Abstract
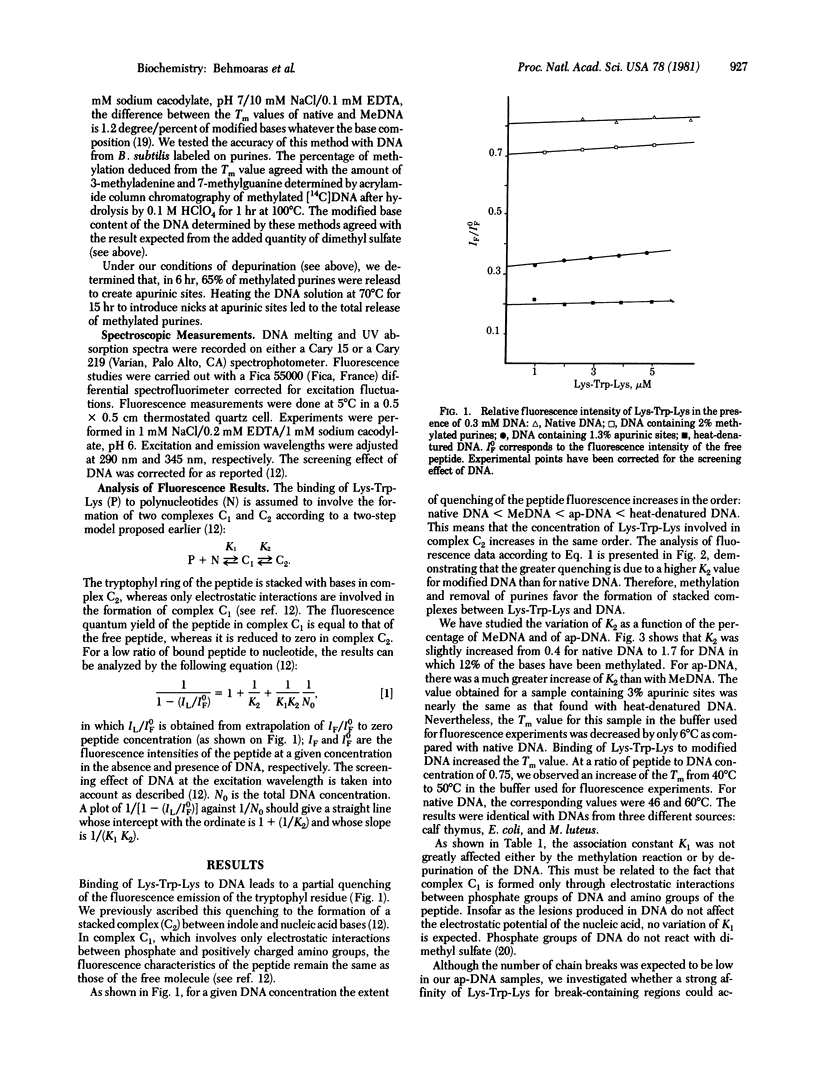
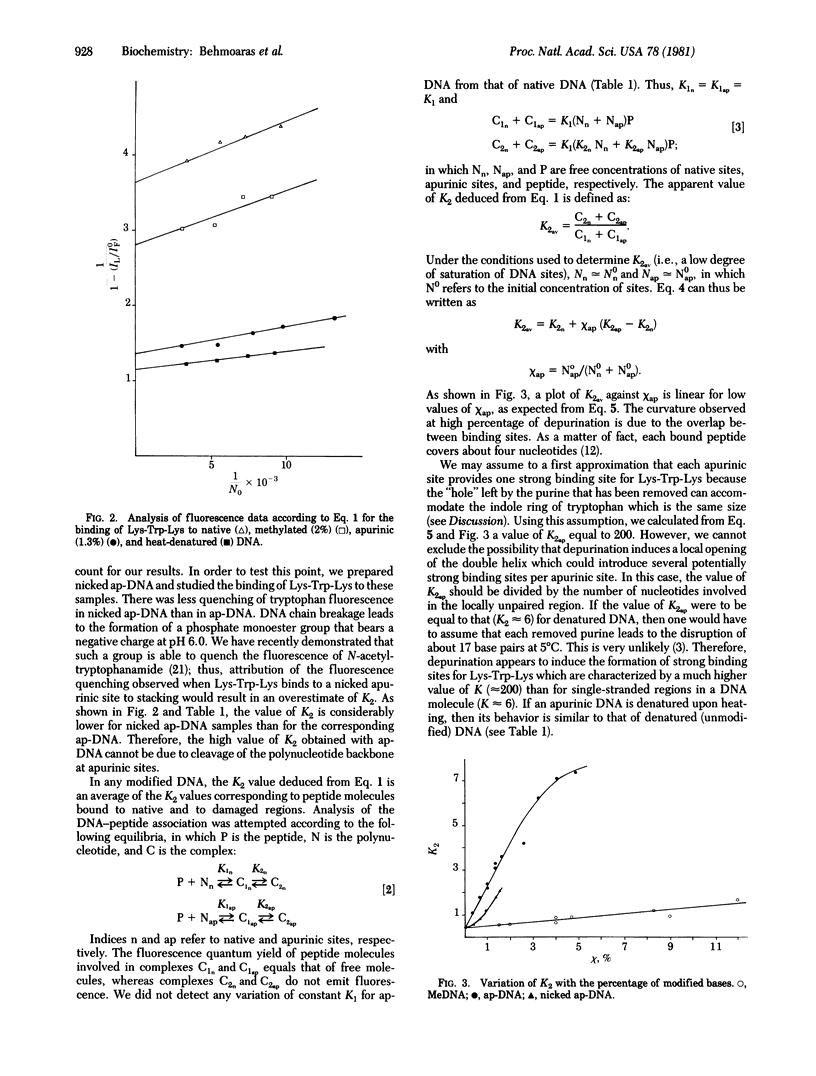
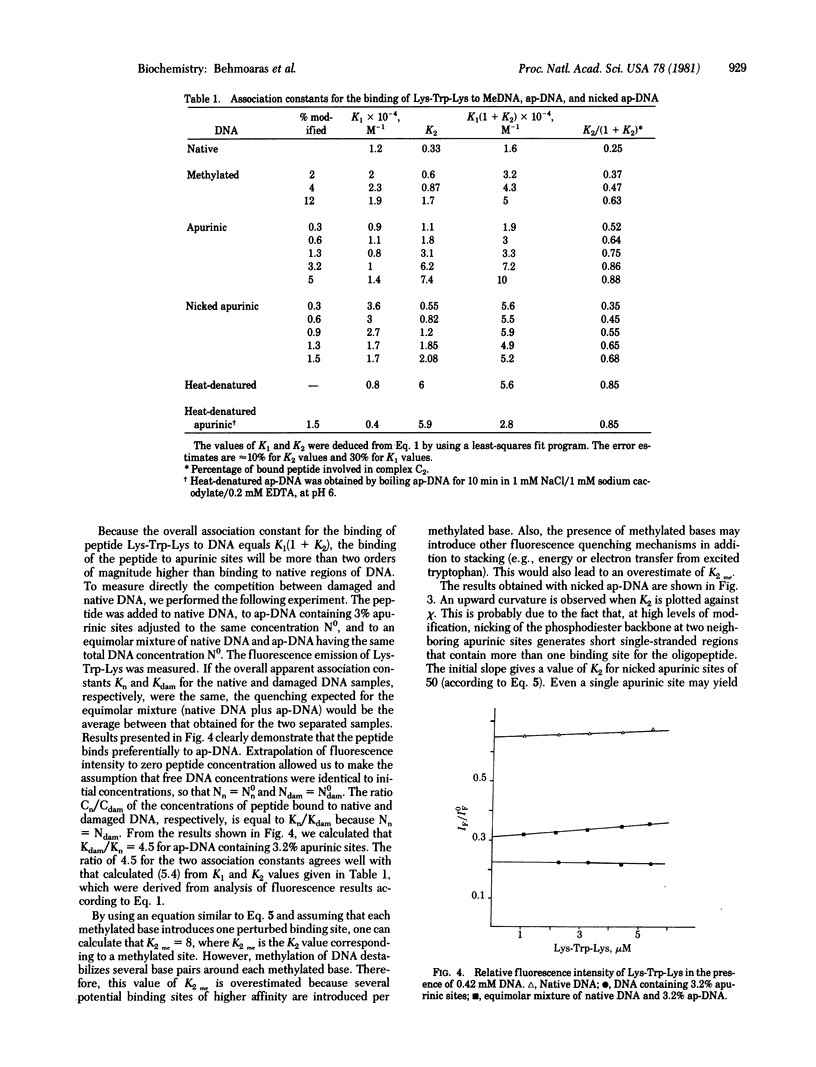
We have used fluorescence spectroscopy to study the binding of lysyltryptophyl-alpha-lysine (Lys-Trp-Lys) to DNA modified by dimethyl sulfate before and after depurination and strand breakage. Quenching of tryptophan fluorescence increased upon association of the peptide with modified DNA as compared with native DNA. We have demonstrated that this quenching is related to a preferential stacking of the indole ring with nucleic acid bases in damaged regions. Stacking increased in the following order: methylated DNA less than DNA with strand breaks at apurinic sites much less than apurinic DNA. For apurinic DNA, the overall association constant of Lys-Trp-Lys was increased by more than two orders of magnitude as compared to native DNA. Enhancement of the affinity of the tripeptide for an apurinic site requires the integrity of the phosphodiester bond. Single-strand cleavage at an apurinic site leads to a marked decrease of the association constant. The peptide Lys-Trp-Lys is therefore able to recognize destabilized regions in the vicinity of a lesion and to discriminate between different configurations of the damaged region. These results are discussed with respect to the role that stacking interactions could play in the specificity of recognition of DNA alterations by enzymes involved in DNA repair mechanisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alev-Behmoaras T., Toulmé J. J., Hélène C. Effect of phosphate ions on the fluorescence of tryptophan derivatives. Implications in fluorescence investigation of protein-nucleic acid complexes. Biochimie. 1979;61(8):957–960. doi: 10.1016/s0300-9084(79)80246-1. [DOI] [PubMed] [Google Scholar]
