Abstract
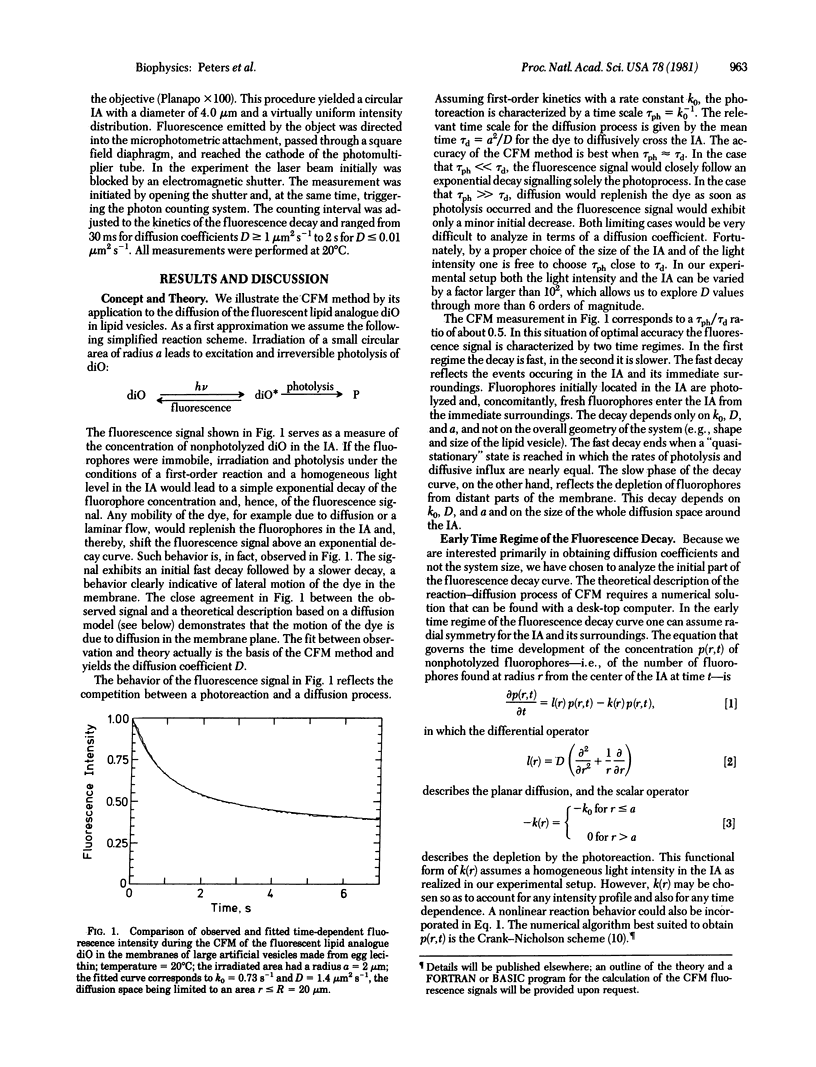
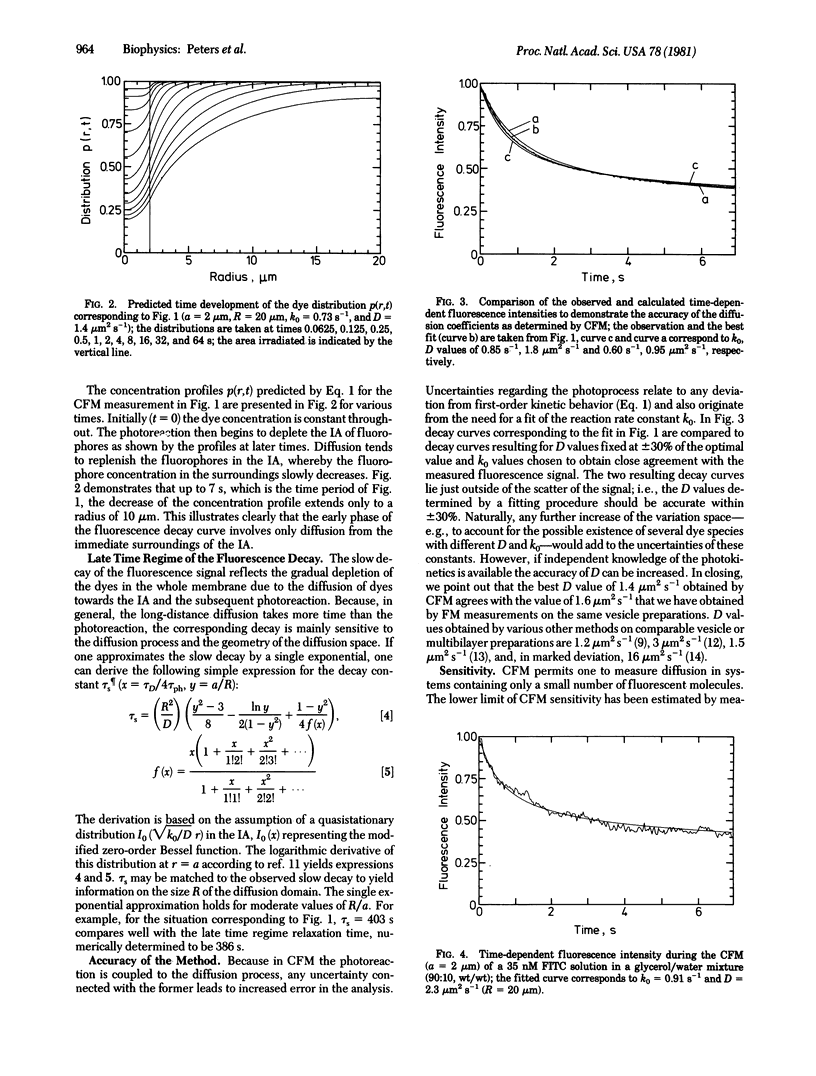
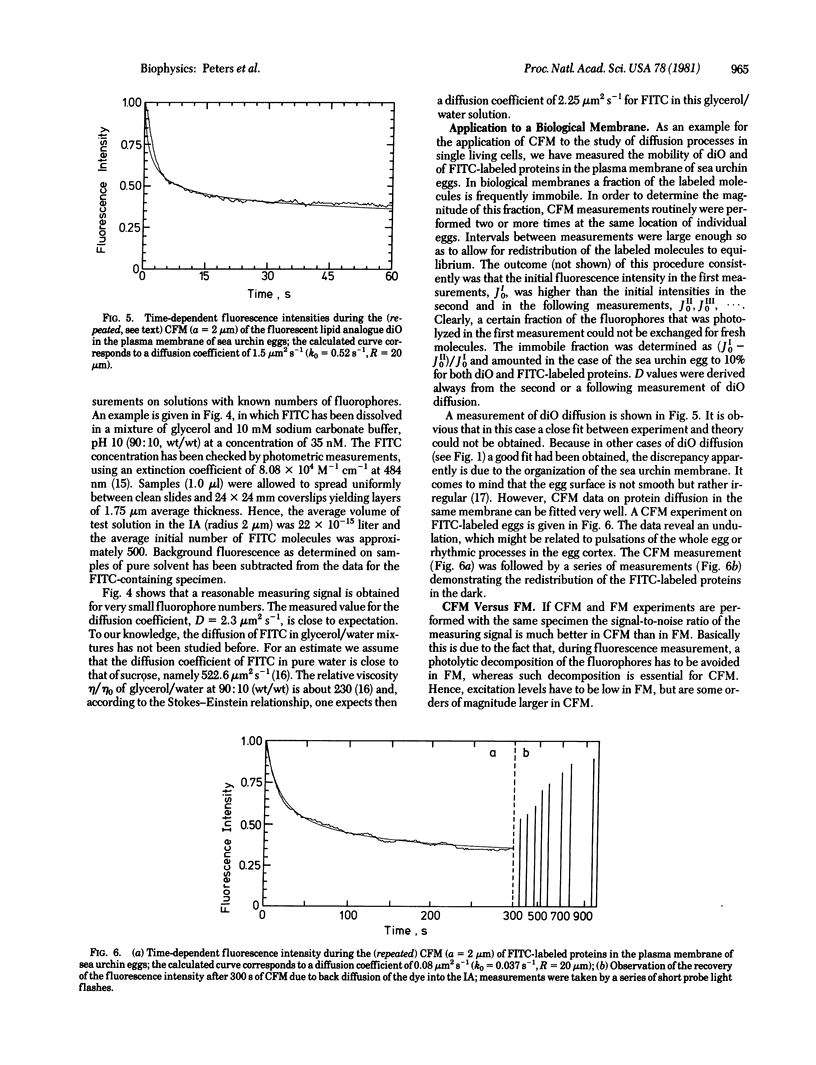
Continuous fluorescence microphotolysis is a sensitive method for the study of translational diffusion in the plasma membrane of single living cells and related systems. In this communication the conceptual basis of the method and its theoretical framework and experimental realization, as well as applications, are reported. In continuous fluorescence microphotolysis a microscopic membrane area of a single fluorescently labeled cell is irradiated by a laser beam while the fluorescence emitted from the area is monitored. The decay of the measuring signal reflects the competition of two processes: (i) the elimination of fluorophores by irreversible photolysis, and (ii) the entrance of new fluorophores into the area by diffusion. Rate constants for the two processes can be derived from the measuring data by mathematical analysis. As compared to our initial approach, fluorescence microphotolysis [Peters, R., Peters, J., Tews, K. H. & Bähr, W. (1974) Biochim. Biophys. Acta 367, 282-294], the main advantage of the method described here is an improvement of data quality and detection limit by orders of magnitude. From the practical point of view the main advantage is a simplification of the experimental setup. Results obtained by this method are encouraging and support the contention that continuous fluorescence microphotolysis may disclose new aspects of diffusion processes in biological systems.
Keywords: diffusion equation, lateral diffusion in membranes, photobleaching, sea urchin eggs
