Abstract
Objective
To identify, in community dwelling elders, the determinants of sustained pain improvement or worsening.
Design
A longitudinal study with two baseline and 11 monthly follow-up interviews was conducted. Pain was assessed monthly using the Parmelee adaptation of the McGill Pain Inventory.
Subjects
Subjects included 109 Caucasian and 132 African American, Philadelphia residing Medicare recipients (65–74 years of age).
Outcome Measures
To identify sustained pain change (≥2 months), the data for each subject were reconfigured to yield 10 overlapping 3-month data segments. Each segment was classified as improved or worsened pain. Other variables included: the Geriatric Depression Scale (GDS), self-rated health (SRH), physical functioning, and number of improved or worsened medical conditions.
Results
Pain experienced (over 3-month periods) was typically stable. Sustained improved pain was more likely than worsened pain. Odds ratios obtained through Generalized Estimation Equation analyses showed that a 1-point increase in GDS scores increased the odds of worsened pain by 1.18 (1.11–1.30). Fair/poor SRH, being female, and having medical conditions worsen increased the odds of worsened pain by 4.04 (2.12–7.70), 1.63 (1.11–2.38), and 2.12 (1.42–3.16), respectively. Observed, statistically significant associations between these variables, except gender, and improved pain were in the opposite direction.
Conclusions
With a 1-month time lag between predictor variable assessment and follow-up pain measures, the study supports temporal associations between depressive symptoms and SRH and subsequent pain change. Clinicians providing care to community dwelling elders are advised to evaluate and attend to both the depressive symptoms and SRH of their patients.
Keywords: Pain, Depression, Self-Rated Health, Aged, Epidemiology, Risk Factor
Introduction
Prevalence rates of pain experienced by older, independently living community dwelling individuals range between 20–80% depending on the specific population studied and the way in which pain was defined and measured [1–12]. Despite the high prevalence of pain, the negative impact of pain on a person’s psychosocial and social well-being and ability to live independently, [2,4,8,12] there have been surprisingly few longitudinal studies of independently living elders recruited from the community that permit an adequate investigation of the epidemiology (etiology, incidence, and natural course) of chronic pain. Moreover, in most relevant studies, questions on pain appear to be part of a larger health status assessment and the published findings represent the secondary analyses of study data collected for another purpose [3,4,7–12]. Though limited, the available evidence indicates that prevalence rates in groups over age 60 change little with advancing age [5–7], but incidence of activity limiting pain increases with advancing age [7]. For example, in a 3-year longitudinal study of community dwelling residents in Staffordshire England, Thomas et al. [7] observed incident life-interfering pain in 12.8% of individuals 60–69 years of age compared to 28% in those 80+ years of age. Pain experienced by older individuals appears remarkably stable over extended time periods [4,8–10]. Mossey and Gallagher [4] observed that 36% of a sample of independently living continuing care retirement community residents reported persistent activity limiting pain over a 2-year period, while an additional 27.8% reported persistent nonlimiting pain. These findings are consistent with those reported by Elliot et al. [9] and Geerlings et al. [10] who investigated the course of chronic pain in community dwelling samples over 4- and 3-year periods, respectively.
Studies have indicated that depression [3,4,9,10], the presence of medical conditions [2–4,13], and gender [4,14–16] can impact the experience and the course of pain. While the association of pain and depression in older individuals is well documented (see Bair et al. [17] for a recent review of this literature), the temporal relationships between the two are not as clear. Physiological similarities between depression and pain provide evidence that temporal associations could develop in either direction [18,19]. Findings from several recent longitudinal studies support the reciprocal nature of the pain–depression relationship [10,11]. Females are more likely to report pain, and when reported, pain is more likely to be chronic and to be of greater severity than that reported by males [14–16]. Although there is considerable evidence of racial disparities in the adequacy of pain assessment and in access to effective pain treatment programs [20–24], few relevant community based studies have included the elderly from racial minority groups. In studies of samples drawn from residents in the United States that included Hispanic and non-Hispanic Black and White older individuals, no racial/cultural effect has been observed for the prevalence of severe or activity limiting pain once socio-economic and medical factors have been controlled [8,25,26].
The ability to draw firm conclusions regarding risk factors, particularly for pain worsening or improvement, however, is limited. There is no uniformity in the operational definitions of pain presence, intensity, activity interference, or chronicity. For example, Thomas et al. [7] define “chronic pain” in terms of the response to a single question, while Mossey and Gallagher [4] define “chronic pain” in terms of the presence of activity limiting pain in 3, 4, or 5 of the five pain assessments obtained consecutively over a 24-month period in their longitudinal study. In addition, the intervals between pain assessments have ranged from several months in some studies [4,10] to many years in others [9]. Even the shortest intervals of 5 or 6 months are prohibitively long to pinpoint the occurrence of incident changes in pain levels. Thus, timely evaluations of temporal associations between potential predictor variables and such changes have not been possible.
The need to develop further understanding of the risk factors and determinants of pain improvement or worsening, especially when it is sustained and/or activity limiting, and to better understand the natural course of pain experienced by elders who reside independently in community settings is compelling. In his comments on the International Association for the Study of Pain (IASP) decision to treat 2007 as the year to focus on “Pain in Older Persons,” Gibson has suggested such individuals may be thought of as the “forgotten majority” (p. 628) [27]. As noted above, this is particularly true for racially diverse elderly populations. The expected large increase in the number of individuals who will continue to reside in the community beyond their 7th decade highlights the urgent need to untangle the epidemiology of pain in this “majority” group.
The study reported here uses longitudinal data obtained at 12, 1-month intervals to address one aspect of the epidemiological puzzle. The presence of repeated pain reports has permitted identification of episodes of sustained worsened and improved pain over short intervals (3 months). With additional data obtained at the same time on medical status and physical, emotional, and social functioning, it has been possible to identify proximately measured predictors of sustained worsened and improved episodes of pain. Based on the aforementioned research findings, the following primary hypotheses were evaluated: Higher depressive symptom levels, fair or poor self-rated health (SRH), and female gender predict subsequent worsened pain that is sustained for at least 2 months. Conversely, male gender, few depressive symptoms, and positive SRH predict sustained pain improvement during a comparable 2-month time frame.
Due to available monthly assessments of depressive symptoms, a secondary hypothesis regarding the independent predictive effect of pain level on subsequent improvement or worsening of depressive symptoms was also evaluated. This article presents and discusses the results of the analyses to test these hypotheses.
Methods
Study Subjects and Recruitment
The Philadelphia-based Healthy Aging Study (NIA Grant R01AG15730) was designed to investigate factors that contributed to global SRH held by older, community dwelling individuals. To ensure adequate sex and race representation of persons with the distinct SRH ratings of “excellent,” “good,” “fair,” and “poor/bad,” to the single question: “How do you rate your health today?”, a stratified quota sample was assembled. Individuals aged 65–74, who represent “young elderly,” were targeted to increase the likelihood of a wide range across the health/illness continuum in the recruited sample. The Health Care Financing Administration ([HCFA] currently the Center for Medicare and Medicaid Services) approved the use of its May 1999 Names and Address file of age eligible individuals who resided in Philadelphia County as the study sampling frame, provided that a “passive recruitment” strategy was used. The HCFA Names and Address File was first divided into four subfiles defined by race (African American/Caucasian) and gender, and individuals in each subfile were randomly assigned a sequence number. During the first recruitment phase, letters on HCFA letterhead were sent to inform individuals that the Healthy Aging Study (HAS) had permission to use the Names and Address list for recruitment purposes and that they could avoid further recruitment by calling the HAS study office. To facilitate data management, 1000 HCFA letters were mailed monthly to 250 individuals, selected according to their sequence number, from each of the four strata. During the second recruitment phase, individuals not asking to be excluded from recruitment were mailed the HAS letter that described the study. Individuals interested in participating were asked to contact the HAS office and provide their telephone number. All such individuals were personally contacted by telephone to discuss study eligibility and enrollment possibilities.
