Abstract
BACKGROUND & AIMS
T-helper (Th)17 cells that secrete interleukin (IL)-22 have immunomodulatory and protective properties in the liver and other tissues. IL-22 induces expression of proinflammatory genes, but is also mitogenic and anti-apoptotic in hepatocytes.
Therefore, it could have multiple functions in the immune response to hepatitis B virus (HBV).
METHODS
We examined the role of IL-22 in regulating liver inflammation in HBV transgenic mice and measured levels of IL-22 in HBV-infected patients.
RESULTS
In HBV transgenic mice, injection of a single dose of IL-22 increased hepatic expression of proinflammatory genes, but did not directly inhibit virus replication. When splenocytes from HBV-immunized mice were transferred into HBV transgenic mice, the severity of the subsequent liver damage was ameliorated by neutralization of IL-22. In this model, IL-22 depletion did not affect interferon-γ–mediated noncytopathic inhibition of virus replication initiated by HBV-specific cytotoxic T cells, but it significantly inhibited recruitment of antigen–non-specific inflammatory cells into the liver. In patients with acute HBV infections, the percentage of Th17 cells in peripheral blood and concentration of IL-22 in serum were significantly increased.
CONCLUSION
IL-22 appears to be an important mediator of the inflammatory response following recognition of HBV by T cells in the liver. These findings might be relevant to the development of cytokine-based therapies for patients with HBV infection.
Keywords: liver disease, immune response, mouse model, anti-viral
Interleukin (IL)-22 is an IL-10 family cytokine produced primarily by Th17, Th22, and NK22 cells 1–3. The IL-22 receptor is highly expressed within specific tissues, including hepatocytes in the liver 4, 5. Signaling through the IL-22 receptor leads to the expression of acute inflammatory proteins 6, 7, activation of proliferative and/or anti-apoptotic programs 8, 9, and induction of antimicrobial genes and cytokines/chemokines 10, 11. Thus, unlike IL-10, which regulates immune cell function, IL-22 appears to control tissue responses to the immune system 12. With regard to the liver, most evidence supports a protective role for IL-22 in preventing hepatocyte damage. Expression of IL-22 in the liver protects hepatocytes from carbon tetrachloride (CCl4), concanavalin A (ConA), and Fas ligand-induced damage 8, 9. IL-22 null mice are highly sensitive to liver injury caused by ConA-mediated hepatitis, which can be lessened by transfer of IL-22-expressing Th17 cells into these animals 13. IL-22 was also recently shown to be protective in a mouse model of alcohol-induced liver injury 14.
However, IL-22 may also have a proinflammatory role in some disease processes. For example, IL-22 induces keratinocyte migration, epidermal hyperplasia, and dermal inflammation, and may therefore be a key mediator in the pathogenesis of psoriasis 6, 11, 15, 16. IL-22 is also upregulated in the brain of multiple sclerosis patients and mediates permeabilization of the blood-brain barrier 17, and has been shown to have a proinflammatory role in a mouse model of arthritis 18. Thus, the potential dual nature of IL-22 in modulating tissue immune responses may depend on the specific context in which the cytokine is expressed 19, 20. In fact, it was recently demonstrated that IL-17A regulates the pathogenic and protective functions of IL-22 in airway inflammation 21.
IL-22 expression is upregulated in the liver in patients with chronic hepatitis B and C virus (HBV, HCV) infection 22, 23. However, the function of IL-22 in protecting the liver during a specific immune response to these viruses has not been examined. Because IL-22 can induce pro-inflammatory gene expression, yet is mainly protective in many disease models, we hypothesized that IL-22 may perform an important role in the immune response to HBV. To test this possibility, we examined the ability of IL-22 to induce antiviral activity, liver disease, and recruitment of inflammatory cells into the liver in HBV transgenic mice, and also examined IL-22 expression in HBV infected patients.
MATERIALS AND METHODS
Mice
The HBV transgenic (Tg) mouse lineage 1.3.32 (inbred C57BL/6, H-2b) encodes a 1.3-overlength copy of the HBV genome (serotype ayw), expresses all of the HBV antigens, and replicates HBV in the liver without any evidence of cytopathology 24. Lineage 1.3.32 mice were injected intravenously (i.v.) or intraperitoneally (i.p.) with 25 μg of recombinant murine IL-22 (Peprotech Inc., Rocky Hill, NJ) diluted in 200 μL of saline. Control animals received the same volume of saline alone. In the splenocyte transfer experiments, lineage 1.3.32 mice were bred one generation with BALB/c mice (H-2d) to produce F1 hybrid recipient animals (designated HBV.CB6F1). In all experiments, mice were matched for age (8–12 weeks), sex (male), and serum HBeAg levels before use. All experiments were performed in accordance with Yale Institutional Animal Care and Use Committee approved procedures.
Immunization of mice and splenocyte transfer
C57BL/6 × BALB/c F1 (CB6F1) mice were injected intramuscularly with 100 μg of plasmid pCMV-S2.S encoding the HBV middle envelope protein in order to prime a CD8+ T cell response to HBsAg. Twenty-eight days later, mice were intranasally infected with 1 × 106 pfu of recombinant vesicular stomatitis virus (VSV) expressing the HBV middle envelope protein (VSV-MS) 25. Seven days after the booster immunization, mice were euthanized, and splenocytes were purified as described previously 25. Two hundred million splenocytes, containing ~107 HBV-specific CD8+ cytotoxic T lymphocytes (CTLs), were injected i.v. into HBV.CB6F1 mice, with or without 100 μg of anti-mouse IL-22 antibody (Ab) (Peprotech). The neutralizing activity of the antibody reported by the manufacturer was confirmed by measuring inhibition of IL-22-induced STAT-3 phosphorylation in cultured immortalized mouse hepatocytes (data not shown). The observed neutralization activity was ≥ that measured for IL-22BP (R&D Systems, Minneapolis, MN), and the antibody did not neutralize the structurally related cytokine IL-28 26 (data not shown). In some experiments, splenocytes were labeled with carboxyfluorescein succinimidyl ester (CFSE, eBioscience, San Diego, CA) before injection. Control mice received the same number of splenocytes purified from unimmunized CB6F1 mice. Groups of four mice were euthanized at day 2 or 5 after transfer, and their livers, spleen, and peripheral blood were harvested. Mice euthanized 5 days post injection received additional 100 μg doses of anti-IL-22 Ab delivered intraperitoneally 2 and 4 days after the initial splenocyte transfer.