- Brun F., Toulmé J. J., Hélène C. Interactions of aromatic residues of proteins with nucleic acids. Fluorescence studies of the binding of oligopeptides containing tryptophan and tyrosine residues to polynucleotides. Biochemistry. 1975 Feb 11;14(3):558–563. doi: 10.1021/bi00674a015. [DOI] [PubMed] [Google Scholar]
- Deutsch W. A., Linn S. DNA binding activity from cultured human fibrolasts that is specific for partially depurinated DNA and that inserts purines into apurinic sites. Proc Natl Acad Sci U S A. 1979 Jan;76(1):141–144. doi: 10.1073/pnas.76.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimicoli J. L., Hélène C. Interactions of aromatic residues of proteins with nucleic acid. II. Proton magnetic resonance studies of the binding of tyramine and tyrosine-containing peptides to poly(adenylic acid) and deoxyribonucleic acid. Biochemistry. 1974 Feb 12;13(4):724–730. doi: 10.1021/bi00701a014. [DOI] [PubMed] [Google Scholar]
- Gabbay E. J., Sanford K., Baxter C. S., Kapicak L. Specific interaction of peptides with nucleic acids. Evidence for a "selective bookmark" recognition hypothesis. Biochemistry. 1973 Oct 9;12(21):4021–4029. doi: 10.1021/bi00745a001. [DOI] [PubMed] [Google Scholar]
- Hadi S. M., Goldthwait D. A. Endonuclease II of Escherichia coli. Degradation of partially depurinated deoxyribonucleic acid. Biochemistry. 1971 Dec 21;10(26):4986–4993. doi: 10.1021/bi00802a024. [DOI] [PubMed] [Google Scholar]
- Laval J. Recent progress in excision repair of DNA. Biochimie. 1978;60(10):1123–1134. doi: 10.1016/s0300-9084(79)80346-6. [DOI] [PubMed] [Google Scholar]
