Abstract
Background: self-rated health (SRH) likely reflects both mental and physical health domains, and is assessed by asking individuals to describe their health status. Poor SRH is associated with disease incidence and subsequent mortality. Changes in SRH across time in persons with different incident diseases are uncharacterised.
Methods: SRH was assessed in the Atherosclerosis Risk in Communities study via annual telephone interviews over a median of 17.6 years. Individual quadratic growth models were used for repeated measures of SRH in persons who remained disease-free during follow-up (n = 11,188), as well as among those who were diagnosed with myocardial infarction (MI; n = 1,071), stroke (n = 809), heart failure (HF; n = 1,592) or lung cancer (n = 433) and those who underwent a cardiac revascularisation procedure (n = 1,340) during follow-up.
Results: among disease-free participants and across time, there was a trend for lowest mean SRH among persons living in low socioeconomic areas and highest mean SRH among persons living in high socioeconomic areas. Factors contributing to the decline in SRH over time included advanced age, lower educational attainment, smoking and obesity.
Conclusion: addressing factors related to poor SRH trajectories among patients pre- and post-incident disease may favourably affect health outcomes among patients regardless of type of disease.
Keywords: socioeconomic status, disease progression, health status, health status disparities, elderly
Introduction
Self-rated health (SRH) is a measure of general health status, which is thought to reflect both mental and physical health domains [1]. SRH is typically obtained by asking individuals to describe their health status on a Likert scale (i.e. excellent, good, fair or poor) [2]. SRH is generally stable until age 50 years [3], and then declines with increasing age [4]. Low SRH is associated with worsening health and mortality [5–7]. Thus, it is hypothesised that low SRH may be able to predict a wide range of adverse health outcomes [7].
Cross-sectional analyses of elderly persons demonstrate that living in disadvantaged neighbourhoods and having low education is associated with self-reported poor health [8–11]. Furthermore, the association between neighbourhood-level socioeconomic status (SES) and SRH remains after taking other individual-level measures—including measures of income, education and occupation—into account [8].
While many studies have investigated factors associated with current SRH [4, 8, 11] or a change in SRH (i.e. from baseline) [6], few studies have reported the trajectory of repeated measures of SRH across a specified time period [12]. To our knowledge, research quantifying the trajectory of repeated measures of SRH among patients with incident disease by SES has not been undertaken, and may provide additional insight into the study of disease progression. Information regarding SRH trajectories that differ by SES could be used to develop interventions which prevent the loss of well-being associated with an incident disease diagnosis, with interventions tailored to address issues pertinent to the specific disease diagnosis.
Methods
Atherosclerosis Risk in Communities (ARIC) cohort participants were enrolled from four US communities beginning in 1987 [13]. ARIC study staff conducts annual follow-up by telephone, and SRH was measured among 15,792 black and white men and women at baseline and at each annual follow-up (1987–2006) for a median of 17.6 (range 1–19) years using the question, ‘Over the past year, compared to other people your age, would you say that your health has been excellent, good, fair or poor?’ SRH data are simple to collect [4], yet may be difficult to interpret, since the SRH scale is not precisely ordinal. SRH data from the Cardiovascular Health Study were transformed to a scale from 100 (perfect health) to 0 (death), representing the probability of being healthy in the future, conditional on the current value of SRH [14]. We transformed SRH accordingly: 95 for excellent, 80 for good, 30 for fair, 15 for poor and 0 for death.
There were 276,200 total SRH observations for members of the cohort; 9,552 (3.4%) were missing. If an observation was missing, and there were complete SRH values for both the previous and subsequent year of follow-up, we imputed the missing observation by averaging the values from the previous and subsequent years. As a result, over half (n = 5,140) of the missing observations were imputed. We assigned a zero for the missing value if it occurred during the year in which the cohort member died. In order to capture the SRH of the entire cohort across time, and not just that of the survivors, we included observations through contact year 19 for members lost to follow-up or who were deceased, and assigned a zero for each follow-up year which occurred after the cohort members' death.
