Abstract
The plasticity of neural stem/progenitor cells allows a variety of different responses to many environmental cues. In the past decade, significant research has gone into understanding the regulation of neural stem/progenitor cell properties, because of their promise for cell replacement therapies in adult neurological diseases. Both endogenous and grafted neural stem/progenitor cells are known to have the ability to migrate long distances to lesioned sites after brain injury and differentiate into new neurons. Several chemokines and growth factors, including stromal cell-derived factor-1 and vascular endothelial growth factor, have been shown to stimulate the proliferation, differentiation, and migration of neural stem/progenitor cells, and investigators have now begun to identify the critical downstream effectors and signaling mechanisms that regulate these processes. Both our own lab and others have shown that the extracellular matrix and matrix remodeling factors play a critical role in directing cell differentiation and migration of adult neural stem/progenitor cells within injured sites. Identification of these and other molecular pathways involved in stem cell homing into ischemic areas is vital for the development of new treatments. To ensure the best functional recovery, regenerative therapy may require the application of a combination approach that includes cell replacement, trophic support, and neural protection. Here we review the current state of our knowledge about endogenous adult and exogenous neural stem/progenitor cells as potential therapeutic agents for central nervous system injuries.
Keywords: adult neurogenesis, neural stem/progenitor cells, stem cell niche, stem cell therapy, stroke, stroke-induced neurogenesis
ADULT NEURAL STEM CELLS
Stem cells are defined by two key abilities: to self-renew and to differentiate into multiple cell types. The adult central nervous system (CNS) contains neural stem cells (NSCs) that can undergo either symmetric division that yields two daughter NSCs, which have identical stem cell properties as the parental cell, or asymmetric division, which gives rise to one identical daughter NSC and one lineage-committed cell. This lineage-committed cell is the precursor of one of the three major cell types in the adult brain: neurons, oligodendrocytes, or astrocytes.
Two regions of the adult CNS are confirmed to have ongoing neurogenesis, a process defined by the production of new neurons. These neurogenic areas include the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus and the subventricular zone (SVZ) bordering the lateral ventricles. Recent publications have shown that the process of neurogenesis in the SGZ originate with slowly dividing or quiescent neural stem cells (qNSCs), which can be identified by a combination of several makers, such as brain lipid binding protein-positive (BLBP), Nestin, and glial fibrillary acidic protein (GFAP) [1]. These qNSCs give rise to the transient and rapidly amplifying neural progenitor cells (NPCs, defined by Nestin and Sox2 expression). The majority of these NPCs differentiate into immature doublecortin-positive (DCX+) neurons (or neuroblasts), which migrate a short distance into the granule cell layer [2, 3]. These neuroblasts mature into NeuN+ neurons and have been shown to integrate into the hippocampal network as fully functional neurons [4]. These new neurons extend their axonal projections along the mossy fiber pathways to form synapses with the CA3 pyramidal neurons of the hippocampus and protrude their dendrites toward the molecular layer to form synapses with neurons in the perforant pathway of the entorhinal cortex [5, 6]. Adult neurogenesis in the hippocampus is suggested to play an important role in adult learning and memory [5–7]; however, most experimental evidence to support this notion has been correlational, and further research is in progress. In the SVZ, the cellular composition and architecture have been illustrated as a three-dimensional interconnected niche consisting of three major cell types [8]. Neuroblasts (type A cells, DCX+) are organized in a network that are ensheathed by the processes of qNSCs (type B cells, Nestin+ and GFAP+) and give rise to rapid amplifying NPCs (type C cells, Nestin+, and GFAP−) [9, 10]. Many of the neuroblasts merge in the anterior and dorsal SVZ, then migrate to the olfactory bulb in a chain configuration through an enclosed path called the rostral migratory stream (RMS) formed by a tunnel of astrocytes [11, 12]. In the olfactory bulb, these neuroblasts develop into functionally mature interneurons that are likely important for processing new information in the olfactory system [13]. Adult qNSCs (type B cells) in the SVZ and the SGZ share basic properties with embryonic radial glia (RG) cells share basic properties, among them are gene expression profiles and long basal processes with elongated/polarized morphology (reviewed by [9, 14]). Adult SVZ NSCs are derived from RG and are believed to retain progenitor function throughout life [15]. Adult SGZ NSCs, on the other hand, may originate from RG, but more experimental evidence is needed. Understanding the origin and the properties of adult NSCs will help to identify the optimal potential of these cells in cell-based therapy.
Despite restricted in vivo neurogenesis, multipotent neural stem/progenitor cells (NSPCs) have been isolated from many regions throughout the adult mammalian brain and utilized for in vitro studies [16]. NSPCs isolated from the rodent fetal brain or adult SVZ, DG, or forebrain can be maintained as multipotent progenitor cells in serum-free media with defined supplemental factors and the presence of the mitogens basic fibroblast growth factor (bFGF or FGF2) and epithelial growth factor (EGF) [6]. Clonal analyses have demonstrated that these NSPCs can be instructed to differentiate into all three major cell lineages of the brain (neurons, astrocytes, and oligodendrocytes) by responding specifically to the exogenous signals administered to the culture [7]. Therefore, the in vitro culture of NSPCs makes not only a good system for studying neurogenesis, but also an excellent source of cells for potential cell-based therapies [17]. Upon transplantation into neurogenic regions of the adult brain, such as the hippocampus and the olfactory bulb, or damaged regions in the CNS, these NSPCs have the ability to differentiate into new neurons based on signals located within the local environment [18–20]. Therefore, the regenerative capacity of not only endogenous NSCs, but also exogenous transplanted NSPCs (described in the later section, Therapeutic Application of Stem Cells), holds great potential for repairing the CNS damaged as a result of stroke, trauma, or neurodegenerative diseases, such as Parkinson’s, Alzheimer’s, and amyotrophic lateral sclerosis.
STROKE
Stroke is one of the leading causes of death and a major cause of disabilities in adults. More than half of stroke victims suffer some type of disability, ranging from different levels of minor weakness in a limb, to a complete loss of mobility in one side of the body. Stroke may also lead to a complete inability to speak [21]. Currently, treatment for stroke requires a stringent rehabilitation program that includes both medical and physical therapy. Nevertheless, two thirds of all survivors will still have some type of difficulty with regular daily activities, including eating, walking, and using their limbs. In this review, we will discuss the effects of stroke-induced damage on the brain, outline the potential mechanisms by which stroke induces neurogenesis, and summarize the current state of stem cell therapies for stroke.
Acute ischemic stroke is caused by cerebral artery occlusion through the loss or the reduction of cerebral blood flow, leading to an infarction of brain tissue. This event triggers two cascades of damage that result in cell death of neurons, astrocytes, and oligodendrocytes in the ischemic region [22]. First, during the initial phase of the infarct, the loss of oxygen or glucose to the brain region results in the failure of cells to conduct their normal physiological cellular functions through mechanisms such as the depletion of intracellular ATP levels, causing these cells to undergo apoptosis. A major cause of neuronal death by oxygen and glucose depletion is through glutamate excitotoxicity, which can result from impaired ion exchange pumps, triggering the reversed extracellular release of glutamate by neurotransmitter transporters [22]. High concentrations of extracellular glutamate act on receptors on the post-synaptic neuron and can lead to calcium influx, failure of the mitochondria, energy depletion, and eventually further neuronal death through apoptosis [22]. Second, some of the damage done to the brain following stroke comes from delayed effects, including the release of nitric oxide, oxygen free radicals, and other reactive oxygen species, which leads to further damage to neurons and the surrounding environment [23]. Besides the harmful effects on neurons, ischemia can damage the integrity of the neurvascular network via release of matrix metalloproteinases (MMPs) and other proteases secreted by endothelial cells that comprise the protective blood-brain barrier (BBB) [23]. The loss of neurovascular structural integrity results in a breakdown of the tight junctions between astrocytes of the brain and endothelial cells of the vascular system, which contributes to cerebral edema in the secondary stage of brain injury. The events described above are important factors to consider not only when treating stroke patients, but also critical issues to be dealt with for cell-based therapies using either endogenous or transplanted stem cells.