Patients
Sixteen patients with acute hepatitis B (AHB), 41 patients with chronic hepatitis B (CHB), and 20 asymptomatic HBV carriers (AsC) were enrolled in the study. The standards for diagnoses were made according to the diagnostic standard of the Chinese National Program for Prevention and Treatment of Viral Hepatitis. All patients were hospitalized or followed-up in Tangdu Hospital from June 2007 to March 2009. Patients who were co-infected with HIV or other hepatitis viruses or concurrently afflicted by immunocompromised diseases and autoimmune diseases were excluded. No one received anti-HBV agents or steroids six months before sampling, and as a control, 16 healthy donors were also selected. The clinical data obtained for the enrolled subjects are listed in Table 1. The human study protocol was approved by the ethics committee of Tangdu Hospital.
Table 1.
Clinical characteristics of studied subjects
| Group | AHB | CHB | AsC | NC |
|---|---|---|---|---|
| Cases | 16 | 41 | 20 | 16 |
| Sex (male: female) | 10: 6 | 27:14 | 13: 7 | 10: 6 |
| Age (years) | 29.3 ± 7.5 | 26.8 ± 8.5 | 27.6 ± 8.2 | 30.1 ± 5.4 |
| ALT (U/L) | 1222 ± 679 | 151 ± 26 | 23.8 ± 7.3 | N.D. |
| HBsAg positive | 16 | 41 | 20 | 0 |
| HBeAg positive | 9 (56.25%) | 41 | 20 | 0 |
| HBeAb positive | 7 (43.75%) | 0 | 0 | 0 |
| HBcAb positive | 16 | 41 | 20 | 0 |
| HBcAb IgM positive | 16 | 0 | 0 | 0 |
| HBV DNA positive | 16 | 41 | 20 | 0 |
Flow cytometry
Intrahepatic leukocytes (IHLs) were purified as described previously 25. Splenocytes and IHLs were surface-stained with anti-mouse CD3, CD4, CD8, NK1.1, CD11b, CD11c, CD19, and/or Gr-1 (BD Bioscience, San Jose, CA) for detection of leukocyte subsets, and analyzed using an LSRII flow cytometer (BD Biosciences). Peripheral blood mononuclear cells (PBMCs) from patients were isolated by Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO) density gradient centrifugation and stimulated with PMA (50 ng/mL) and ionomycin (1 μg/mL), with monensin (10 μg/mL) for 5 hours. Anti-human CD3, CD4, CD8 were used for surface staining, and anti-human IL-17 and IL-22 (eBioscience) were used for intracellular staining. Data were acquired using a FACS Calibur flow cytometer (BD Biosciences). All data were analyzed using FlowJo software version 8.6 (Tree Star Inc., Ashland, OR).
HBV DNA and RNA analyses
HBV replicative DNA intermediates and viral RNA were detected by Southern and Northern blot analysis of total genomic liver DNA and RNA, respectively, as previously described 27, 28.
Biochemical and histopathological examination of liver disease
Hepatocellular injury was monitored by measuring serum alanine aminotransferase (ALT) activity at day 2 and 5 after treatment using Infinity ALT reagent (Thermo Electron, Louisville, CO). Mice were euthanized by carbon dioxide asphyxiation and livers were harvested for histopathological examination. Livers were fixed in 10% neutral buffered formalin, processed, embedded in paraffin, sliced at 5 μm, and stained with hematoxylin and eosin (HE) using routine methods. Livers were examined for histopathological changes (by C.J.B.) blind to experimental group using light microscopy. Livers were scored for the presence and severity of extramedullary hematopoiesis (EMH), inflammation, and necrosis. Each histopathological parameter was initially assessed at low power and scored separately at high power by using a semiquantitative criterion-based methodology 29, 30. Severity scores range from 0 to 5, and numeric values of 0 (within normal limits, absent), 1 (minimal, <10%), 2 (mild, 10%–20%), 3 (moderate, 20%–30%), 4 (marked, 30%–40%), and 5 (severe, >40%) were assigned according to presence and severity.
Real-time reverse-transcription PCR (RT-PCR)
Total liver RNA was reverse transcribed with random hexamers using the TaqMan reverse transcription kit (Applied Biosystems, Foster, CA) following the manufacturer’s instruction. Real-time PCR was performed using an Applied Biosystems 7500 real-time PCR system with SYBR-Green detection. Relative gene expression was quantified by the ΔΔCT method using 7500 System Sequence Detection software (Applied Biosystems).
Enzyme linked immunosorbent assay (ELISA)
IL-17 and IL-22 concentration were measured by ELISA (R&D Systems, Minneapolis, MN). Mouse serum amyloid A (SAA) was also measured by ELISA (Invitrogen, Carlsbad, CA).
Statistical analyses
Statistical significance was determined by Dunn’s multiple comparison test, Student’s t test, or Spearman correlation analysis using SPSS version 12.0 for Windows (SPSS, Chicago, IL). P values of <0.05 were considered to indicate a significant difference.