Of the original 15,792 cohort members, 754 were excluded due to missing neighbourhood-level median household income (nINC) (n = 13,030 SRH observations), resulting in 263,170 SRH observations available for these analyses. We analysed mean SRH at discrete time points and trajectories of SRH across time among participants who were free of the selected diseases of interest as described below (‘disease-free’) at baseline and disease-free throughout follow-up (n = 11,188), as well as among those disease-free at baseline and receiving a diagnosis of incident myocardial infarction (MI; n = 1,071), stroke (n = 809), heart failure (HF; n = 1,592) or lung cancer (n = 433), and those undergoing cardiac revascularisation procedures (n = 1,340) during follow-up. We assessed SRH data through the end of 2006, and incident events through the end of 2005, in order to give each cohort member at least 1 year of follow-up post-event.
For comparison purposes, each member of the disease-free group was assigned a random ‘event’ date [12]. As a result, the pre-event and post-event trajectories from the incident disease groups could be compared with those of the group which remained healthy throughout follow-up, to determine if the SRH trajectories among the diseased differ from the trajectory of SRH that would be expected due to disease-free aging.
Participants' baseline place of residence (1987–89) was geocoded to the level of the census tract as described elsewhere [15]. nINC was obtained from the 1990 US Census and averaged across all ARIC study communities. Participants were assigned a tertile of nINC [low (<$24,777), medium (≤$24,777 to <36,071) or high (≥$36,071)] based upon their address at baseline.
Additional demographic factors influencing pre- and post-event trajectories were of interest: age (centred at 65 years) and age squared at the time of the annual follow-up contact were included in statistical models, along with gender and race/study community. Health status and behaviour variables assessed at baseline included body mass index (BMI), classified into normal (referent, <25 kg/m2), overweight (25 to <30 kg/m2) or obese (≥30 kg/m2); hypertension, present if systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or if taking hypertensive medication within the previous 2 weeks; current drinker and current smoker. Educational attainment was assessed at baseline and categorised as less than 11 years, high school graduate and greater than high school (referent). We accounted for period effects [1987–92 (referent), 1993–99 and 2000–06] at each annual follow-up contact in order to capture secular trends [16] which may influence the nINC–SRH relationship, such as changes in health behaviours and disease treatments occurring in the ARIC communities over time. We used an indicator variable, accounting for the presence of disease (yes/no) at each annual follow-up, which represented the change in SRH from pre- to post-disease, while an interaction term (time*indicator variable) reflected the change in slope pre- to post-disease.
We regressed incident disease-specific SRH at each time point of interest (i.e. baseline; 3, 2 and 1 year prior to the event; event year and 1, 2, 3, 4 and 5 years post-event) on study covariates to generate estimated adjusted SRH values and standard errors (PROC GLM, SAS 9.1.3, Cary, NC, USA). We used the change in adjusted SRH between the year of event and 1 year later to calculate how much of the decline in SRH post-event was due to death for each incident disease group. We fit individual quadratic growth models separately to data by incident disease group, accounting for repeated measures of SRH (PROC MIXED, SAS 9.1.3, Cary, NC, USA). Effect measure modification of the nINC–SRH relationship was assessed (disease-free: P interaction < 0.01; other disease: P interaction < 0.05) by demographic, medical history and health behaviour variables.
Results
Disease-free participants were more likely to reside in high nINC areas (36.1%) at baseline (Table 1), be female (59.2%) and have greater than a high school education (37.7%). Among participants who were not disease-free over the course of follow-up, participants receiving a cardiac revascularisation procedure were more likely to be male (67.3%). Stroke patients were more likely to be living in low nINC areas (43.8%) and HF patients were most likely to have less than a high school education (39.2%).
Table 1.