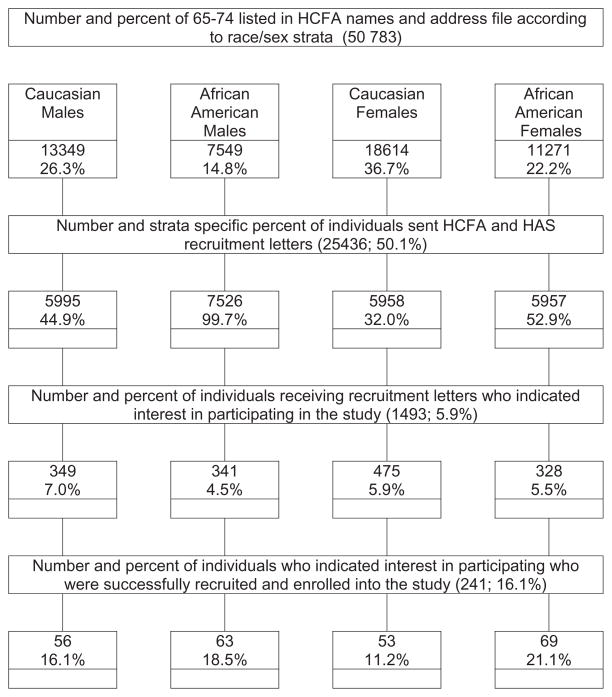
During the recruitment, 25,500 HCFA letters were mailed. Figure 1 shows the yield from the different recruitment and enrollment stages. The unequal initial race/sex strata recruitment probabilities reflected differences in the rate of expressed interest in the study and the speed with which the 16 study cells (four race/sex and four SRH ratings) were populated. Of the “interested” 1433 individuals, 21% were found to be ineligible, primarily due to cognitive impairment as indicated by a score of 8 or more on the widely used and validated Orientation Memory Concentration Test [28], a 6-item version of the Blessed-Roth Mental Status Test [29]. Another 56% were not enrolled because their referent stratum was already populated. Of the 328 study eligible individuals who were invited to enroll, 26.5% refused. Within this group, Black Males were over-represented (31.5% refusal) and White Males were under-represented (18.8% refusal).
Figure 1.
Flow chart of study recruitment and enrollment. HCFA = Health Care Financing Administration; HAS = Healthy Aging Study.
The 241 individuals who comprised the final study sample signed consent forms that had been approved by the Medical College of Pennsylvania (now Drexel University) Institutional Review Board. Subsequent to a telephone screening interview and an in-person baseline interview, follow-up telephone interviews were conducted at monthly intervals for 11 months. At their start, the interviewer stated: “I will be asking you questions about your health … since your last interview.” Eighty-six percent of interviews were conducted within 31 ± 7 days of the previous interview, with an additional 7% of interviews conducted within 31 ± 11 days. Variables measured included the baseline measures for pain, depressive symptoms, SRH, physical function, and health care utilization. As well, questions to assess such variables as change in medical conditions and presence of selected symptoms were asked.
One hundred and sixty-eight individuals (69.7%) had complete data for all follow-up interviews, and an additional 48 (19.9%) had complete data for 9 or 10 of the follow-up interviews. In analyses comparing the 73 noncompleters to those with complete data, the only statistically significant difference observed was for age (70.8 [SD 3.2] vs 71.8 [SD 3.1]). No differences were seen for race, gender, living alone, marital status, depressive symptom scores, presence of pain, self-rated health, or physical functioning at baseline.
Primary Study Variables
Pain
At baseline and each monthly interview, subjects were asked three items from the 6-item Likert scale to assess pain in the elderly adapted from the McGill Pain Questionnaire by Parmelee et al. [30]. This scale has a reported standardized α of 0.91 for the composite scale [30] and a test–retest reliability of 0.84 [31]. Two of the questions asked during the baseline interview were: 1) How much bodily pain have you had in the past few weeks? (0 = none, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe, or 5 = very severe) and 2) How much are you bothered by the pain when it is at its worst? (1 = not at all, 2 = a little bit, 3 = moderately, 4 = quite a bit, or 5 = extremely). At each monthly interview, the reference time frame for question 1 was changed from “in the past few weeks” to “in the past week”; question 2 was repeated verbatim. These two baseline and follow-up questions were used to construct, for each assessment period, a composite pain measure that reflected both pain severity and intensity. The five categories were: 1 = no pain, 2 = pain with little bother, 3 = pain with moderate bother, 4 = very mild or mild pain with quite a bit or extreme bother, and 5 = moderate to very severe pain with quite a bit or extreme bother.
Sustained Pain Improvement and Worsening
Responses to the above composite pain measure for each month were used to determine the presence of sustained improved pain and worsened pain. For both improved and worsened pain, sustained change was defined as a change that occurred and lasted for at least 2 months. To maximize the opportunity to observe episodes of sustained improvement or worsening, the longitudinal experience of each individual was divided into 10 consecutive, overlapping 3-month segments beginning with the baseline interview month. Sustained worsening was observed when the pain score of the first month had a lower value than scores for both the second and third months in the segment. Conversely, sustained improvement was defined as a higher pain score in the first month compared to both the second and third months. A new variable for each 3-month segment was created with the following categories: 1 = the same pain level for all 3-months, 2 = sustained improved pain, 3 = sustained worsened pain, and 4 = fluctuating pain levels not meeting the definition of sustained improved or worsened pain.
Depressive Symptoms
Depressive symptom levels at baseline were assessed with the 30-item, dichotomously coded Geriatric Depression Scale (GDS). The shorter 15-item GDS was administered at each monthly interview. The respective scale scores represent the sum of depressive responses. High correlations between the 15- and 30-item versions of the GDS have been observed [32,33]. For these analyses, the baseline GDS scores were recalculated based only on responses to the questions included in the 15-item version. The long and short versions of the GDS have been used extensively to assess depressive symptoms in older populations [34]. While scores above 11 on the 30-item scale and 5 on the 15-item scale are considered to indicate a high risk for major depression [35], the GDS is not appropriate as an indicator of major depression. Despite this, there is extensive evidence demonstrating independent, positive associations between depressive symptom levels, as indicated by the GDS or another depressive symptom scale (e.g., the Center for Epidemiologic Depression Scale [36] or the Beck Depression Inventory [37]), and poor health outcomes such as mortality, poor physical function, incomplete recovery from an illness or accident, and high use of health services. Following the recommendations of Judd and Akiskal [38,39] that depressive symptom levels are best characterized as a continuum that over time, may range from no symptoms through subsyndromal, or subthreshold symptom levels to syndromal, major depression, depressive symptom level was treated, here, as a continuous variable.