A second characteristic of stroke injury is brain inflammation driven by the involvement of peripherally derived cytokines. After brain injury, the BBB is permeable to cells of the immune system, such as mononuclear phagocytes, T-lymphocytes, natural killer cells, and polymorphonuclear leukocytes [24, 25]. All these cells produce and secrete cytokines that contribute to CNS inflammation and gliosis after brain injury. In support of this notion, microvessels in ischemic brain regions are filled with leukocytes, unlike microvessels in the healthy brain, which are clear of inflammatory cells [25]. Ameboid microglia, a form of reactive microglia, can be identified within several hours of ischemia [26]. This form of brain injury is linked with the expression of inflammatory factors, including cytokines (e.g., interluekin (IL)-1 and tumor necrosis factor (TNF)-α) and chemokines (e.g., IL-8, monocyte chemotactic protein (MCP)-1, chemokine (C-C motif) ligand 5, leukotriene B4 (LTB4), and CXCL10). The upregulation of cell adhesion receptors, such as intercellular adhesion molecule (ICAM)-1 and selectins, supports leukocyte adherence to the endothelium [27]. TNF-α and IL-1β predispose or "prime" endothelium for cellular adherence [28]. Additionally, adhesion molecules, such as CD11/CD18 integrins, are also thought to be pivotal in this inflammatory process [29]. The exact nature of the signaling mechanisms in brain inflammation remains unanswered but undoubtedly involves chemotactic cytokines (e.g., chemokines such as TNF-α, IL-1β, and IL-8), as well as adhesion molecules and proteinases, which together promote cell adherence and infiltration and enhance the permeability of brain endothelium [27]. The enhanced SVZ neurogenesis into the damaged region, as well as neurogenic-supporting angiogenesis, is triggered by these cytokines and growth factors secreted into the surrounding environment of the adult neural stem cells (discussed in further detail in the section Stroke-Induced Neurogenesis).
Although some of the molecular targets leading to cell death have been identified, with clinical therapies developed to block these targets, current therapeutics are inefficient, and there is no effective treatment for stroke-induced brain damage. Recombinant tissue plasminogen activator (TPA), a thrombolytic drug agent, is now the only approved agent for treating stroke patients. TPA can break up blood clots during the initial phase of an ischemic stroke, but it also raises the risk of intracranial bleeding [30], perhaps mediated by the increased expression of MMPs; therefore, its success varies in individual patients [30–32]. Currently, clinical trials for the treatment of the acute stages of stroke are focused on a combination of introducing TPA and regulating the neurovascular proteases [32]. However, administration of TPA is time-sensitive, and there can be other complications, such as increased infarct size and brain hemorrhage, which limit the success rate [33]. Therefore, other methods like stem cell therapy for stroke injury are being avidly pursued by researchers.
STROKE-INDUCED NEUROGENESIS
Recent experimental findings raise the possibility that functional improvement after stroke may be achieved through neural replacement by endogenous NSCs residing in the adult brain, such as in the SVZ. Using an embolic middle cerebral artery occlusion (MCAO) model in adult rodents, many studies have shown that, within the first week after focal ischemic insult and pronounced loss of striatal and cortical neurons, there is a major increase in NSC proliferation within the SVZ. Increased NSC proliferation has also been seen in both the hippocampus and the SVZ after trauma, seizures, and global ischemia during the first week after injury, but the rates of proliferation return to normal after several weeks [34, 35]. At two weeks after the injury, newly generated neuroblasts re-route from the SVZ and RMS into the damaged area (up to 2 mm in distance), where some of these cells are found to express mature neuronal markers at later time points [36, 37]. In addition, SVZ-derived neuroblasts can replace damaged neurons after brain injury within the hippocampus, striatum, neocortex, and other damaged regions of the CNS [38–40]. Ultimately, though, the increased number of migrating neuroblasts in the hippocampus seems to remain in the dentate granule layer, where the majority of these cells and other neuroblasts migrated to other regions will not survive [41, 42]. Therefore, injury-induced neurogenesis is suspected of being regulated by extrinsic factors secreted from reactive cells within the infract regions [36, 43]. However, the number of neurons generated from endogenous NSPCs is extremely low (∼0.2% of the striatal cells lost), and the survival of these new neurons in the lesioned area is minimal [44].
Therefore, modulating endogenous NSPCs for brain repair is a critical issue facing stem cell therapy. Comprehending this knowledge will require more research into NSPCs interaction with the surrounding microenvironment, called the stem cell niche. In the next sections of this review, we will discuss some of the key elements in the environment surrounding NSPCs that regulate NSPC properties in the normal and injured brain.
NEURAL STEM CELL NICHE IN THE NORMAL AND INJURED BRAIN
Cell Migration
In recent years, interest in understanding the physiological and pathological processes of stem cell migration has flourished, due largely to the discovery of neural progenitor cell migration in the adult brain under both normal and injured conditions. In both embryonic and adult brains, neural cells demonstrate two unique types of mobility: radial migration and tangential migration (for more details, see Barkho and Zhao, Book Chapter 2010 [45]). In radial migration, newly born neurons in the neocortex migrate along radial glial processes, whereas tangential migration is defined as a non-radial, glia-guided neuronal translocation. Radial migration is seen mainly during embryonic cortical development, as well as in restricted areas of the cerebellum and the olfactory bulb. Also, although tangential migration is found throughout developing brains, it only persists in the RMS of adult brains, where migrating neuroblasts travel long distances through a glial tunnel formed by astrocytes from the SVZ to the olfactory bulb. Upon arrival at the olfactory bulb, neuroblasts switch to radial migration to reach their final destination. Interestingly, such a change of migration pattern from tangential to radial migration also happens in response to cortical injury [46]; therefore, these cells have the capacity to adapt migration patterns in response to changes in their surrounding environment.
Cytokines are a group of signaling molecules that stimulate cellular function through autocrine, paracrine, or endocrine mechanisms [47–49]; chemokines (chemotactic cytokines) are a family of small secreted cytokines defined by the arrangement of the conserved cysteine residues that are known to induce directed chemotaxis of responsive cells [50, 51]. Some chemokines are considered proinflammatory and can be induced during an immune response, whereas others are present to establish homeostasis. Several of these chemokines, such as stromal cell-derived factor 1 (SDF-1α), are known to mediate cell migration during normal angiogenesis, as well as cancer cell metastasis. In the normal CNS, chemokines are typically known for their role in cell migration during brain development. For example, SDF-1α has an obligate role in neuronal migration during the formation of the granule-cell layer of the cerebellum [52]. Just recently, SDF-1α signaling was found to play a role in directing the migration of neuroblast within the RMS [53]. However, upon neuroinflammatory injury, as occurs in multiple sclerosis and stroke, chemokines are produced by reactive cells, such as astrocytes and immune cells within the lesioned area. For instance, in the mouse ischemic model, astrocytes and endothelial cells express higher levels of chemokines, such as SDF-1α [54] and VEGF [55]. These injury-induced chemokines are reported to attract inflammatory cells and cause cell death in diseased or injured regions; however, chemokines can also direct NSCs and neuroblasts to re-route toward an injured region [56]. We and others have shown that NSPCs express the SDF-1α receptor, CXCR4 [53, 57, 58], which regulates the migration of these cells toward a stroke-induced injury (for more details, see section Stroke-Induced Neurogenesis). Several other factors, such as monocyte chemoattractant protein (MCP)-1 and macrophage inflammatory protein (MIP)-1 [59, 60], are also upregulated in injured brains and are believed to play a role in stimulating neurogenesis after stroke [61]. CNS injuries drastically change the types and concentrations of cytokines and chemokines in the brain, thereby significantly altering the environment that NSCs encounter (for more details about cell migration, the role of cytokines and chemokines, and the function of MMPs in normal and damaged brains, see Barkho and Zhao 2010 [45]). The brief summary on cell migration here aims to give a better understanding of the plasticity of adult neural stem cells. For the remainder of the discussion, we will focus on the mechanisms underlying cell fate and the migration of these cells in response to environmental cues.