Baseline characteristics (%) of participants by incident disease status: the ARIC Study (1987–2005)
| Disease-free (n = 11,188) | Cardiac revascularisation procedure (n = 1,340) | Myocardial infarction (n = 1,071) | Lung cancer (n = 433) | Stroke (n = 809) | Heart failure (n = 1,592) | |
|---|---|---|---|---|---|---|
| Age, mean (years) | 54.2 | 55.7 | 56.0 | 57.5 | 54.7 | 57.1 |
| nINC | ||||||
| Low | 29.5 | 21.8 | 25.8 | 31.6 | 43.8 | 42.6 |
| Medium | 34.4 | 44.5 | 40.0 | 40.2 | 29.0 | 33.3 |
| High | 36.1 | 33.7 | 34.2 | 28.2 | 27.2 | 24.1 |
| Female | 59.2 | 32.7 | 43.0 | 36.7 | 55.3 | 46.8 |
| Race/study community | ||||||
| Black/NC | 3.1 | 2.7 | 2.8 | 3.2 | 3.6 | 3.3 |
| Black/MS | 22.7 | 10.6 | 24.4 | 19.2 | 39.9 | 31.0 |
| White/NC | 22.4 | 29.2 | 24.3 | 26.1 | 17.1 | 19.8 |
| White/MN | 27.4 | 27.5 | 21.9 | 24.5 | 17.2 | 17.6 |
| White/MD | 24.4 | 30.0 | 26.6 | 27.0 | 22.2 | 28.3 |
| Hypertensive | 31.2 | 40.2 | 48.6 | 37.9 | 42.3 | 54.0 |
| Overweight or obese | 65.0 | 65.9 | 74.8 | 55.9 | 70.2 | 78.1 |
| Current smoker | 23.0 | 30.5 | 37.4 | 65.8 | 28.1 | 37.0 |
| Current drinker | 57.3 | 57.1 | 49.6 | 64.0 | 48.7 | 45.4 |
| Educational attainment | ||||||
| Less than HS | 21.0 | 23.5 | 32.6 | 33.5 | 27.9 | 39.2 |
| HS or equivalent | 41.1 | 42.7 | 37.9 | 43.0 | 42.4 | 36.9 |
| Greater than HS | 37.7 | 33.7 | 29.4 | 23.5 | 29.4 | 23.6 |
Each type of incident disease was treated separately, thus it was possible for cohort participants to belong to more than one incident disease group, with the exception of disease-free participants. The largest overlap occurred between incident MI and cardiac revascularisation procedure, which shared 12% of participants. All other types of incident disease co-occurred at either a rate of 5% (i.e. HF and cardiac revascularisation procedure, and HF and MI) or ≤1% (data not shown). Participants aged 60–64 years at baseline tended to report lower SRH and demonstrate a steeper decline in SRH across study follow-up when compared with participants aged 45–49 years at baseline, regardless of incident disease status. Those with lung cancer had the lowest SRH across all age groups, followed by those with HF (please see the figure Appendix 1 in the Supplementary data available in Age and Ageing online).
At baseline, 33.2% of participants reported excellent, 46.8% good, 16.6% fair and 3.4% poor SRH. The internal validity of the SRH measure in this cohort was high, as participants reporting excellent health at baseline were least likely (and those reporting poor health at baseline were most likely) to be hypertensive, overweight/obese or to be deceased by the end of follow-up (please see the table Appendix 3 in the Supplementary data available in Age and Ageing online).
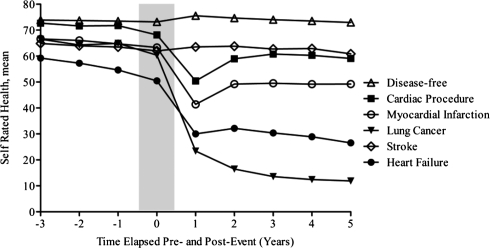
Figure 1 shows mean SRH by type of incident disease pre- and post-event, adjusting for age, race/study community, gender, hypertensive status, BMI, current smoking, current drinking, educational attainment and period effects. Average SRH 3 years prior to the incident event was highest among disease-free participants (73.9) and lowest among those developing HF (59.2). Between 3 years pre-event and the event year, the smallest average decline in SRH occurred among disease-free individuals (−1.4), while the largest average decline occurred among those developing HF (−8.2). Large declines in SRH were seen during the 1 year following the event among MI, cardiac revascularisation procedure, lung cancer and HF patients. The percent of the decline in SRH due to death among these patients was 16, 8, 24 and 32%, respectively. A gradual increase in SRH post-event was seen among participants surviving events that did not have as high of a burden of mortality as HF and lung cancer (Figure 1).
Figure 1.
Trajectory of SRH pre- and post-event by incident disease status: the ARIC Study (1987–2006). (Adjusted for nINC, age, race/study community, gender, hypertensive status, body mass index, current smoking, current drinking, educational attainment and period effects.)