Sustained Improvement or Worsening in Depressive Symptom Level
Consistent with the approach used to define sustained improved or worsened pain, sustained change in depressive level was determined by comparing the depression symptom level reported at the first of the 3 months in each 3-month segment with the depressive symptom level reported in the last two segment months. Sustained, worsened depressive symptoms were observed when the depressive symptom level in the first segment month was lower than the levels reported in the subsequent 2 months. Conversely, sustained improved depressive symptoms were characterized by a decrease in depressive symptom level during the second and third segment months compared to the symptom level reported in the first month of the 3-month segment.
Physical Functioning
Physical functioning was assessed at each interview using an adaptation of the self-report scale developed by Nagi [40]. Subjects were asked to rate their ability to perform eight distinct activities including standing, sitting, kneeling, and lifting. Response options ranged from 0 = no difficulty to 3 = unable to do that activity. Responses were summed across all activities to obtain a scale score ranging from 0 = no difficulty with any activity to 24 = unable to do any activity.
SRH
To assess their health, subjects were asked the global SRH question previously indicated. Those responding “excellent” or “good” were further asked: “If you could have chosen between excellent, very good or good, which one would have best represented how you rate your health today?” For these analyses, SRH was coded as excellent, very good, good, fair, and poor/bad. Extensive research on this measure [41,42] has shown it to be correlated with objective measures of health (e.g., number of medical conditions) and other subjective measures (e.g., pain experience and depressive symptom level).
Number of Health Conditions
During the baseline interview, subjects were asked to report: “yes” or “no” to whether they had had or currently had any of 10 medical condition categories: high blood pressure, diabetes, cancer, stroke, heart attack, any other heart trouble, neurological diseases, stomach ulcer, emphysema or chronic lung disease, or problems with circulation in the arms or legs. These condition categories were selected because they are among the 10 leading causes of death for individuals 65–74 years of age [43] and they were included in the National Health Interview Schedule adult questionnaire, or they were included in the Multi-level Assessment Instrument, a multidimensional instrument to assess the health and physical functioning of older individuals [44,45]. A variable reflecting the number of conditions endorsed was computed.
Improved or Worsened Health Conditions
At each of the monthly interviews, subjects were asked: 1) “Were you told you have a new medical problem since we talked with you [date of last interview]?” 2) “Have any of your medical problems gotten worse since [date of last interview],” and 3) “Do you think any of your medical problems have gotten better since we talked with you [date of last interview]?” Data from these questions were combined to form two separate dichotomous variables: medical conditions improved and medical conditions worsened, with codes 0 = no and 1 = yes.
Analysis Methods
As noted previously, episodes of improved and worsened pain and improved or worsened depressive symptoms were operationally defined in terms of sustained change based on the value of the respective variable at the first interview of a 3-month segment compared with the values reported for the last two segment months. To ensure that all episodes of sustained change were documented, the 12-study months were divided into overlapping 3-month segments. This yielded, for each of the 241 study participants, 10 3-month segments. For analysis purposes, a new data file was constructed that contained one data record for each complete 3-month data segment. This yielded 2,410 3-month segment records. In addition to the variables indicating improved or worsened pain or depressive symptoms, each 3-month segment record contained the monthly composite pain variables, depressive symptoms, physical functioning, self rated health, and improved or worsened medical condition variables. Table 1 includes the variable name, definition, and the interview during which the variables included in these analyses were measured.
Table 1.
Study variable names, definition, and interview measured (baseline or month of 3-month segment)
| Variable Name | Definition | Baseline | M1 | M2 | M3 |
|---|---|---|---|---|---|
| M1 pain score | Composite severity/intensity Pain score |
X | |||
| Improved pain | Improved pain observed M2 M1 pain score > M2 and M3 scores |
X | X | X | |
| Worsened pain | Worsened pain observed M2 M1 pain score < M2 and M3 score |
X | X | X | |
| M1 depressive symptoms (M1 GDS score) | 15-item GDS score | X | |||
| Improved depressive symptoms (GDS score) | Improved GDS observed M2 M1 GDS score > M2 and M3 scores |
X | X | X | |
| Worsened depressive symptoms (GDS score) | Worsened GDS observed M2 M1 GDS score < M2 and M3 scores |
X | X | X | |
| Self-rated health | Global self-rated health question | X | |||
| Sociodemographic variables | Age, race, gender, marital status, living arrangement | X | |||
| Baseline medical conditions | Number of 10 medical conditions endorsed | X | |||
| Improved medical conditions | 1 + medical conditions improved in past month | X | |||
| Worsened medical conditions | 1 + medical conditions worsened in past month | X |
GDS = Geriatric Depression Scale.
After eliminating 259 records where the 3-month segment variables were incomplete, 2151 3-month segments comprised the analysis sample. Statistically significant (P < 0.05) differences at baseline between the 2,151 usable segments and the 259 excluded segments included age (71.5 ± 3.2 SD vs 71.0 ± 3.2 SD), marital status (47.1 vs 54.5% currently married), and race (44.6 vs 50.5% white). No statistically significant differences were seen for gender, living alone, or physical functioning at baseline. Additionally, no statistically significant differences were seen for M1 pain scores, M1 depressive symptom level, or SRH.
Person-specific as well as segment-specific descriptive statistics were obtained for baseline health and demographic characteristics. Univariate analyses were conducted to determine if SRH, depressive symptoms, and gender were associated with improved or worsened pain and to identify potential confounders of the observed associations. Three-month segments characterized by improved pain (N = 306) were compared with all others (N = 1,320) after excluding those where the M1 pain score of “0” (no pain) precluded improvement. Likewise, the 234 segments during which worsened pain was observed, were compared to all other segments for which such pain change was actually a possibility (N = 1703). Similar methods were used to determine univariate associations for improved and worsened depressive symptoms.
Pearson chi-square tests and student’s t-tests were used to compare demographic characteristics or health variables of the improved or worsened pain groups. Variables for which there was a statistically significant association (P < 0.1) with a sustained change in pain were considered eligible to be included in respective multivariate models for improved and worsened pain. Comparable analyses were conducted to identify candidate variables for multivariate analyses to model improved and worsened depressive symptoms. These preliminary analyses were conducted using SPSS version 15 (SPSS Inc., Chicago, IL).
Although the improved and worsened pain and depression variables were dichotomous, the use of logistic regression methods for the multivariate hypothesis testing analyses was precluded due to the presence of up to 10 records for each individual. Instead, General Estimating Equations (GEE) methods were used. GEE is an appropriate analytic choice in situations where, as here, the observations are not independent of each other [46]. The SAS GENMOD program has a GEE option that adjusts for the correlations between clustered observations (Statistical Analysis Software version 9, SAS Institute, Cary, NC). For these analyses, the individual represented the “cluster” variable. To adjust for potential “regression to the mean” effects, the M1 pain score was entered first into the equation, backward elimination procedures were used to evaluate the relative importance of the remaining variables and an α level = 0.05 determined inclusion of variables in the final models. The presence of interactions with race and sex were evaluated. Comparable analyses were undertaken to evaluate the hypotheses related to improved and worsened depressive symptom levels.
