathology. Tumors of the Central Nervous System. 4th ed. Washington, DC: American registry of pathology; 2007. pp. 33–208. [Google Scholar]
- 7.Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. doi:10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 8.van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715–2722. doi: 10.1200/JCO.2005.04.6078. doi:10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 9.Mariani L, Deiana G, Vassella E, et al. Loss of heterozygosity 1p36 and 19q13 is a prognostic factor for overall survival in patients with diffuse WHO grade 2 gliomas treated without chemotherapy. J Clin Oncol. 2006;24:4758–4763. doi: 10.1200/JCO.2006.05.9238. doi:10.1200/JCO.2006.05.9238. [DOI] [PubMed] [Google Scholar]
- 10.McDonald JM, See SJ, Tremont IW, et al. The prognostic impact of histology and 1p/19q status in anaplastic oligodendroglial tumors. Cancer. 2005;104:1468–1477. doi: 10.1002/cncr.21338. doi:10.1002/cncr.21338. [DOI] [PubMed] [Google Scholar]
- 11.Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003;62:1118–1128. doi: 10.1093/jnen/62.11.1118. [DOI] [PubMed] [Google Scholar]
- 12.Perry A, Fuller CE, Banerjee R, Brat DJ, Scheithauer BW. Ancillary FISH analysis for 1p and 19q status: preliminary observations in 287 gliomas and oligodendroglioma mimics. Front Biosci. 2003;8:a1–a9. doi: 10.2741/896. doi:10.2741/896. [DOI] [PubMed] [Google Scholar]
- 13.Burton EC, Lamborn KR, Feuerstein BG, et al. Genetic aberrations defined by comparative genomic hybridization distinguish long-term from typical survivors of glioblastoma. Cancer Res. 2002;62:6205–6210. [PubMed] [Google Scholar]
- 14.Scheie D, Cvancarova M, Mork S, et al. Can morphology predict 1p/19q loss in oligodendroglial tumors? Histopathology. 2008;53:578–587. doi: 10.1111/j.1365-2559.2008.03160.x. doi:10.1111/j.1365-2559.2008.03160.x. [DOI] [PubMed] [Google Scholar]
- 15.Scheie D, Andresen PA, Cvancarova M, et al. Fluorescence in situ hybridization (FISH) on touch preparations: a reliable method for detecting loss of heterozygosity at 1p and 19q in oligodendroglial tumors. Am J Surg Pathol. 2006;30:828–837. doi: 10.1097/01.pas.0000213250.44822.2e. doi:10.1097/01.pas.0000213250.44822.2e. [DOI] [PubMed] [Google Scholar]
- 16.Miller CR, Dunham CP, Scheithauer BW, Perry A. Significance of necrosis in grading of oligodendroglial neoplasms: a clinicopathologic and genetic study of newly diagnosed high-grade gliomas. J Clin Oncol. 2006;24:5419–5426. doi: 10.1200/JCO.2006.08.1497. doi:10.1200/JCO.2006.08.1497. [DOI] [PubMed] [Google Scholar]
- 17.Shaw EG, Scheithauer BW, O'Fallon JR, Davis DH. Mixed oligoastrocytomas: a survival and prognostic factor analysis. Neurosurgery. 1994;34:577–582. doi: 10.1227/00006123-199404000-00002. doi:10.1227/00006123-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Helseth R, Helseth E, Johannesen TB, et al. Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol Scand. 2010;122:159–167. doi: 10.1111/j.1600-0404.2010.01350.x. doi:10.1111/j.1600-0404.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- 19.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. doi:10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 20.He J, Mokhtari K, Sanson M, et al. Glioblastomas with an oligodendroglial component: a pathological and molecular study. J Neuropathol Exp Neurol. 2001;60:863–871. doi: 10.1093/jnen/60.9.863. [DOI] [PubMed] [Google Scholar]
- 21.Homma T, Fukushima T, Vaccarella S, et al. Correlation among pathology, genotype, and patient outcomes in glioblastoma. J Neuropathol Exp Neurol. 2006;65:846–854. doi: 10.1097/01.jnen.0000235118.75182.94. doi:10.1097/01.jnen.0000235118.75182.94. [DOI] [PubMed] [Google Scholar]
- 22.Kraus JA, Lamszus K, Glesmann N, et al. Molecular genetic alterations in glioblastomas with oligodendroglial component. Acta Neuropathol. 2001;101:311–320. doi: 10.1007/s004010000258. [DOI] [PubMed] [Google Scholar]