Extracellular Matrix
The extracellular matrix (ECM) contains a complex set of molecules that are tightly regulated. In the nervous system, the ECM plays a pivotal role in neural development, including cell survival, migration, differentiation, axon growth, and synapse formation [62]. Some of these molecules are transiently expressed at particular time points during development and downregulated during adulthood. The major ECM molecules of the CNS are fibrous matrix proteins (e.g., collagens, fibronectin, and vitronectin), basement membrane proteins (e.g., laminin), tenascins, and proteoglycans. The interactions between cells and ECM molecules are dynamic and mediated via cellular receptors and molecules. These interactions promote cell adhesion, activate the intracellular signaling pathways, and modulate the activity of several growth factors [62]. The cell surface receptors for the ECM are the integrins, a large family of α- and β-subunits that can form over 20 different receptors [63]. These receptors can interact with many ligands and cell surface molecules, such as tyrosine kinases (RTKs), G-protein coupled receptors (GPCRs), growth factor receptors, L1-CAM, or members of the tetraspanin family of proteins. When integrin receptors are directly or indirectly activated, they transduce signals through several pathways, including focal adhesion kinase (FAK), the Src family kinase Fyn, MAP kinase, protein phosphatases, SH2-SH3 adaptors, Rho-family GTPases, and phospholipid mediators. The activation of these signaling cascades ultimately results in a number of changes in the characteristics of integrins, such as plasma membrane localization, internalization, ligand affinity, intracellular protein effectors, interaction with the cytoskeleton [64]. These changes can also directly affect the dynamics of cell-cell and cell-ECM interactions. For example, after ischemic injury, DCX+ neuroblasts are in close proximity to the endothelial cells of vasculature [65], suggesting a possible mechanism by which the neuroblasts migrate along the endothelial cells trail to reach the lesioned area. Since the basal membrane of the vasculature consists of laminin, it has been shown that MMPs secreted in the environment of migrating neuroblasts degrade laminin to mediate cell migration [66, 67]. Consistent with this notion, migrating neuroblasts within the RMS are found to express the laminin receptor, β1 integrin, and deficiency in this integrin inhibits the interaction among neighboring neuroblasts and abolishes their migration towards the olfactory bulb [68]. Furthermore, our laboratory and other investigators have found that laminin is the most effective ECM component for mediating the cell migration of NSPCs in in vitro migration assays [58, 69].
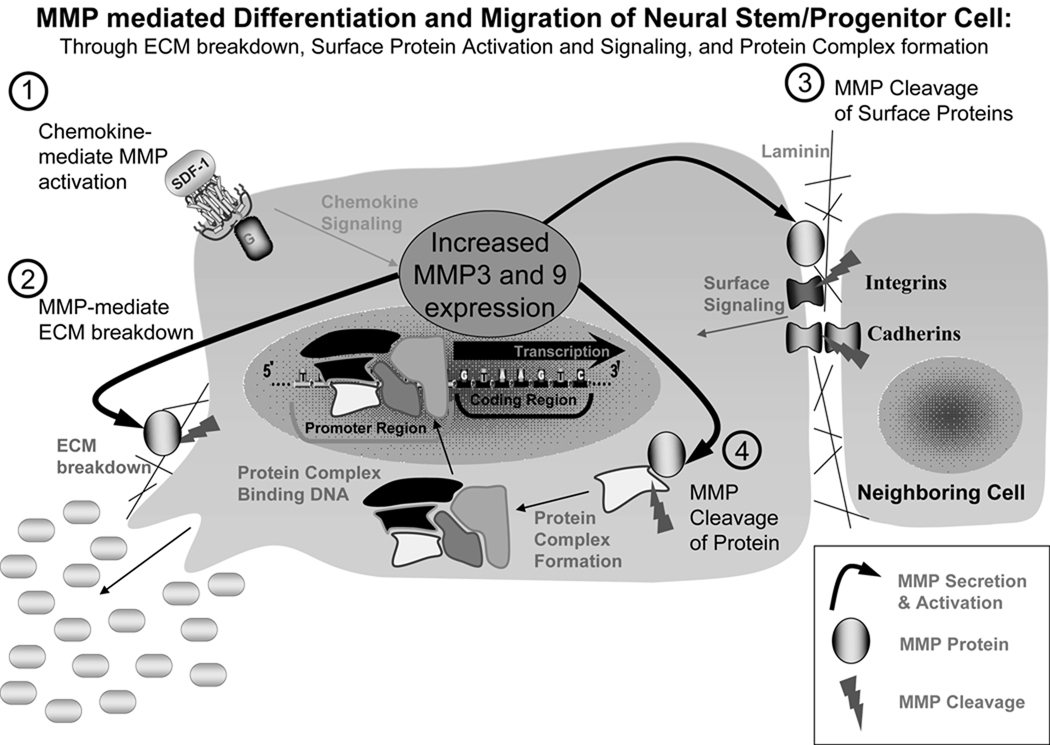
Chemokine-induced cell migration requires the remodeling of the ECM. The chemotaxic functions of stromal cell-derived factor-1 (SDF-1) and vascular endothelial growth factor (VEGF) are known to be mediated by the activation of MMPs [70] [Fig. (1)]. MMPs are a family of enzymes that collectively are able to degrade all the components of the ECM [71, 72]. MMPs participate in a host of important physiological processes, including CNS development, embryological remodeling, wound healing, and angiogenesis, and their role in cancer cell metastasis has been studied extensively [73, 74]. Although MMPs have been investigated for their involvement in ischemic brain injuries, such as neuronal death and blood-brain barrier breakdown [75], their role in the neurogenic response of adult NSPCs after ischemic insults has only recently been considered. Neuroblast migration is known to require ECM remodeling [76], and MMP-9 immunoreactivity is colocalized with migrating neuroblasts [67]. Furthermore, MMP-2 and MMP-9 expressed by endothelial cells promote neuroblast migration [66]. This notion has been suggested to be regulated through the degradation of the ECM via extrinsic activation of MMPs [Fig. (1)]. Furthermore, we have found that MMP-3 and MMP-9 not only play a role in the migration of the neuroblasts, but are also involved in NSPC differentiation. Acute knockdown of MMP-3 or MMP-9 in NSPCs using siRNAs results in reduced neuronal differentiation in response to chemokines, such as SDF-1 and VEGF [58]. However, the functions of these endogenous MMPs in regulating adult NSPC differentiation, proliferation, survival, and migration are still unclear. It has been suggested that MMPs, with their ability to cleave the interaction between integrins expressed on the cell surface and the ECM, can regulate the differentiation of adult NSPCs [77] [Fig. (1)]. Therefore, future studies in this area will greatly enhance our understanding of the roles the ECM and proteases play in brain injuries and repair.
Figure 1. Potential roles of MMPs in adult NSPC migration and differentiation.
1) Extracellular chemokines, such as SDF-1, from either the surrounding niche within a normal brain or an injured region, signal the activation of MMP-3 and MMP-9. 2) MMPs can be secreted locally to promote the breakdown of the ECM and drive the migration and differentiation of the NSPC toward the concentration gradient of chemokines. 3) MMP can cleave cell surface proteins, such as integrins and cadherins, to stimulate a signaling cascade to activate the pathways for migration and differentiation of NSPCs. 4) MMP-3 and MMP-9 have been suggested to cleave intracellular proteins that participate in the formation of transcriptional complexes or degrading protein inhibitors, which will drive the expression of specific genes involved in the migration and differentiation of NSPCs.
Cell-Cell Contact and Communication
The gap junction proteins and cadherins (members of the adherens junction proteins) are also known to play a role in NSPC proliferation and differentiation [78, 79]; however, the function of these proteins in the neurogenic response to ischemic injury has not been investigated. Gap junctions play critical roles during embryogenesis, such as providing cell-cell communication for signaling pathways and allowing the intercellular transfer of ions, second messengers, and morphogens. Impairment or alteration of gap junctions may lead to changes in the stem cell niche and push NSPCs to leave the proliferative state and initiate differentiation [80, 81]. For example, Connexin 43 (Cx43), a gap junction protein, is expressed by embryonic NSPCs to form contact between the stem cells and astrocytes, and Cx43 phosphorylation regulates the differentiation of NSPCs [82]. The expression switch of Cx43 to Cx33 or Cx40 on the cell surface of embryonic hippocampal progenitor cells is also suggested to promote neuronal differentiation [83]. Recently, the first evidence of gap junctions in adult neurogenesis has been uncovered. The Cx43 protein was found to be expressed in the radial glial-like cells in the adult hippocampus, and the ablation of Cx43 in these cells led to reduced adult neurogenesis [84]. In addition, Gap junctions have been shown to mediate cell-cell communication through the expression of α1 connexins, and either gain or loss of function of this protein results in altered neural crest cell migration during embryonic development [85]. Therefore, cell-cell communication through gap junctions is crucial for stem cell migration and differentiation, and although no evidence has been found to connect gap junctions with stroke-induced neurogenesis, grasping the mechanisms will be significant to understand cell damage after injury and the potential of repair.