Individual quadratic growth models controlling for all available covariates indicated that advancing age and lower educational attainment were predictors of the decline in SRH over time regardless of type of incident disease (Table 2). For the majority of incident disease types, being hypertensive, obese and a current smoker contributed to significant declines in SRH over time. Among disease-free participants, as well as participants undergoing cardiac revascularisation procedures, living in low nINC areas at baseline was a predictor of SRH decline. Notable among disease-free participants was a significant interaction between nINC and educational attainment, indicating that the combination of low nINC and lower educational attainment conferred a statistically significant excess risk of an additional decrease in the trajectory of SRH over time. Statistically significant declines in SRH occurred pre- to post- event, and statistically significant changes in slope pre- and post- event occurred for all incident disease types except stroke.
Table 2.
Predictorsa of the change in SRH over time [β estimate (SE)] by incident disease status: the ARIC Study (1987–2006)
| Disease-free (n = 11,188) | Cardiac revascularisation procedure (n = 1,340) | Myocardial infarction (n = 1,071) | Lung cancer (n = 433) | Stroke (n = 809) | Heart failure (n = 1,592) | |
|---|---|---|---|---|---|---|
| Intercept | 87.7 (0.8)* | 84.8 (2.2)* | 78.5 (2.9)* | 79.7 (4.6)* | 81.3 (3.4)* | 71.2 (2.5)* |
| Age, centred at 65 | −0.5 (0.04)* | −0.3 (0.1)* | −0.6 (0.1)* | −0.3 (0.2) | −0.9 (0.1)* | −0.3 (0.1)* |
| Age2 | −0.03 (0.002)* | −0.02 (0.004)* | −0.03 (0.005)* | −0.02 (0.01)* | −0.03 (0.01)* | −0.03 (0.005)* |
| nINC (vs. High) | ||||||
| Low | −2.3 (0.8)* | −5.2 (1.7)* | −5.0 (2.3)* | 2.0 (5.0) | 1.8 (2.1) | −0.04 (1.6) |
| Medium | −1.2 (0.6)* | −0.9 (1.3) | 1.2 (1.9) | 6.2 (3.8) | −0.6 (1.9) | 0.5 (1.4) |
| Female (vs. Male) | −1.0 (0.3)* | −3.7 (1.0)* | −1.9 (1.2) | −0.6 (1.7) | 1.7 (1.3) | 0.01 (1.0) |
| Race/study community (vs. White/MD) | ||||||
| Black/NC | −6.5 (0.9)* | −1.3 (2.8) | −7.4 (3.5)* | −6.1 (5.3) | −9.1 (3.7)* | −8.0 (2.8)* |
| Black/MS | −8.7 (0.6)* | −3.7 (1.8)* | −6.1 (2.0)* | −5.5 (2.9) | −10.7 (2.2)* | −7.5 (1.6)* |
| White/NC | −0.8 (0.4) | −0.6 (1.1) | 0.1 (1.6) | 1.9 (2.2) | −2.1 (2.1) | −3.6 (1.4)* |
| White/MN | 0.1 (0.5) | 2.1 (1.3) | 4.6 (1.8)* | 1.6 (2.6) | 2.6 (2.2) | 2.4 (1.6) |
| Hypertensive | −4.9 (0.3)* | −4.4 (0.9)* | −5.6 (1.2)* | −2.4 (1.8) | −7.9 (1.4)* | −4.2 (1.0)* |
| BMI (vs. Normal) | ||||||
| Obese | −4.8 (0.4)* | −6.8 (1.2)* | −5.8 (1.5)* | −5.4 (2.1)* | −7.1 (1.7)* | −2.8 (1.3)* |
| Overweight | −0.7 (0.3)* | −2.8 (1.1)* | −1.3 (1.4) | −1.1 (1.9) | −3.6 (1.6)* | 0.9 (1.3) |
| Current smoker | −4.0 (0.4)* | −3.8 (1.0)* | −4.2 (1.2)* | −4.7 (1.8)* | −7.8 (1.4)* | −3.8 (1.0)* |