- Laval J. Two enzymes are required from strand incision in repair of alkylated DNA. Nature. 1977 Oct 27;269(5631):829–832. doi: 10.1038/269829a0. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Lethbridge J. H., Edwards P. A., Shooter K. V. Inactivation of bacteriophage T7 by mono- and difunctional sulphur mustards in relation to cross-linking and depurination of bacteriophage DNA. J Mol Biol. 1969 Jan 14;39(1):181–198. doi: 10.1016/0022-2836(69)90341-6. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Thatcher C. J. Methylation of deoxyribonucleic acid in cultured mammalian cells by N-methyl-N'-nitro-N-nitrosoguanidine. The influence of cellular thiol concentrations on the extent of methylation and the 6-oxygen atom of guanine as a site of methylation. Biochem J. 1970 Feb;116(4):693–707. doi: 10.1042/bj1160693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3618–3623. doi: 10.1021/bi00769a019. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Livneh Z., Elad D., Sperling J. Enzymatic insertion of purine bases into depurinated DNA in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1089–1093. doi: 10.1073/pnas.76.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R., Toulme F., Montenay-Garestier T., Helene C. The role of tyrosine in the association of proteins and nucleic acids. Specific recognition of single-stranded nucleic acids by tyrosine-containing peptides. J Biol Chem. 1979 Jan 10;254(1):75–82. [PubMed] [Google Scholar]
- Ramstein J., Héléne C., Leng M. A study of chemically methylated deoxyribonucleic acid. Eur J Biochem. 1971 Jul 15;21(1):125–136. doi: 10.1111/j.1432-1033.1971.tb01448.x. [DOI] [PubMed] [Google Scholar]