Cadherins form cell-cell interactions through homotypic bonds across the extracellular space, and the cleavage of cadherin interaction between cells through β-catenin signaling pathway activation is clearly one of the molecular mechanisms behind cell differentiation [86, 87]. For example, cadherins are known to be expressed by cultured adult NSPCs and are suspected of regulating the properties of the adult NSPC niche [88]. This process involves cleavage by MMPs and the ADAMs (A Disintegrin And Metalloprotease) of cadherin bonds, which causes a conformational change in these molecules and the release of intracellular β-catenin, which is known to play a role in maintaining adult NSPCs within their niche [79]. A recent report shows that proliferative NSPCs in the hippocampus are clustered together and are associated with each other via a cell adhesion molecule, N-cadherin. As these cells differentiate and migrate out of the cluster, the N-cadherin expression/β-catenin signaling is reduced, and the expression of E-cadherin is upregulated [89]. These experiments suggest that the NSPCs have a specific cellular arrangement and intercellular communication with neighboring cells, allowing these cells to either stay in the stem cell state within the cluster or to differentiate and migrate as they move out of the stem cell cluster. As described earlier, stroke-induced chemokines promote adult NSPCs to express MMPs, and these MMPs are secreted into the microenvironment of the stem cell niche. Gap junctions and cadherins are direct targets of cleavage by these MMPs, leading to activation of cell surface proteins and downstream signaling events that promote the migration and differentiation of adult NSPCs. Further investigation is underway to determine the role of both gap junctions and cadherins in the NSPC niche, results of which will help understand the mechanisms that regulate adult NSPCs and their surroundings in response to injury.
THERAPEUTIC APPLICATION OF STEM CELLS
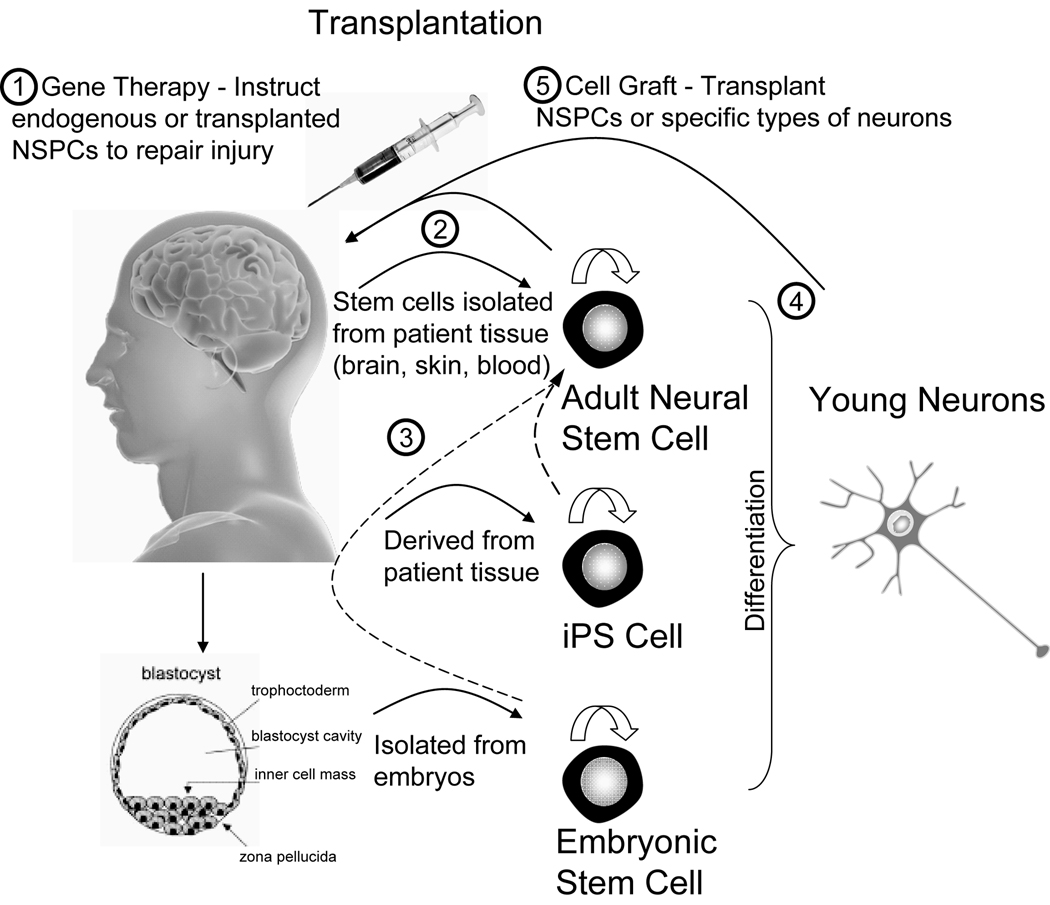
Since short-term pharmaceutical treatments for brain injury has had limited success, stem cell-based therapies are now the subject of intense investigation. The discovery of injury-induced neurogenesis holds the opportunity for neuronal replacement by endogenous NSPCs residing in the adult brain for functional improvement after stroke [54]. This is supported by recent findings of increased neurogenesis in rodent models after brain injury, as described above (see section, Stroke-Induced Neurogenesis; [6]). Although NSPC-based therapies for brain disorders, such as stroke, Parkinson's disease, Huntington’s disease, multiple sclerosis, and spinal cord injury, have been vigorously pursued, most have been tested only in experimental animal models. There are important issues that must be resolved before such promising therapies can be used in humans. Here we will discuss the optimal cell sources for transplantation (embryonic vs. adult; endogenous vs. exogenous cells), as well as the best route for cell administration (local vs. systemic transplantations); see Fig. (2). Early in stem cell therapy studies, researchers tried several experimental strategies for brain repair, such as promoting endogenous NSPCs to home into a damaged region and instructing them to differentiate into the desired cell types using growth factors or signaling molecules. Several key methods have even investigated cell delivery routes and directed cell differentiation using gene manipulation of stem cells or modification of the local stem cell niche (refer to reviews [90–92] for more details). More recently, studies have aimed to transplant NSPCs obtained from patient-derived adult NSPCs, induced pluripotent stem (iPS) cells, or embryonic stem (ES) cells [93] [Fig. (2)]. Although we have gained valuable knowledge from recent experiments, these attempts have had limited success at actually improving function and have yielded insufficient evidence for a definitive stem cell-based procedure [94]. Therefore, in this section, we will discuss different potential applications of different types of stem cells for stroke-induced injury repair.
Figure 2. Potential of stem cells for cell-based therapies for brain injuries.
1) Growth factors and gene therapies can potentially be used to instruct either endogenous adult NSPCs or transplanted stem cells to repair the injured brain. 2) Cells might be isolated from patient tissues (e.g., biopsied from brain, skin, or blood), and these cells then differentiated into either NSPCs or neurons that are used for autologously grafting into an individual patients. 3) Both embryonic stem cells (ES cells, derived from fertilized embryos) and induced pluripotent stem cells (iPS cells, reprogrammed from patient somatic cells to prevent graft rejection) can be directed to generate NSPCs (dotted lines), which can then be used for transplantation. 4) Like NSPCs, ES cells and iPS cells can be differentiated into young neurons; however, how to direct differentiation into pure and specific types of new neurons remains unknown. 5) Other studies have focused on grafting specific types of neurons or an intermediate cell population (precursor to the desired cell type) to replace lost neurons by directed differentiation of different types of stem cells (such as CD34+ or HSC). Finally, it is important to determine whether a combination of gene therapy and cell transplantation will further enhance brain repair and the recovery of function for patients.
Endogenous Stem Cell Therapy
Endogenous adult NSPCs residing in the neurogenic niche may be beneficial for brain repair, as a result of their ability to support neurogenesis and gliogenesis during adulthood. However, after a variety of brain injuries as described above, the microenvironment surrounding the NSPCs changes drastically due to the reactivity of neighboring cells, such as astrocytes, endothelial cells, and microglia. The release of cytokines and chemokines from these cells changes the microenvironment and therefore the niche for endogenous adult NSPCs. For example, as mentioned above, adult NSPCs in the SVZ initially experience a massive proliferative response within the first week of ischemic stroke in response to an increase of TNF-α [95]. However, the majority of these cells either fail to survive or differentiate into glial cells stimulated by the high concentrations of inflammatory cytokines secreted by neighboring cells.