| Current drinker | 1.6 (0.3)* | 3.6 (0.9)* | 3.4 (1.2)* | 0.9 (1.8) | 2.5 (1.4) | 2.8 (1.0)* |
| Educational attainment (vs. Greater than HS) | ||||||
| Less than HS | −9.5 (1.0)* | −9.7 (1.3)* | −10.3 (1.5)* | −11.5 (5.2)* | −12.9 (1.7)* | −9.5 (1.3)* |
| HS or equivalent | −3.2 (0.5)* | −3.4 (1.0)* | −5.0 (1.3)* | 4.7 (3.2) | −6.0 (1.5)* | −3.9 (1.2)* |
| Contact year | −0.3 (0.04)* | −0.2 (0.1) | 0.1 (0.1) | −0.6 (0.2)* | 0.1 (0.2) | −0.4 (0.1)* |
| Disease status | −1.7 (0.3)* | −14.9 (1.1)* | −19.5 (1.2)* | −44.7 (2.4)* | −4.8 (1.3)* | −26.3 (1.2)* |
| 1987−92 (vs. 2000−06) | −0.6 (0.4) | 0.9 (1.1) | 1.4 (1.5) | −1.3 (2.1) | 3.0 (1.3)* | 5.0 (1.0)* |
| 1993−99 (vs. 2000−06) | −0.01 (0.2) | 0.04 (0.7) | −0.03 (1.0) | −0.8 (1.3) | 2.3 (0.7)* | 3.2 (0.6)* |
| Age, centred at 65*1987−92 | −0.1 (0.05)* | NS | NS | NS | 0.03 (0.2) | NS |
| Age, centred at 65*1993−99 | 0.05 (0.03) | NS | NS | NS | 0.3 (0.1)* | NS |
| Low nINC*Less than HS | −3.9 (1.2)* | NS | NS | 1.9 (6.8) | NS | NS |
| Low nINC*HS or equivalent | −2.7 (0.8)* | NS | NS | −12.4 (5.6)* | NS | NS |
| Medium nINC*Less than HS | −1.5 (1.2) | NS | NS | −4.6 (6.3) | NS | NS |
| Medium nINC*HS or equivalent | −0.3 (0.8) | NS | NS | −8.2 (4.6) | NS | NS |
| Low nINC*1987−92 | 2.0 (0.4)* | 1.8 (1.3) | 3.3 (1.6)* | NS | NS | NS |
| Low nINC*1993−99 | 0.6 (0.3) | −0.3 (1.0) | 2.1 (1.2) | NS | NS | NS |
| Medium nINC*1987−92 | 0.4 (0.4) | 0.5 (1.1) | −0.6 (1.6) | NS | NS | NS |
| Medium nINC*1993−99 | 0.3 (0.3) | 1.5 (0.8)* | 1.0 (1.2) | NS | NS | NS |
| Contact year*Disease status | 0.2 (0.03)* | 0.4 (0.1)* | −0.6 (0.1)* | 0.6 (0.2)* | 0.2 (0.1) | 0.8 (0.1)* |
aSignificant predictors (P < 0.05) are indicated with an (*); NS, interaction not significant for this model.
Discussion and conclusion
To our knowledge, this is the first study to characterise trajectories of change in SRH status pre- and post- incident disease diagnosis. Values of SRH tended to be lower at baseline and declined at a greater rate prior to the disease occurrence among participants who were disease-free at baseline but developed a disease over the course of follow-up compared with healthy members of the cohort. The largest pre-event decline occurred in the HF group. A positive stepwise association between nINC and SRH persisted across 19 years of follow-up of the entire cohort, regardless of incident disease status. While nINC contributed to the decline in SRH among members of the cohort with selected types of incident disease, it was not a contributing factor to SRH decline over time for all participants, unlike age, educational attainment, current smoking and obesity. With exception of lung cancer, being hypertensive contributed to significant declines in SRH over time.