Results
The characteristics of the sample are displayed in Table 2. All participants were between the ages of 65 and 74 with a mean (SD) of 71.5 (3.2) years. Reflecting the quota recruitment strategies, there were nearly equal numbers of men (49%) and women (51%) and slightly more African Americans (55%) than Caucasians (45%). At baseline, 26% of all individuals reported no pain. Of those with pain, 52% reported associated activity limitations and 54% were extremely bothered by the pain. A considerable minority (16%) of the sample had elevated (≥5) GDS scores at baseline. The mean (SD) GDS for those with elevated scores (GDS ≥ 5) was 13.8 (5.6) compared with 3.5 (4.0) for those with low scores. Thirty-five percent of individuals reported excellent or very good health at baseline, and 14% reported poor or bad health. A majority lived with others and nearly half were married.
Table 2.
Baseline health and sociodemographic characteristics of the 241 individuals who contributed 3-month segments to the analysis sample
| Variable | Mean (SD) |
|---|---|
| Age | 71.5 (3.2) |
| Blessed exam | 3.2 (2.6) |
| Medical conditions | 2.1 (1.6) |
| Physical function | 4.2 (4.3) |
| Variable | % |
|---|---|
| Gender | |
| Male | 49.4 |
| Female | 50.6 |
| Race | |
| White | 45.2 |
| Black | 4.8 |
| Pain and limitations | |
| No pain* | 26.2 |
| Pain, no limitations | 36.3 |
| Pain, limitations | 37.6 |
| Pain score | |
| No pain* | 26.5 |
| Pain, little bother | 14.5 |
| Pain, moderate bother | 19.7 |
| Mild pain, extreme bother | 23.5 |
| Moderate—severe pain, extreme bother | 15.8 |
| GDS score | |
| <5 | 84.1 |
| ≥5 | 15.9 |
| Self-rated health | |
| Excellent | 10.5 |
| Very good | 24.7 |
| Good | 18.4 |
| Fair | 32.6 |
| Poor/bad | 13.8 |
| Lived alone | |
| Yes | 36.3 |
| No | 63.8 |
| Marital status | |
| Never married | 5.0 |
| Married | 47.9 |
| Widowed | 30.4 |
| Separated/divorced | 16.7 |
Percentages vary for no pain groups due to small numbers of missing data.
GDS = Geriatric Depression Scale.
Improved and Worsened Pain
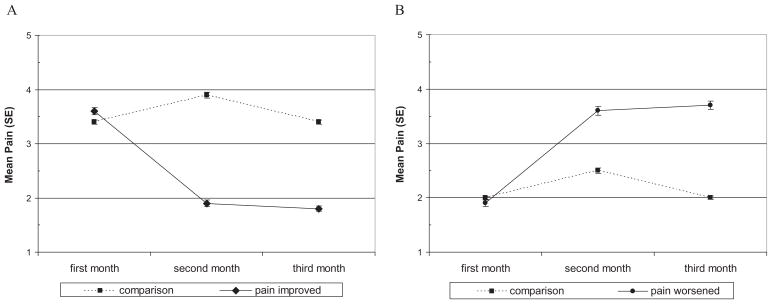
Table 3 shows the distribution of the pain experienced during the 3-month segments. As seen here, over the 11-month study, 306 episodes of sustained improved pain (14.2% of all 3-month segments) were observed. These episodes were experienced by 170 of the sample members. In contrast, 234 episodes of sustained worsened pain (10.9% of the segments) were experienced by 147 individuals. Consistent with other studies [4,8–10], stable pain levels were most frequently observed with 20.1, 17.9, and 20.7% of the segments characterized, respectively, as “no pain,” “persistent low pain,” and “persistent high pain.” The mean pain scores for each month for the improved and worsened pain groups and the respective comparison groups are displayed in Figure 2A,B. As per definition, the changes in pain for those experiencing an episode of improved or worsened pain persist. As evident, respective differences between the first segment month and the subsequent two segment months for improved and worsened pain groups are statistically significant (P < 0.000) and clinically important. As a group, ratings for improved pain segments decreased from just above “pain with moderate bother” to a level lower than “pain with little bother,” and reflect the change from an elevated M1 pain score to a report of “no pain” for 44.5% of the improved segments. Change of a comparable magnitude was observed for worsened pain with the mean M1 pain score, “pain with little bother” increasing to very close to a rating of “very mild or mild pain with quite a bit or extreme bother.” The majority of episodes of worsened pain (56.8%) did not represent an episode of incident pain but rather a worsening of already existing pain.
Table 3.
Pain and depressive symptom experience of the 3-month segments included in these analyses
| Pain Classification | N | % |
|---|---|---|
| Stable pain | ||
| No pain | 433 | 20.1 |
| Persistent low | 386 | 17.9 |
| Persistent high | 445 | 20.8 |
| Improved pain | 306 | 14.2 |
| Worsened pain | 234 | 10.9 |
| Fluctuating | 347 | 16.1 |
| Total | 2151 | 100.0 |
| GDS score classification | ||
| All equal GDS | ||
| Scores = 0 | 791 | 36.6 |
| Scores ≥1 | 18 | 0.8 |
| Improved GDS | 378 | 17.5 |
| Worsened GDS | 300 | 13.9 |
| Fluctuating GDS | 674 | 31.2 |
| Total | 2161* | 100.0 |
Differences in the number of 3-month segments with valid GDS scores and those with valid pain scores reflect missing pain data.
GDS = Geriatric Depression Scale.
Figure 2.
Mean (SE) pain intensity during three-month segments. (A) Improved pain group vs comparison group; (B) worsened pain group vs comparison group. A pain intensity of 1 represents no pain and 5 represents moderate to severe pain with extreme bother.
Statistically significant associations, in the hypothesized direction, were observed between the occurrence of worsened pain and gender, M1 depressive symptom level, and SRH. Statistically significant predictors of worsened pain also included number of baseline medical conditions, physical functioning, and worsened medical conditions. As hypothesized, M1 depressive symptom level and SRH, but not gender, had statistically significant associations with improved pain. Additional variables that had statistically significant associations with improved pain included improved medical conditions and M1 pain scores. Notably, statistically significant associations between race and either the occurrence of improved or worsened pain were not observed.
In analyses not shown here, statistically significant associations were also seen between all of the following variables: gender, M1 depressive symptom level and SRH, physical function, number of baseline medical conditions, and improved and worsened medical conditions.
Based on the results of the univariate analyses, separate multivariate equations were generated to evaluate the hypothesized associations between gender, M1 depressive symptoms, and SRH with episodes of improved and worsened pain. In addition to the M1 pain score, physical function, number of baseline medical conditions, and worsened and improved medical conditions variables were included in the respective equations. The statistically significant variables retained in the final models for improved and worsened pain are shown in Table 4. Controlling for the effects of the M1 pain score, two hypothesized variables, M1 depressive symptoms and SRH, were retained in the final equations for both dependent variables. A statistically significant main effect for gender was observed only for worsened pain. Worsened medical condition was the only other variable in the final equations for both improved and worsened pain.
Table 4.