RESULTS
IL-22 does not have direct antiviral effects against HBV in vivo
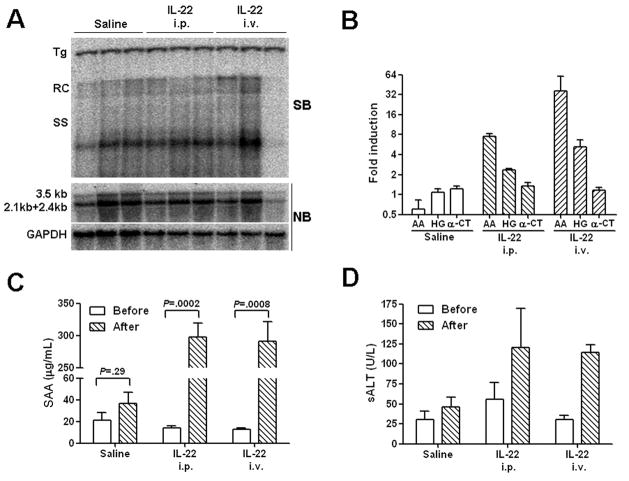
We previously found in cell culture that IL-22 has only minimal direct effects on HBV replication 31. However, it was possible that the in vivo activity of the cytokine might differ due to either differences in hepatocyte differentiation, or indirect effects through other cell types, either of which might not be accurately reflected in cell culture. Administration of cytokines to HBV Tg mice can disrupt virus replication through direct antiviral activity in hepatocytes (IFN-γ) or through indirect activation of other cells (IL-12) 32, 33. As shown in Fig. 1A, HBV DNA replication was not inhibited by intravenous or intraperitoneal administration of 25 μg IL-22 to HBV Tg mice, a dose previously shown to induce an acute-phase response in normal mice 34. There was also no significant change in the level of HBV 3.5- and 2.1-kb mRNA compared to GAPDH (Fig. 1A), or in the release of the secreted viral antigens HBsAg and HBeAg into the serum (data not shown). Under these conditions, IL-22 injection induced an increase in intrahepatic acute-phase gene expression (including amyloid A and haptoglobin; Fig. 1B), and also a statistically significant elevation of circulating serum amyloid A (Fig. 1C), confirming a response to the cytokine in the liver. Furthermore, administration of IL-22 caused only a mild elevation in serum ALT levels (Fig. 1D) with no histopathologic evidence of inflammation or other pathologic changes in the liver (data not shown).
Figure 1. IL-22 induces an acute-phase like response in the liver of HBV Tg mice but does not inhibit virus replication.
(A) Groups of age, sex, and serum HBeAg matched transgenic mice (3 mice per group) were injected intravenously or intraperitoneally with a single dose of 25 μg of IL-22, and analyzed 1 day post injection. HBV replication in the liver was measured by Southern blot (SB) analysis of relaxed-circle (RC) and single-stranded (SS) DNA replication forms, and compared to levels in control animals that did not receive the cytokine. HBV 3.5 kb and 2.1+2.4 kb mRNA expression from total liver RNA was examined by northern blot (NB) analysis, and compared to the housekeeping gene GAPDH. (B) Intrahepatic expression of amyloid A (AA), haptoglobin (HB), and anti-chymotrypsin (α-CT) were measured by quantitative real-time RT-PCR (qRT-PCR). Results are displayed as fold differences relative to one control mouse, and normalized to GAPDH expression. (C) Serum amyloid A (SAA) levels from the same mice were measured by ELISA. (D) The level of serum alanine aminotransferase (sALT) was measured before and after injection. The data represent mean ± SD of three mice.
IL-22 neutralization does not affect the IFN-γ-mediated inhibition of HBV but reduces the severity of liver disease
Following transfer of HBsAg-specific CTLs or immunized splenocytes into HBV transgenic mice, virus-specific T cells traffic to the liver and produce IFN-γ, which non-cytopathically inhibits HBV replication 27, 28. This process triggers the subsequent recruitment of other non-specific inflammatory cells into the liver through a mechanism that requires chemokines 35, neutrophils 36, and matrix metalloproteinases 37, leading to an increase in liver inflammation and ALT elevation. Therefore, using this model, we examined the role of IL-22 in the inflammatory processes that follow HBV antigen recognition in the liver.