- Singer B. The chemical effects of nucleic acid alkylation and their relation to mutagenesis and carcinogenesis. Prog Nucleic Acid Res Mol Biol. 1975;15(0):219–284. [PubMed] [Google Scholar]
- Swenson D. H., Lawley P. D. Alkylation of deoxyribonucleic acid by carcinogens dimethyl sulphate, ethyl methanesulphonate, N-ethyl-N-nitrosourea and N-methyl-N-nitrosourea. Relative reactivity of the phosphodiester site thymidylyl(3'-5')thymidine. Biochem J. 1978 Jun 1;171(3):575–587. doi: 10.1042/bj1710575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé F., Hélène C., Fuchs R. P., Daune M. Binding of a tryptophan-containing peptide (lysyltryptophyllysine) to deoxyribonucleic acid modified by 2-(N-acetoxyacetylamino)fluorene. Biochemistry. 1980 Mar 4;19(5):870–875. doi: 10.1021/bi00546a007. [DOI] [PubMed] [Google Scholar]
- Toulmé J. J., Charlier M., Héléne C. Specific recognition of single-stranded regions in ultraviolet-irradiated and heat-denatured DNA by tryptophan-containing peptides. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3185–3188. doi: 10.1073/pnas.71.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé J. J., Hélène C. Specific recognition of single-stranded nucleic acids. Interaction of tryptophan-containing peptides with native, denatured, and ultraviolet-irradiated DNA. J Biol Chem. 1977 Jan 10;252(1):244–249. [PubMed] [Google Scholar]
- Uhlenhopp E. L., Krasna A. I. Alterations in the structure of deoxyribonucleic acid on chemical methylation. Biochemistry. 1971 Aug 17;10(17):3290–3295. doi: 10.1021/bi00793a020. [DOI] [PubMed] [Google Scholar]
- Verly W. G., Rassart E. Purification of Escherichia coli endonuclease specific for apurinic sites in DNA. J Biol Chem. 1975 Oct 25;250(20):8214–8219. [PubMed] [Google Scholar]