Sociodemographic and health characteristics of 3-month segments classified as sustained improved pain and worsened pain
| Predictor Variables | Improved Pain
|
Worsened Pain
|
||
|---|---|---|---|---|
| Yes (N = 306) | No (N = 1320) | Yes (N = 234) | No (N = 1703) | |
| Gender—female (%) | 53.1 | 54.8 | 55.8 | 46.8*** |
| Race—white (%) | 43.8 | 46.3 | 45.7 | 44.4 |
| Self-rated health—excellent or very good (%) | 29.5 | 21.5*** | 26.1 | 38.9*** |
| M1 pain score | ||||
| Little-moderate bother (%) | 47.7 | 60.9*** | 51.7 | 48.1 |
| Worsened conditions | ||||
| One or more (%) | 11.1 | 19.0*** | 19.7 | 8.9*** |
| Improved conditions | ||||
| One or more (%) | 24.5 | 19.7 | 21.4 | 21.5 |
| Baseline medical conditions (range 0–10) | 2.2 | 2.4* | 2.3 | 2.0*** |
| M1 GDS score (mean) range (0–15) | 1.9 | 2.8*** | 2.1 | 1.3*** |
| Physical function (mean) (range 0–24) | 3.6 | 5.4*** | 4.6 | 2.5*** |
P ≤ 0.5;
P ≤ 0.01;
P ≤ 0.001.
GDS = Geriatric Depression Scale.
As seen in Table 5, compared to males, females were 1.63 (1.11, 2.38) times more likely to report an episode of worsened pain. For each 1 point increase in M1 GDS scores, the odds of worsened pain increased by a factor of 1.05 (1.01, 1.10). The odds of worsened pain increased as SRH ratings became less positive. Those reporting their health to be fair, poor, or bad were 4.04 (2.12, 7.70) times more likely than those with excellent SRH to experience an episode of worsened pain. Worsened medical conditions also increased the odds of worsened pain.
Table 5.
AORs (95% CI) for predictors of a sustained improvement or a sustained worsening of pain in a 3-month segment
| Predictor Variables | Improved Pain
|
Worsened Pain
|
||||
|---|---|---|---|---|---|---|
| AOR | 95% CI
|
AOR | 95% CI
|
|||
| L | U | L | U | |||
| M1 pain score | ||||||
| 1 point change | 2.41 | 1.91 | 3.05 | 0.59 | 0.49 | 0.71 |
| Gender | ||||||
| Males (Ref) | ||||||
| Females | 2.24 | 0.79 | 6.37* | 1.63 | 1.11 | 2.38 |
| M1 GDS score | ||||||
| 1 point increase | 0.85 | 0.79 | 0.91 | 1.05 | 1.01 | 1.10 |
| Self-rated health | ||||||
| Excellent (Ref) | ||||||
| Very good | 0.59 | 0.36 | 0.98 | 1.89 | 1.01 | 3.52 |
| Good | 0.51 | 0.31 | 0.86 | 2.07 | 1.10 | 3.89 |
| Fair/poor/bad | 0.41 | 0.23 | 0.73 | 4.04 | 2.12 | 7.70 |
| Worsened conditions | ||||||
| None (Ref) | ||||||
| One+ | 0.61 | 0.47 | 0.81 | 2.12 | 1.42 | 3.16 |
| M1 pain score × Female gender | 0.68 | 0.52 | 0.91 | — | ||
AOR with 95% confidence intervals that include 1.0 are not statistically significant. Gender was retained in the model for Improved Pain due to the presence of the interaction term, M1 pain score × female gender.
GDS = Geriatric Depression Scale; AOR = adjusted odds ratios; OR = odds ratios.
All ORs are adjusted for all other variables in the model. Unless noted, the final models were limited to variables with statistically significant AORs.
With the exception of gender, the predictors of an episode of improved pain were similar, but the associations were in the opposite directions. Each 1-point increase in the M1 GDS score reduced the odds of improved pain by a factor of 0.85 (0.79, 0.91). Reporting a poorer SRH score decreased the likelihood of an episode of improved pain. Compared to those who reported “excellent” SRH, the odds of improved pain for those with a fair-poor rating was 0.41 (0.23–0.73). Likewise, reporting worsened medical conditions decreased the odds of improved pain by a factor of 0.67 (0.51, 0.88). The presence of a statistically significant interaction between gender and M1 pain score indicates that compared to men, the increased likelihood of improved pain associated with a higher M1 pain score was diminished among women. It is not clear why this is the case.
Sustained Improved and Worsened Depressive Symptom Level (GDS Scores)
The distribution of depressive symptom levels over the 3-month segments shown in Table 3 reveals a pattern similar to that observed for pain scores over the same time interval. GDS scores remained at “0” during the largest proportion of the segments (46.3%). GDS scores improved more frequently than worsened (23.3 vs 18.4%). For the 182 and 149 individuals who experienced, improved and worsened GDS scores, respectively, the average difference between the M1 GDS score and the mean (SD) of the second and third month scores was 1.9 (1.3) for both groups. The range of GDS score changes were 1–8.5 for the improved GDS group, and 1–9.5 for the worsened GDS group. Of clinical relevance, for 17% of the segments characterized by worsened depressive symptoms, GDS scores increased over time from < 5 at month 1 to ≥5. In contrast, of the 3-month segments where the month 1 GDS score was ≥5, 48.1% improved to a GDS score <5.
Table 6 displays the results of univariate analyses to evaluate the associations between sociodemographic and health variables, and improved and worsened depressive symptoms. As hypothesized, statistically significant univariate associations were observed between the M1 pain score and improved and worsened depressive symptoms. Comparable associations were evident for SRH. A statistically significant association with gender was observed only for worsened depressive symptoms. Race was unrelated to either dependent variable.
Table 6.
Health and sociodemographic characteristics of 3-month segments classified according to sustained improvement in depressive symptom (GDS) score and sustained worsening in depressive symptom
| Predictor Variables | Depressive Symptoms Sustained Improvement |
Depressive Symptoms Sustained Worsening |
||
|---|---|---|---|---|
| Yes (N = 378) | No (N = 1737) | Yes (N = 300) | No (N = 1861) | |
| Gender—female (%) | 54.5 | 51.6 | 59.3 | 49.6*** |
| Race—White (%) | 46.3 | 44.9 | 45.6 | 42.3 |
| Self-rated health—excellent or very good (%) | 16.0 | 12.5** | 12.7 | 33.7*** |
| M1 pain score mean (SD) | 3.2 (1.5) | 2.9 (1.5)* | 3.0 (1.5) | 2.5 (1.5)*** |
| Worsened conditions | ||||
| One or more (%) | 16.7 | 19.6 | 23.7 | 11.9*** |
| Improved conditions | ||||
| One or more (%) | 22.2 | 20.4 | 16.0 | 21.2* |
| Baseline medical conditions mean (SD) (range 0–10) | 2.6 (1.6) | 2.5 (1.6) | 2.7 (1.6) | 2.1 (1.6)*** |
| M1 GDS score mean (SD) | 4.3 (3.2) | 2.9 (2.6)*** | 2.3 (2.6) | 1.9 (2.7)* |
| Physical Function mean (SD) (range 0–24) | 5.6 (4.9) | 5.4 (4.6) | 5.4 (4.6) | 3.6 (4.3)*** |
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001.
GDS = Geriatric Depression Scale.