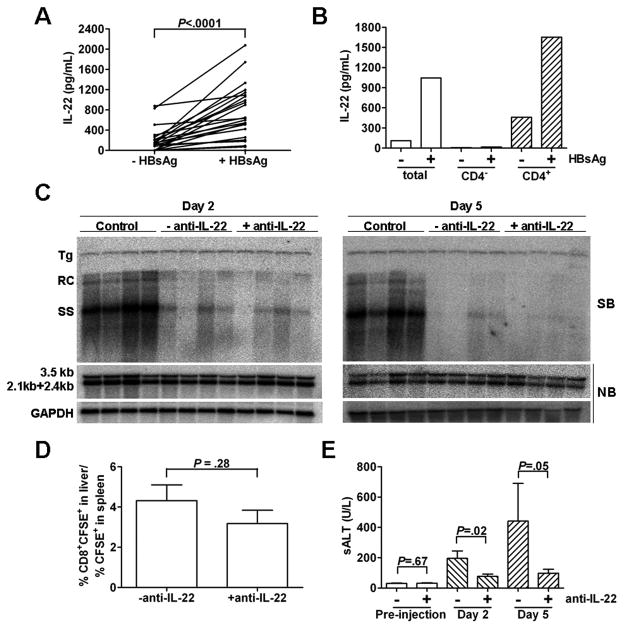
We first determined that splenocytes from HBs-immunized mice produced the Th17 cytokines IL-17 (data not shown) and IL-22 (Fig. 2A) after stimulation with HBsAg, confirming the presence of both antigen-specific cells, as well as cells capable of producing IL-22 either directly or as a consequence of bystander activation. Furthermore, IL-22 production from the immunized splenocytes was strictly dependent on the presence of CD4+ T cells (Fig. 2B). Following injection with the immunized splenocytes, the level of HBV DNA replication intermediates in the liver of the HBV Tg mice were reduced within 2 days after transfer and remained suppressed for at least 5 days (Fig. 2C). Administration of anti-IL-22 Ab did not alter the IFN-γ-induced inhibition of HBV replication in the liver observed on both days 2 and 5 (Fig. 2C). In addition, viral RNA expression was not decreased on either day 2 or day 5 (Fig. 2C). We next determined whether the depletion of IL-22 affected the initial trafficking of HBV-specific CD8 T cells into the liver. The immunized splenocytes were stained with CFSE before transfer, and the recruitment of the transferred CTLs into the liver was quantified by measuring the number of intrahepatic CD8+CFSE+ cells. After normalization with the percentage of CFSE+ cells in the spleen, the proportion of CD8+CFSE+ cells in the liver was very similar in the liver of mice that were euthanized on day 2 with or without anti-IL-22 Ab (Fig. 2D), indicating that IL-22 depletion did not affect their initial recruitment into the liver. The liver damage in mice that received immunized splenocytes was moderate 2 days after injection as measured by serum ALT, and was further increased at day 5 (Fig. 2E). Surprisingly, administration of anti-IL-22 Ab diminished the severity of liver disease at both time points by approximately 60% when compared with that of animals that received splenocytes without anti-IL-22 (Fig. 2E).
Figure 2. IL-22 depletion does not affect IFN-γ mediated inhibition of HBV replication, but ameliorates subsequent liver damage following transfer of immunized splenocytes.
(A) IL-22 expression in immunized splenocytes was measured by ELISA after a 4-day incubation in vitro with or without HBsAg stimulation. Matched data points indicate splenocytes from an individual animal. (B) IL-22 expression from pooled total, CD4-depleted (Miltenyi Biotec; CD4−), or CD4-purified (CD4+) splenocytes. (C) HBV.CB6F1 mice (4 per group) were injected with 2×108 immunized splenocytes with or without anti-IL-22 Ab. Mice were euthanized at days 2 and 5 postinjection, and total hepatic DNA was analyzed for HBV replication by Southern blot (SB). Bands corresponding to integrated transgene (Tg), relaxed-circular (RC), and single-stranded (SS) HBV DNA replication intermediates are indicated. Northern blot (NB) was used for analysis of HBV 3.5 kb and 2.1+2.4 kb mRNA expression, normalized to GAPDH. Results were compared to control livers from another four matched transgenic littermates injected with unimmunized splenocytes. (D) HBV.CB6F1 mice (4 per group) were injected with 2×108 CFSE-stained immunized splenocytes with or without anti-IL-22 Ab. The livers and spleens were harvested at day 2 after splenocyte transfer, and the percentages of CFSE+CD8+ cells in the liver and CFSE+ cells in the spleen were analyzed by flow cytometry. The data represent mean ± SD of four mice. (E) The mean sALT activity (± SD), measured 1 day pre-injection and at euthanasia 2 or 5 days post transfer, is indicated for each group.
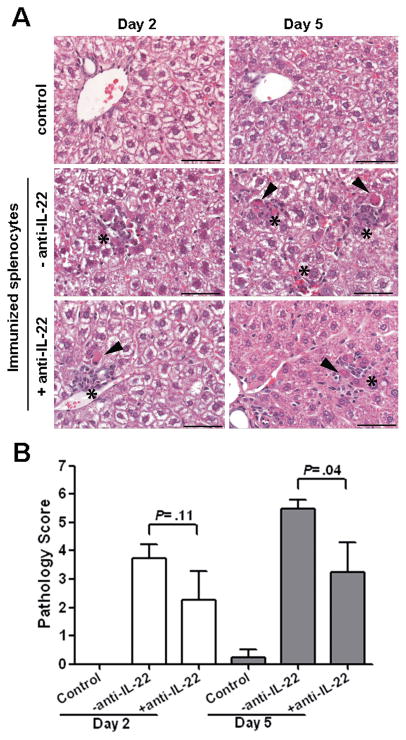
Consistent with the ALT levels, in control mice that received unimmunized splenocytes, there was no evidence of significant hepatitis or other pathology in the liver (Fig. 3A, upper panel). In contrast, histological examination of the liver from animals injected with immunized splenocytes revealed scattered foci of single cell necrosis with associated recruitment of inflammatory cells throughout the liver parenchyma. These livers have more frequent foci of necrotic hepatocytes surrounded by inflammation at day 5 (Fig. 3A, middle right panel) than day 2 (Fig. 3A, middle left panel). Administration of anti-IL-22 reduced the frequency of necrotic foci and inflammation at day 5 (Fig. 3A, lower right panel) compared to mice given immunized splenocytes alone. A quantitative measure of liver histopathology was significantly reduced by anti-IL-22 Ab administration by day 5 post splenocyte transfer (P=0.04), but not by day 2 (P=0.11) (Fig. 3B).
Figure 3. IL-22 neutralization reduces liver inflammation and pathology following transfer of immunized splenocytes.