- 23.Engelhard HH, Stelea A, Mundt A. Oligodendroglioma and anaplastic oligodendroglioma: clinical features, treatment, and prognosis. Surg Neurol. 2003;60:443–456. doi: 10.1016/s0090-3019(03)00167-8. doi:10.1016/S0090-3019(03)00167-8. [DOI] [PubMed] [Google Scholar]
- 24.Kros JM, Pieterman H, Van Eden CG, Avezaat CJ. Oligodendroglioma: the Rotterdam-Dijkzigt experience. Neurosurgery. 1994;34:959–966. doi: 10.1227/00006123-199406000-00002. doi:10.1227/00006123-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Shaw EG, Scheithauer BW, O'Fallon JR, Tazelaar HD, Davis DH. Oligodendrogliomas: the Mayo Clinic experience. J Neurosurg. 1992;76:428–434. doi: 10.3171/jns.1992.76.3.0428. doi:10.3171/jns.1992.76.3.0428. [DOI] [PubMed] [Google Scholar]
- 26.Zlatescu MC, TehraniYazdi A, Sasaki H, et al. Tumor location and growth pattern correlate with genetic signature in oligodendroglial neoplasms. Cancer Res. 2001;61:6713–6715. [PubMed] [Google Scholar]
- 27.Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. doi:10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 28.Griffin CA, Burger P, Morsberger L, et al. Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol. 2006;65:988–994. doi: 10.1097/01.jnen.0000235122.98052.8f. doi:10.1097/01.jnen.0000235122.98052.8f. [DOI] [PubMed] [Google Scholar]
- 29.Ducray F, Idbaih A, de RA, et al. Anaplastic oligodendrogliomas with 1p19q codeletion have a proneural gene expression profile. Mol Cancer. 2008;7:41. doi: 10.1186/1476-4598-7-41. doi:10.1186/1476-4598-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrer-Luna R, Mata M, Nunez L, et al. Loss of heterozygosity at 1p-19q induces a global change in oligodendroglial tumor gene expression. J Neurooncol. 2009;95:343–354. doi: 10.1007/s11060-009-9944-y. doi:10.1007/s11060-009-9944-y. [DOI] [PubMed] [Google Scholar]
- 31.von Deimling A, Louis DN, von Ammon K, et al. Evidence for a tumor suppressor gene on chromosome 19q associated with human astrocytomas, oligodendrogliomas, and mixed gliomas. Cancer Res. 1992;52:4277–4279. [PubMed] [Google Scholar]
- 32.von Deimling A, Bender B, Jahnke R, et al. Loci associated with malignant progression in astrocytomas: a candidate on chromosome 19q. Cancer Res. 1994;54:1397–1401. [PubMed] [Google Scholar]
- 33.Giannini C, Burger PC, Berkey BA, et al. Anaplastic oligodendroglial tumors: refining the correlation among histopathology, 1p 19q deletion and clinical outcome in Intergroup Radiation Therapy Oncology Group Trial 9402. Brain Pathol. 2008;18:360–369. doi: 10.1111/j.1750-3639.2008.00129.x. doi:10.1111/j.1750-3639.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinojima N, Kochi M, Hamada J, et al. The influence of sex and the presence of giant cells on postoperative long-term survival in adult patients with supratentorial glioblastoma multiforme. J Neurosurg. 2004;101:219–226. doi: 10.3171/jns.2004.101.2.0219. doi:10.3171/jns.2004.101.2.0219. [DOI] [PubMed] [Google Scholar]
- 35.Kros JM, Van Eden CG, Stefanko SZ, et al. Prognostic implications of glial fibrillary acidic protein containing cell types in oligodendrogliomas. Cancer. 1990;66:1204–1212. doi: 10.1002/1097-0142(19900915)66:6<1204::aid-cncr2820660621>3.0.co;2-a. doi:10.1002/1097-0142(19900915)66:6<1204::AID-CNCR2820660621>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 36.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. doi:10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 37.Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103:1227–1233. doi: 10.1002/cncr.20867. doi:10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 38.Johannesen TB, Langmark F, Lote K. Progress in long-term survival in adult patients with supratentorial low-grade gliomas: a population-based study of 993 patients in whom tumors were diagnosed between 1970 and 1993. J Neurosurg. 2003;99:854–62. doi: 10.3171/jns.2003.99.5.0854. doi:10.3171/jns.2003.99.5.0854. [DOI] [PubMed] [Google Scholar]
- 39.Kros JM. Oligodendrogliomas: clinicopathological correlations. J Neurooncol. 1995;24:29–31. doi: 10.1007/BF01052654. doi:10.1007/BF01052654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.