Table 7, shows the results of the GEE analyses used to evaluate the hypothesis that M1 pain was an independent predictor of improved or worsened depressive symptoms. Variables included in Model 1 were limited to the M1 GDS and pain scores. As seen here, after controlling for depressive symptoms, the association between M1 pain and improved depressive symptoms was not statistically significant. While the adjusted association between M1 pain and worsened depressive symptom scores remained statistically significant (Model 1), when SRH was included in the equation (Model 2), the association between pain and worsened depressive symptom disappeared. Addition of other independent variables into the regression equation did not alter these findings.
Table 7.
AORs (95% CI) for predictors of a sustained improvement or a sustained worsening (at least 2 months duration) in GDS scores in a 3-month segment
| Predictor Variables | Model 1
|
Model 2
|
||||
|---|---|---|---|---|---|---|
| AOR | 95% CI
|
AOR | 95% CI
|
|||
| L | U | L | U | |||
| Improved GDS | ||||||
| M1 pain score | — | — | ||||
| 1 point change | ||||||
| M1 GDS score | ||||||
| 1 point increase | 1.22 | 1.14 | 1.30 | 1.35 | 1.24 | 1.46 |
| Worsened conditions | ||||||
| None (Ref) | — | — | — | |||
| One+ | — | — | — | 0.57 | 0.40 | 0.81 |
| Self-rated health | ||||||
| Excellent (Ref) | ||||||
| Very good | — | — | — | 0.56 | 0.27 | 1.18* |
| Good | — | — | — | 0.39 | 0.20 | 0.77 |
| Fair/poor/bad | — | — | — | 0.37 | 0.18 | 0.74 |
| Worsened GDS | ||||||
| M1 pain score | 1.25 | 1.12 | 1.39 | — | ||
| 1 point change | ||||||
| M1 GDS score | ||||||
| 1 point increase | 0.86 | 0.79 | 0.93 | 0.80 | 0.73 | 0.87 |
| Worsened conditions | ||||||
| None (Ref) | ||||||
| One+ | — | — | — | 1.86 | 1.26 | 2.74 |
| Self-rated health | ||||||
| Excellent (Ref) | ||||||
| Very good | — | — | — | 5.51 | 1.72 | 17.72 |
| Good | — | — | — | 9.68 | 3.11 | 30.11 |
| Fair/poor/bad | — | — | — | 22.69 | 7.17 | 71.84 |
AOR with 95% confidence intervals that include 1.0 are not statistically significant.
GDS = Geriatric Depression Scale; AOR = adjusted odds ratios; OR = odds ratios.
Model 1 includes pain and GDS score only. Model 2 includes all variables listed. All ORs are adjusted for all other variables. Unless noted, the final models were limited to variables with statistically significant AORs.
Discussion
This study was unique in that it permitted investigation of predictors of incident episodes of sustained improved and worsened pain in older community dwelling individuals that occurred over months rather than years and where the predictor variable measurement was proximate to the episode onset. A high degree of stability in the pain experience reported by study participants was observed and accounted for 58.7% of the 3-month segments included in the analyses. When an episode of sustained change occurred, improved pain was observed more often than worsened pain. As hypothesized, SRH and M1 depressive symptoms independently increased the risk of observing an increase or decrease in pain. The hypothesized association between gender and improved and worsened pain was only partially supported with females more likely to experience an episode of sustained worsened pain, but no more likely than males to experience an episode of sustained improved pain. Other factors related to improvement or worsening of pain pertained more directly to the individual’s physical health state and reflected, as expected, initial pain level and worsening or improvement in medical conditions.
While directly comparable studies of short-term pain experiences have not been identified, our findings are generally consistent with those from other longitudinal studies that included longer time intervals. The absence of associations between improved or worsened pain and race has been observed in three large longitudinal studies of racially diverse groups of community dwelling individuals [8,25,26]. The observed importance of the more medically specific factors of worsened medical conditions and initial pain level as predictors is also comparable to findings reported by other investigators [11,25,47].
The increased prevalence and severity of many types of pain have consistently been shown to be higher among women than men [4,14–16,48]. Coping strategies have also been shown to differ between men and women [15]. These differences may explain our finding that women were more likely to experience a sustained worsened pain.
Depressive symptoms in the present study preceded and predicted changes in pain. Depression has been shown to predict pain occurrence and chronicity, regardless of the anatomical sight of pain [6,9,10,49]. For example, in their 48-month longitudinal study, Elliot et al. [9] observed membership in the lowest quartiles of the mental health and global health SF36 domain scores increased the risk of new episodes of pain, while individuals in the highest quartiles of these measures were more likely to recover from pain. In a 12-month study of individuals over age 74, Donald and Foy [6] observed that reporting sadness increased the odds of incident pain by a factor of 1.37. These associations may result from one or more of several purported mechanisms of the relationship of chronic pain and depression: shared pathophysiological mechanisms in the central nervous system; the effects of depression on pain threshold or tolerance; or lowered perceived control and self-efficacy among those with depression. As reviewed by Delgado [19], the serotonergic and noradrenergic neurotransmitters are involved in the pathophysiology of both chronic pain and elevated depressive symptoms. Changes caused by the onset of one symptom set could impact the onset or worsening of the other.
There has been much discussion in the literature regarding the directionality of associations between chronic pain and depressive symptoms and disorders. Data from several longitudinal studies of community dwelling populations support the presence of a reciprocal relationship between these conditions [10,11]. Findings from this study do not support a reciprocal relationship. While the M1 pain score level was related to a sustained change in depressive symptoms in the univariate analyses, the statistically significant associations between M1 pain level and improved or worsened depressive symptoms disappeared when SRH and/or M1 GDS scores were included in the analyses. The absence of predictive relationships between pain and changes in depressive symptoms in this study may reflect the designation of 3-month segments as the units of analyses. This short time period may be of insufficient length for the development or resolution of depressive symptoms. Studies by Atkinson et al. [50] and Dohrenwend et al. [47] suggest that chronicity, not onset, of pain predicts onset of major depression, although depression does worsen pain severity reports. This study supports the finding that depressive symptoms increase pain symptom severity and intensity but do not cause the onset of pain itself [51].
The observed prognostic significance of excellent and very good SRH for improved pain and of fair and poor health ratings for worsened pain is comparable to that observed by Blyth et al. in a cross-sectional study of a randomly selected sample of Australian adults [52], and Ericksen et al. [53] in a 6-year longitudinal study of the adult Danish population. It is unclear what aspects of SRH were contributing to these associations. Reflecting the consistently observed predictive importance of SRH for mortality, recovery, and illness onset, such ratings are thought to measure a complex of health-related attributes [41,54].
When considering the findings reported here, several aspects of the study require particular attention. Although the HCFA 1999 Names and Address file served as the study sampling frame and recruitment letters were mailed according to randomly determined sequence numbers, the required passive recruitment strategy adversely affected the representativeness of the assembled sample because no enrollment steps could be taken until the older community resident had agreed in writing or by telephone. The overall 6% response rate provides some indication of the potential for selection bias in the sample. Although this response rate is consistent with what is known about similarly derived samples [55,56], a somewhat healthier group of Philadelphia County elderly expressed interest in the study; however, the need to fill the fair and poor/bad SRH strata resulted in the wide range of health states that was observed in the assembled sample. The restriction of the study sample to individuals 65–74 years of age, limits the relevance of the study findings to all but the “young old.”