(A) Representative sections of liver from control HBV Tg mice given splenocytes from non-immunized mice (upper panels), immunized splenocytes alone (middle panels) and with anti-IL-22 Ab (lower panels) at day 2 (left) and day 5 (right) post administration. The overall morphology of control livers (upper panels) was normal. Mice with HBV-specific splenocyte induced hepatic injury alone had more frequent foci of necrotic hepatocytes (arrowheads) and inflammation (asterisks) at day 5 (middle right panel) than day 2 (middle left panel). Mice with HBV-specific splenocyte induced hepatic injury given anti-IL-22 Ab had fewer foci of necrosis and inflammation (lower panels) than mice not given anti-IL-22 Ab. Scale Bars = 50 microns. (B) Histopathology scores for necrosis and inflammation were significantly reduced at day 5 in anti-IL-22 treated mice.
Depletion of IL-22 blocks the recruitment of inflammatory cells into the liver
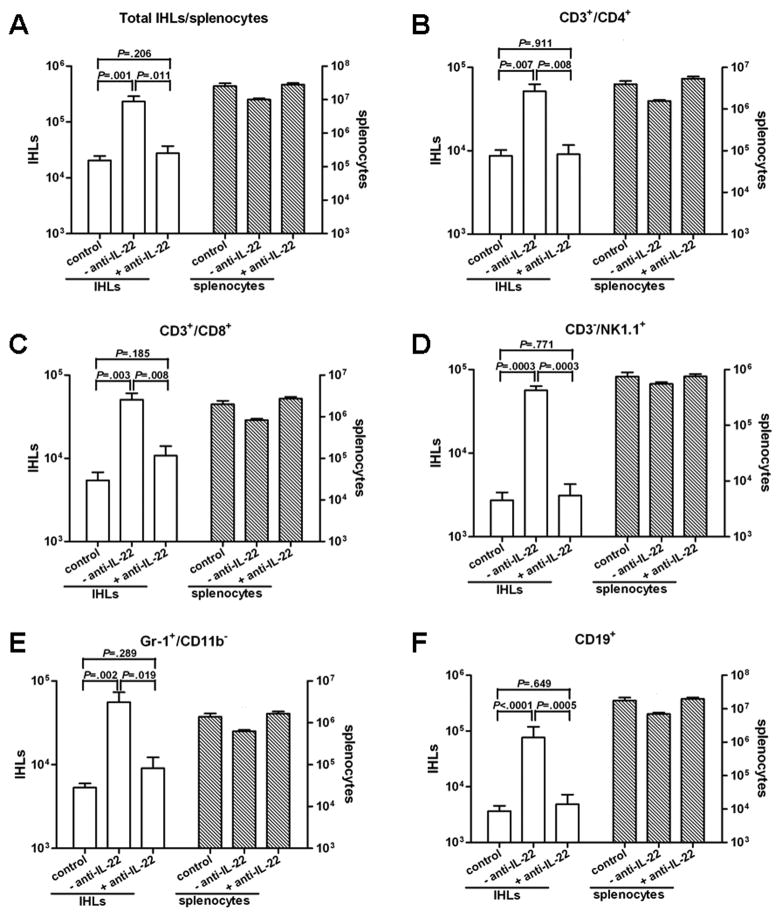
To determine the cellular makeup of the inflammatory infiltrate in the liver, the absolute numbers of IHLs and splenocytes recovered were quantified at day 5 post transfer, and cell surface marker expression on the recruited inflammatory cells was determined by flow cytometry. When compared with controls, there were no significant differences in the total number, and in the specific subsets, of cells in the spleen of recipient mice with or without anti-IL-22 Ab (Fig. 4). However, the total number of IHLs, as well as all cell subsets, increased in the liver of mice injected with immunized splenocytes. The total number of IHLs increased over 11-fold on day 5 after immunized splenocyte transfer compared to controls (Fig. 4A), corresponding with an increase in: T helper cells (CD3+/CD4+; Fig. 4B), CTLs (CD3+/CD8+; Fig. 4C), NK cells (CD3−/NK1.1+; Fig. 4D), neutrophils (Gr-1+/CD11b−; Fig. 4E), and B cells (CD19+; Fig 4F). Similar increases were also observed in NKT cells (CD3+/NK1.1+; 17.2-fold), lymphoid dendritic cells (CD11b−/CD11c+; 24.4-fold), myeloid dendritic cells (CD11b+/CD11c+; 31.4-fold), and macrophages (Gr-1−/CD11b+/CD11c−; 14.5-fold) (data not shown). As also shown in Fig. 4A, anti-IL-22 Ab administration reduced the number of total IHLs by 8.5-fold. The number of all cell subsets recruited was reduced in these mice such that their numbers were similar to or only slightly higher than those detected in controls (Fig. 4B–F and data not shown). These results indicate that in addition to the reduction in sALT elevation and liver pathology, neutralization of IL-22 also blocked the recruitment of all leukocyte subsets into the liver.
Figure 4. Depletion of IL-22 blocks the recruitment of antigen-nonspecific cells into the liver.
Livers from HBV Tg mice receiving splenocytes from non-immunized mice (control) or immunized mice in the absence (-anti-IL-22) or presence (+anti-IL-22) of IL-22 Ab were weighed at the time of euthanasia, and IHLs were isolated from two liver lobes of a similar weight and analyzed by flow cytometry. The indicated numbers of total IHLs or splenocytes and cell subsets represent the absolute numbers in the liver or spleen, respectively. (A) total IHL and splenocyte number, (B) CD4 T cells, (C) CD8 T cells, (D) NK cells, (E) neutrophils, and (F) B cells. Similar patterns were observed for NKT cells, lymphoid dendritic cells, myeloid dendritic cells, and macrophages (not shown).