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Cullis P. R. Lateral diffusion rates of phosphatidylcholine in vesicle membranes: effects of cholesterol and hydrocarbon phase transitions. FEBS Lett. 1976 Nov;70(1):223–228. doi: 10.1016/0014-5793(76)80762-4. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Webb W. W. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry. 1978 Jul 25;17(15):3046–3053. doi: 10.1021/bi00608a016. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- Koppel D. E. Fluorescence redistribution after photobleaching. A new multipoint analysis of membrane translational dynamics. Biophys J. 1979 Nov;28(2):281–291. doi: 10.1016/S0006-3495(79)85176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Koppel D. E. Membrane damage caused by irradiation of fluorescent concanavalin A. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3314–3317. doi: 10.1073/pnas.76.7.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegner M. J., Epel D. Sea urchin sperm-egg interactions studied with the scanning electron microscope. Science. 1973 Feb 16;179(4074):685–688. doi: 10.1126/science.179.4074.685. [DOI] [PubMed] [Google Scholar]
- Träuble H., Sackmann E. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. 3. Structure of a steroid-lecithin system below and above the lipid-phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4499–4510. doi: 10.1021/ja00768a015. [DOI] [PubMed] [Google Scholar]
- Wolf D. E., Edidin M., Dragsten P. R. Effect of bleaching light on measurements of lateral diffusion in cell membranes by the fluorescence photobleaching recovery method. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2043–2045. doi: 10.1073/pnas.77.4.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]