While pain assessments in this study are unlikely to suffer from greater measurement error than in other studies, the exclusive use of self-report for data collection brings with it the potential for reporting bias in all measures. The presence of such biases in terms of the pain assessments may be diminished because in the HAS, pain was not the stated topic of investigation. Subjects had no reason to consciously or unconsciously modify their pain assessments to meet the perceived expectations or subtle pressures of the study team.
The designation of 3-month segments as the unit of analyses also warrants comment. This approach was taken to identify all possible discrete episodes of change in pain or depressive symptoms where the change persisted for at least 2 months, and to ensure that the measurement of the potential predictor variables proceeded, but was as proximate as possible to the observed change in pain level. Although 3-month segments represent the experiences of the 241 study subjects, the study findings cannot directly be attributed to individuals. Moreover, because data from a given month are included in more than one 3-month segment, the observations are not entirely independent of each other; however, use of GEE analyses accounts for this dependence.
Conclusions
Several important conclusions are warranted by the study findings. First, the predictive significance of depressive symptoms and SRH for self-reported pain improvement or worsening appears more robust than previously known, in that the statistically significant associations observed here, with only a 1-month time lag between assessment of predictor variables and follow-up pain levels, are almost identical to those observed in other studies despite time lags of 5–72 months. Second, although causality can not be proved by these results, the short time lag between assessment of the predictor variables and the occurrence of a change in pain level provides support for a causal hypothesis that changes in mood cause a worsening of pain symptoms, but not the pain condition itself. Thus, clinicians should consider whether elevated depressive symptoms, even those not meeting DSM-IV® criteria for major depression, are contributing to a worsening of pain symptoms in patients with the chronically painful conditions common in older adults (e.g., arthritis, diabetic neuropathy). A clinical decision would follow to treat depression, rather than the usual response, which may include increasing analgesics and co-analgesics with their potential medical complications [51,57]. Third, while routine screening for depressive symptoms has been recommended to clinicians providing primary medical care to community dwelling older individuals, and this recommendation is further supported by our findings, the routine assessment of their patient’s SRH has not been recommended. Although there is no clear consensus regarding what influences an individual’s SRH, a single question appears—all that is required to obtain information that has substantial prognostic significance, potentially serving as a “red flag” to identify those whose pain might be anticipated to worsen in future months [41,42,54]. The findings from this study support a recommendation to assess SRH at all patient visits whether for primary or specialty care. Last, while it was possible to use uniquely rich data collected for another purpose for the secondary data analyses reported here, epidemiological studies designed to investigate further the risk factors, course and consequences of pain in older community dwelling elders continue to be an urgent need. Findings from such studies would benefit both the older individual and the clinicians who provide them with care and guidance.
References
- 1.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (norstOP) Pain. 2004;110(1–2):361–8. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Onder G, Landi F, Gambassi G, et al. Association between pain and depression among older adults in Europe: Results from the aged in home care (AdHOC) project: A cross-sectional study. J Clin Psychiatry. 2005;66(8):982–8. doi: 10.4088/jcp.v66n0804. [DOI] [PubMed] [Google Scholar]
- 3.Von Korff M, Crane P, Lane M, et al. Chronic spinal pain and physical-mental comorbidity in the United States: Results from the national comorbidity survey replication. Pain. 2005;113(3):331–9. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Mossey JM, Gallagher RM. The longitudinal occurrence and impact of comorbid chronic pain and chronic depression over two years in continuing care retirement community residents. Pain Med. 2004;5(4):335–48. doi: 10.1111/j.1526-4637.2004.04041.x. [DOI] [PubMed] [Google Scholar]
- 5.Scudds RJ, Ostbye T. Pain and pain-related interference with function in older Canadians: The Canadian study of health and aging. Disabil Rehabil. 2001;23(15):654–64. doi: 10.1080/09638280110043942. [DOI] [PubMed] [Google Scholar]
- 6.Donald IP, Foy C. A longitudinal study of joint pain in older people. Rheumatology (Oxford) 2004;43(10):1256–60. doi: 10.1093/rheumatology/keh298. [DOI] [PubMed] [Google Scholar]
- 7.Thomas E, Mottram S, Peat G, Wilkie R, Croft P. The effect of age on the onset of pain interference in a general population of older adults: Prospective findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2007;129(1–2):21–7. doi: 10.1016/j.pain.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Bryant LL, Grigsby J, Swenson C, Scarbro S, Baxter J. Chronic pain increases the risk of decreasing physical performance in older adults: The San Luis Valley Health and Aging Study. J Gerontol a Biol Sci Med Sci. 2007;62(9):989–96. doi: 10.1093/gerona/62.9.989. [DOI] [PubMed] [Google Scholar]
- 9.Elliott AM, Smith BH, Hannaford PC, Smith WC, Chambers WA. The course of chronic pain in the community: results of a 4-year follow-up study. Pain. 2002;99(1–2):299–307. doi: 10.1016/s0304-3959(02)00138-0. [DOI] [PubMed] [Google Scholar]
- 10.Geerlings SW, Twisk JW, Beekman AT, Deeg DJ, van Tilburg W. Longitudinal relationship between pain and depression in older adults: Sex, age and physical disability. Soc Psychiatry Psychiatr Epidemiol. 2002;37(1):23–30. doi: 10.1007/s127-002-8210-2. [DOI] [PubMed] [Google Scholar]
- 11.Chou KL. Reciprocal relationship between pain and depression in older adults: Evidence from the English longitudinal study of ageing. J Affect Disord. 2007;102(1–3):115–23. doi: 10.1016/j.jad.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Peters TJ, Sanders C, Dieppe P, Donovan J. Factors associated with change in pain and disability over time: A community-based prospective observational study of hip and knee osteoarthritis. Br J Gen Pract. 2005;55(512):205–11. [PMC free article] [PubMed] [Google Scholar]
- 13.Bingefors K, Isacson D. Epidemiology, co-morbidity, and impact on health-related quality of life of self-reported headache and musculoskeletal pain—a gender perspective. Eur J Pain. 2004;8(5):435–50. doi: 10.1016/j.ejpain.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: A world health organization study in primary care. JAMA. 1998;280(2):147–51. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 15.Keefe FJ, Affleck G, France CR, et al. Gender differences in pain, coping, and mood in individuals having osteoarthritic knee pain: A within-day analysis. Pain. 2004;110(3):571–7. doi: 10.1016/j.pain.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Rustoen T, Wahl AK, Hanestad BR, et al. Gender differences in chronic pain—findings from a population-based study of Norwegian adults. Pain Manag Nurs. 2004;5(3):105–17. doi: 10.1016/j.pmn.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: A literature review. Arch Intern Med. 2003;163(20):2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 18.Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: A diathesis–stress framework. Psychol Bull. 1996;119(1):95–110. [Google Scholar]