IL-22 depletion reduces intrahepatic chemokine expression
A number of factors, including the chemokines CXCL9 (MIG) and CXCL10 (IP-10) are known to be important for recruitment of cells into the liver of HBV Tg mice following transfer of HBV-specific CTLs 35. Therefore, we examined the influence of IL-22 on intrahepatic chemokine expression (Fig. 5). Consistent with the changes in inflammatory cell recruitment (Fig. 4), expression of CXCL9 and CXCL10 were both reduced in the presence of the IL-22 antibody (Fig. 5), indicating that IL-22 is likely functioning upstream of chemokine expression to recruit inflammatory cells into the liver.
Figure 5. IL-22 depletion reduces chemokine expression in the liver.
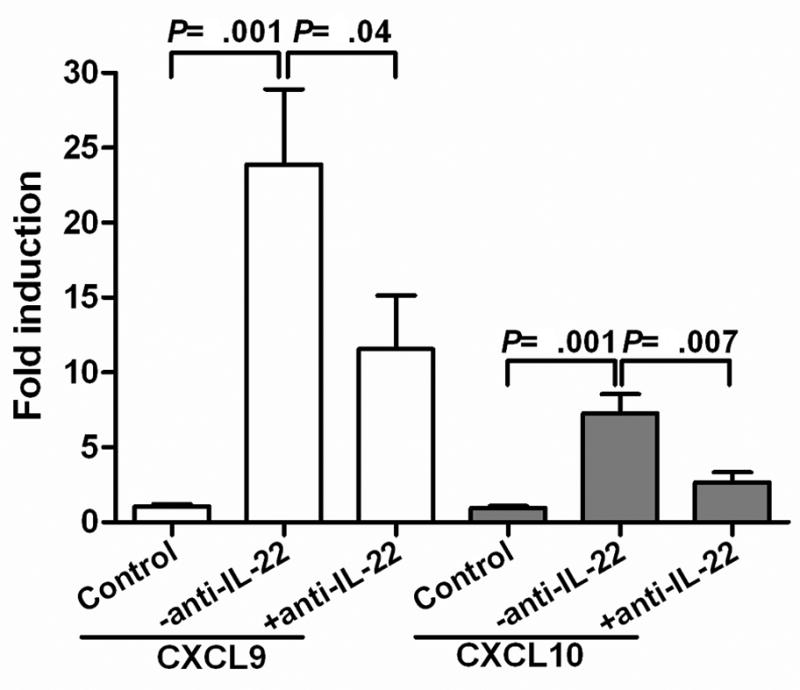
Expression of CXCL9 and CXCL10 in the liver of HBV Tg mice 2 days after receiving splenocytes from non-immunized mice (control; n=6) or immunized mice in the absence (−anti-IL-22; n=8) or presence (+anti-IL-22; n=6) of IL-22 Ab. RNA expression was measured by qRT-PCR, and results are displayed as fold differences relative to the control group, normalized to GAPDH.
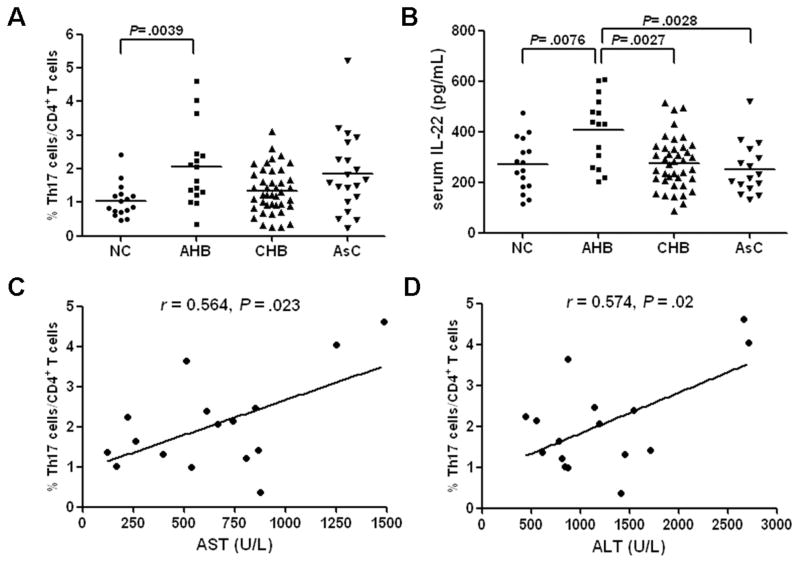
Increased peripheral Th17 cells and IL-22 expression in patients with AHB
Finally, we determined whether the Th17 cell subsets and IL-22 levels in the serum differed in various disease states in HBV-infected patients. After stimulation with PMA and ionomycin, the percentage of Th17 cells in stimulated CD4+ cells from AHB patients were significantly higher than those from NCs (2.06 ± 1.18% vs 1.05 ± 0.50%, P = 0.0039, Fig. 6A). However, circulating Th17 cell frequencies revealed no significant differences in CHB patients (1.35 ± 0.68%) and AsCs (1.86 ± 1.18%) compared to healthy donors (P > 0.05, Fig. 6A). Moreover, serum IL-17 and IL-22 were detected in all subjects. A low concentration of IL-17 was detected in serum of all patients and control subjects, and IL-17 levels in serum showed no significant difference among the four groups (data not shown). However, IL-22 concentration in AHBs (407.42 ± 137.41 pg/mL) was notably higher compared with concentrations in NCs (269.04 ± 104.27 pg/mL), CHBs (275.77 ± 102.72 pg/mL), and AsCs (252.54 ± 102.25 pg/mL) (P<0.01, Fig. 6B). Intracellular cytokine staining of PBMC from AHB and CHB patients showed that IL-17 and IL-22 were predominately produced by the CD3+CD8− cell population (mostly CD4 T cells; data not shown), and revealed the presence of IL17+/IL-22-, IL-17-/IL-22+, and IL-17+/IL-22+ cell populations (Supplemental Figure S1). Bivariate correlation revealed that the percentage of Th17 cells among CD4+ T cells is directly associated with both ALT and AST level in AHB patients (r = 0.574, P = 0.02 and r = 0.564, P = 0.023, respectively, Fig. 6C and D).