Even though endogenous adult NSPCs have the capacity to replace lost neurons in animal models of cerebral ischemia [44, 96, 97], the potential for functional recovery in humans remains uncertain. In the rodent stroke model, the efficiency of endogenous adult NSPCs at generating new neurons that will survive and repair the damaged area is extremely low [36]; therefore, one current strategy focuses on promoting NSPC recruitment to the injured area using chemokines and growth factors within the lesioned area [Fig (2)]. These factors can attract large numbers of endogenous NSPCs to the injured region, and some of these may participate in brain regeneration. Growth factors or cytokines, such as BDNF [98] and VEGF, [99] have been used, with some promise of success; however, the functional recovery of animals was limited. More recently, it has been suggested that a combination of these factors might have a beneficial outcome in promoting endogenous stem cell therapy and improve brain function [100, 101]. For example, simultaneous promotion of neurogenesis and angiogenesis could result in a better therapeutic outcome. In the penumbra area of stroke brains, an increase of microvessel density has been observed, and an increase in angiogenesis has been correlated with longer patient survival rates. In rodent, endothelial cell proliferation triggered by elevated expression of VEGF from astrocytes and neurons is seen as early as one day after brain injury and is suggested to enhance angiogenesis and decrease the infarct region [102–104]. Results have also shown that treatment with erythropoietin (EPO) leads to a significant increase in VEGF and BDNF levels in the brain in a rodent stroke model, enhancing neurogenesis and angiogenesis, as well as improving neurological function after stroke [105]. Additional studies have found that the coupling of these processes promotes endogenous NSPC neurogenesis in brain injury, indicating that both angiogenesis and neurogenesis might have to work simultaneously to support the integration of endogenous NSPCs [106, 107]. Nevertheless, VEGF treatment and increased angiogenesis can also lead to greater permeability of the BBB after the acute stages of stroke insult [104], making it important to define an optimal time period and specific dosage for any cytokine treatment. Thus, the most recent course pursued by researchers is to define the molecular mechanisms underlying the beneficial effects of angiogenesis on endogenous adult NSPCs and neurogenesis to repair brain injury. From a better understanding of the basic biology of these adult NSPCs, we will be able to develop more effective therapeutic strategies using these cells.
Exogenous Embryonic and Adult Neural Stem Cell Therapy
As described above, in brain injuries, either acute or chronic effects of cytokines and chemokines may alter the NSPC niche, perturb stem cell properties, and interfere with the capability of endogenous NSPCs to repair the brain. Currently, the use of endogenous adult NSPCs has led to only limited recovery for patients after stroke injury. Therefore, exogenous stem cell transplantation is now being intensely investigated. Isolated embryonic or adult NSPCs are known to be capable of brain repair because of their abilities to proliferate, migrate, and differentiate after grafting into the adult brain [108]. Recent experiments have shown NSPCs can also be derived from embryonic stem (ES) cells, induced pluripotent stem (iPS) cells, and adult stem cells isolated from different tissue, such as skin and blood [109, 110]. Therefore, in this review we will briefly discuss all these stem cell types, because of their significance to the field and their advantages and disadvantages in therapeutics.
Embryonic Stem (ES) Cells
ES cells are pluripotent cells because they can differentiate into all cell types found in the organs of the human body. Adult stem cells, in contrast, are generally limited to differentiation into the cell types of their tissue of origin. For decades, mixed fetal brain cells have been evaluated as a means to treat Parkinson's and Huntington’s disease [111, 112] and have been transplanted into human patients [113]; however, results in clinical trials were disappointing. The advantage of ES cells is that large numbers of them can be expanded in culture and specific neuronal types can be produced for transplantation, whereas adult stem cells are rare in mature tissues, and methods for expanding them in cell culture remain somewhat primitive [114–116]. This is an important issue, because large numbers of cells are needed for stem cell replacement therapies. The pluripotency of ES cells in generating any type of adult neurons is also an advantage over adult NSPCs. However, despite their great characteristics for therapeutics, ES cells have only recently been approved by the FDA for their first clinical trial, for spinal cord injury. The delay in using these cell lines arises mainly from the ethical considerations, but there are still several key issues to resolve before applying these cells as a complex therapy to the brain. Transplantation studies of ES cells in animal models have shown promising results; however, further knowledge is required before translating these studies to human therapy [117]. The major disadvantages of grafting exogenous ES cells are that introducing these foreign cells into a patient could cause transplantation rejection or the formation of tumors, but whether recipients would reject donor ES cells has not been determined in humans.
Recently, human ES cell differentiation into retinal pigment epithelium (RPE) cells has been a major success and is regarded as a promising therapeutic application for retinal disease [118, 119]. These human ES cell-derived RPE cells have been transplanted into many animal models, resulting in repair of retinal structure and subsequent vision recovery [119, 120]. This has led to a possibility of clinical trials of ES cell therapy for Stargardt's disease, a leading cause of juvenile blindness [121]. ES cells, with their ability to self-renew indefinitely in vitro, maintain the ability to differentiate into all three germ layers, are used not only to differentiate specific cell types for transplantation studies, but are also held as the gold standard for any alternative pluripotent stem cells under study [122]. Thus, the study of ES cells is a critical component of the stem cell field. As discussed below, even though induced pluripotent cells (iPSCs) derived from terminally differentiated somatic cells express genes that are typical of ES cells, iPSCs have not been completely reversed to the state of embryonic development. In the field of regenerative medicine, the development of other stem cell types may be necessary for different diseases and injuries based on clinical needs, but further studies of any such stem cells compared with ES cells are necessary to determine the optimal stem cells for specific diseases.
Induced Pluripotent Stem (iPS) Cells
Within the last four years, a new discovery has led to optimism about the potential of stem cell therapy. Several groups found that the ectopic expression of only four transcription factors (Oct4, Sox2, Myc, and Klf4) was sufficient to reprogram the differentiation state of somatic cells into the pluripotent state of ES cells [123]. In this study, murine embryonic and adult fibroblasts were infected with virus expressing candidate pluripotency genes, followed by selection for the expression of a pluripotent stem cell marker. After a few weeks, ES cell-like pluripotent stem cells, so-called induced pluripotent stem (iPS) cells, were obtained. Other studies have shown that transduction of different sets of pluripotency factors (e.g., Oct4, Sox2, Nanog, and LIN28) [124, 125], as well as certain small molecules [126, 127], could have similar reprogramming effects in both mouse and human cell lines. In all these cases, the iPS cells generated have properties similar to ES cells, in that they can be maintained to self-renew and can be differentiated into every cell type of the organism. This discovery has given fresh impetus to stem cell therapy, because iPS cells can be generated from individual patients. Recently, iPS cells were derived from an 82-year-old patient with the familial form of amyotrophic lateral sclerosis, and were shown to differentiate in vitro into all major cell types of the body, but more specifically into motor neurons expressing HB9 and Islet 1/2 [128]. Therefore, iPS cells represent not only a way to avoid the use of ES cells, but also have the advantage of being patient-derived cells, which can be grafted without immunological rejection, regardless of the age or the origin of the tissue. Nonetheless, the derivation and analyses of iPS cells are in the early stages. Some of the major problems include the extremely low efficiency of reprogramming and the risks of using viral infections to produce iPS cells; tumor formation after transplantation also remains as a major concern. Further knowledge of iPS cells, especially how they compare with ES cells, has become a prerequisite for iPS cell-based stem cell therapy.
Exogenous Adult Stem Cells
As for adult stem cells, hematopoietic stem cells (HSCs) are the only type that have been used to successfully treat human diseases, where they have proved their clinical potential in the treatment of diabetes [129], amyloidosis [130], and some cancers, including renal cell carcinoma [131]. This means they have great potential for stroke treatment. For example, delivery of circulating CD34+ subpopulations from human umbilical cord blood cells into a rodent stroke model can enhance angiogenesis and neurogenesis, with some restoration of cortical tissue, as well as functional recovery [132, 133]. Cells obtained from placental or cord blood possess an additional advantage, in that they require less strict human leukocyte antigen (HLA) matching criteria and improve the likelihood of cell graft reception in patients with donor mismatch [134–136]. Placental or cord blood also contains mesenchymal stem cells (MSCs), which are currently under study for their ability to promote angiogenesis [137–140]. There are also more human clinical stem cells trials underway now for treating other human diseases, including CNS tumors, mastocytosis, and chronic granulomatous disease, to name but a few (www.clinicaltrails.gov).