Trends in SRH differ for different types of incident disease. Previous studies have shown a decline in health status pre-event for diseases such as cancer, MI and HF, as well as a relationship between health-related quality of life and the risk of hospital readmission or death post-event [12, 17]. The steep decline in SRH post-event, followed by a levelling-off period, has been noted in other studies [12]. This phenomenon is at least in part due to the fact that all participants experiencing an incident event had to be alive prior to the event, after which they could die. The percent of decline during the first year post-event varied by type of incident disease, with a high proportion of deaths affecting the lung cancer and HF groups the most and cardiac revascularisation and MI patients the least. Whether the disease is acute-onset, such as stroke, or of slower onset, such as HF, may have implications for the duration and speed of change in SRH.
Strengths of our study include a large number of SRH observations, due to nearly 20 years of follow-up. We imputed SRH values if there were SRH values immediately prior to or following the missing value, allowing for a more complete picture of SRH across time. In addition, due to the thorough medical history obtained at baseline, we excluded participants with prevalent disease from study, thus restricting our analysis to incident events only. Our results are consistent with an Israeli study which reported poor income, low education and obesity as independent predictors of a decline in SRH among MI patients [18].
It was important to account for death in the analyses in order to get an accurate picture of the trajectory of SRH among members of the cohort. For example, if only live participants were considered, SRH may have been shown to improve after a sentinel health event, since the sickest patients (i.e. those with fair or poor SRH) would have disproportionately died [14]. Similarly, had we attributed a zero to the participants' year of death, but counted SRH values as missing thereafter, we would have tracked the post-event SRH trajectories of the disease survivors, and ignored the experience of the entire cohort.
SRH is a good indicator of overall health status; however, multiple measurements of other subjective health indicators were not collected annually from participants. Thus, the extent to which a poor SRH rating was associated with poor physical health, mental health, cognitive status, functional status or a combination of these or other measures, such as positive or negative affect [19], cannot be ascertained from these data. Better functional status, for example, may improve one's ability to perform disease self-management techniques, which has been shown to have a positive impact on health status of HF patients [20]. There is evidence that different pathways may link SES to distinct domains of health status [21].
Repeated measures data provide additional insight into the study of disease progression. In this study, significant predictors of the decline in SRH over a 20-year period included age, educational attainment, current smoking and obesity. As SRH is associated with subsequent risk of morbidity and mortality, addressing factors related to poor SRH trajectories among patients pre- and post-incident disease may favourably affect health outcomes among patients regardless of type of incident disease.
Key points.
SRH is a subjective measure of health which correlates well with objective measures.
Individuals tend to self-report poorer health status as they age.
The decline in self-rated health, both pre- and post-disease, differs between incident disease types.
Factors contributing to a steeper decline in self-rated health include educational attainment, smoking and obesity.
Conflicts of interest
None declared.
Supplementary data
Supplementary data mentioned in the text is available to subscribers in Age and Ageing online.
Funding
This work was supported by National Institutes of Health; National Heart, Lung and Blood Institute and National Research Service Award training grant 5-T32-HL007055. The ARIC study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021 and N01-HC-55022.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
References