Figure 6. Th17 cells and IL-22 expression in HBV-infected patients.
The percentage of circulating Th17 cells (A) and concentration of IL-22 in serum (B) were measured by flow cytometry or ELISA in healthy controls (n=16), patients with acute hepatitis B (AHB, n=16), chronic hepatitis B (CHB, n=41), and asymptomatic HBV carriers (AsC, n=20). Horizontal bars indicate the mean value of each subset. The individual frequency for each subject is shown. Significance was calculated using the Dunn’s multiple comparison test. (C and D) Spearman correlation analysis of Th17 percentage with serum AST and ALT in sixteen AHB patients.
DISCUSSION
IL-22 induces the expression of antimicrobial proteins that are needed for defense against pathogens 5, 11. We found that a single dose injection of IL-22 does not reduce the levels of either HBV DNA replication intermediates or HBV mRNA expression in the liver of HBV transgenic mice, consistent with our previous findings in cell culture that IL-22 has only minimal direct effects on HBV replication 31. Interestingly, a recent study demonstrated that circulating IL-22 induces changes in systemic physiology indicative of an acute-phase response 34. We also observed an increase in amyloid A and haptoglobin gene expression in the liver and SAA in peripheral blood in response to IL-22 administration. These results indicated that IL-22 induced acute phase like gene expression in the liver of HBV transgenic mice, which may potentially modulate the inflammatory processes in HBV infection, but was not sufficient to directly inhibit virus replication in vivo.
Due to the potential context-dependent pro- and anti-inflammatory nature of IL-22, we examined which function of IL-22 may predominate in the intrahepatic inflammatory response that follows recognition of HBV antigen in the liver. We found that after transfer of immunized splenocytes into transgenic mice replicating HBV in their hepatocytes, there was a reduction in HBV DNA replicative intermediates, consistent with the known inhibitory effect of IFN-γ produced by recruited intrahepatic HBV-specific CTLs 27, 28. HBV replication was also strongly inhibited in the animals that received anti-IL-22 Ab, further reiterating that IL-22 does not have a direct antiviral activity against virus, nor does it regulate the antiviral potential of virus-specific CTLs in a transgenic mouse system. In contrast, neutralization of IL-22 significantly reduced chemokine expression and the subsequent recruitment of inflammatory cells into the liver. In certain contexts, IL-22 may therefore directly or indirectly contribute to liver disease pathogenesis by promoting the migration of inflammatory cells, which are known to amplify CTL-induced hepatocyte injury, into the liver 35.
It should be pointed out that the pro-inflammatory pathogenic role of IL-22 in this model system contrasts with the generally protective role of the cytokine found in other models of hepatitis or liver injury 9, 13. One important distinction between these models lies in the fact that in the HBV adoptive transfer system, liver inflammation and subsequent ALT elevation is potentiated by recruitment of inflammatory cells into the liver, which requires specific cellular and protein mediators including neutrophils, chemokines, and matrix metalloproteinases 35–37. Interestingly, IL-22 has been shown to induce chemokine and MMP expression 10 and mobilize neutrophils 34, which may account for its proinflammatory effect in this model. IL-22 has also been shown to increase the pathological activity of TNF-α 1, which is also expressed in the liver after transfer of HBV-specific splenocytes 27. Nevertheless, based on other studies showing a general protective role of IL-22 in the liver, it is likely that even in this system there are also hepatoprotective effects of the cytokine, and the overall detrimental net effect of IL-22 is likely a reflection of the predominance of the pathological over the protective functions of the cytokine in this model.
We also observed that Th17 cells from AHB patients were approximately 2-fold higher in peripheral blood than those from healthy donors and further correlated with ALT and AST, and found that serum IL-22, but not IL-17, was markedly increased in patients with AHB. These findings are generally consistent with other recent patient studies. Zhang et al. found that Th17 cells correlated with the severity of liver disease in CHB patients 38. Although this study found a strong association in CHB that is not immediately apparent in our population, it is important to note that our patients had generally lower mean ALT levels (151 vs 258), and both studies found an association between Th17 frequency and ALT, indicating that the level of liver inflammation may be a more important correlate than infection status. Dambacher et al. reported a slight increase in serum IL-22 in acute HBV patients, but this did not reach statistical significance in their small patient population 22. Recently, Park et al. found that chronic HBV and HCV patients have a high percentage of inflammatory cells in the liver that express IL-22, which further positively correlated with liver inflammation grade and serum AST 23. In total these studies suggest that Th17 cells may take part in the disease process of acute and/or chronic HBV infection, and that IL-22 may be an important cytokine in disease pathogenesis.
In summary, we found that IL-22 is dispensable for the noncytopathic inhibition of HBV induced by IFN-γ, but may potentiate the intrahepatic recruitment of antigen-nonspecific cells that can increase subsequent liver injury. A similar role of IL-22 may also be played in the pathogenesis of acute viral hepatitis in humans. The potential context-dependent pathological and protective effects of this cytokine may be significant for the development of new therapeutic approaches to treat acute or chronic HBV infection.
Supplementary Material
Acknowledgments
Grant Support:
This work was supported by grants from NIH National Cancer Institute (CA137067) and National Science and Technology Major Project of China (2008ZX10002-006). Y.Z. was also supported by a training grant from China Scholarship Council (2009659001), and M.A.C. was supported by NIH training grant T32GM007324.