Transplantation of adult NSPCs has been considered for a variety of human brain injuries, particularly stroke. Though studies of other types of stem cells have generated considerable excitement for exogenous stem cell-based therapy, as discussed above, the utility of these cells will depend largely on our understanding of both endogenous and grafted NSPCs in adult brains [Fig. (2)]. Initial studies have shown the regenerative capacities of both rodent and human embryonic or fetal-derived NSPCs grafted into injured brains [141–143]. In recent years, since isolation of NSPCs from the adult brain has become a standard technique and adult NSPCs are the original precursors of neurons, astrocytes, and oligodendrocytes in the adult brain, transplantation studies have shifted from fetal cells to adult NSPCs for therapeutic applications. For example, one study investigated the effects of adult SVZ-derived NSPCs transplanted into the brains of a rat stroke model at different time points post-experimental MCAO. These grafted NSPCs were pre-labeled with magnetic particles, allowing their migration to be tracked by magnetic resonance imaging. The results showed that grafted adult NSPCs survived and migrated toward the ischemic area, and the treated rats experienced better functional recovery [144]. More recently, researchers have found that human NSPCs transplanted into the adult rodent brain can migrate to the perilesional zone and can proliferate and differentiate into mature neurons after transient forebrain ischemia [145]. Other rodent transplantation studies have also shown the remarkable potential of human NSPCs in treating stroke [146, 147], as well as some other neurological diseases [148, 149]. However, a major disadvantage of adult NSPCs is their limited self-renewal ability in vitro, which challenges our ability to expand and maintain their multipotency in culture for therapeutic applications. Furthermore, isolating adult NSPCs from human patients at the present time is impracticable. Therefore, some transplantation studies have focused on deriving progenitor cells with neuronal differentiation potentials from more accessible patient tissues, such as blood, skin, and hair [109, 110, 150]. Although none of these methods and experiments has yielded perfect results, these studies have brought us closer to utilizing adult stem cell-based therapies to repair the brain.
CONCLUSION
As described above, the ability of both transplanted ES cells and adult endogenous progenitor cells to differentiate into the major neural cell types holds out great promise for the use of stem cells to repair injured adult brains. Understanding the molecular basis of stem cell plasticity will help in the quest for more effective therapeutic treatments for neural injuries, such as ischemic stroke, but first we need to resolve several critical issues before cell-based therapies can be explored for human patients. Past research into stroke-induced neurogenesis has left us with challenging questions, such as how to precisely control the migration and differentiation of transplanted or endogenous stem cells. In addition, several important issues remain to be decided, such as what should be the source of stem cells, the administration route of stem cells, the combination of trophic factors, an immunosuppression method to prevent graft rejection, and the prevention of tumor formation. To further our knowledge in these areas, we must ensure that a wide range of cell types, from ES cells to adult-derived cells, are well studied. Furthermore, translating studies performed in animals into humans is a critical step in therapeutic development. For example, one challenge that remains is exactly how to administer NSPCs for stroke treatment; therefore, investigators are currently analyzing many methods to determine both the potentials and limitations of all stem cells. These methods include but are not limited to cell delivery routes, gene manipulation of stem cells prior to grafting, and co-grafting with growth factors. Better knowledge of stem cell behavior in both healthy and damaged brains will be critical for optimizing stem cell-based therapies to repair the damaged CNS.
ACKNOWLEDGEMENT
We thank M.C. Wilson, L.A. Cunningham, P.G. McGuire, and R.L. Pfeiffer for helpful discussions and critical reading of the manuscript. Author X.Z. is supported by NIH Grants MH080434, MH078972, and P20RR15636, and B.Z.B. was supported by American Heart Association Pre-doctoral Fellowship 0810123Z.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003 Nov;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 2.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008 Feb;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 4.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002 Feb 28;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008 Feb 22;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 7.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007 Mar;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 8.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997 Jul 1;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001 Apr;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 10.Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009 Jun;19:672–682. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996 Dec 10;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996 Feb 16;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 13.Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002 Apr 1;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004 Dec 14;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gage FH. Mammalian neural stem cells. Science. 2000 Feb 25;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 17.Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000 Oct 26;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- 18.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000 Jun 22;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 19.Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002 Aug 23;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 20.Kolb B, Morshead C, Gonzalez C, et al. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab. 2007 May;27:983–997. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- 21.Prevention CoDCCa. Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morbidity and Mortality Weekly Report. 2001 Feb 23;50:120–125. [PubMed] [Google Scholar]
- 22.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999 Sep;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 23.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003 May;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 24.Tsuchihashi Y, Kitamura T, Fujita S. Immunofluorescence studies of the monocytes in the injured rat brain. Acta Neuropathol. 1981;53:213–219. doi: 10.1007/BF00688024. [DOI] [PubMed] [Google Scholar]
- 25.Giulian D, Chen J, Ingeman JE, George JK, Noponen M. The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J Neurosci. 1989 Dec;9:4416–4429. doi: 10.1523/JNEUROSCI.09-12-04416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.del Zoppo GJ, Milner R, Mabuchi T, et al. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007 Feb;38:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 27.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999 Aug;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Feuerstein G, Wang X, Barone FC. Cytokines in brain ischemia--the role of TNF alpha. Cell Mol Neurobiol. 1998 Dec;18:695–701. doi: 10.1023/A:1020690004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70:427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Nagai N, Yamakawa K, Kawakami J, Lijnen HR, Umemura K. Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood. 2009 Oct 8;114:3352–3358. doi: 10.1182/blood-2009-02-203919. [DOI] [PubMed] [Google Scholar]
- 31.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002 Mar;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 32.Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008 Jun;7:243–253. doi: 10.2174/187152708784936608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilic E, Bahr M, Hermann DM. Effects of recombinant tissue plasminogen activator after intraluminal thread occlusion in mice: role of hemodynamic alterations. Stroke. 2001 Nov;32:2641–2647. doi: 10.1161/hs1101.097381. [DOI] [PubMed] [Google Scholar]
- 34.Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. Feb;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parent JM, Valentin VV, Lowenstein DH. Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J Neurosci. 2002 Apr 15;22:3174–3188. doi: 10.1523/JNEUROSCI.22-08-03174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003 Feb;13:127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- 37.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004 Jul;10 Suppl:S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 38.Jin K, Sun Y, Xie L, et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003 Sep;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 39.Kuge A, Takemura S, Kokubo Y, Sato S, Goto K, Kayama T. Temporal profile of neurogenesis in the subventricular zone, dentate gyrus and cerebral cortex following transient focal cerebral ischemia. Neurol Res. 2009 Nov;31:969–976. doi: 10.1179/174313209X383312. [DOI] [PubMed] [Google Scholar]
- 40.Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 41.Takasawa K, Kitagawa K, Yagita Y, et al. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2002 Mar;22:299–307. doi: 10.1097/00004647-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Tonchev AB, Yamashima T, Sawamoto K, Okano H. Enhanced proliferation of progenitor cells in the subventricular zone and limited neuronal production in the striatum and neocortex of adult macaque monkeys after global cerebral ischemia. J Neurosci Res. 2005 Sep 15;81:776–788. doi: 10.1002/jnr.20604. [DOI] [PubMed] [Google Scholar]