- 1.Singh-Manoux A, Martikainen P, Ferrie J, Zins M, Marmot M, Goldberg M. What does self rated health measure? Results from the British Whitehall II and French Gazel cohort studies. J Epidemiol Community Health. 2006;60:364–72. doi: 10.1136/jech.2005.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh-Manoux A, Dugravot A, Shipley MJ, et al. The association between self-rated health and mortality in different socioeconomic groups in the GAZEL cohort study. Int J Epidemiol. 2007;36:1222–8. doi: 10.1093/ije/dym170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCullough ME, Laurenceau JP. Gender and the natural history of self-rated health: a 59-year longitudinal study. Health Psychol. 2004;23:651–5. doi: 10.1037/0278-6133.23.6.651. [DOI] [PubMed] [Google Scholar]
- 4.McFadden E, Luben R, Bingham S, Wareham N, Kinmonth A-L,, Khaw K-T. Social inequalities in self-rated health by age: cross-sectional study of 22 457 middle-aged men and women. BMC Public Health. 2008;8:230. doi: 10.1186/1471-2458-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy BS, Kasl SV, Vaccarino V. Repeated hospitalizations and self-rated health among the elderly: a Multivariate Failure Time Analysis. Am J Epidemiol. 2001;153:232–41. doi: 10.1093/aje/153.3.232. [DOI] [PubMed] [Google Scholar]
- 6.Wolinsky FD, Miller TR, Malmstrom TK, et al. Self-rated health: changes, trajectories, and their antecedents among African Americans. J Aging Health. 2008;20:143–58. doi: 10.1177/0898264307310449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardage C, Isacson D, Pedersen NL. Self-rated health as a predictor of mortality among persons with cardiovascular disease in Sweden. Scand J Public Health. 2001;29:13–22. [PubMed] [Google Scholar]
- 8.Wight RG, Cummings JR, Miller-Martinez D, Karlamangla AS, Seeman TE, Aneshensel CS. A multilevel analysis of urban neighborhood socioeconomic disadvantage and health in late life. Soc Sci Med. 2008;66:862–72. doi: 10.1016/j.socscimed.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Power C, Rodgers B, Hope S. U-shaped relation for alcohol consumption and health in early adulthood and implications for mortality. Lancet. 1998;352:877. doi: 10.1016/S0140-6736(98)23937-7. [DOI] [PubMed] [Google Scholar]
- 10.Kunst AE, Bos V, Lahelma E, et al. Trends in socioeconomic inequalities in self-assessed health in 10 European countries. Int J Epidemiol. 2005;34:295–305. doi: 10.1093/ije/dyh342. [DOI] [PubMed] [Google Scholar]
- 11.Daponte-Codina A, Bolivar-Munoz J, Toro-Cardenas S, Ocana-Riola R, Benach-Rovira J, Navarro-Lopez V. Area deprivation and trends in inequalities in self-rated health in Spain, 1987–2001. Scand J Public Health. 2008;36:504–15. doi: 10.1177/1403494807088454. [DOI] [PubMed] [Google Scholar]
- 12.Diehr P, Williamson J, Patrick DL, Bild DE, Burke GL. Patterns of self-rated health in older adults before and after sentinel health events. J Am Geriat Soc. 2001;49:36–44. doi: 10.1046/j.1532-5415.2001.49007.x. [DOI] [PubMed] [Google Scholar]
- 13.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–33. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 14.Diehr P, Patrick DL. Trajectories of health for older adults over time: accounting fully for death. Ann Intern Med. 2003;139(5_Part_2):416–20. doi: 10.7326/0003-4819-139-5_part_2-200309021-00007. [DOI] [PubMed] [Google Scholar]
- 15.Whitsel EA, Rose KM, Wood JL, et al. Accuracy and repeatability of commercial geocoding. Am J Epidemiol. 2004;160:1023–9. doi: 10.1093/aje/kwh310. [DOI] [PubMed] [Google Scholar]
- 16.Rice NE, Lang IA, Henley W, Melzer D. Baby boomers nearing retirement: the healthiest generation? Rejuvenation Res. 2010;13:105–14. doi: 10.1089/rej.2009.0896. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Artalejo F, Guallar-Castillon P, Pascual CR, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165:1274–9. doi: 10.1001/archinte.165.11.1274. [DOI] [PubMed] [Google Scholar]
- 18.Gerber YP, Benyamini YP, Goldbourt UP, Drory YMD for the Israel Study Group on First Acute Myocardial Infarct. Prognostic importance and long-term determinants of self-rated health after initial acute myocardial infarction. Medical Care. 2009;47:342–9. doi: 10.1097/MLR.0b013e3181894270. [DOI] [PubMed] [Google Scholar]
- 19.Winter L, Lawton MP, Langston CA, Ruckdeschel K, Sando R. Symptoms, affects, and self-rated health: evidence for a subjective trajectory of health. J Aging Health. 2007;19:453–69. doi: 10.1177/0898264307300167. [DOI] [PubMed] [Google Scholar]
- 20.Suwanno J, Petpichetchian W, Riegel BD, Issaramalai S-A. A model predicting health status of patients with heart failure. J Cardiovas Nursing. 2009;24:118–26. doi: 10.1097/JCN.0b013e318197a75c. [DOI] [PubMed] [Google Scholar]
- 21.Barbareschi G, Sanderman R, Kempen GIJM, Ranchor AV. Socioeconomic status and the course of quality of life in older patients with coronary heart disease. Int J Behav Med. 2009;16:197–204. doi: 10.1007/s12529-008-9010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.