We thank the volunteers who generously participated in this study. We also thank Dr. Francis V. Chisari (The Scripps Research Institute) for providing the HBV transgenic mice, and Lauren Zenewicz (Yale University) for helpful discussion.
Abbreviations
- Ab
antibody
- AHB
acute hepatitis B
- AsC
asymptomatic HBV carrier
- CFSE
carboxyfluorescein succinimidyl ester
- CHB
chronic hepatitis B
- CTL
cytotoxic T lymphocyte
- ELISA
enzyme linked immunosorbent assay
- HIV
human immunodeficiency virus
- IFN
interferon
- IHL
intrahepatic leukocyte
- IL
interleukin
- PBMC
peripheral blood mononuclear cells
- Tg
transgenic
- TNF
tumor necrosis factor
- VSV
vesicular stomatitis virus
Footnotes
Potential conflict of interest: Nothing to report.
Author Roles:
Y.Z. and M.A.C. performed the study, Y.Z., M.A.C., J.Q.L., C.X.H., C.J.B., X.F.B., and M.D.R. analyzed and interpreted the data, Y.Z., M.A.C, C.J.B., and M.D.R. prepared the manuscript, and X.F.B. and M.D.R. supervised the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin–22. Blood. 2009;113:4008–10. doi: 10.1182/blood-2008-12-192443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–9. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 5.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 7.Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–9. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- 8.Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004;1:43–9. [PubMed] [Google Scholar]
- 9.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–42. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 10.Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–84. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 11.Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 12.Zenewicz LA, Flavell RA. IL-22 and inflammation: leukin’ through a glass onion. Eur J Immunol. 2008;38:3265–8. doi: 10.1002/eji.200838655. [DOI] [PubMed] [Google Scholar]
- 13.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–59. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 16.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–5. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, Matthys P. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 2009;60:390–5. doi: 10.1002/art.24220. [DOI] [PubMed] [Google Scholar]
- 19.Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. IL-17 and IL-22: siblings, not twins. Trends Immunol. 2010;31:354–61. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9:447–53. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dambacher J, Beigel F, Zitzmann K, Heeg MH, Goke B, Diepolder HM, Auernhammer CJ, Brand S. The role of interleukin-22 in hepatitis C virus infection. Cytokine. 2008;41:209–16. doi: 10.1016/j.cyto.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, Ki SH, Yoo SH, Dooley S, Wang FS, Young HA, Gao B. In vivo consequences of liver-specific interleukin-22 expression: Implications for human liver disease progression. Hepatology. doi: 10.1002/hep.24339. ePub Apr 4 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidotti LG, Matzke B, Schaller H, Chisari FV. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158–69. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cobleigh MA, Buonocore L, Uprichard SL, Rose JK, Robek MD. A vesicular stomatitis virus-based hepatitis B virus vaccine vector provides protection against challenge in a single dose. J Virol. 2010;84:7513–22. doi: 10.1128/JVI.00200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gad HH, Dellgren C, Hamming OJ, Vends S, Paludan SR, Hartmann R. Interferon-lambda is functionally an interferon but structurally related to the interleukin-10 family. J Biol Chem. 2009;284:20869–75. doi: 10.1074/jbc.M109.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 28.Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–63. doi: 10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Montgomery RR, Booth CJ, Wang X, Blaho VA, Malawista SE, Brown CR. Recruitment of macrophages and polymorphonuclear leukocytes in Lyme carditis. Infect Immun. 2007;75:613–20. doi: 10.1128/IAI.00685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H, Booth CJ, Pinus AB, Chen P, Lee A, Qiu M, Whitlock M, Murphy PS, Constable RT. Induced hepatic fibrosis in rats: hepatic steatosis, macromolecule content, perfusion parameters, and their correlations--preliminary MR imaging in rats. Radiology. 2008;247:696–705. doi: 10.1148/radiol.2473070605. [DOI] [PubMed] [Google Scholar]
- 31.Pagliaccetti NE, Chu EN, Bolen CR, Kleinstein SH, Robek MD. Lambda and alpha interferons inhibit hepatitis B virus replication through a common molecular mechanism but with different in vivo activities. Virology. 2010;401:197–206. doi: 10.1016/j.virol.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavanaugh VJ, Guidotti LG, Chisari FV. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71:3236–43. doi: 10.1128/jvi.71.4.3236-3243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guidotti LG, Morris A, Mendez H, Koch R, Silverman RH, Williams BR, Chisari FV. Interferon-regulated pathways that control hepatitis B virus replication in transgenic mice. J Virol. 2002;76:2617–21. doi: 10.1128/JVI.76.6.2617-2621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang SC, Nickerson-Nutter C, Pittman DD, Carrier Y, Goodwin DG, Shields KM, Lambert AJ, Schelling SH, Medley QG, Ma HL, Collins M, Dunussi-Joannopoulos K, Fouser LA. IL-22 induces an acute-phase response. J Immunol. 2010;185:5531–8. doi: 10.4049/jimmunol.0904091. [DOI] [PubMed] [Google Scholar]
- 35.Kakimi K, Lane TE, Wieland S, Asensio VC, Campbell IL, Chisari FV, Guidotti LG. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J Exp Med. 2001;194:1755–66. doi: 10.1084/jem.194.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sitia G, Isogawa M, Kakimi K, Wieland SF, Chisari FV, Guidotti LG. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 2002;99:13717–22. doi: 10.1073/pnas.172521999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sitia G, Isogawa M, Iannacone M, Campbell IL, Chisari FV, Guidotti LG. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J Clin Invest. 2004;113:1158–67. doi: 10.1172/JCI21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L, Fu JL, Shi F, Shi M, Wang HF, Wang FS. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81–91. doi: 10.1002/hep.23273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.