- 43.Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006 Jan;26:1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- 44.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002 Sep;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 45.Barkho B, Zhao X. Neural stem cell migration: Roles of chemokines and proteases. In: Jin K, editor. Adult Neurogenesis and Central Nervous System Diseases. Research Signpost; 2010. pp. 65–90. [Google Scholar]
- 46.Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004 Jan 23;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 47.Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol. 2005 Apr;18:117–123. doi: 10.1097/01.wco.0000162851.44897.8f. [DOI] [PubMed] [Google Scholar]
- 48.Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008 May 1;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- 49.Vitkovic L, Bockaert J, Jacque C. "Inflammatory" cytokines: neuromodulators in normal brain? J Neurochem. 2000 Feb;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- 50.Laing KJ, Secombes CJ. Chemokines. Dev Comp Immunol. 2004 May 3;28:443–460. doi: 10.1016/j.dci.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Laing KJ, Secombes CJ. Trout CC chemokines: comparison of their sequences and expression patterns. Mol Immunol. 2004 Jul;41:793–808. doi: 10.1016/j.molimm.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 52.Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999 Nov;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- 53.Kokovay E, Goderie S, Wang Y, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. Aug 6;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thored P, Arvidsson A, Cacci E, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006 Mar;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 55.Zhang ZG, Zhang L, Tsang W, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002 Apr;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Wiltrout C, Lang B, Yan Y, Dempsey RJ, Vemuganti R. Repairing brain after stroke: a review on post-ischemic neurogenesis. Neurochem Int. 2007 Jun;50:1028–1041. doi: 10.1016/j.neuint.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004 Apr 1;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- 58.Barkho BZ, Munoz AE, Li X, Li L, Cunningham LA, Zhao X. Endogenous Matrix Metalloproteinase (MMP)-3 and MMP-9 Promote the Differentiation and Migration of Adult Neural Progenitor Cells in Response to Chemokines. Stem Cells. 2008 Sep 25; doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JS. Cytokines and adhesion molecules in stroke and related diseases. J Neurol Sci. 1996 May;137:69–78. doi: 10.1016/0022-510x(95)00338-3. [DOI] [PubMed] [Google Scholar]
- 60.Otto VI, Gloor SM, Frentzel S, et al. The production of macrophage inflammatory protein-2 induced by soluble intercellular adhesion molecule-1 in mouse astrocytes is mediated by src tyrosine kinases and p42/44 mitogen-activated protein kinase. J Neurochem. 2002 Mar;80:824–834. doi: 10.1046/j.0022-3042.2001.00748.x. [DOI] [PubMed] [Google Scholar]
- 61.Hallbergson AF, Gnatenco C, Peterson DA. Neurogenesis and brain injury: managing a renewable resource for repair. J Clin Invest. 2003 Oct;112:1128–1133. doi: 10.1172/JCI20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venstrom KA, Reichardt LF. Extracellular matrix. 2: Role of extracellular matrix molecules and their receptors in the nervous system. Faseb J. 1993 Aug;7:996–1003. doi: 10.1096/fasebj.7.11.8370483. [DOI] [PubMed] [Google Scholar]
- 63.Takada Y, Kamata T, Irie A, Puzon-McLaughlin W, Zhang XP. Structural basis of integrin-mediated signal transduction. Matrix Biol. 1997 Oct;16:143–151. doi: 10.1016/s0945-053x(97)90002-0. [DOI] [PubMed] [Google Scholar]
- 64.Schmid RS, Anton ES. Role of integrins in the development of the cerebral cortex. Cereb Cortex. 2003 Mar;13:219–224. doi: 10.1093/cercor/13.3.219. [DOI] [PubMed] [Google Scholar]
- 65.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006 Dec 13;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, Zhang ZG, Zhang RL, et al. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006 May 31;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SR, Kim HY, Rogowska J, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006 Mar 29;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belvindrah R, Hankel S, Walker J, Patton BL, Muller U. Beta1 integrins control the formation of cell chains in the adult rostral migratory stream. J Neurosci. 2007 Mar 7;27:2704–2717. doi: 10.1523/JNEUROSCI.2991-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kearns SM, Laywell ED, Kukekov VK, Steindler DA. Extracellular matrix effects on neurosphere cell motility. Exp Neurol. 2003 Jul;182:240–244. doi: 10.1016/s0014-4886(03)00124-9. [DOI] [PubMed] [Google Scholar]
- 70.Pufe T, Harde V, Petersen W, Goldring MB, Tillmann B, Mentlein R. Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J Pathol. 2004 Mar;202:367–374. doi: 10.1002/path.1527. [DOI] [PubMed] [Google Scholar]
- 71.Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002 Oct;14:624–632. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- 72.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003 Jul;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 73.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001 Nov;11:S37–S43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mannello F, Tonti GA, Bagnara GP, Papa S. Role and function of matrix metalloproteinases in the differentiation and biological characterization of mesenchymal stem cells. Stem Cells. 2006 Mar;24:475–481. doi: 10.1634/stemcells.2005-0333. [DOI] [PubMed] [Google Scholar]
- 75.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005 Jun;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 76.Bovetti S, Bovolin P, Perroteau I, Puche AC. Subventricular zone-derived neuroblast migration to the olfactory bulb is modulated by matrix remodelling. Eur J Neurosci. 2007 Apr;25:2021–2033. doi: 10.1111/j.1460-9568.2007.05441.x. [DOI] [PubMed] [Google Scholar]
- 77.Streuli C. Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol. 1999 Oct;11:634–640. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 78.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 79.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003 Oct;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 80.Giaume C, Venance L. Gap junctions in brain glial cells and development. Perspect Dev Neurobiol. 1995;2:335–345. [PubMed] [Google Scholar]
- 81.Dermietzel R, Spray DC. Gap junctions in the brain: where, what type, how many and why? Trends Neurosci. 1993 May;16:186–192. doi: 10.1016/0166-2236(93)90151-b. [DOI] [PubMed] [Google Scholar]
- 82.Duval N, Gomes D, Calaora V, Calabrese A, Meda P, Bruzzone R. Cell coupling and Cx43 expression in embryonic mouse neural progenitor cells. J Cell Sci. 2002 Aug 15;115:3241–3251. doi: 10.1242/jcs.115.16.3241. [DOI] [PubMed] [Google Scholar]
- 83.Rozental R, Morales M, Mehler MF, et al. Changes in the properties of gap junctions during neuronal differentiation of hippocampal progenitor cells. J Neurosci. 1998 Mar 1;18:1753–1762. doi: 10.1523/JNEUROSCI.18-05-01753.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kunze A, Congreso MR, Hartmann C, et al. Connexin expression by radial glia-like cells is required for neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2009 Jul 7;106:11336–11341. doi: 10.1073/pnas.0813160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang GY, Cooper ES, Waldo K, Kirby ML, Gilula NB, Lo CW. Gap junction-mediated cell-cell communication modulates mouse neural crest migration. J Cell Biol. 1998 Dec 14;143:1725–1734. doi: 10.1083/jcb.143.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001 May 4;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 87.Shapiro L. beta-catenin and its multiple partners: promiscuity explained. Nat Struct Biol. 2001 Jun;8:484–487. doi: 10.1038/88532. [DOI] [PubMed] [Google Scholar]
- 88.Lobo MV, Alonso FJ, Redondo C, et al. Cellular characterization of epidermal growth factor-expanded free-floating neurospheres. J Histochem Cytochem. 2003 Jan;51:89–103. doi: 10.1177/002215540305100111. [DOI] [PubMed] [Google Scholar]
- 89.Seki T, Namba T, Mochizuki H, Onodera M. Clustering, migration, and neurite formation of neural precursor cells in the adult rat hippocampus. J Comp Neurol. 2007 May 10;502:275–290. doi: 10.1002/cne.21301. [DOI] [PubMed] [Google Scholar]
- 90.Little L, Healy KE, Schaffer D. Engineering biomaterials for synthetic neural stem cell microenvironments. Chem Rev. 2008 May;108:1787–1796. doi: 10.1021/cr078228t. [DOI] [PubMed] [Google Scholar]
- 91.Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007 Aug;11:381–387. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robertson MJ, Gip P, Schaffer DV. Neural stem cell engineering: directed differentiation of adult and embryonic stem cells into neurons. Front Biosci. 2008;13:21–50. doi: 10.2741/2558. [DOI] [PubMed] [Google Scholar]
- 93.Rossi F, Cattaneo E. Opinion: neural stem cell therapy for neurological diseases: dreams and reality. Nat Rev Neurosci. 2002 May;3:401–409. doi: 10.1038/nrn809. [DOI] [PubMed] [Google Scholar]
- 94.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001 Mar 8;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 95.Katakowski M, Chen J, Zhang ZG, Santra M, Wang Y, Chopp M. Stroke-induced subventricular zone proliferation is promoted by tumor necrosis factor-alpha-converting enzyme protease activity. J Cereb Blood Flow Metab. 2007 Apr;27:669–678. doi: 10.1038/sj.jcbfm.9600390. [DOI] [PubMed] [Google Scholar]