- 19.Delgado PL. Common pathways of depression and pain. J Clin Psychiatry. 2004;65(suppl 12):16–9. [PubMed] [Google Scholar]
- 20.Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277–94. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen M, Ugarte C, Fuller I, Haas G, Portenoy RK. Access to care for chronic pain: Racial and ethnic differences. J Pain. 2005;6(5):301–14. doi: 10.1016/j.jpain.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 22.McCracken LM, Matthews AK, Tang TS, Cuba SL. A comparison of blacks and whites seeking treatment for chronic pain. Clin J Pain. 2001;17(3):249–55. doi: 10.1097/00002508-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Green CR, Baker TA, Ndao-Brumblay SK. Patient attitudes regarding healthcare utilization and referral: A descriptive comparison in African- and Caucasian Americans with chronic pain. J Natl Med Assoc. 2004;96(1):31–42. [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Differences in expectations of outcome mediate African American/White patient differences in “willingness” to consider joint replacement. Arthritis Rheum. 2002;46(9):2429–35. doi: 10.1002/art.10494. [DOI] [PubMed] [Google Scholar]
- 25.Reyes-Gibby CC, Aday LA, Todd KH, Cleeland CS, Anderson KO. Pain in aging community-dwelling adults in the United States: Non-Hispanic Whites, non-Hispanic Blacks, and Hispanics. J Pain. 2007;8(1):75–84. doi: 10.1016/j.jpain.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: Differences among white, African American, and Hispanic subjects. J Pain. 2004;5(6):317–28. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Gibson SJ. IASP global year against pain in older persons: Highlighting the current status and future perspectives in geriatric pain. Expert Rev Neurother. 2007;7(6):627–35. doi: 10.1586/14737175.7.6.627. [DOI] [PubMed] [Google Scholar]
- 28.Katzman R, Brown T, Fuld P, et al. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–9. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 29.Blessed G, Tomlinson BE, Roth M. Blessed-roth dementia scale (DS) Psychopharmacol Bull. 1988;24(4):705–8. [PubMed] [Google Scholar]
- 30.Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol. 1991;46(1):P15–21. doi: 10.1093/geronj/46.1.p15. [DOI] [PubMed] [Google Scholar]
- 31.Parmelee PA, Smith B, Katz IR. Pain complaints and cognitive status among elderly institution residents. J Am Geriatr Soc. 1993;41(5):517–22. doi: 10.1111/j.1532-5415.1993.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 32.Sheikh J, Yesavage JA. Geriatric depression scale (GDS) recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–72. [Google Scholar]
- 33.Alden D, Austin C, Sturgeon R. A correlation between the geriatric depression scale long and short forms. J Gerontol. 1989;44(124):124–5. doi: 10.1093/geronj/44.4.p124. [DOI] [PubMed] [Google Scholar]
- 34.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982–1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 35.Lesher EL, Berryhill JS. Validation of the geriatric depression scale—short form among inpatients. J Clin Psychol. 1994;50(2):256–60. doi: 10.1002/1097-4679(199403)50:2<256::aid-jclp2270500218>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 36.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1(385):385–401. [Google Scholar]
- 37.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;44(561):561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 38.Judd LL, Akiskal HS. Delineating the longitudinal structure of depressive illness: Beyond clinical subtypes and duration thresholds. Pharmacopsychiatry. 2000;33(3):3–7. doi: 10.1055/s-2000-7967. [DOI] [PubMed] [Google Scholar]
- 39.Judd LL, Akiskal HS, Maser JD, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55(694):694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- 40.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54(4):439–67. [PubMed] [Google Scholar]
- 41.Mossey JM, Shapiro E. Self-rated health: A predictor of mortality among the elderly. Am J Public Health. 1982;72(8):800–8. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Idler EL, Russell LB, Davis D. Survival, functional limitations, and self-rated health in the NHANES I epidemiologic follow-up study, 1992. First national health and nutrition examination survey. Am J Epidemiol. 2000;152(9):874–83. doi: 10.1093/aje/152.9.874. [DOI] [PubMed] [Google Scholar]
- 43.Minino AM, Heron MP, Murphy SL, Kochanek K. Washington, DC: US Department of Health and Human Services; 2007. [accessed May 20, 2008]. National Vital Statistics Report: Table 9: Death rates by age, and age adjusted death rates for the 15 leading causes of death in 2004: United States, 1999–2004 [homepage on the Internet] Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr55/nvsr55_19.pdf. [Google Scholar]
- 44.Lawton MP, Moss M, Fulcomer M, Kleban MH. A research and service-oriented multilevel assessment instrument. J Gerontol. 1982;37(91):91–9. doi: 10.1093/geronj/37.1.91. [DOI] [PubMed] [Google Scholar]
- 45.National Center for Health Statistics. Questionnaires from the National Health Interview Survey, 1985–1989. Hyattsville, MD: National Center for Health Statistics; 1993. Report No.: Series 1, No. 31, Publication No. (PHS) 93–1307. [Google Scholar]
- 46.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 47.Dohrenwend BP, Raphael KG, Marbach JJ, Gallagher RM. Why is depression comorbid with chronic myofascial face pain? A family study test of alternative hypotheses. Pain. 1999;83(2):183–92. doi: 10.1016/s0304-3959(99)00100-1. [DOI] [PubMed] [Google Scholar]
- 48.Keefe FJ, Lefebvre JC, Egert JR, et al. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: The role of catastrophizing. Pain. 2000;87(3):325–34. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 49.Von Korff M, Le Resche L, Dworkin SF. First onset of common pain symptoms: A prospective study of depression as a risk factor. Pain. 1993;55(2):251–8. doi: 10.1016/0304-3959(93)90154-H. [DOI] [PubMed] [Google Scholar]
- 50.Atkinson JH, Slater MA, Patterson TL, Grant I, Garfin SR. Prevalence, onset, and risk of psychiatric disorders in men with chronic low back pain: A controlled study. Pain. 1991;45(2):111–21. doi: 10.1016/0304-3959(91)90175-W. [DOI] [PubMed] [Google Scholar]
- 51.Gallagher RM, Verma S. Mood and anxiety disorders in chronic pain. In: Dworkin R, Brieghtbart W, editors. Psychosocial and Psychiatric Aspects of Pain: A Handbook For Health Care Providers. Progress In Pain Research and Management. Seattle, WA: ISAP Press; 2004. [Google Scholar]
- 52.Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: A prevalence study. Pain. 2001;89(2–3):127–34. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 53.Eriksen J, Ekholm O, Sjogren P, Rasmussen NK. Development of and recovery from long-term pain. A 6-year follow-up study of a cross-section of the adult Danish population. Pain. 2004;108(1–2):154–62. doi: 10.1016/j.pain.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 54.Singh-Manoux A, Martikainen P, Ferrie J, et al. What does self rated health measure? Results from the British Whitehall II and French Gazel cohort studies. J Epidemiol Community Health. 2006;60(4):364–72. doi: 10.1136/jech.2005.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cosgrove N, Borhani NO, Bailey G, et al. Mass mailing and staff experience in a total recruitment program for a clinical trial: the SHEP experience. Control Clin Trials. 1999;19(133):133–48. doi: 10.1016/s0197-2456(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 56.Silagy CA, Campion K, McNeil JJ, et al. Comparison of recruitment strategies for a large-scale clinical trial in the elderly. J Clin Epidemiol. 1991;44(1105):1105–14. doi: 10.1016/0895-4356(91)90013-y. [DOI] [PubMed] [Google Scholar]
- 57.Gallagher RM, Verma S. Managing pain and comorbid depression: A public health challenge. Semin Clin Neuropsychiatry. 1999;4(203):203–20. doi: 10.153/SCNP00400203. [DOI] [PubMed] [Google Scholar]