- 96.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002 Dec;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 97.Haas S, Weidner N, Winkler J. Adult stem cell therapy in stroke. Curr Opin Neurol. 2005 Feb;18:59–64. doi: 10.1097/00019052-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 98.Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001 Sep 1;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002 Sep 3;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cairns K, Finklestein SP. Growth factors and stem cells as treatments for stroke recovery. Phys Med Rehabil Clin N Am. 2003 Feb;14:S135–S142. doi: 10.1016/s1047-9651(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 101.Toth ZE, Leker RR, Shahar T, et al. The combination of granulocyte colony-stimulating factor and stem cell factor significantly increases the number of bone marrow-derived endothelial cells in brains of mice following cerebral ischemia. Blood. 2008 Jun 15;111:5544–5552. doi: 10.1182/blood-2007-10-119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000 Mar;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hayashi T, Watabe H, Kudomi N, et al. A theoretical model of oxygen delivery and metabolism for physiologic interpretation of quantitative cerebral blood flow and metabolic rate of oxygen. J Cereb Blood Flow Metab. 2003 Nov;23:1314–1323. doi: 10.1097/01.WCB.0000090506.76664.00. [DOI] [PubMed] [Google Scholar]
- 104.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000 Oct;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004 Jul;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 106.Arai K, Jin G, Navaratna D, Lo EH. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J. 2009 Sep;276:4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007 Feb;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 108.Goh EL, Ma D, Ming GL, Song H. Adult neural stem cells and repair of the adult central nervous system. J Hematother Stem Cell Res. 2003 Dec;12:671–679. doi: 10.1089/15258160360732696. [DOI] [PubMed] [Google Scholar]
- 109.Morrison SJ. Stem cell potential: can anything make anything? Curr Biol. 2001 Jan 9;11:R7–R9. doi: 10.1016/s0960-9822(00)00033-6. [DOI] [PubMed] [Google Scholar]
- 110.Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat Med. 2001 Apr;7:393–395. doi: 10.1038/86439. [DOI] [PubMed] [Google Scholar]
- 111.Lindvall O. Clinical application of neuronal grafts in Parkinson's disease. J Neurol. 1994 Dec;242:S54–S56. doi: 10.1007/BF00939243. [DOI] [PubMed] [Google Scholar]
- 112.Fisher LJ, Gage FH. Intracerebral transplantation: basic and clinical applications to the neostriatum. FASEB J. 1994 May;8:489–496. doi: 10.1096/fasebj.8.8.8181667. [DOI] [PubMed] [Google Scholar]
- 113.Langston JW. The promise of stem cells in Parkinson disease. J Clin Invest. 2005 Jan;115:23–25. doi: 10.1172/JCI24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Srivastava AS, Malhotra R, Sharp J, Berggren T. Potentials of ES cell therapy in neurodegenerative diseases. Curr Pharm Des. 2008;14:3873–3879. doi: 10.2174/138161208786898617. [DOI] [PubMed] [Google Scholar]
- 115.Menendez P, Wang L, Bhatia M. Genetic manipulation of human embryonic stem cells: a system to study early human development and potential therapeutic applications. Curr Gene Ther. 2005 Aug;5:375–385. doi: 10.2174/1566523054546198. [DOI] [PubMed] [Google Scholar]
- 116.Shufaro Y, Reubinoff BE. Therapeutic applications of embryonic stem cells. Best Pract Res Clin Obstet Gynaecol. 2004 Dec;18:909–927. doi: 10.1016/j.bpobgyn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 117.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006 May;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 118.Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009;4:811–824. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- 119.Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009 Oct 2;5:396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 120.Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009 Sep;27:2126–2135. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- 121.Garber K. Biotech in a blink. Nat Biotechnol. Apr;28:311–314. doi: 10.1038/nbt0410-311. [DOI] [PubMed] [Google Scholar]
- 122.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. Jun 10;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 124.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007 Dec 21;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 125.Muller T, Fleischmann G, Eildermann K, et al. A novel embryonic stem cell line derived from the common marmoset monkey (Callithrix jacchus) exhibiting germ cell-like characteristics. Hum Reprod. 2009 Jun;24:1359–1372. doi: 10.1093/humrep/dep012. [DOI] [PubMed] [Google Scholar]
- 126.Lyssiotis CA, Foreman RK, Staerk J, et al. Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc Natl Acad Sci U S A. 2009 Jun 2;106:8912–8917. doi: 10.1073/pnas.0903860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008 Nov;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 128.Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008 Aug 29;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 129.Skyler JS. Cellular therapy for type 1 diabetes: has the time come? Jama. 2007 Apr 11;297:1599–1600. doi: 10.1001/jama.297.14.1599. [DOI] [PubMed] [Google Scholar]
- 130.Seldin DC, Anderson JJ, Skinner M, et al. Successful treatment of AL amyloidosis with high-dose melphalan and autologous stem cell transplantation in patients over age 65. Blood. 2006 Dec 1;108:3945–3947. doi: 10.1182/blood-2006-06-029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Childs R, Chernoff A, Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000 Sep 14;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 132.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004 Aug;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Peterson DA. Umbilical cord blood cells and brain stroke injury: bringing in fresh blood to address an old problem. J Clin Invest. 2004 Aug;114:312–314. doi: 10.1172/JCI22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Haylock DN, Nilsson SK. Expansion of umbilical cord blood for clinical transplantation. Curr Stem Cell Res Ther. 2007 Dec;2:324–335. doi: 10.2174/157488807782793745. [DOI] [PubMed] [Google Scholar]
- 135.Ballen KK. New trends in umbilical cord blood transplantation. Blood. 2005 May 15;105:3786–3792. doi: 10.1182/blood-2004-10-4125. [DOI] [PubMed] [Google Scholar]
- 136.Ballen KK. Advances in umbilical cord blood transplantation. Curr Stem Cell Res Ther. 2006 Sep;1:317–324. doi: 10.2174/157488806778226858. [DOI] [PubMed] [Google Scholar]
- 137.Liao W, Xie J, Zhong J, et al. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation. 2009 Feb 15;87:350–359. doi: 10.1097/TP.0b013e318195742e. [DOI] [PubMed] [Google Scholar]
- 138.Bieback K, Kluter H. Mesenchymal stromal cells from umbilical cord blood. Curr Stem Cell Res Ther. 2007 Dec;2:310–323. doi: 10.2174/157488807782793763. [DOI] [PubMed] [Google Scholar]
- 139.Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006 Nov;24:2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- 140.Cho GW, Koh SH, Kim MH, et al. The neuroprotective effect of erythropoietin-transduced human mesenchymal stromal cells in an animal model of ischemic stroke. Brain Res. Sep 24;1353:1–13. doi: 10.1016/j.brainres.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 141.Aoki H, Onodera H, Yae T, Jian Z, Kogure K. Neural grafting to ischemic CA1 lesions in the rat hippocampus: an autoradiographic study. Neuroscience. 1993 Sep;56:345–354. doi: 10.1016/0306-4522(93)90336-e. [DOI] [PubMed] [Google Scholar]
- 142.Farber SD, Onifer SM, Kaseda Y, et al. Neural transplantation of horseradish peroxidase-labeled hippocampal cell suspensions in an experimental model of cerebral ischemia. Prog Brain Res. 1988;78:103–107. doi: 10.1016/s0079-6123(08)60272-1. [DOI] [PubMed] [Google Scholar]
- 143.Kelly S, Bliss TM, Shah AK, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004 Aug 10;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang ZG, Jiang Q, Zhang R, et al. Magnetic resonance imaging and neurosphere therapy of stroke in rat. Ann Neurol. 2003 Feb;53:259–263. doi: 10.1002/ana.10467. [DOI] [PubMed] [Google Scholar]
- 145.Wennersten A, Meier X, Holmin S, Wahlberg L, Mathiesen T. Proliferation, migration, and differentiation of human neural stem/progenitor cells after transplantation into a rat model of traumatic brain injury. J Neurosurg. 2004 Jan;100:88–96. doi: 10.3171/jns.2004.100.1.0088. [DOI] [PubMed] [Google Scholar]
- 146.Olstorn H, Moe MC, Roste GK, Bueters T, Langmoen IA. Transplantation of stem cells from the adult human brain to the adult rat brain. Neurosurgery. 2007 Jun;60:1089–1098. doi: 10.1227/01.NEU.0000255461.91892.0D. discussion 98–9. [DOI] [PubMed] [Google Scholar]
- 147.Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003 Sep;34:2258–2263. doi: 10.1161/01.STR.0000083698.20199.1F. [DOI] [PubMed] [Google Scholar]
- 148.Takeuchi H, Natsume A, Wakabayashi T, et al. Intravenously transplanted human neural stem cells migrate to the injured spinal cord in adult mice in an SDF-1- and HGF-dependent manner. Neurosci Lett. 2007 Oct 16;426:69–74. doi: 10.1016/j.neulet.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 149.Lee ST, Chu K, Park JE, et al. Intravenous administration of human neural stem cells induces functional recovery in Huntington's disease rat model. Neurosci Res. 2005 Jul;52:243–249. doi: 10.1016/j.neures.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 150.Le Belle JE, Svendsen CN. Stem cells for neurodegenerative disorders: where can we go from here? BioDrugs. 2002;16:389–401. doi: 10.2165/00063030-200216060-00001. [DOI] [PubMed] [Google Scholar]